Abstract
Circadian rhythms are ~24-hour cycles of physiology and behavior that are synchronized to environmental cycles, such as the light-dark cycle. During the 20th century, most research focused on establishing the fundamental properties of circadian rhythms and discovering circadian pacemakers that were believed to reside in the nervous system of animals. During this time, studies that suggested the existence of circadian oscillators in peripheral organs in mammals were largely dismissed. The discovery of a single-locus circadian pacemaker in the nervous system of several animals affirmed the single-oscillator model of the circadian system. However, the discovery of the genes that constituted the molecular timekeeping system provided the tools for demonstrating the existence of bona fide circadian oscillators in nearly every peripheral tissue in animals, including rodents, in the late 1990s and early 2000s. These studies led to our current understanding that the circadian system in animals is a hierarchical multi-oscillatory network, composed of master pacemaker(s) in the brain and oscillators in peripheral organs. Further studies showed that altering the temporal relationship between these oscillators by simulating jet-lag and metabolic challenges in rodents caused adverse physiological outcomes. Herein we review the studies that led to our current understanding of the function and pathology of the hierarchical multi-oscillator circadian system.
Keywords: peripheral clock, multi-oscillatory, circadian system, mammals, Drosophila, rhythms
Introduction
Circadian rhythms, which are 24-hour cycles of behavior and physiology (e.g. sleep/wake and core body temperature cycles), are ubiquitous and observed in most organisms. Before the 1960s, scientists established the three fundamental properties of circadian rhythms. First, the circadian rhythm must be self-sustained and free-run with a period of ~24 hours (i.e. the 24h rhythm persists in constant conditions). Second, the circadian rhythm should entrain to (be synchronized by) environmental cycles, such as the light-dark cycle. Third, the circadian rhythm should maintain a ~24h period across a physiological range of temperatures (i.e. it is temperature-compensated). After these formal properties were established, the next goal was to discover the locus of the self-sustained circadian oscillator.
During the 1960s and 1970s, scientists searched for the location of the circadian pacemaker in multicellular organisms. At this time, it was believed that a pacemaker(s) located in the brain or in a neuroendocrine gland drives overt circadian rhythms in behavior and physiology. The approaches used were to lesion, transplant, and culture candidate pacemaker tissues. For an organ to qualify as the circadian pacemaker, it had to adhere to the fundamental circadian properties. Lesioning should abolish the overt circadian rhythms and transplantation of that tissue should restore the circadian rhythms. Moreover, the tissue must express a circadian rhythm in vitro in constant conditions. Using these methods, the locus of the circadian pacemaker was identified in the central nervous system of several animals—the optic lobe in cockroaches (lesion [1], transplant [2], in vitro [3]), the pineal gland in house sparrows (lesion [4], transplant [5,6]) and the suprachiasmatic nucleus (SCN) of the hypothalamus in rodents (lesion [7,8], in vivo isolation [9], transplant [10-13], in vitro [14-16]). It was later shown that the pacemakers in non-mammalian vertebrate species are distributed in a circadian axis and include the retina, SCN, and pineal gland. The dominant pacemaker in the axis varies in each species [17-19].
Although the single-pacemaker model of the circadian system was the prevailing view in the 20th century, scientists were also making discoveries suggesting that circadian oscillators could be located in peripheral tissues. This hierarchical multi-oscillator view of the circadian system was not widely accepted. More than 40 years after the initial discoveries that implicated peripheral tissues as circadian clocks, it is now dogma in the field of chronobiology that the circadian system is a hierarchical multi-oscillator network of circadian clocks. Herein we review the studies that led to our current understanding of the hierarchical multi-oscillator circadian system.
Earliest Studies of Peripheral Circadian Rhythms: First Evidence of Multi-Oscillator Circadian Systems
In 1958, Erwin Bünning reported that intestines cultured from golden hamsters expressed ~24h rhythms in peristalsis under a range of temperatures (20-39°C) [20]. After Bünning’s report, several studies by G. Edgar Folk and others in the 1960s and 1970s showed that cultured mammalian tissues (adrenal, heart, and liver) exhibited circadian rhythms in metabolism, hormonal secretion, or enzyme activity [21-29]. These studies were published when mammalian circadian biologists were trying to identify a solitary circadian pacemaker in the central nervous system. Therefore, in the field of mammalian circadian rhythms, the existence of circadian oscillators in peripheral organs was not widely acknowledged. In contrast, chronobiologists studying insects had already developed a multi-oscillator model of the insect circadian system after an elegant study by Jaga Giebultowicz and her colleagues showed that the isolated testis-seminal ducts complex from gypsy moths contained a functional self-sustained circadian oscillator that was entrained by light [30].
