Abstract
Molecular circadian clocks align daily behavioral and metabolic rhythms with the external day-night cycle. Priming energy metabolism for recurring changes on a 24-hour basis, these clocks are deeply interlinked with metabolic homeostasis and health. Circadian rhythm disruptions, as occurring in shift work or sleep disorders, are often accompanied by metabolic disturbances – from the promotion of overweight and type-2 diabetes to the development of the metabolic syndrome. An important indicator of the adverse outcomes of overweight seems to be a systemic low-grade inflammation which is initially observed in adipose tissues and is promoted by circadian misalignment. Interestingly, the genetic disruption of circadian clocks in rodents leads to metabolic dysregulations very comparable to what is observed in shift workers and with the development of tissue specific clock gene knockout mice, the importance of single-tissue clocks for the metabolic regulation was further deciphered. In this review, we summarize the current knowledge on the role of mistimed behavior in metabolic health and outline behavioral interventions aiming at reducing the metabolic ramifications of chronodisruption.
Keywords: circadian clocks, chronodisruption, adipose tissue, metabolism, inflammation
Circadian Influence on Adipose Metabolism, Overweight, and Metaflammation
Today’s society is facing an overweight pandemic with an obesity rate of more than 30 percent in adults and a similarly high obesity rate of 16 percent in children in the United States [1]. In the last decades a profound progression towards a sedentary lifestyle in combination with over-nutrition increased the prevalence of obesity and its complications [2]. Metabolic health and body weight regulation are deeply interlinked with the endogenous circadian clock system that orchestrates 24-hour rhythms of behavior and metabolic regulation [3]. Perturbation of this regulatory system might amplify metabolic dysregulation and the adverse effects of typical modern-day and lifestyle factors such as shift work, long active hours, and reduced overall sleep, which all have the capability to disrupt daily behavioral routines. In combination with a constant food availability, this challenged metabolic regulation is further stressed, and weight gain and impaired glucose tolerance might arise [4]. In this review we evaluate the interaction of modern 24-hour lifestyles, circadian clocks, and their role in metabolic regulation and health.
The Fat Century
Today’s permanent and ubiquitous availability of food changed human nutrition to a state where – in stark contrast to all times up to now – the worldwide probability of being overweight has become higher than that of suffering from undernutrition [5]. Particularly during the last few decades overweight and obesity have steadily increased throughout the population and in line with this weight increase, the risk was elevated for sequelae like type-2 diabetes, cardiovascular disorders, and the metabolic syndrome [6]. These metabolic alterations are often associated with a modern lifestyle; shift workers in particular have an elevated risk of developing at least one metabolic disorder in their lifespan with risk ratios being directly proportional to the time period spent in shift work [7,8]. In shift work at least three factors are combined that already each on its own can impact health and metabolism: changed sleep patterns, nocturnal light exposure, and mistimed food intake. Roenneberg and Merrow developed a comprehensive model for the interaction of these factors in regard to the circadian misalignment and the development of obesity and other health issues [9]. Humans are diurnal species sleeping mainly during the dark hours of the night. Habitual sleep lasting around seven hours per day on average associates with the lowest death risk in comparison to shorter or longer sleep hours where the mortality rate is higher, probably due to an increased systemic inflammation or an altered overall health status [10]. Outcomes of short sleep times worsen if sleep duration is further decreased or mistimed [11]. In school children, for example, not only the lack of sleep, but also a shift to later sleeping hours is associated with increased weight gain during the summer holidays [12]. Nocturnal wake episodes or night shift work often involve increased light exposure during the dark, which further disrupts molecular circadian rhythms. In mice, already low levels of light at night shift behavioral and metabolic rhythms and lead to increased weight gain [13,14]. Shortened sleep is also associated with reduced levels of the satiety-inducing hormone leptin and elevated levels of the hunger-promoting peptide ghrelin [15]. With regard to glucose metabolism, postprandial insulin release from the pancreas is decreased close to the rest phase compared to the beginning of the activity phase in humans [16]. Thus, late meals towards the end of the active phase, i.e. the evening in humans, the morning in nocturnal rodents, lead to prolonged high serum glucose concentrations that are more likely to be stored as triglycerides (TGs) in adipose tissues [16,17].
Natural sleep and meal rhythms are often severely disrupted by shift work. Mimicking such conditions in the laboratory, in subjects following a 3-day behavioral and environmental aligned (day work) or misaligned (night work) schedule circadian misalignment resulted in a pre-diabetic state of impaired glucose tolerance and decreased insulin sensitivity [18]. Along with metabolic changes and disrupted circadian rhythms, the immune system is affected in shift workers resulting in altered numbers of circulating lymphocytes and natural killer cells and increased inflammatory cytokines like interleukin 6 (IL-6) and tumor necrosis factor α (TNF-α) that contribute to a pro-inflammatory phenotype [19-23]. Nocturnal light exposure also directly leads to a drop of the epiphyseal hormone melatonin [24]. Low melatonin levels – or light pulses at night – are associated with an increased breast cancer risk in night shift working nurses [24]. With regard to energy metabolism, melatonin supplementation was able to improve body weight regulation and metabolism in a rat model for obesity-related type-2 diabetes [25]. Oral supplementation in humans with melatonin deficiency increases volume and activity of brown adipose tissue (descripted later) and improves total cholesterol and triglyceride levels without affecting body weight [26]. Melatonin can reset the circadian sleep cycle and has beneficial short-term effects on shift workers [27]. Its stabilizing effect on sleep-wake regulation is further improved when combined with bright light exposure in the morning [28,29]. Interestingly, so far, no long-term effects of melatonin supplementation have been investigated.
