Abstract
The activity/rest rhythm of mammals reflects the output of an endogenous circadian oscillator entrained to the solar day by light. Despite detailed understanding of the neural and molecular bases of mammalian rhythms, we still lack practical tools for achieving rapid and flexible adjustment of clocks to accommodate shift-work, trans-meridian jet travel, or space exploration. Efforts to adapt clocks have focused on resetting the phase of an otherwise unaltered circadian clock. Departing from this tradition, recent work has demonstrated that bifurcation of circadian waveform in mice facilitates entrainment to extremely long and short zeitgeber periods. Here we evaluate the formal nature of entrainment to extreme non-24 h days in male Syrian hamsters. Wheel-running rhythms were first bifurcated into a 24 h rest/activity/rest/activity cycle according to established methods. Thereafter the 24 h lighting cycle was incrementally adjusted over several weeks to 30 h or to 18 h. Almost without exception, wheel-running rhythms of hamsters in gradually lengthened or shortened zeitgebers remained synchronized with the lighting cycle, with greater temporal precision observed in the former condition. Data from animals transferred abruptly from 24 h days to long or short cycles suggested that gradual adaptation facilitates but is not necessary for successful behavioral entrainment. The unprecedented behavioral adaptation following waveform bifurcation reveals a latent plasticity in mammalian circadian systems that can be realized in the absence of pharmacological or genetic manipulations. Oscillator interactions underlying circadian waveform manipulation, thus, represent a tractable target for understanding and enhancing circadian rhythm resetting.
Keywords: entrainment, circadian, oscillator interactions, T cycles, hamster
Introduction
There exists a lamentable mismatch between the demands for productive human labor around the clock and a circadian pacemaker mechanism that is resistant to rapid or permanent adjustment to a phase other than that dictated by the natural day/night cycle [1,2]. The annual cost of shift-work – manifest as low productivity, high accident rates and poor health – has been estimated to exceed $200 billion just in the United States [3]. Applying principles of phase-resetting by light pulses elaborated in animal studies, chronobiological efforts to mitigate costs of human shift-work have focused largely on adjusting circadian phase through exposure to strictly controlled light schedules [4-8]. This strategy entails numerous challenges including the inherent relative weakness of human circadian phase-resetting, inconsistent work schedules, and avoidance of competing entrainment cues from the environment [4].
Alternative targets for circadian manipulation – e.g., pacemaker amplitude or waveform – have received considerably less attention than phase (but see [9,10]). With regard to waveform, plasticity in the shape of the daily oscillation likely reflects an adaptation to seasonally changing day lengths, and circadian pacemakers of many animals, including those of humans, are capable of flexibly adjusting the relative lengths of subjective day and night [11,12]. This seasonal modulation of waveform, moreover, alters the sensitivity and responsiveness of the pacemaker to phase-resetting actions of light [13-19]. Other, if less ecologically relevant, changes in circadian waveform include the appearance of anti-phase bimodality (i.e., “splitting”) that occurs in animals following prolonged exposure to constant conditions [12,20] and the additive expression in some species of both entrained and free-running components near the limits of entrainment (e.g., on a 22 h day) [21-23].
We have previously described an alternative waveform manipulation in three rodent genera that affords flexible entrainment configurations [24]. Strongly facilitated by the addition of extremely dim illumination during each scotophase [25], exposure to a 24 h light:dark:light:dark (LDLD) cycle can rapidly and reliably bifurcate the circadian waveform into a rest/activity/rest/activity rhythm in nearly all experimental subjects. This entrainment pattern represents a temporal reorganization of oscillatory components within the neural pacemaker, the suprachiasmatic nuclei (SCN) [26-28], generates bimodal circadian control of melatonin secretion and body temperature [29-31], and can be maintained under a variety of LDLD conditions [30,32,33]. The bifurcated mouse SCN exhibits lower amplitude rhythms of Per2 expression and enhanced phase resetting measured in ex vivo hypothalamic slices [28]. While consequences for health and sleep have yet to be rigorously evaluated, bifurcated animals continue to breed well and outperform experimentally jet-lagged mice in a fear-conditioning protocol [34].
Furthermore, once bifurcated under dim light, hamsters will rapidly re-entrain to standard 24 h LD cycle of any phase, reducing behavioral jet-lag by approximately 70 percent compared to standard entrainment [35]. Bifurcated mice, moreover, are capable of instantaneous and stable entrainment to LDLD cycles as long as 30 h or LD cycles as short as 18 h, well beyond conventional limits of entrainment [36,37]. Apart from bifurcation, the same dim nocturnal scotophase illumination that facilitates rhythm bifurcation also accelerates re-entrainment to shifts in the LD cycle and expands the upper and lower range of entrainment [38-40].
The investigation of experimental protocols that facilitate control of daily activity outside of a traditional 24 h day should improve our basic understanding of circadian plasticity and its translational potential. In the present study, entrainment of the bifurcated circadian waveform of male hamsters was comprehensively examined under gradually lengthening or shortening non-24 h days to characterize the degree and nature of period and phase control. With LDLD cycles of alternating bright and dim light to manipulate waveform and period simultaneously, rhythms of hamsters were entrained so that rest and activity were reliably and predictably distributed across the 24 h clock cycle, a prima facie goal in shift-work scheduling. The results demonstrate a latent plasticity in mammalian circadian systems that can be realized in the absence of pharmacological or genetic manipulations. The behavior of the system in response to various analytical probes was additionally characterized. Differences between rodent and human circadian systems notwithstanding, the results suggest a close connection between circadian waveform and entrainability that may be fruitfully explored for its potential to mitigate the harms associated with industrial demands on humans at all hours of the day and night.