The Molecular Biology Era: Using Reporter Technology to Demonstrate Self-Sustained Circadian Rhythms in Peripheral Organs
Successful cloning of circadian genes in Drosophila, zebrafish, and mammals in the 1980s and 1990s provided the tools to observe molecular rhythms in tissues outside of the central nervous system. Giebultowicz and Hege observed ~24h cycles of expression of the circadian proteins, PERIOD and TIMELESS, in Malpighian tubules in headless Drosophila housed in constant dark or entrained to the light-dark cycle [31,32]. Steve Kay and colleagues generated transgenic Drosophila in which the promotor of the period gene drove firefly luciferase reporter gene expression. Using these transgenic flies, they measured circadian gene transcription from living flies [33], and also measured light emission from cultured tissues [34]. Surprisingly, nearly every tissue, including the antenna, proboscis, wing, and leg, exhibited self-sustained circadian rhythms that entrained to environmental light-dark cycles.
In vertebrates, Tosini and Menaker found that cultured neural retinas from golden hamsters exhibited a circadian rhythm of melatonin release that entrained to light [35]. One year later, in 1997, two mammalian genes, Clock and Period, were cloned by forward and reverse genetics, respectively [36-39]. Both Clock and Period were expressed in the central nervous system and in many other peripheral organs in mice and humans. Schibler and colleagues also found that Period2 and other circadian genes cycled in an immortalized rat fibroblast cell line [40]. At the same time, Sassone-Corsi and colleagues discovered that organs cultured from zebrafish showed rhythmic expression of circadian genes, and later those rhythms were shown to directly entrain to environmental light-dark cycles [41,42].
In 2000, our group was the first to demonstrate self-sustained circadian gene expression in cultured peripheral tissues in mammals [43]. We generated a transgenic rat which carried the Period1-luciferase transgene, in which the Period1 promotor controlled the expression of luciferase. Consistent with the original observation that Period1 mRNA was expressed in many peripheral organs, tail snips submerged in luciferin were bioluminescent. The first tissues we attempted to culture were the SCN and muscle. We found that cultured muscle exhibited two cycles of a circadian rhythm of bioluminescence (the first Period1-bioluminescence recording from cultured muscle is shown in Figure 1B). As expected based on lesion and transplant studies, the Period1-bioluminesence circadian rhythm was robust in cultured SCN explants (Figure 1A). In addition, most tissues we cultured also exhibited circadian rhythms. This study transformed our understanding of the mammalian circadian system and demonstrated that it is composed of multiple circadian oscillators, similar to Drosophila and zebrafish. In contrast to Drosophila and zebrafish, mammalian peripheral oscillators are not light-sensitive and only tissues in the eye (e.g. retina, cornea, retinal pigment epithelium-choroid) have been shown to entrain to light-dark cycles in vitro [44-48].
Figure 1.
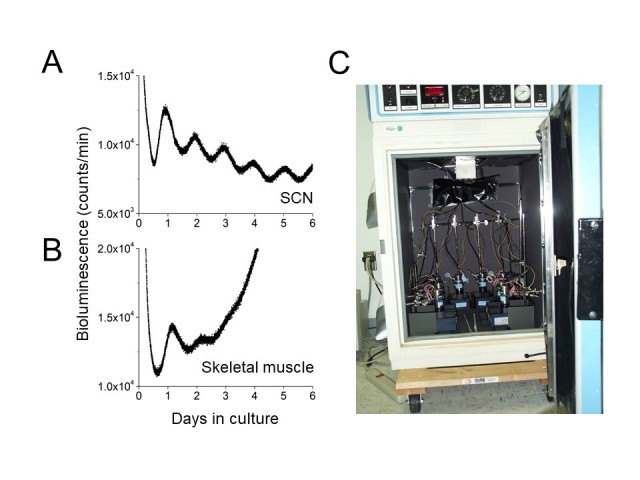
First successful recording of a circadian promoter-driven luminescence rhythm in cultured rodent peripheral tissue. On May 19, 1999, the SCN and skeletal muscle were explanted from a 15 day-old Period1-luciferase rat (L1-line) and cultured with 0.1 mM luciferin. This was our second attempt to record the luminescence rhythm.Bioluminescence was continuously monitored from the SCN (A) and muscle (B) by photo-multiplier tubes (HC135, Hamamatsu) maintained in the incubator at 36°C. Photon counts were recorded at 1-min intervals. C: Photo of the original set-up for bioluminescence recording. It had only two photo-multiplier tubes that were extended to 8 channels.
To investigate how the hierarchical multi-oscillatory mammalian circadian system entrained to the environmental light-dark cycle, we subjected Period1-luciferase transgenic rats to a jet-lag protocol (shifting the time of lights-on 6h earlier to simulate eastward travel or 6h later to simulate westward travel). We found that the SCN circadian rhythm adapted to the new light-dark cycle quickly, but it took several days for peripheral tissue rhythms to entrain to the new light-dark cycle [43]. Importantly, the speed of entrainment was different in each peripheral organ.
Because food availability is a cyclic environmental factor, we next fed Period1-luciferase rats only during the daytime. In the presence of the light-dark cycle, rats were given access to food for only 4h during the light phase. Since rats are nocturnal and normally eat during the night, the daytime restricted feeding provided two conflicting environmental cues, light and food. We found that the SCN rhythm entrained to the light-dark cycle (and was unaffected by restricted feeding), while the liver rhythm entrained to feeding time [49]. Two other groups independently observed the same phenomenon using conventional mRNA measurements [50,51].