Snack the Weight Up – Mistimed Food Intake and its Metabolic Consequences
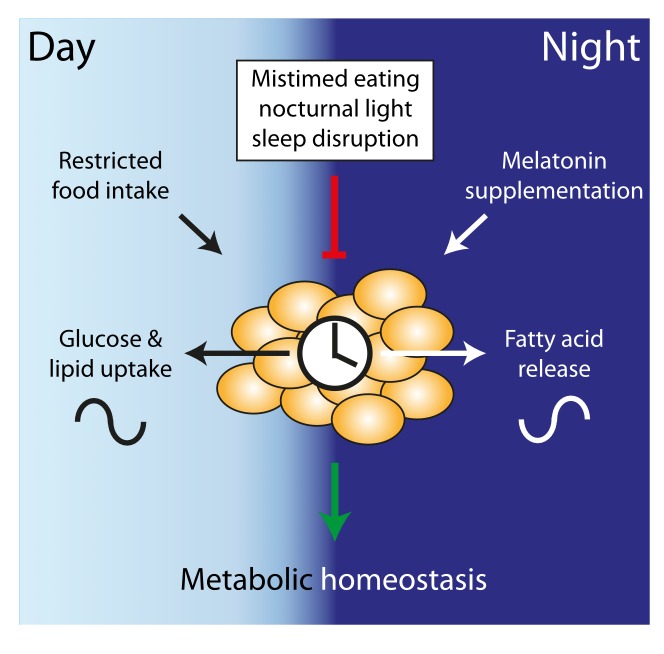
Being active in the normal rest phase – e.g. during shiftwork – is often accompanied by mistimed food intake which not only increases the total caloric intake, thereby promoting weight gain, but also has adverse effects on energy metabolism (see Figure 1). This was first observed in mice that were fed exclusively during their inactive phase (i.e. the day), gaining more weight than their active phase-fed littermates already after nine days [30]. The inactive phase fed group shifted circadian rhythms in metabolic active organs. Moreover, overall caloric intake was elevated with the majority of food being consumed shortly after it became available [30,31]. Lifestyle influences on everyday eating behavior and bodyweight homeostasis in humans have been observed. In a small-scale study by the Panda group, non-shift workers ate at almost random intervals over a time window of 14-15 hours of the day. If overweight participants from this group reduced their daily eating timespan to 10 hours, without any other restrictions, they not only lost weight but also improved their sleep rhythm [32]. Food is a strong Zeitgeber for peripheral organs and the timing of food intake can disrupt or amplify the coordinated clock system [33]. The importance of meal timing is also seen in mice with access to a high-fat diet (HFD). If provided ad libitum, HFD weakens rhythmic behavior with mice eating more during their inactive phase, promoting hyperphagy and obesity [34]. However, when HFD is only provided during the nighttime (i.e. the active phase in mice), mice stay slim and healthy [35,36]. Interestingly, especially the late phase of the activity phase may be crucial for regulating energy homeostasis. Mice that are offered an HFD snack at the end of their active phase show increased adiposity and hypoleptinemia compared to those receiving an early active phase snack [31]. In a repeated jet lag experiment (6 hours of phase advance every week) mice with ad-libitum food access become overweight within 10 days. In this setting obesity is prevented by restricting the food availability to the dark phase [37].
Figure 1.
Diurnal adipose tissue homeostasis. During active/ daytime hours food is consumed and white adipose tissue stores Glucose and lipids. During the dark and fasting night fatty acids are released, supported by melatonin. Undisturbed cycles stabilize the metabolic homeostasis (green). Mistimed eating, nocturnal light and sleep disruption inhibit this timed processes (red).
Adipose Tissue Circadian Clocks in the Regulation of Energy Metabolism
Adverse outcomes of misaligned behavior have attributed to disruptions of the endogenous circadian clock apparatus. Circadian clocks have evolved to anticipate predictable changes in environmental conditions, priming the organism for optimal metabolic function and nutrient utilization [38]. On the molecular level, circadian timekeeping is based on 24-hour oscillations of interlocked transcriptional-translational feedback loops that regulate cell type-specific programs of clock-controlled genes by binding to circadian enhancer elements [39,40]. At the center of this molecular clock apparatus are the transcription factors BMAL1 (Brain and Muscle Arnt-like protein-1) and CLOCK (Circadian locomotor Output Cycles Kaput). Circadian clock oscillations are self-sustained but remain sensitive to resetting or entraining signals and can thereby adapt to changing environmental conditions and time zones [41]. Strong entrainment signals are light exposure and food intake that during specific phases reset central and peripheral clock, respectively, thus aligning the endogenous rhythm with external time. Like most tissues, adipose tissues harbor circadian clocks and local metabolic processes like carbohydrate and lipid metabolism are rhythmically regulated in adipocytes [42].