Methods
Animals: All procedures were approved by the UCSD Institutional Animal Care and Use Committee. Male Syrian hamsters (HsdHan: AURA, Harlan, Indianapolis, IN), 5-6 weeks were provided with food and water ad libitum. Photophases were illuminated with fluorescent bulbs that generated intensities of 30 – 100 lux inside the cages. Scotophases were very dimly illuminated by low voltage LEDs that produced an intensity of < 0.1 lux, with peak and half bandwidths of 561 and 23 nm, respectively, an irradiance of < 1.3 x 10-8 W/cm2 and photon flux of <5 x 1010 photons/cm2sec.
Procedure: At the start of the experiment, 18 hamsters were transferred to individual running wheel cages (48 x 27 x 20 cm equipped with 17 cm diameter wheels). To induce bifurcation, they were held for 2 weeks in LD20:4 and then transferred to LDLD8:4:8:4. After 7 weeks, the LDLD cycle was increased or decreased incrementally to T30 (n=8) or T18 (n=10), respectively. In both cases, the scotophases remained 4 h in duration, and only the length of the photophase was altered. T cycles were adjusted by 30 minutes approximately every 7-10 days as specified in Table 1. Due to a built-in symmetry in the selected LDLD cycles, the T30, T24, and T18 LDLD cycles can be more parsimoniously described as T15, T12, and T9 LD cycles, respectively. Because prior work strongly suggested that the T12 rhythm derives mechanistically from two 24 h oscillations in anti-phase [24,26,41], the LDLD designations have been preferred for this report.
Table 1. Schedule and photoperiod of LDLD T cycles used in the experiment.
| Long Ts | Short Ts | |||||
| T (h) | Dark duration (h) | # cycles | T (h) | Dark duration (h) | # cycles | |
| T24.0 | 4 | 48 | T24.0 | 4 | 48 | |
| T24.5 | 4 | 7.5 | T23.5 | 4 | 7.5 | |
| T25.0 | 4 | 8 | T23.0 | 4 | 9 | |
| T25.5 | 4 | 9.5 | T22.5 | 4 | 9.5 | |
| T26.0 | 4 | 8.5 | T22.0 | 4 | 10 | |
| T26.5 | 4 | 9 | T21.5 | 4 | 11 | |
| T27.0 | 4 | 9 | T21.0 | 4 | 11.5 | |
| T27.5 | 4 | 8.5 | T20.5 | 4 | 11.5 | |
| T28.0 | 4 | 11.5 | T20.0 | 4 | 16 | |
| T28.84 | 4 | 9.5 | T19.5 | 4 | 14.5 | |
| T29.0 | 4 | 7 | T19.0 | 4 | 10 | |
| T29.5 | 4 | 8.5 | T18.5 | 4 | 14.5 | |
| T30.0 | 4 | 50.5 | T18.0 | 4 | 84 | |
| T30.0 | 6 | 16.5 | T18.0 | 6 | 27.5 | |
| T30.0 | 8 | 14 | T18.0 | 8 | 23 | |
| T30.0 | 10 | 13 |
The two dark and two light phases were always equal in length. Changes in T values were achieved by lengthening or shortening the light phases.
Because of the apparent robustness of behavioral entrainment as the experiment progressed, a preliminary assessment of the role of entrainment history in adapting to these T cycles was conducted post hoc. Accordingly, six additional age-matched male hamsters were transferred directly from LD14:10 into the long or short T cycles at the point in the experiment when T=28.8 h (n=4) and T=19.5 h (n=2), respectively. Thereafter, these additional animals were exposed to identical conditions as the other experimental subjects.
To assess whether light was directly suppressing wheel-running activity after prolonged exposure to T30 and T18, we introduced dark pulses of 3 h and 2 h duration, respectively, in the middle of chosen photophases on three occasions (n=12 in T30 and T18, respectively). Durations were chosen with hopes that they would be sufficiently long to detect de-masking effects but not interfere with ongoing entrainment. In a further effort to evaluate entrainment versus masking effects of light, scotophase duration was progressively increased in 2 h increments from 4 h to 8 h in both T18 and T30 with corresponding symmetric reductions of the photophases (Table 1). Subsequently, the T30 group was exposed to an additional increase in scotophase duration to 10 h. A computer failure resulted in loss of data and lighting for 16 and 24 h, respectively when animals were in T18 and T30 with 4 h scotophases. Restoration of the light cycle was arranged to coincide with the next scheduled light onset.
Analysis: Wheel-running activity was recorded with the VitalView monitoring system using 6 min bins, and analyzed in ClockLab (Actimetrics, Wilmette, IL) and Excel (Microsoft, Redmond, WA) software. For quantification of phase angles of entrainment, activity onsets were identified as the first time point meeting an arbitrarily selected threshold of 30 counts/min following a 90 min interval of inactivity, and the onsets associated with the two alternating dark periods were processed separately. For each sequence, variance in onsets was calculated separately for each scotophase of LDLD consisting of seven LDLD cycles. Where there was no activity in a scotophase, no activity onset was recorded and no value entered in the variance estimate.
To complement analysis of phase angles, steady-state entrainment in T18 and T30 was evaluated with respect to period control with an entrainment quotient (EQ) based on Lomb-Scargle periodograms calculated over 16-21 day intervals of uninterrupted data collection in each LDLD photoperiod in T18 and T30. Periodogram amplitude (arbitrary units) matching T (9 h/15 h rather than 18 h/30 h because of the cosinar basis of Lomb-Scargle calculations) was noted and expressed relative to the sum of periodogram period at T and the peak power in the circadian (i.e., 22-26 h) range. Thus, EQ = Power @ T/(Power @ T + Peak Power @ 22-26 h). If EQ approaches 1.0, then the behavioral rhythm matches the period of the external cycle and there is no free-running rhythmicity. Values near 0 indicate that no rhythmicity matches the imposed lighting cycle. Whereas EQ does not incorporate an assay of behavior in DD – an important criterion for entrainment – EQ measures have been shown to predict phase control upon release into constant conditions in prior studies of mice [36,37].