Emergence of the Circadian Misalignment Concept
The discovery of peripheral circadian oscillators in mammals was paradigm-shifting; we came to view the mammalian circadian system as a hierarchical multi-oscillatory system rather than a system controlled by one pacemaker structure in the SCN. This new paradigm afforded a series of experiments that measured how development, aging, and metabolic challenges (e.g. high-fat diet, exercise) altered the rhythms in peripheral tissues [52-55]. Numerous studies also described the adverse physiological consequences of disruption of the multi-oscillator circadian system. A striking consequence of this disruption was our study that showed aged mice and rats in the jet-lag protocol had increased mortality [56,57]. We found that only 47 percent of aged mice survived repeated 6-h advances of the light-dark cycle, compared to 83 percent survival of aged mice in a typical static light-dark cycle. Circadian misalignment also adversely affects physiology in humans. For example, healthy adult subjects who were forced to sleep and eat on a 28-h cycle became prediabetic when their circadian rhythms were misaligned with the environmental cycle [58].
As the concept of “circadian misalignment” has gained momentum, so has the complexity and diversity of the definitions and experimental paradigms in investigating this concept. For example, circadian misalignment can be internal (e.g. desynchrony among peripheral oscillators or among central and peripheral oscillators) or external (e.g. the light-dark cycle is not aligned with the internal rhythm), or a combination of these factors (as seen during jet-lag). There can even be misalignment within a pacemaker structure. For example, groups of cellular oscillators within the SCN (e.g. the right and left SCN or the ventral and dorsal SCN) can dissociate under certain environmental conditions [59,60].
Functional Significance of Peripheral Oscillators
After the new hierarchical multi-oscillator model of the circadian system was established, the next obvious question became: what is the role of a peripheral circadian oscillator? In gypsy moths, it was shown that the circadian oscillator in the testis-seminal ducts complex controlled sperm release [30]. This question was addressed in mammals by generating tissue/cell type-specific circadian gene knockout animals. The Period and other circadian genes have multiple paralogs and single gene knockout does not cause arrhythmicity. Bmal1 is the only single-gene knockout that disabled the circadian oscillator. As a result, most studies have used Cre-lox technology to knock out Bmal1 and make clock-less tissues. But, somewhat surprisingly, a significant portion of genes, including some circadian genes, continued to cycle in Bmal1 knockout (or knock down) tissues in vivo, because systemic circadian hormonal and physiological signals drove rhythmicity in tissues [61]. Regardless, most of the studies summarized in Table 1 support the hypothesis that circadian oscillators in peripheral tissues control local physiology. For instance, the ERG b-wave rhythm was lost in retina-specific Bmal1-knockout mice [62]. Metabolic defects were found in the mice in which Bmal1 was knocked out in tissues related to metabolism (liver [63], skeletal muscle [64], pancreas [65,66]). Probably the most severe phenotype in tissue-specific Bmal1 knockouts is shortened life span in cardiomyocyte-specific knockouts [67]. Knocking out Bmal1 in ovarian steroidogenic cells or theca cells decreased fertility and litter size [68,69]. An interesting finding in tissue-specific knockouts is that the effects of disabling the clock in a tissue can extend beyond the function of that tissue. Paul and colleagues found changes in the total amount of non-REM sleep in the mouse when Bmal1 was knocked out in muscle [70]. This could be due, in part, to the heterogeneous functions of BMAL1 both in the output of the circadian oscillator and the non-circadian roles of BMAL1. Bmal1 has a paralog, Bmal2, which is down-regulated in Bmal1-knockout tissues [71]. CLOCK/NPAS2 and BMAL1/BMAL2 are transcription factors that activate thousands of E-box-containing genes. Therefore, knocking out Bmal1 in a tissue not only disables the circadian oscillator, but also causes an array of other genes to be aberrantly regulated. Therefore, the tissue-specific functions of peripheral clocks must be confirmed by knocking out other circadian genes that are not transcription activators (e.g. Period1/2 or Cryptochrome1/2 double knockouts).
Table 1. Physiological Consequences of Tissue-Specific Bmal1 Deletion.