White adipose tissue (WAT) presents the major fat mass in the mammalian body, but in the last decades brown adipose tissue (BAT) depots regained scientific and public interest. In contrast to white adipocytes that isolate and store energy in form of TGs, the primary task of BAT is active mitochondrial heat production characterized by uncoupling protein 1 (UCP1) expression [43]. BAT harbors intrinsic clocks and in rodents UCP1 expression follows a diurnal pattern [44-46]. Interestingly, mice lacking UCP1 remain lean at room temperature but gain weight at thermoneutrality [47,48], while genetic BAT ablation directly leads to the development of obesity [49]. Circadian BAT activity and energy expenditure can directly be modulated by Bmal1 deletion in the murine ventromedial hypothalamus circumventing SCN signaling, leaving the local BAT clock intact but leading to a reduced body weight in mice [50]. In humans, BAT occurrence was originally believed to be restricted to infants. Recent studies, however, have shown that significant amounts of BAT can be detected in adults. BAT activity is stimulated by acute cold exposure, but correlates negatively with age and BMI [51-56]. Human BAT utilizes glucose for heat production with a thermogenic, but still glucose responsive circadian rhythm [57]. High BAT abundance is correlated with low glycemia, but with regard to potential therapeutic benefits of BAT in obesity and type-2 diabetes, it has to be considered that even with upregulated glucose uptake due to cold exposure, BAT mediated energy turnover remains minor compared to that of skeletal muscle [57-59].
Molecular Clock Disruption and Metabolic Outcomes
Dysfunctional circadian clocks and rhythms are associated with impaired metabolic homeostasis (see Table 1). A mutation of the clock core component, Clock, in mice causes blunted rhythmic behavior and elevated food intake during the inactive light phase and increased weight gain compared to age-matched wildtype animals [60]. Already on a (low-fat) normal-chow diet provided ad libitum, these mutant mice show symptoms of the metabolic syndrome with elevated adiposity and blood triglyceride, cholesterol, and 24-hour glucose levels [60]. Mice with a deletion of the CLOCK partner protein BMAL1 show a diverse adipose phenotype. On one hand, they fail to establish sufficient amounts of adipose depots and display no ectopic fat formation in liver or muscle tissue but instead they suffer from elevated levels of circulating fatty acids with high blood triglycerides, free fatty acids, and cholesterol [61]. The inability of fat storage in Bmal1-/- mice persists if they are fed HFD [61]. Tissue-specific deletion of Bmal1 has been widely used to delete molecular clock function in metabolic tissues. Abolished clock function in liver interferes with the stabilization of blood glucose levels, leading to hypoglycemic states during the diurnal fasting periods [62]. While the liver clock drives a rhythmic hepatic glucose release, pancreatic beta-cell clock ablation leads to a loss of insulin release after glucose stimuli and a hypoinsulinemic state in mice and Bmal1 deletion in muscles leads to weight gain [62-64]. Adipose tissue cells are programmed to take up energy during the active/feeding and release it during the rest/fasting phase [42]. If adipose clocks are genetically disabled in mice, plasma concentrations of triglycerides and free fatty acids during the day are elevated, feeding rhythms attenuated and animals gain excessive amounts of adipose tissue [65].
Table 1. Adipose phenotypes of clock disruption in mice.
| Mouse/Mutation/Experiment | Phenotype | Reference |
| Light exposure at night | Metabolic and behavioral phase shifts, weight gain | [13,78] |
| Mistimed food intake | Tissue clock phase shifts, weight gain | [30,31] |
| High-fat diet ad libitum | Blunted behavioral and tissue clock rhythms, weight gain, hyperphagy | [34] |
| Chronic jet lag (6h advance/week) | Weight gain | [37] |
| Constant light exposure | Weight gain | [101] |
| SCN lesion | Loss of behavioral and molecular rhythms, obesity, hyperphagy | [102] |
| Clock mutation | Blunted behavioral rhythms, weight gain, hyperphagy | [60] |
| Bmal1 KO | Lean, hyperlipidemia | [61] |
| Per1/2 double KO | Leptin resistance | [103] |
| Per2 KO | Altered lipid metabolism, hypotriglyceridemia, weight gain under HFD | [104,105] |
| Per3 KO | Increased fat mass | [106] |
| Cry1 KO | Resistant to diet induced obesity through HFD (not Cry2 KO) | [107] |
| Cry1/2 double KO | Loss of behavioral rhythms, hyperinsulinemia, weight gain | [108-110] |
| Reverbα/β double KO | Loss of behavioral rhythms, hyperlipidemia, hyperglycemia, hepatic steatosis | [111-113] |
| Liver Bmal1 KO | Hypoglycemia during fasting period | [62] |
| Pancreas Bmal1 KO | Hypoinsulinemia | [63] |
| Adipose tissue Bmal1 KO | Weight gain | [65] |
| Muscle Bmal1 KO | Weight gain | [64] |
Metabolic Inflammation