Statistics comparisons were made with within-subjects repeated measures (ANOVA), paired t-tests or between-subjects t-tests with α set to 0.05.
Results
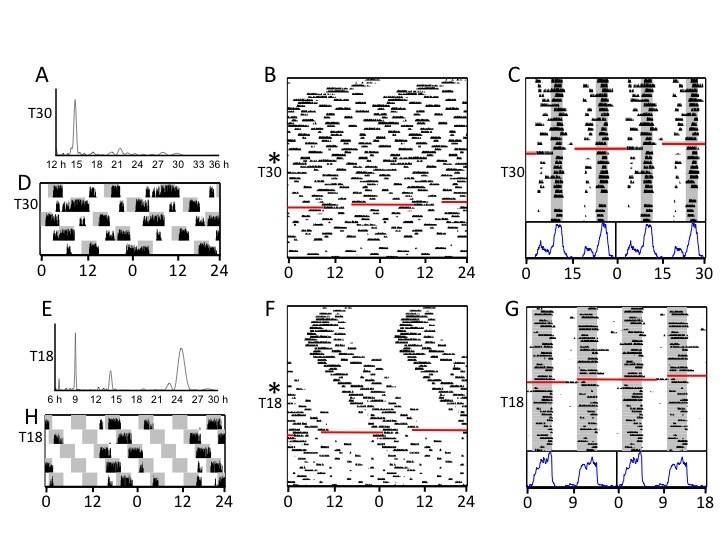
Each of 18 male Syrian hamsters exhibited bifurcated activity rhythms in LDLD8:4:8:4 (T24) with dimly illuminated (< 0.1 lux) scotophases. Subsequently, each of eight animals that were exposed to incrementally lengthening LDLD cycles maintained a clearly bifurcated activity pattern that evidenced no visually obvious loss of entrainment even as the LDLD cycle reached T30 (Figure 1A). The remaining 10 hamsters experienced gradually shortening LDLD cycles that eventually reached T18 (Table 1). As in the representative individual running wheel record shown (Figure 1B), they too retained bifurcated rhythms with activity confined to dark periods, although, in the shortest T cycles, activity was periodically absent from individual dark phases, suggesting a compromised degree of period and phase control (see below).
Figure 1.
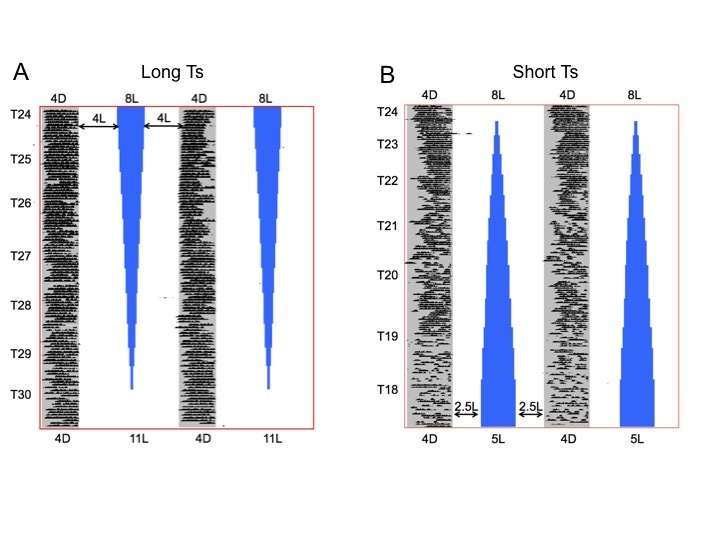
Representative single-plotted wheel-running actograms of individual hamsters exposed to LDLD cycles incrementally lengthening or shortening from T=24 h to T=30 h (A) or T=18 h (B), respectively. In both cases, the length of each of the two dark phases was fixed at 4 h. To align the entire actogram vertically and highlight the changes in entrainment with respect to dark onset, the representation of intervening light phases is systematically distorted. White areas represent light while blue areas are space holders that represent no time but allow alignment of the scotophases throughout the transition from T24 to T30 or T18, respectively. In all LDLD cycles in this study scotophases (D) were dimly illuminated (561 nm LEDs provided constant dim illumination of < 0.1 lux). The actograms illustrate the robust control of activity throughout the experiment in both groups, the more variable pattern of activity onsets in T18 versus T30 and the absence of apparent free-running rhythmicity.
Figure 2 displays the mean phase angle difference measured between activity onset and dark onset (ψA,D) of the alternating activity bouts for each condition. In contradiction of non-parametric entrainment theory based on the generalized prediction from the species phase response curve (PRC) and free-running period (τ) in darkness, activity onsets occurred progressively later relative to light offset as T lengthened throughout the experiment (Figure 2A). Also, as T lengthened, day-to-day within-subjects variability in the phase angle of activity (ψA,D) increased, indicating reduced phase control (Figure 2C). In shortening T cycles, the predicted delay in activity onsets relative to lights off occurred only until T23 (Figure 2B). At that point, the two activity bouts exhibited transiently different onset phase angles before establishing a similar and stable phase relationship to light offset. Within-subjects variability in the phase of activity onset also increased as T shortened and was markedly greater than observed in long Ts. Both of these unexpected effects, on phase angle of entrainment and on within-subject variability, are evident in the individual representative actograms (e.g., Figure 1A, B), illustrating that they are not artifacts of between-subject averaging.