| Tissue Bmal1 deleted / Cre Driver | Key Results | References |
| Retina / CHX10-Cre | ERG b-wave rhythm was lost | Storch et al. (2007) [62] |
| Liver / Albumin-Cre | Hypoglycemia during fasting phase | Lamia et al. (2008) [63] |
| Liver / Albumin-Cre | Increased expression of lipoprotein lipase mRNA | Shimba et al. (2011) [81] |
| Pancreatic islet / PDX1-Cre | Impaired glucose tolerance / hyperglycemia | Marcheva et al. (2010) [65] |
| Pancreatic islet / PDX-CreER* | Impaired glucose tolerance / hyperglycemia / hypoinsulinemia | Perelis et al. (2015) [66] |
| Adipocyte / adipocyte protein 2-Cre or adiponectin-Cre | Obese / reduced amplitude of food intake rhythm / reduced energy expenditure | Paschos et al. (2012) [82] |
| Skeletal muscle / muscle creatine kinase-Cre | No phenotype | Shimba et al. (2011) [81] |
| Skeletal muscle / human skeletal actin-MerCreMer* | Disrupted glucose metabolism / hyperglycemia in non-fasting / glucose intolerance / altered body composition / increased amount of non-REM sleep | Hodge et al. (2015) [83] Harfmann et al. (2016) [64] Ehlen et al. (2017) [70] |
| Cardiomyocyte / αMHC-Cre | Shortened life span / accelerated age-dependent-dilated cardiomyopathy | Young et al. (2014) [67] Ingle et al. (2015) [84] |
| Smooth muscle / SM22α-Cre | Reduced amplitude blood pressure rhythms | Xie et al. (2015) [85] |
| Perivascular adipose tissue (Brown adipocyte) / UCP1-Cre | Reduced blood pressure during resting phase | Chang et al. (2018) [86] |
| Adrenal / MC2R# | No alteration corticosterone rhythm under light-dark cycle, but amplitude of rhythm is diminished under constant darkness | Son et al. (2008) [87] |
| Adrenal / aldosterone synthase-Cre | No alteration in corticosterone rhythm under regular light-dark cycle (12:12) | Engeland et al., (2018) [88] |
| Renal tubular cell / Pax8-rtTA/LC1φ | Small kidney size / increased plasma urea level | Nikolaeva et al. (2016) [89] |
| Ovarian steroidogenic cell / SF1-Cre | Impaired uterine implantation / worsened fertility | Liu et al. (2014) [68] |
| Ovarian theca cell / Cyp17-Cre | Abolished daily rhythm of oocyte release in response to eLH / small litter size (subfertile) | Mereness et al. (2016) [69] |
| Ovarian granulosa cell / Cyp19-Cre | No abnormality was observed | Mereness et al. (2016) [69] |
| Pituitary gonadotrope cell / GnRHR-internal ribosome entry site-Cre | Increased estrous cycle length variability / no changes in litter size | Chu et al. (2013) [90] |
| Myeloid / LysM-Cre | Increased size of atherosclerotic lesion in Apoe-/- background | Huo et al. (2017) [91] |
*tamoxifen inducible; #knockdown by Bmal1 antisense; φdoxycycline inducible
Hierarchical Organization of the Multi-Oscillator System: Circadian Pacemaker(s) at the Top of the Hierarchy
The central circadian pacemaker, the SCN, is necessary and sufficient for circadian rhythms in behavior and physiology. To understand the relationship between the SCN and peripheral oscillators, the function of the SCN was disabled by either lesion or by Bmal1 knockout in the brain (this knockout included, but was not exclusive to, the SCN). These studies support the hypothesis that the SCN coordinates the phases of peripheral oscillators. Peripheral clocks remained rhythmic in both SCN-lesioned and brain-Bmal1 knockout mice, but the phase relationship between peripheral oscillators was disrupted [72-74]. Together, many studies have contributed to the metaphor of the mammalian circadian system as a symphony, where the SCN is the conductor and the peripheral oscillators are the musicians (Figure 2) [75].
Figure 2.
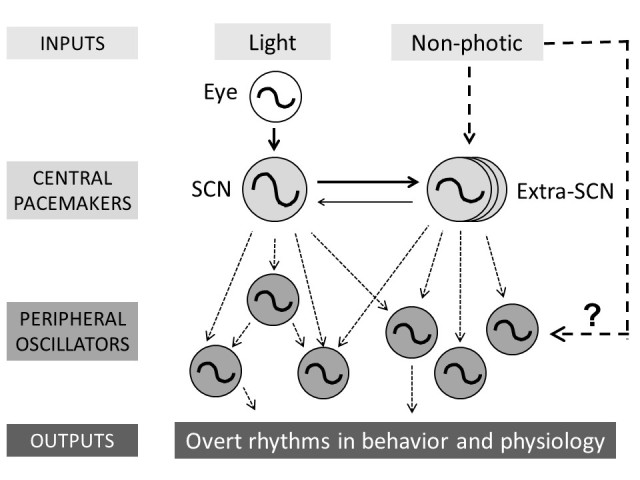
Current working model of the hierarchical multi-oscillatory mammalian circadian system. Light (via the eye) entrains the SCN and non-photic inputs (e.g. food, palatable meal, wheel-running) entrain (via unknown pathways) extra-SCN pacemakers. The SCN and extra-SCN pacemakers coordinate the phases of peripheral oscillators. The output pathways that control overt rhythms of behavior and physiology are largely unknown. Dotted lines represent unknown pathways.