Adipose tissue is not only involved in metabolic, but also in inflammatory responses through secretion of pro- and anti-inflammatory cytokines [66]. Under obesogenic conditions, immune cells are recruited to adipose stores which can further amplify inflammatory responses [67]. Increased body weight is not inevitably accompanied by further metabolic disorders such as cardiovascular diseases or type-2 diabetes, but a so-called “healthy overweight state” can transform into the pathogenic obese phenotype [6,68]. A hallmark for this shift is the adipose immune state and systemic low-grade inflammation is a key attribute of the metabolic syndrome [69]. During inflammation processes neutrophils are the first innate immune cells recruited to the tissue and later cleared by tissue resident macrophages [70]. In mice, the number of adipose tissue infiltrating neutrophils increases already after a short exposure to HFD [71,72]. Neutrophil intrinsic clocks drive cellular aging and regulate tissue infiltration and defense behavior [73]. In the obese state the number of pro-inflammatory adipose tissue resident immune cells rises rapidly, increasing and the risk for developing systemic inflammation [74]. Neutrophils contribute to impaired insulin sensitivity in adipose tissue with the release of the proteolytic enzyme elastase that continues to affect the tissue, even after the number of neutrophils is again reduced [72]. Further innate immune cells resident in WAT are macrophages which contribute to systemic inflammation with rhythmic secretion of TNF-α and IL-6 [75]. This secretion rhythm is depended on a functional intrinsic clock of the macrophages [75]. In shift workers, which have an elevated risk to develop the metabolic syndrome, leukocyte counts are elevated [76]. In rats, chronic advanced but not delayed shifts of the light-dark cycle lead to an increase of inflammation [77]. Further, dim light at night amplifies TNF-α expression in WAT in mice under a high-fat diet [78]. In adipose tissue, this inflammation directly leads to alterations in triglyceride and glucose homeostasis [79,80]. Treatment with hormones involved in circadian system organization can affect adipose inflammation suggesting that adipose clocks may be involved in determining adipocyte inflammatory state. A supplementation with glucocorticoids can dampen adipose tissue inflammation and human microarray studies revealed that dexamethasone treatment alters immune and inflammatory response pathways in adipocytes [81,82]. Additionally, glucocorticoids inhibit further immune cell recruitment to adipose tissue in obesity [83]. The effect of melatonin supplementation varies from pro- to anti-inflammatory properties, depending on the concentration, setting, and the measured parameter [84]. In young Zucker diabetic fatty rats melatonin treatment improves their phenotypic low-grade inflammation and reduces plasma IL-6 and TNF-α levels by about 10 percent [85]. In leptin deficient ob/ob mice, melatonin supplementation improves adipose tissue inflammation [86]. Melatonin partly counteracts the 24-h endocrine rhythm disruption caused by an HFD [87], inhibits the development of metabolic syndrome under different obesogenic light regimes [88] (see Table 1), and normalizes biochemical parameters in mild diet induced inflammation in rats [89]. In humans, melatonin treatment in metabolic syndrome improves blood pressure values and lipid profiles [90] and reduces TNF-α concentrations [91]. In obese women, melatonin treatment decreases inflammatory parameters [92] while, in contrast, in healthy women glucose tolerance is impaired under melatonin treatment [93]. Another player in metabolic adipose tissue inflammation is the gut microbiome, which displays a diurnal rhythm in species composition, is highly influenced by clock genes [94-96], and vice versa, can also modulate the circadian clock [97]. Further dysregulations of the microbiome are associated with pro-inflammatory metabolic states like type-2 diabetes and obesity [98]. Germ-free mice display alterations in the rhythmic transcriptome and metabolites. Interestingly, in these mice rhythmic cytokine signaling in WAT is ablated [99] promoting low-grade WAT inflammation [100].
Conclusion
The demands of modern societies promote a health impairing lifestyle with disrupted sleep and eating patterns. Natural circadian regulation is weakened, and averse metabolic outcomes may be the consequence. With increasing knowledge on rhythm interfering factors and their mechanisms of action, the next goal of chronobiology and medicine will be the implementation of this knowledge into daily life and clinical practice. It may be naïve to strive for an abolishment of shiftwork in a globalized economy, but – if shiftwork cannot be avoided – chronotherapeutic interventions aiming at stabilizing the circadian clock network, such as scheduled meal timing, timed light exposure/avoidance, or melatonin supplementation, may become useful tools to reduce adverse health effects and rebalance energy metabolism under chronodisruptive conditions and generally improve metabolic and glucose homeostasis and fitness.
Acknowledgments
This work was funded by grants of the German Research Foundation (DFG; GRK-1957 & OS353-7/1) and a Lichtenberg Fellowship of the Volkswagen Foundation (HO).
Glossary
- TGs
triglycerides
- BMAL1
Brain and Muscle Arnt-like protein-1
- CLOCK
Circadian locomotor Output Cycles Kaput
- WAT
White Adipose Tissue
- BAT
Brown Adipose Tissue
- HFD
High Fat Diet
- IL-6
Interleukin 6
- TNF-α
Tumor Necrosis Factor α
Author Contributions
IK and HO conceptualized the paper. IK and HO wrote and revised the manuscript.