Figure 2.
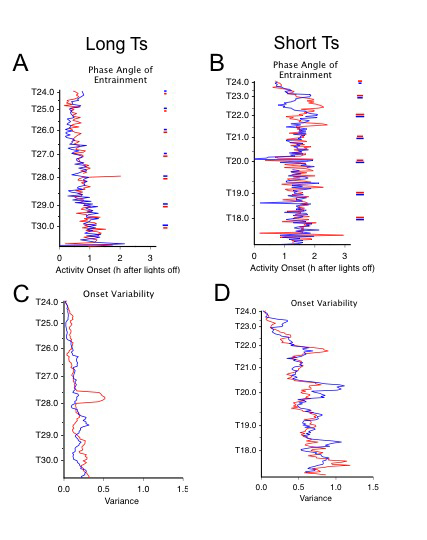
Mean phase angle of entrainment (A, B) and its within-subject variance (C, D) are depicted for the activity onsets associated with each of the two bifurcated activity bouts (blue and red lines) under lengthening (A, C; n=8) and shortening (B, D; n=10) LDLD T cycles. For phase angle of entrainment, activity onset is plotted in hours relative to dark onset (ψA,D) such that activity onsets following dark onset are indicated by positive values. The between-subjects variability in this measure (one standard error of the mean) is represented by correspondingly colored horizontal lines to the far right in panels A and B, displayed only at hourly values of T. The variance in phase angle represented in C and D was calculated over 5-7 days for each bout in each animal and then averaged across subjects. According to classical entrainment theory, entrainment results when the daily phase shift (Δϕ) equals the difference between the free-running period and zeitgeber period (τ - T). Consequently, progressively larger phase delays and advances are required when T lengthens and shortens, respectively. Based on the extensively characterized light pulse PRC in hamsters, increased phase delays under lengthening Ts should be achieved by light falling later into subjective night: i.e. if the L/D transition is equivalent in effect to the light pulse used to derive a PRC, activity onsets should occur progressively earlier relative to dark onset. Despite successful entrainment, this predicted relationship is not observed. Similarly, under shortening Ts, increased phase advances should be achieved by light falling progressively earlier into late subjective night, and activity onsets should increasingly lag dark onset, so that phase advances will increase as the timing of the D/L transition moves earlier in the subjective night. Such a relationship is apparent only up until ~ T = 22 h.
The quality of behavioral entrainment was more thoroughly examined, both subjectively and objectively, during an extended interval (54 and 32 cycles in T18 and T30, respectively) that was unchanging but for a computer failure that resulted in loss of data for ~16 h and loss of lighting for ~24 h. Actograms from two representative animals (different from those in Figure 1) are double-plotted modulo-T (Figure 3C, G) as well as modulo-24 h (Figure 3B, D, F, H). The modulo-T format especially facilitates visualization of entrainment to the prevailing T; the modulo-24 h plots serve to render more visually apparent any free-running rhythmicity in the circadian range (i.e., near 24 h). In T30, this representative hamster, like all eight hamsters, exhibited rhythms with activity uniformly divided between alternating scotophases (Figure 3C). The periodograms (Figure 3A) and visual inspection of actograms, whether plotted modulo-24 h (Figure 3B) or modulo-T (Figure 3C), failed to reveal any subjective evidence for activity bouts free-running with periods close to 24 h. In T18, in contrast, all 10 hamsters showed a visually apparent, but often transient, non-entrained circadian rhythm (Figure 3F), manifest as a short inactive period that recurred at ~24 h or slightly longer periods. This was not always apparent in the periodogram analysis (Figure 3E). In two of 10 cases, this appeared only following the lighting failure, but in the majority of cases the appearance of this ~ 24 h rhythmicity anteceded the lighting malfunction (Figure 3F).
Figure 3.
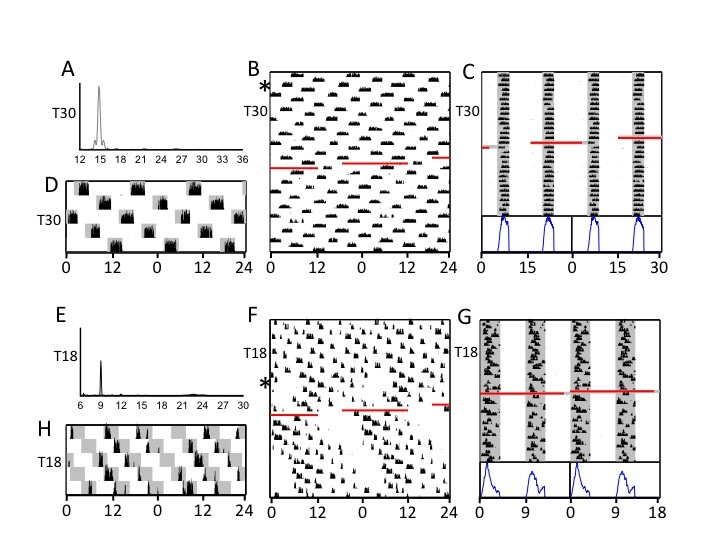
Representative activity data from individual hamsters during exposure to T30 (A-D) and T18 (E-H). Approximately 6 weeks of data under these conditions are double-plotted on a 24 h abscissa (B, D, F, H) or modulo-T (C, G). Curves at the bottom of panels C and G are educed waveforms of activity counts folded at modulo-T and averaged across all cycles depicted in actograms above. Lomb-Scargle periodogram analyses (A, E) were conducted on the first 19 days of the record prior to a short interval of missing data, indicated by a red line. Also shown (D, H) is 120 h of double-plotted activity modulo-24 h with shading representative of dark periods for assessment of light masking. Asterisks (in B, F) indicate starting points of 120 h blow-up plots (D, H). The actograms and periodograms illustrate the robust steady-state entrainment of hamsters in T30 versus the more variable entrainment in T18.