The SCN is not the only circadian pacemaker that is capable of acting as the conductor of the symphony. It is known that at least two SCN-independent circadian pacemakers exist in rodents [76,77]. One is the food-entrainable oscillator (FEO), which controls food anticipatory activity during time-restricted feeding. The second is the methamphetamine-sensitive circadian oscillator (MASCO), whose behavior rhythm (MASCO-driven activity rhythm) appears when low-dose methamphetamine is chronically administered to rodents. Interestingly, both the FEO and MASCO do not depend on canonical circadian genes to keep time [76,77]. By measuring the phases of luminescence rhythms from ex vivo tissues, it was shown that both the FEO and MASCO can substitute for the SCN and coordinate the phases of peripheral oscillators when the SCN is lesioned or the circadian clock in the SCN is disabled [73,78]. However, the anatomical loci and the roles of the extra-SCN pacemakers under normal conditions (i.e. without restricted feeding and without methamphetamine), when the SCN is present and functional, remain to be elucidated.
The Next Frontier
Although the hierarchical multi-oscillatory nature of the circadian system is now well established, many questions remain to be answered. We still have much to learn about the physiological significances of peripheral oscillators. Perhaps the most understudied aspect of the mammalian circadian system is the output pathways of the SCN. For example, although we know the SCN is necessary and sufficient for the circadian rhythm of locomotor activity, we know very little about the neural circuitry downstream of the SCN that controls this rhythm. Neuroanatomical studies have shown that the SCN primarily projects to the subparaventricular zone (SPZ) and dorsomedial nucleus of the hypothalamus (DMH). Lesion studies have shown that the SPZ and DMH participate in regulation of circadian rhythms of sleep, locomotor activity, eating, and body temperature, but the specific neural and hormonal output/modulatory pathways remain to be elucidated [79]. In the reciprocal direction, we have shown that the eyes and the SCN are coupled and stabilize the locomotor activity rhythm of the hamster in constant darkness [80]. Understanding the ways that the SCN and peripheral oscillators interact will reveal the network architecture of the circadian system and further elucidate the physiological functions of peripheral oscillators.
Acknowledgments
We would like to thank Helmut Krämer for translating an article written in German and Bill Schwartz for sharing his collection of historical literature. We also would like to thank Mike Menaker, Terry Page, and Martha Gillette for clarifying historical studies.
Glossary
- SCN
suprachiasmatic nucleus
- FEO
food-entrainable oscillator
- MASCO
methamphetamine-sensitive circadian oscillator
- SPZ
subparaventricular zone
- DMH
dorsomedial nucleus of the hypothalamus
Author Contributions
AJB, JSP and SY wrote the manuscript; SY performed literature searches and outlined the manuscript. JSP is supported by National Institutes of Health grants DK098321, DK107851, P30GM127211, P30DK020579, and the University of Kentucky. SY is supported by National Institutes of Health grant R21 NS099809. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Nishiitsutsuji-Uwo J, Pittendrigh CS. Central nervous control of circadian rhythmicity in the cockroach. III. The optic lobes, locus of the driving oscillation? Z Vgl Physiol. 1968;58:1–46. [Google Scholar]
- Page TL. Transplantation of the cockroach circadian pacemaker. Science. 1982. April;216(4541):73–5. [DOI] [PubMed] [Google Scholar]
- Page TL. Circadian organization and the representation of circadian information in the nervous systems of invertebrates. In: Hekkens, Kerkhof, Rietveld Trends in Chronobiology Oxford: Pergamon Press; 1988. p. 67–79. [Google Scholar]
- Gaston S, Menaker M. Pineal function: the biological clock in the sparrow? Science. 1968. June;160(3832):1125–7. [DOI] [PubMed] [Google Scholar]
- Zimmerman NH, Menaker M. Neural connections of sparrow pineal: role in circadian control of activity. Science. 1975. October;190(4213):477–9. [DOI] [PubMed] [Google Scholar]
- Zimmerman NH, Menaker M. The pineal gland: a pacemaker within the circadian system of the house sparrow. Proc Natl Acad Sci USA. 1979. February;76(2):999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972. July;42(1):201–6. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA. 1972. June;69(6):1583–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye ST, Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc Natl Acad Sci USA. 1979. November;76(11):5962–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaki Y, Nihonmatsu I, Kawamura H. Transplantation of the neonatal suprachiasmatic nuclei into rats with complete bilateral suprachiasmatic lesions. Neurosci Res. 1984. February;1(1):67–72. [DOI] [PubMed] [Google Scholar]