References
- OECD Obesity-Update-2017-OECD. 2017 Available from: https://www.oecd.org/els/health-systems/Obesity-Update-2017.pdf .
- Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–9. [DOI] [PubMed] [Google Scholar]
- Marcheva B, Ramsey KM, Peek CB, Affinati A, Maury E, Bass J. Circadian Clocks and Metabolism. Handb Exp Pharmacol. 2013:127–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly JJ, El-Hamdouchi A, Diouf A, Monyeki A, Somda SA. Determining the worldwide prevalence of obesity. Lancet. 2018;391:1773–4. [DOI] [PubMed] [Google Scholar]
- Roos V, Elmståhl S, Ingelsson E, Sundström J, Ärnlöv J, Lind L. Metabolic Syndrome Development During Aging with Special Reference to Obesity Without the Metabolic Syndrome. Metab Syndr Relat Disord. 2017;15:36–43. [DOI] [PubMed] [Google Scholar]
- Niedhammer I, Lert F, Marne MJ. Prevalence of overweight and weight gain in relation to night work in a nurses’ cohort. Int J Obes Relat Metab Disord J Int Assoc Study Obes. 1996;20:625–33. [PubMed] [Google Scholar]
- Nakamura K, Shimai S, Kikuchi S, Tominaga K, Takahashi H, Tanaka M, et al. Shift work and risk factors for coronary heart disease in Japanese blue-collar workers: serum lipids and anthropometric characteristics. Occup Med (Lond). 1997;47:142–6. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Merrow M. The Circadian Clock and Human Health. Curr Biol. 2016;26:R432–43. [DOI] [PubMed] [Google Scholar]
- Hall MH, Smagula SF, Boudreau RM, Ayonayon HN, Goldman SE, Harris TB, et al. Association between Sleep Duration and Mortality Is Mediated by Markers of Inflammation and Health in Older Adults: The Health, Aging and Body Composition Study. Sleep (Basel). 2015;38:189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itani O, Kaneita Y, Murata A, Yokoyama E, Ohida T. Association of onset of obesity with sleep duration and shift work among Japanese adults. Sleep Med. 2011;12:341–5. [DOI] [PubMed] [Google Scholar]
- Moreno JP, Crowley SJ, Alfano CA, Hannay KM, Thompson D, Baranowski T. Potential circadian and circannual rhythm contributions to the obesity epidemic in elementary school age children. Int J Behav Nutr Phys Act. 2019;16:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borniger JC, Maurya SK, Periasamy M, Nelson RJ. Acute dim light at night increases body mass, alters metabolism, and shifts core body temperature circadian rhythms. Chronobiol Int. 2014;31:917–25. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Aubrecht TG, Meléndez-Fernández OH, Weil ZM, Nelson RJ. Dim light at night disrupts molecular circadian rhythms and increases body weight. J Biol Rhythms. 2013;28:262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad A, Dalla Man C, Nandy DK, Levine JA, Bharucha AE, Rizza RA, et al. Diurnal Pattern to Insulin Secretion and Insulin Action in Healthy Individuals. Diabetes. 2012;61:2691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Strubbe JH. Circadian control of insulin secretion is independent of the temporal distribution of feeding. Physiol Behav. 1998;63:553–8. [DOI] [PubMed] [Google Scholar]
- Morris CJ, Purvis TE, Mistretta J, Scheer FA. Effects of the Internal Circadian System and Circadian Misalignment on Glucose Tolerance in Chronic Shift Workers. J Clin Endocrinol Metab. 2016;101:1066–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renegar KB, Floyd R, Krueger JM. Effect of sleep deprivation on serum influenza-specific IgG. Sleep. 1998;21:19–24. [PubMed] [Google Scholar]
- Everson CA. Clinical assessment of blood leukocytes, serum cytokines, and serum immunoglobulins as responses to sleep deprivation in laboratory rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1054–63. [DOI] [PubMed] [Google Scholar]
- Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–26. [DOI] [PubMed] [Google Scholar]
- Shearer WT, Reuben JM, Mullington JM, Price NJ, Lee BN, Smith EO, et al. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107:165–70. [DOI] [PubMed] [Google Scholar]
- Blask DE, Brainard GC, Dauchy RT, Hanifin JP, Davidson LK, Krause JA, et al. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res. 2005;65:11174–84. [DOI] [PubMed] [Google Scholar]
- Jimenéz-Aranda A, Fernández-Vázquez G. Mohammad A-Serrano M, Reiter RJ, Agil A. Melatonin improves mitochondrial function in inguinal white adipose tissue of Zücker diabetic fatty rats. J Pineal Res. 2014;57:103–9. [DOI] [PubMed] [Google Scholar]
- Halpern B, Mancini MC, Bueno C, Barcelos IP, de Melo ME, Lima MS, et al. Melatonin Increases Brown Adipose Tissue Volume and Activity in Patients With Melatonin Deficiency: A Proof-of-Concept Study. Diabetes. 2019;68:947–52. [DOI] [PubMed] [Google Scholar]
- Pallesen S, Bjorvatn B, Magerøy N, Saksvik IB, Waage S, Moen BE. Measures to counteract the negative effects of night work. Scand J Work Environ Health. 2010;36:109–20. [DOI] [PubMed] [Google Scholar]