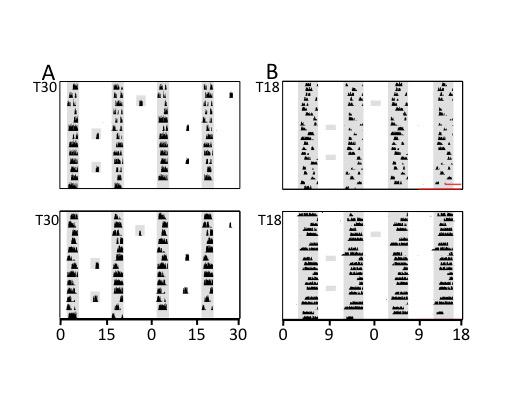
When age-matched hamsters without a lengthy history of bifurcation and gradually changing T cycles were transferred directly from LD14:10 into the long or short T cycles at T=28.8 h (n=4) and T=19.5 h (n=2), respectively, they took varying amounts of time to match the behavior of the pre-established groups. Two of the former hamsters adopted activity patterns indistinguishable from those of their gradually entrained counterparts after just a single LDLD cycle (Figure 4A,B); a third bifurcated after one cycle and was rapidly entrained with an unusually positive phase angle (wheel running onset came in advance of lights out for several weeks, Figure 4C; 5A-D). The fourth took more than a week to bifurcate, thereafter running more in darkness, but also during photophases (Figure 4D). In T19.5 and shorter cycles, one of two hamsters was transiently bifurcated in the first week (Figure 4E), whereas the other retained a conventional unbifurcated rhythm for several weeks with strong negative masking of activity by the photophases (Figure 4F, 5E-H). Thus, despite a visually obvious free-running rhythm with τ ~ 24.6 h (Figure 5F), the activity profile creates an illusory impression of entrainment, presumably due to a strong masking response (Figure 5G).
Figure 4.
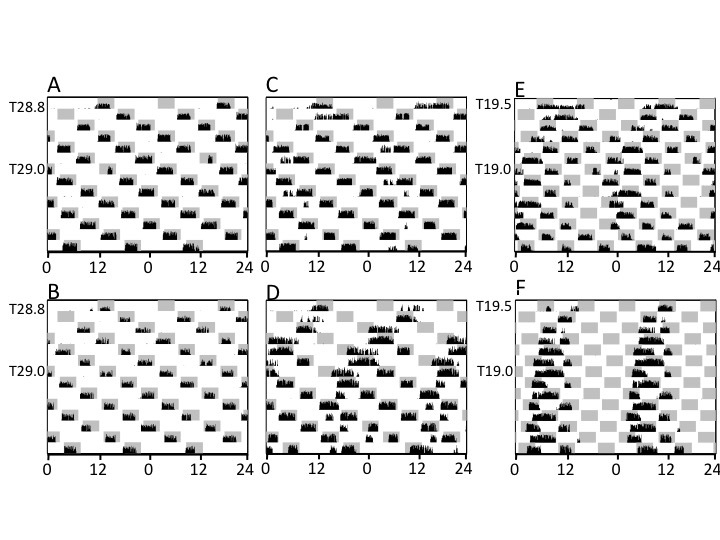
Double-plotted modulo-24 h actograms of all 6 hamsters commencing at the abrupt transfer from LD14:10 to long (A-D; T28.8) and short (E, F; T19.5) LDLD cycles. Conventions as in Figure 3. Actograms illustrate the diversity of responses to abrupt exposure to extreme T cycles and the close association of immediate bifurcation with high quality entrainment.
Figure 5.
Representative activity data from individual hamsters transferred abruptly from LD14:10 to long (A-D) and short (E-H) T cycles. Data double-plotted modulo-24 h (B, F) span the time from the initial transfer to T28.8 or T19.5 until they had been in T30 or T18 for 6 weeks. Data plotted modulo-T (C, G) include only intervals under final T cycles. Periodogram analyses (A, E) were conducted on the first 19 days of T30 or T18. The 120 h blow-up plots (D, H) correspond to beginning of the exposure to T30 and T18, respectively. The actograms illustrate the atypically entrained or free-running patterns of behavior resulting from abrupt transfer to extreme T cycles.
The entrainment configuration under T30 and T18 was further assessed by administering three dark probes (3 h in T30, 2 h in T18) timed to coincide with the middle of a scheduled light phase. Dark probes administered in T30 induced robust wheel-running activity in every instance (Figure 6A; 6485 ± 536 counts/3 h, mean ± sem). For each of three identical probes, activity onsets during the darkness occurred significantly later than in the previous, regularly scheduled dark phases (probes 1-3: t(11) = 2.9, 6.5 and 3.1, respectively; p < 0.05 for each; Cohen’s d = 0.8, 1.9 and 0.9, respectively; overall means = 1.8 vs 1.2 h for probes vs prior dark phases). The probes also perturbed activity in the regular dark period that followed it: Following all three probes, activity levels in the next scheduled scotophase were significantly reduced compared to the scotophase prior (probes 1-3: t(11) = 5.0, 3.9, 7.3, respectively; p < 0.01 for each; Cohen’s d = 1.4, 1.1, and 2.1, respectively; overall means = 4627 vs 8738 counts/4 h for pre- and post-probe dark phases). Moreover, activity onsets post-probe were delayed after two of three probes (probes 1-3: t(11) = 4.0, 1.6, and 6.7, respectively; p < 0.01 for probes 1, 3, p = 0.13 for probe 2; Cohen’s d = 1.1, 0.5, and 1.9, respectively; overall means = 2.0 vs 1.2 h for probes vs prior dark phases). This pattern did not persist beyond the first bout post-probe, suggesting a rapid return to the pre-existing state of entrainment. In contrast, dark probes administered in T18 failed to induce any activity in almost all cases (Figure 6B), and wheel-running counts > 1000 were detected in only two of 36 possible instances (not shown).