- Drucker-Colín R, Aguilar-Roblero R, García-Hernández F, Fernández-Cancino F, Bermudez Rattoni F. Fetal suprachiasmatic nucleus transplants: diurnal rhythm recovery of lesioned rats. Brain Res. 1984. October;311(2):353–7. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J Neurosci. 1987. June;7(6):1626–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990. February;247(4945):975–8. [DOI] [PubMed] [Google Scholar]
- Green DJ, Gillette R. Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Res. 1982. August;245(1):198–200. [DOI] [PubMed] [Google Scholar]
- Groos G, Hendriks J. Circadian rhythms in electrical discharge of rat suprachiasmatic neurones recorded in vitro. Neurosci Lett. 1982. December;34(3):283–8. [DOI] [PubMed] [Google Scholar]
- Shibata S, Oomura Y, Kita H, Hattori K. Circadian rhythmic changes of neuronal activity in the suprachiasmatic nucleus of the rat hypothalamic slice. Brain Res. 1982. September;247(1):154–8. [DOI] [PubMed] [Google Scholar]
- Underwood H. The pineal and melatonin: regulators of circadian function in lower vertebrates. Experientia. 1990. January;46(1):120–8. [DOI] [PubMed] [Google Scholar]
- Menaker M, Moreira LF, Tosini G. Evolution of circadian organization in vertebrates. Braz J Med Biol Res. 1997. March;30(3):305–13. [DOI] [PubMed] [Google Scholar]
- Tamai TK, Vardhanabhuti V, Arthur S, Foulkes NS, Whitmore D. Flies and fish: birds of a feather. J Neuroendocrinol. 2003. April;15(4):344–9. [DOI] [PubMed] [Google Scholar]
- Bünning E. Das Weiterlaufen der “physiologischen Uhr” im Säugerdarm ohne zentrale Steuerung. Naturwissenschaften. 1958;45:68. [Google Scholar]
- Andrews RV, Folk GE., Jr Circadian metabolic patterns in cultured hamster adrenal glands. Comp Biochem Physiol. 1964. April;11:393–409. [DOI] [PubMed] [Google Scholar]
- Andrews RV, Shiotsuka R. The effect of actinomycin D on the in vitro adrenal secretory rhythm of the hamster. Comp Biochem Physiol. 1970. September;36(2):353–63. [DOI] [PubMed] [Google Scholar]
- Andrews RV. Circadian rhythms in adrenal organ cultures. Gegenbaurs Morphol Jahrb. 1971;117(1):89–98. [PubMed] [Google Scholar]
- Shiotsuka R, Jovonovich J, Jovonovich J. Circadian and ultradian corticosterone rhythms in adrenal organ cultures. Chronobiologia. 1974. September;1 Suppl 1:109–21. [PubMed] [Google Scholar]
- Tharp GD, Folk GE., Jr Rhythmic changes in rate of the mammalian heart and heart cells during prolonged isolation. Comp Biochem Physiol. 1965. February;14:255–73. [DOI] [PubMed] [Google Scholar]
- Langner R, Rensing L. Circadian rhythm of oxygen consumption in rat liver suspension culture: changes of pattern. Z Naturforsch B. 1972. September;27(9):1117–8. [DOI] [PubMed] [Google Scholar]
- Hardeland R. Circadian rhythmicity in cultured liver-cells. 1. Rhythms in tyrosine aminotransferase activity and inducibility and in [H-3] leucine incorporation. Int J Biochem. 1973;4(24):581–90. [Google Scholar]
- Hardeland R. Circadian rhythmicity in cultured liver-cells. 2. Reinduction of rhythmicity in tyrosine aminotransferase activity. Int J Biochem. 1973;4(24):591–5. [Google Scholar]
- Hardeland R. Further evidence for a post-transcriptional component in regulation of circadian rhythmicity in cultured liver-cells - Possible significance of RNA processing. J Interdiscipl Cycle Res. 1976;7(4):291–7. [Google Scholar]
- Giebultowicz JM, Riemann JG, Raina AK, Ridgway RL. Circadian system controlling release of sperm in the insect testes. Science. 1989. September;245(4922):1098–100. [DOI] [PubMed] [Google Scholar]
- Giebultowicz JM, Hege DM. Circadian clock in Malpighian tubules. Nature. 1997. April;386(6626):664. [DOI] [PubMed] [Google Scholar]
- Hege DM, Stanewsky R, Hall JC, Giebultowicz JM. Rhythmic expression of a PER-reporter in the Malpighian tubules of decapitated Drosophila: evidence for a brain-independent circadian clock. J Biol Rhythms. 1997. August;12(4):300–8. [DOI] [PubMed] [Google Scholar]
- Brandes C, Plautz JD, Stanewsky R, Jamison CF, Straume M, Wood KV, et al. Novel features of drosophila period Transcription revealed by real-time luciferase reporting. Neuron. 1996. April;16(4):687–92. [DOI] [PubMed] [Google Scholar]
- Plautz JD, Kaneko M, Hall JC, Kay SA. Independent photoreceptive circadian clocks throughout Drosophila. Science. 1997. November;278(5343):1632–5. [DOI] [PubMed] [Google Scholar]
- Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996. April;272(5260):419–21. [DOI] [PubMed] [Google Scholar]
- Antoch MP, Song EJ, Chang AM, Vitaterna MH, Zhao Y, Wilsbacher LD, et al. Functional identification of the mouse circadian Clock gene by transgenic BAC rescue. Cell. 1997. May;89(4):655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, et al. Positional cloning of the mouse circadian clock gene. Cell. 1997. May;89(4):641–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tei H, Okamura H, Shigeyoshi Y, Fukuhara C, Ozawa R, Hirose M, et al. Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature. 1997. October;389(6650):512–6. [DOI] [PubMed] [Google Scholar]
- Sun ZS, Albrecht U, Zhuchenko O, Bailey J, Eichele G, Lee CC. RIGUI, a putative mammalian ortholog of the Drosophila period gene. Cell. 1997. September;90(6):1003–11. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998. June;93(6):929–37. [DOI] [PubMed] [Google Scholar]