- Burke TM, Markwald RR, Chinoy ED, Snider JA, Bessman SC, Jung CM, et al. Combination of light and melatonin time cues for phase advancing the human circadian clock. Sleep (Basel). 2013;36:1617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touitou Y, Bogdan A. Promoting adjustment of the sleep-wake cycle by chronobiotics. Physiol Behav. 2007;90:294–300. [DOI] [PubMed] [Google Scholar]
- Bray MS, Ratcliffe WF, Grenett MH, Brewer RA, Gamble KL, Young ME. Quantitative Analysis of Light-Phase Restricted Feeding Reveals Metabolic Dyssynchrony in Mice. Int J Obes. 2005;2013(37):843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray MS, Tsai JY, Villegas-Montoya C, Boland BB, Blasier Z, Egbejimi O, et al. Time-of-day-dependent dietary fat consumption influences multiple cardiometabolic syndrome parameters in mice. Int J Obes. 2005;2010(34):1589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S, Panda S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab. 2015;22:789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–21. [DOI] [PubMed] [Google Scholar]
- Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring). 2009;17:2100–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, et al. Time-Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High-Fat Diet. Cell Metab. 2012;15:848–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oike H, Sakurai M, Ippoushi K, Kobori M. Time-fixed feeding prevents obesity induced by chronic advances of light/dark cycles in mouse models of jet-lag/shift work. Biochem Biophys Res Commun. 2015;465:556–61. [DOI] [PubMed] [Google Scholar]
- Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AB, Karp NA, Maywood ES, Sage EA, Deery M, O’Neill JS, et al. Circadian orchestration of the hepatic proteome. Curr Biol. 2006;16:1107–15. [DOI] [PubMed] [Google Scholar]
- Tsang AH, Barclay JL, Oster H. Interactions between endocrine and circadian systems. J Mol Endocrinol. 2014;52:R1–16. [DOI] [PubMed] [Google Scholar]
- Takahashi JS. Molecular components of the circadian clock in mammals. Diabetes Obes Metab. 2015;17 Suppl 1:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shostak A, Meyer-Kovac J, Oster H. Circadian regulation of lipid mobilization in white adipose tissues. Diabetes. 2013;62:2195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. [DOI] [PubMed] [Google Scholar]
- Friedrichs M, Kolbe I, Seemann J, Tsang AH, Cherradi L, Klein J, et al. Circadian clock rhythms in different adipose tissue model systems. Chronobiol Int. 2018;35:1543–52. [DOI] [PubMed] [Google Scholar]
- Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G, et al. Characterization of peripheral circadian clocks in adipose tissues. Diabetes. 2006;55:962–70. [DOI] [PubMed] [Google Scholar]
- Gerhart-Hines Z, Feng D, Emmett MJ, Everett LJ, Loro E, Briggs ER, et al. The nuclear receptor Rev-erbα controls circadian thermogenic plasticity. Nature. 2013;503:410–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enerbäck S. Brown adipose tissue in humans. Int J Obes. 2005;2010(34 Suppl 1):S43–6. [DOI] [PubMed] [Google Scholar]
- Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–9. [DOI] [PubMed] [Google Scholar]
- Lowell BB, S-Susulic V, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993. December;366(6457):740–2. 10.1038/366740a0 [DOI] [PubMed] [Google Scholar]
- Orozco-Solis R, Aguilar-Arnal L, Murakami M, Peruquetti R, Ramadori G, Coppari R, et al. The Circadian Clock in the Ventromedial Hypothalamus Controls Cyclic Energy Expenditure. Cell Metab. 2016;23:467–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC, et al. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab. 2011;96:192–9. [DOI] [PubMed] [Google Scholar]
- Pfannenberg C, Werner MK, Ripkens S, Stef I, Deckert A, Schmadl M, et al. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes. 2010;59:1789–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers NH, Landa A, Park S, Smith RG. Aging leads to a programmed loss of brown adipocytes in murine subcutaneous white adipose tissue. Aging Cell. 2012;11:1074–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhang M, Xu M, Gu W, Xi Y, Qi L, et al. Brown adipose tissue activation is inversely related to central obesity and metabolic parameters in adult human. PLoS One. 2015;10:e0123795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Bova R, Schofield L, Bryant W, Dieckmann W, Slattery A, et al. Brown Adipose Tissue Exhibits a Glucose-Responsive Thermogenic Biorhythm in Humans. Cell Metab. 2016;23:602–9. [DOI] [PubMed] [Google Scholar]