Figure 6.
Representative actograms of 2 hamsters in T30 (A) or T18 (B) including exposure to 3 h (T30) or 2 h (T18) dark probes (shaded areas) interpolated in the middle of scheduled light phases. Dark probes illustrate that light pulses are having actively suppressive effects on activity in T30 but not in T18.
Finally, exposure to a sufficiently long duration light phase was critical for maintaining phase and period control. Plotted modulo-T and modulo-24 h, respectively, Figures 7A and 7B illustrate the change in the activity rhythm of a T30 hamster as the duration of the dark phase is lengthened (and photophase shortened) in 2 h increments. In this representative subject, period control persists through LDLD 9:6:9:6 and LDLD 7:8:7:8. Only in LDLD 5:10:5:10 is there evidence of a typical circadian division between subjective day and night (Figure 7B) and any emergence of periodogram power in the circadian range (Figure 7C). Representing the T18 condition, the activity record depicted in Figures 7D and 7E began with good period control in LDLD 5:4:5:4, but rhythmicity emerged at a period near 26 h in LDLD 3:6:3:6, and a conventional unimodal rest/activity rhythm with period just longer than 24 h appeared in LDLD1:8:1:8 (Figure 7F). Considering the entire dataset, EQ values in T30 LDLD are initially very close to unity, indicating strong period control of the rhythm by the zeitgeber. These values are reduced significantly but only very incrementally as scotophase duration is lengthened from 4 to 8 h (Figure 8A). EQs in T30, however, decrease abruptly with 10 h nights. Likewise, EQ values are initially high in T18 LDLD but decline markedly with increases in scotophase duration from 4 to 6 h and from 6 to 8 h. When plotted in terms of the dark to T ratio, EQ values for T18 and T30 LDLD are very similar (Figure 8B). When darkness comprises 2/3 of each cycle EQ values do not differ significantly for T18 vs T30.
Figure 7.
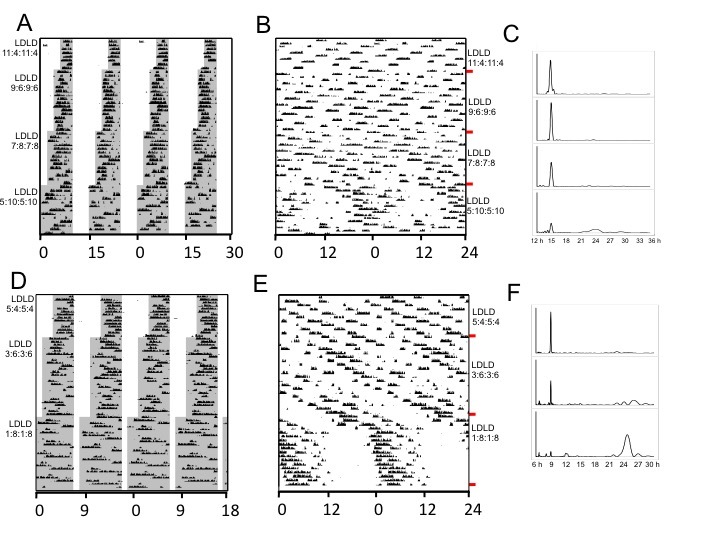
Modulo-T and 24 h double-plotted actograms of representative hamsters in T30 (A, B) or T18 (D, E) under conditions of decreasing proportion of light to dark. Periodogram plots (C, F) for each photoperiod manipulation are shown for the corresponding portions of the actograms (red lines mark photoperiod transitions. These actograms and periodograms demonstrate that entrainment to T30 and T18 is only seriously compromised when the fraction of light to dark is substantially decreased.
Figure 8.
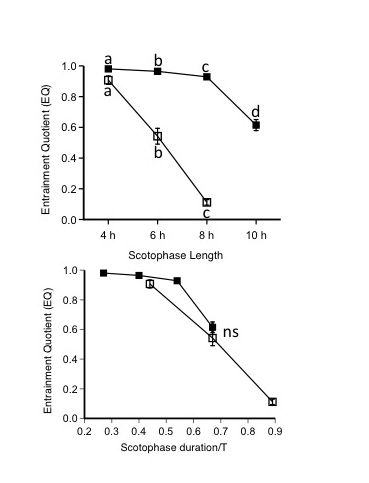
Mean ± sem EQ values for hamsters maintained under T18 (open symbol) and T30 (filled symbol) LDLD conditions plotted as a function of scotophase duration in hours (A) and as a fraction of T (B). Different letters represent significantly different within-group values. Objective measures of the period control indicate that entrainment is compromised as the light:dark ratio decreases.
Discussion
The present study contributes to a small but growing literature that establishes that simple light manipulations may enable extraordinary behavioral entrainment in rodents. As elaborated below, the study a) rigorously demonstrates impressive control of activity in extreme T-cycles; b) illustrates a transition between non-parametric and parametric entrainment mechanisms as T cycles deviate progressively from 24 h; c) suggests that rapid adoption of extraordinary behavioral entrainment is possible and d) excludes a simple masking interpretation of this behavior as evident by responses to de-masking dark pulses and manipulation of photoperiod in T18 and T30.