- Whitmore D, Foulkes NS, Strähle U, Sassone-Corsi P. Zebrafish Clock rhythmic expression reveals independent peripheral circadian oscillators. Nat Neurosci. 1998. December;1(8):701–7. [DOI] [PubMed] [Google Scholar]
- Whitmore D, Foulkes NS, Sassone-Corsi P. Light acts directly on organs and cells in culture to set the vertebrate circadian clock. Nature. 2000. March;404(6773):87–91. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000. April;288(5466):682–5. [DOI] [PubMed] [Google Scholar]
- Ruan GX, Allen GC, Yamazaki S, McMahon DG. An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA. PLoS Biol. 2008. October;6(10):e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba K, Sengupta A, Tosini M, Contreras-Alcantara S, Tosini G. Circadian regulation of the PERIOD 2:LUCIFERASE bioluminescence rhythm in the mouse retinal pigment epithelium-choroid. Mol Vis. 2010. December;16:2605–11. [PMC free article] [PubMed] [Google Scholar]
- Buhr ED, Van Gelder RN. Local photic entrainment of the retinal circadian oscillator in the absence of rods, cones, and melanopsin. Proc Natl Acad Sci USA. 2014. June;111(23):8625–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr ED, Yue WW, Ren X, Jiang Z, Liao HW, Mei X, et al. Neuropsin (OPN5)-mediated photoentrainment of local circadian oscillators in mammalian retina and cornea. Proc Natl Acad Sci USA. 2015. October;112(42):13093–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calligaro H, Coutanson C, Najjar RP, Mazzaro N, Cooper HM, Haddjeri N, et al. Rods contribute to the light-induced phase shift of the retinal clock in mammals. PLoS Biol. 2019. March;17(3):e2006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001. January;291(5503):490–3. [DOI] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000. December;14(23):2950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara R, Wan K, Wakamatsu H, Aida R, Moriya T, Akiyama M, et al. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells. 2001. March;6(3):269–78. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Yoshikawa T, Biscoe EW, Numano R, Gallaspy LM, Soulsby S, et al. Ontogeny of circadian organization in the rat. J Biol Rhythms. 2009. February;24(1):55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci USA. 2002. August;99(16):10801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergast JS, Branecky KL, Yang W, Ellacott KL, Niswender KD, Yamazaki S. High-fat diet acutely affects circadian organisation and eating behavior. Eur J Neurosci. 2013. April;37(8):1350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff G, Esser KA. Scheduled exercise phase shifts the circadian clock in skeletal muscle. Med Sci Sports Exerc. 2012. September;44(9):1663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. Chronic jet-lag increases mortality in aged mice. Curr Biol. 2006. November;16(21):R914–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Yamazaki S, Arble DM, Menaker M, Block GD. Resetting of central and peripheral circadian oscillators in aged rats. Neurobiol Aging. 2008. March;29(3):471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009. March;106(11):4453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Iglesia HO, Cambras T, Schwartz WJ, Díez-Noguera A. Forced desynchronization of dual circadian oscillators within the rat suprachiasmatic nucleus. Curr Biol. 2004. May;14(9):796–800. [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Meyer J, Carpino A, Jr, Schwartz WJ. Antiphase oscillation of the left and right suprachiasmatic nuclei. Science. 2000. October;290(5492):799–801. [DOI] [PubMed] [Google Scholar]
- Kornmann B, Schaad O, Bujard H, Takahashi JS, Schibler U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol. 2007. February;5(2):e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch KF, Paz C, Signorovitch J, Raviola E, Pawlyk B, Li T, et al. Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell. 2007. August;130(4):730–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA. 2008. September;105(39):15172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfmann BD, Schroder EA, Kachman MT, Hodge BA, Zhang X, Esser KA. Muscle-specific loss of Bmal1 leads to disrupted tissue glucose metabolism and systemic glucose homeostasis. Skelet Muscle. 2016. March;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010. July;466(7306):627–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelis M, Marcheva B, Ramsey KM, Schipma MJ, Hutchison AL, Taguchi A, et al. Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science. 2015. November;350(6261):aac4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ME, Brewer RA, Peliciari-Garcia RA, Collins HE, He L, Birky TL, et al. Cardiomyocyte-specific BMAL1 plays critical roles in metabolism, signaling, and maintenance of contractile function of the heart. J Biol Rhythms. 2014. August;29(4):257–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Johnson BP, Shen AL, Wallisser JA, Krentz KJ, Moran SM, et al. Loss of BMAL1 in ovarian steroidogenic cells results in implantation failure in female mice. Proc Natl Acad Sci USA. 2014. September;111(39):14295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mereness AL, Murphy ZC, Forrestel AC, Butler S, Ko C, Richards JS, et al. Conditional deletion of Bmal1 in ovarian theca cells disrupts ovulation in female mice. Endocrinology. 