- Blondin DP, Labbé SM, Phoenix S, Guérin B, Turcotte ÉE, Richard D, et al. Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men. J Physiol. 2015;593:701–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé SM, Caron A, Bakan I, Laplante M, Carpentier AC, Lecomte R, et al. In vivo measurement of energy substrate contribution to cold-induced brown adipose tissue thermogenesis. FASEB J. 2015;29:2046–58. [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimba S, Ogawa T, Hitosugi S, Ichihashi Y, Nakadaira Y, Kobayashi M, et al. Deficient of a clock gene, brain and muscle Arnt-like protein-1 (BMAL1), induces dyslipidemia and ectopic fat formation. PLoS One. 2011;6:e25231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA. 2008;105:15172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Moulik M, Fang Z, Saha P, Zou F, Xu Y, et al. Bmal1 and β-Cell Clock Are Required for Adaptation to Circadian Disruption, and Their Loss of Function Leads to Oxidative Stress-Induced β-Cell Failure in Mice. Mol Cell Biol. 2013;33:2327–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyar KA, Ciciliot S, Wright LE, Biensø RS, Tagliazucchi GM, Patel VR, et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol Metab. 2014;3:29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschos GK, Ibrahim S, Song WL, Kunieda T, Grant G, Reyes TM, et al. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat Med. 2012;18:1768–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Vela ME, Torres N, Tovar AR. White adipose tissue as endocrine organ and its role in obesity. Arch Med Res. 2008;39:715–28. [DOI] [PubMed] [Google Scholar]
- Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–7. [DOI] [PubMed] [Google Scholar]
- Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49:1894–903. [DOI] [PubMed] [Google Scholar]
- Talukdar S, Oh DY, Bandyopadhyay G, Li D, Xu J, McNelis J, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrover JM, Del Fresno C, Crainiciuc G, Cuartero MI, Casanova-Acebes M, Weiss LA, et al. A Neutrophil Timer Coordinates Immune Defense and Vascular Protection. Immunity. 2019;50:390–402.e10. [DOI] [PubMed] [Google Scholar]
- Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front Endocrinol. 2016;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, et al. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci USA. 2009;106:21407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sookoian S, Gemma C, Fernández Gianotti T, Burgueño A, Alvarez A, González CD, et al. Effects of rotating shift work on biomarkers of metabolic syndrome and inflammation. J Intern Med. 2007;261:285–92. [DOI] [PubMed] [Google Scholar]
- Herrero L, Valcarcel L, da Silva CA, Albert N, Diez-Noguera A, Cambras T, et al. Altered circadian rhythm and metabolic gene profile in rats subjected to advanced light phase shifts. PLoS One. 2015;10:e0122570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Lieberman RA, Weil ZM, Nelson RJ. Dim light at night exaggerates weight gain and inflammation associated with a high-fat diet in male mice. Endocrinology. 2013;154:3817–25. [DOI] [PubMed] [Google Scholar]
- Hui X, Zhang M, Gu P, Li K, Gao Y, Wu D, et al. Adipocyte SIRT1 controls systemic insulin sensitivity by modulating macrophages in adipose tissue. EMBO Rep. 2017;18:645–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JJ, Zuriaga MA, Ngo DT, Farb MG, Aprahamian T, Yamaguchi TP, et al. Noncanonical Wnt signaling promotes obesity-induced adipose tissue inflammation and metabolic dysfunction independent of adipose tissue expansion. Diabetes. 2015;64:1235–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Gong DW, Burkey BF, Fried SK. Pathways regulated by glucocorticoids in omental and subcutaneous human adipose tissues: a microarray study. Am J Physiol Endocrinol Metab. 2011;300:E571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Pramyothin P, Karastergiou K, Fried SK. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim Biophys Acta. 2014;1842:473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsouris D, Neels JG, Fan W, Li PP, Nguyen MT, Olefsky JM. Glucocorticoids and thiazolidinediones interfere with adipocyte-mediated macrophage chemotaxis and recruitment. J Biol Chem. 2009;284:31223–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radogna F, Diederich M, Ghibelli L. Melatonin: a pleiotropic molecule regulating inflammation. Biochem Pharmacol. 2010;80:1844–52. [DOI] [PubMed] [Google Scholar]
- Agil A, Reiter RJ, Jiménez-Aranda A, Ibán-Arias R, Navarro-Alarcón M, Marchal JA, et al. Melatonin ameliorates low-grade inflammation and oxidative stress in young Zucker diabetic fatty rats. J Pineal Res. 2013;54:381–8. [DOI] [PubMed] [Google Scholar]
- Favero G, Stacchiotti A, Castrezzati S, Bonomini F, Albanese M, Rezzani R, et al. Melatonin reduces obesity and restores adipokine patterns and metabolism in obese (ob/ob) mice. Nutr Res N Y N. 2015;35:891–900. [DOI] [PubMed] [Google Scholar]
- Ríos-Lugo MJ, Cano P, Jiménez-Ortega V, Fernández-Mateos MP, Scacchi PA, Cardinali DP, et al. Melatonin effect on plasma adiponectin, leptin, insulin, glucose, triglycerides and cholesterol in normal and high fat-fed rats. J Pineal Res. 2010;49:342–8. [DOI] [PubMed] [Google Scholar]
- Vinogradova I, Anisimov V. Melatonin prevents the development of the metabolic syndrome in male rats exposed to different light/dark regimens. Biogerontology. 2013;14:401–9. [DOI] [PubMed] [Google Scholar]