According to classical circadian theory, entrainment control of rhythm period and phase occurs when a zeitgeber induces a phase shift in each cycle equal to the difference between the period of the circadian pacemaker (τ) and that of the zeitgeber (T) [42]. Accordingly, the equation τ-T = Δϕ predicts the non-parametric phase shift (Δϕ) necessary for entrainment. Further, the entrained phase angle between rhythm and zeitgeber can be predicted from the phase response curve (PRC) because the PRC describes the size and direction of phase shifts elicited in response to light stimuli applied at all different times (phases) of the free-running rhythm. For entrainment to occur, theory therefore demands that the light stimulus must coincide with the phase of the endogenous rhythm where the phase shift corrects for the difference in pacemaker and zeitgeber periods (Δϕ = τ-T) and where the slope of the PRC enhances stability and cycle-to-cycle phase corrections [42,43]. Additionally, the limits of entrainment (range of T) to which a circadian rhythm may successfully entrain may be estimated from the maximum advance and delay responses measured by the PRC. Empirical studies of these relationships in several rodent species under typical laboratory conditions, including completely dark nights, have revealed good qualitative and reasonably close quantitative agreement of observation with prediction [15,43]. Nonetheless, species PRCs are not static, and other studies demonstrate that mammalian phase shift responses to light are modulated by entrainment history, especially by photoperiod and the entrained duration of the subjective night: Thus, in Syrian and Siberian hamsters, and perhaps in humans, a longer duration subjective night (longer active phase in rodents, longer sleep in humans) is associated with larger delay and advance phase shifts [16,17,44,45]. The impact that such photoperiodic modulation of PRC amplitude and waveform might have on entrainment phase angle and limits of entrainment remains largely unexplored.
In mammals, the phase-response curves (PRCs) to light are of lower amplitude and the resulting limits of entrainment are markedly more narrow than those of some other taxa (e.g., invertebrates, prokaryotes) [46,47]. Empirically, hamsters with conventional, unbifurcated rhythms have been observed to break free of entrainment upon increases in T to 25 h [15,48,49] and decreases to 23 h [15,47,49] or 22.5 h [48], and only moderately greater ranges are generally found in rats and mice [46,47,50,51]. In contexts quite distinct from the bifurcated circadian organization studied here, however, the upper and lower limits of entrainment can vary substantially depending on the conditions under which they are assessed [39,52]. Incorporation of twilight transitions in light intensity, for example, extends the entrainment range of hamsters by approximately one hour in each direction [48]. Dim illumination throughout the scotophase likewise facilitates entrainment to more extreme T cycles [39,40] as sometimes can availability of a running wheel [53].
The present results under long T cycles compare with the most extreme examples known to us of entrainment in mammals. Female Syrian hamsters were previously judged to be entrained to T32 (LD16:16) on the basis of their averaged activity profiles, significant periodogram power at T32, and clustering of phases upon release in DD [53]. As masking of a free-running rhythm may produce very similar activity profiles and substantial statistical power at the period of the T cycle (cf. Figure 5E, G), the present study determinedly assayed for rhythmicity in the typical range of free-running circadian rhythms (i.e., 22-26 h). Essentially none was found until the final stage of the experiment when the fractional length of the light phase was markedly reduced. Moreover, the T30 actograms of the present study appear less variable than published examples for T30, T31, and T32 [53] suggesting superior zeitgeber control under the present manipulations. Entrainment to very long T cycles by male hamsters was also reported using dim illumination at night but with LDLD cycles that were not designed to induce rhythm bifurcation [40]. In that study, which employed the same hamster stock and comparable light sources as the current one, entrainment first failed near T27 and only 25 percent of hamsters met the criteria for entrainment at T30. The present results, with uniform adaptation to T30, thus demonstrate an impressive extension of the upper limit of period control of presumed endogenous circadian oscillators by a zeitgeber. Although characterized less extensively, we reported similarly impressive entrainment in mice [36].
By multiple criteria, including increased variability of activity onsets (Figure 2), visually apparent free-running rhythmicity (e.g. Figure 3F) and lower EQ scores (Figure 8), entrainment to shortening T cycles was less successful than to longer Ts. Despite the poorer quality of entrainment, the observed patterns must be distinguished from simple masking of a consolidated free-running rhythm, as previously described for hamsters in LD1:1 or LD3.5:3.5 [54,55]. In this study, such a clear masking pattern was evident only in one hamster transferred abruptly from LD14:10 to T19.5 (Figure 5F), and was categorically distinct from the entire cohort of experimental subjects exposed to incrementally shortened T cycles. Masking by light of a free-running rhythm would likewise predict that dark pulses would elicit activity only when they coincided with a subjective night. If the pacemaker were free-running, these should coincide roughly half the time and not generate the nearly uniform presence or absence of activity observed in T30 and T18, respectively. Finally, a masking mechanism would predict that free-running behavior in darkness would be similar regardless of scotophase duration. On the contrary, we did not observe emergence of free-running activity until the photophase duration was markedly reduced, strongly suggestive of an entraining action.