2016. February;157(2):913–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlen JC, Brager AJ, Baggs J, Pinckney L, Gray CL, DeBruyne JP, et al. Bmal1 function in skeletal muscle regulates sleep. eLife. 2017. July;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Hida A, McGuinness OP, Wasserman DH, Yamazaki S, Johnson CH. Circadian clock gene Bmal1 is not essential; functional replacement with its paralog, Bmal2. Curr Biol. 2010. February;20(4):316–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, et al. PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004. April;101(15):5339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumo M, Pejchal M, Schook AC, Lange RP, Walisser JA, Sato TR, et al. Differential effects of light and feeding on circadian organization of peripheral clocks in a forebrain Bmal1 mutant. eLife. 2014. December;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara Y, Kuroda H, Saito K, Nakajima Y, Kubo Y, Ohnishi N, et al. In vivo monitoring of peripheral circadian clocks in the mouse. Curr Biol. 2012. June;22(11):1029–34. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Yamazaki S, Menaker M. SCN: ringmaster of the circadian circus or conductor of the circadian orchestra? Novartis Found Symp. 2003;253:110–21; discussion 121–5, 281–4. [PubMed] [Google Scholar]
- Pendergast JS, Yamazaki S. Extra-SCN Circadian Pacemakers In: Honma K, Honma S. Biological Clocks with reference to suprachiasmatic nucleus. Sapporo: Hokkaido University Press; 2017. pp. 141–52. [Google Scholar]
- Pendergast JS, Yamazaki S. The Mysterious food-entrainable oscillator: insights from mutant and engineered mouse models. J Biol Rhythms. 2018. October;33(5):458–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezuk P, Mohawk JA, Yoshikawa T, Sellix MT, Menaker M. Circadian organization is governed by extra-SCN pacemakers. J Biol Rhythms. 2010. December;25(6):432–41. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005. October;437(7063):1257–63. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Alones V, Menaker M. Interaction of the retina with suprachiasmatic pacemakers in the control of circadian behavior. J Biol Rhythms. 2002. August;17(4):315–29. [DOI] [PubMed] [Google Scholar]
- Shimba S, Ogawa T, Hitosugi S, Ichihashi Y, Nakadaira Y, Kobayashi M, et al. Deficient of a clock gene, brain and muscle Arnt-like protein-1 (BMAL1), induces dyslipidemia and ectopic fat formation. PLoS One. 2011;6(9):e25231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschos GK, Ibrahim S, Song WL, Kunieda T, Grant G, Reyes TM, et al. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat Med. 2012. December;18(12):1768–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge BA, Wen Y, Riley LA, Zhang X, England JH, Harfmann BD, et al. The endogenous molecular clock orchestrates the temporal separation of substrate metabolism in skeletal muscle. Skelet Muscle. 2015. May;5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle KA, Kain V, Goel M, Prabhu SD, Young ME, Halade GV. Cardiomyocyte-specific Bmal1 deletion in mice triggers diastolic dysfunction, extracellular matrix response, and impaired resolution of inflammation. Am J Physiol Heart Circ Physiol. 2015. December;309(11):H1827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Su W, Liu S, Zhao G, Esser K, Schroder EA, et al. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J Clin Invest. 2015. January;125(1):324–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Xiong W, Zhao X, Fan Y, Guo Y, Garcia-Barrio M, et al. Bmal1 in perivascular adipose tissue regulates resting-phase blood pressure through transcriptional regulation of angiotensinogen. Circulation. 2018. July;138(1):67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son GH, Chung S, Choe HK, Kim HD, Baik SM, Lee H, et al. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc Natl Acad Sci USA. 2008. December;105(52):20970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeland WC, Massman L, Mishra S, Yoder JM, Leng S, Pignatti E, et al. The adrenal clock prevents aberrant light-induced alterations in circadian glucocorticoid rhythms. Endocrinology. 2018. December;159(12):3950–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaeva S, Ansermet C, Centeno G, Pradervand S, Bize V, Mordasini D, et al. Nephron-specific deletion of circadian clock gene Bmal1 alters the plasma and renal metabolome and impairs drug disposition. J Am Soc Nephrol. 2016. October;27(10):2997–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu A, Zhu L, Blum ID, Mai O, Leliavski A, Fahrenkrug J, et al. Global but not gonadotrope-specific disruption of Bmal1 abolishes the luteinizing hormone surge without affecting ovulation. Endocrinology. 2013. August;154(8):2924–35. [DOI] [PubMed] [Google Scholar]
- Huo M, Huang Y, Qu D, Zhang H, Wong WT, Chawla A, et al. Myeloid Bmal1 deletion increases monocyte recruitment and worsens atherosclerosis. FASEB J. 2017. March;31(3):1097–106. [DOI] [PMC free article] [PubMed] [Google Scholar]