- Cano Barquilla P, Pagano ES, Jiménez-Ortega V, Fernández-Mateos P, Esquifino AI, Cardinali DP. Melatonin normalizes clinical and biochemical parameters of mild inflammation in diet-induced metabolic syndrome in rats. J Pineal Res. 2014;57:280–90. [DOI] [PubMed] [Google Scholar]
- Koziróg M, Poliwczak AR, Duchnowicz P, Koter-Michalak M, Sikora J, Broncel M. Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. J Pineal Res. 2011;50:261–6. [DOI] [PubMed] [Google Scholar]
- Akbari M, Ostadmohammadi V, Tabrizi R, Lankarani KB, Heydari ST, Amirani E, et al. The effects of melatonin supplementation on inflammatory markers among patients with metabolic syndrome or related disorders: a systematic review and meta-analysis of randomized controlled trials. Inflammopharmacology. 2018;26:899–907. [DOI] [PubMed] [Google Scholar]
- Mesri Alamdari N, Mahdavi R, Roshanravan N, Lotfi Yaghin N, Ostadrahimi AR, Faramarzi E. A double-blind, placebo-controlled trial related to the effects of melatonin on oxidative stress and inflammatory parameters of obese women. Horm Metab Res. 2015;47:504–8. [DOI] [PubMed] [Google Scholar]
- Rubio-Sastre P, Scheer FA, Gómez-Abellán P, Madrid JA, Garaulet M. Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. Sleep (Basel). 2014;37:1715–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Bushman FD, FitzGerald GA. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc Natl Acad Sci USA. 2015;112:10479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159:514–29. [DOI] [PubMed] [Google Scholar]
- Voigt RM, Summa KC, Forsyth CB, Green SJ, Engen P, Naqib A, et al. The Circadian Clock Mutation Promotes Intestinal Dysbiosis. Alcohol Clin Exp Res. 2016;40:335–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherji A, Kobiita A, Ye T, Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell. 2013;153:812–27. [DOI] [PubMed] [Google Scholar]
- Winer DA, Luck H, Tsai S, Winer S. The Intestinal Immune System in Obesity and Insulin Resistance. Cell Metab. 2016;23:413–26. [DOI] [PubMed] [Google Scholar]
- Weger BD, Gobet C, Yeung J, Martin E, Jimenez S, Betrisey B, et al. The Mouse Microbiome Is Required for Sex-Specific Diurnal Rhythms of Gene Expression and Metabolism. Cell Metab. 2019;29:362–382.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani PD, Bäckhed F. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab. 2015;22:658–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomans CP, van den Berg SA, Houben T, van Klinken JB, van den Berg R, Pronk AC, et al. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J. 2013;27:1721–32. [DOI] [PubMed] [Google Scholar]
- Coomans CP, van den Berg SA, Lucassen EA, Houben T, Pronk AC, van der Spek RD, et al. The suprachiasmatic nucleus controls circadian energy metabolism and hepatic insulin sensitivity. Diabetes. 2013;62:1102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettner NM, Mayo SA, Hua J, Lee C, Moore DD, Fu L. Circadian Dysfunction Induces Leptin Resistance in Mice. Cell Metab. 2015;22:448–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi B, Bellet MM, Katada S, Astarita G, Hirayama J, Amin RH, et al. PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab. 2010;12:509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Liu A, Weidenhammer A, Cooksey RC, McClain D, Kim MK, et al. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology. 2009;150:2153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa MJ, So AY, Kaasik K, Krueger KC, Pillsbury ML, Fu YH, et al. Circadian rhythm gene period 3 is an inhibitor of the adipocyte cell fate. J Biol Chem. 2011;286:9063–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Ravinet-Trillou C, Beeské S, Avenet P, Pichat P. Mice Deficient in Cryptochrome 1 (Cry1−/−) Exhibit Resistance to Obesity Induced by a High-Fat Diet. Front Endocrinol. 2014;5: 10.3389/fendo.2014.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst GT, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–30. [DOI] [PubMed] [Google Scholar]
- Vitaterna MH, Selby CP, Todo T, Niwa H, Thompson C, Fruechte EM, et al. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad Sci USA. 1999;96:12114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay JL, Shostak A, Leliavski A, Tsang AH, Jöhren O, Müller-Fielitz H, et al. High-fat diet-induced hyperinsulinemia and tissue-specific insulin resistance in Cry-deficient mice. Am J Physiol Endocrinol Metab. 2013;304:E1053–63. [DOI] [PubMed] [Google Scholar]
- Bugge A, Feng D, Everett LJ, Briggs ER, Mullican SE, Wang F, et al. Rev-erbα and Rev-erbβ coordinately protect the circadian clock and normal metabolic function. Genes Dev. 2012;26:657–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, et al. Regulation of Circadian Behavior and Metabolism by Rev-erbα and Rev-erbβ. Nature. 2012;485:123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 2012;485:62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]