According to entrainment theory, the time of activity onset relative to the entraining zeitgeber (phase angle difference, Ψ) should progressively lead as T increases (i.e., activity onsets should occur earlier and later relative to dark onset in increasing and decreasing Ts, respectively). This relationship has been amply documented for a variety of species [46] including Syrian hamsters as circadian rhythms entrain to short and long T cycles at the extremes of inherent capability [40,52]. For rodent wheel-running, phase angle difference is conventionally expressed as the time difference between activity onset and lights off, a practice that makes particular sense when considering a system where light at dusk induces phase delays to effect entrainment. Using this traditional definition, however, the phase angle difference measured in the present study throughout the range of entrainment exhibited a dependence counter to that predicted (the same holds true when rendered in angular degrees). The entrainment theory prediction can, nonetheless, be rescued by choosing an alternative, perhaps more meaningful, zeitgeber phase marker (i.e. a point midway or later in the following photophase). This post-hoc maneuver, if valid, implies a critical role of light at circadian times other than that predicted for non-parametric entrainment. The results suggest instead a parametric, likely continuous, entraining action of light throughout the duration of each photophase. Indeed, probes with darkness support this possibility as their introduction into the middle of the photophase induced activity that was comparable in its uniformity and intensity to that in the normally scheduled dark periods. Thus, despite impressive phase and period control and no evidence of a masked, free-running rhythm, this system does not act as a classical non-parametrically entrained oscillator. The possibility of such systems had been anticipated by Aschoff [56] who wrote that as the circadian system “is ‘stretched’ or ‘compressed’ by long or short zeitgeber periods, respectively, [it] may lose its capability to produce self-sustaining oscillations. This phenomenon would result in extremely large or even ‘unlimited’ ranges of entrainment.” In other words, under such conditions, the circadian clock may become essentially a directly driven system. Unfortunately, in the present study, our bifurcated entrainment protocol was not taken to greater extremes to test this idea further. The observation that the timing of activity in probe dark pulses differs somewhat from that in the regular scotophases suggests that activity is not purely driven by the light:dark cycle and that some, but perhaps very weakened, endogenous temporal organization remains.
Under shortening T cycles, the predicted increasing phase lag of the activity rhythm relative to lights off was obtained but only until T22-T23 at which point a constant mean phase angle difference was maintained. Estimates of the mean entrained phase of activity onset, however, did not take account of scotophases in which no activity was expressed. In such cases, the absence of activity could conceivably reflect activity bouts so delayed that they would occur after lights on and therefore be not apparent due to negative masking by light. Arguing against such an interpretation, when animals in T18 were probed with dark pulses, there was little evidence of any negative masking occurring during the photophases. The most salient aspect of the rhythms in shortening T was the increase in day-to-day variation in the phase of activity observed in individual subjects, a feature shared in the wheel-running actogram from the shortest previously published T-cycle (T19.6) known to us [52]. Comparable effects on within-subjects variability in the entrained phase of bifurcated rhythms have been experimentally induced in T24 by changing only the phase relationship between bouts: specifically, when the bouts are moved closer together, the bout that closely follows the other (i.e., the phase lagging bout) exhibits more variable onset times [32].
Regardless of the role of parametric versus non-parametric influences of light, functional, temporal, and environmental parallels all suggest that this extreme entrainment derives from rhythm bifurcation. Functionally, entrainment to extreme T cycles was never observed without activity being divided between the two scotophases. Temporally, the induction of bifurcation and entrainment appear to coincide following abrupt transfer of animals to T28.8 or T19.5: animals that bifurcated quickly also entrained quickly, while animals that did not bifurcate continued to free-run. Entrainment and bifurcation similarly share a requirement for exposure to sufficiently long light phases for their maintenance, although longer photo-fractions can facilitate entrainment separately from any bifurcation in rats [57]. Both phenomena are strongly facilitated by exposure to dim light at night [25,40]. None of these observations alone establishes a causal relationship between bifurcation and enhanced entrainability, but combined with the observation that the relationship between T and phase angle of entrainment is altered in the bifurcated state, this interpretation may be the most parsimonious.
The several month history of gradual entrainment to lengthening T cycles, although generating a uniform response among subjects, was not an absolute necessity for entrainment to the long LDLD T cycles, because two animals adopted the stable, entrained, and bifurcated pattern after just a single LDLD cycle. The small sample size obviously limits some conclusions as does the sole use of male subjects throughout. Further, we cannot exclude the possibility that increasing age contributes to the changes observed as the experiment progressed. These limitations notwithstanding, the results nevertheless raise the strong possibility that an optimized protocol could induce an instant and reliable entrainment to these extreme T cycles, just as refinements have increased the incidence of bifurcation in 24 h LDLD cycles [26,32,58]. It should be stressed that entrainment to T18 and T30 is formally equivalent to a 6 h advance and delay from the solar day, respectively, effected daily. Similar shifts with standard (T=24h) LD cycles typically require 7-11 days in this species, although drugs or lighting variations can speed re-entrainment by 50 percent [38,59]. Only the 5HT1A partial agonist NAN-190 has comparable accelerating effects where 80% of hamsters showed complete 6 h advances in a single cycle [60]. If manipulations of circadian waveform can release similar entrainment flexibility in humans, much more rapid adjustment to shift-work and time zone travel could be an attainable goal. With these extreme LDLD cycles, activity (or rest) can be entrained to occur at any given clock time within just a few cycles. This contrasts starkly with standard unbifurcated rhythms where alignment of activity with a particular clock time can require many days or even weeks.
Acknowledgments
The authors thank Antonio Mora for providing excellent animal care and Thijs Walbeek for comments on the manuscript.
Glossary
- EQ
Entrainment quotient
- LD
light:dark
- LDLD
light:dark:light:dark
- SCN
Suprachiasmatic nuclei
Author Contributions
MRG and JAE designed, conducted and analyzed experiments; MRG drafted manuscript and JAE edited manuscript. Supported by UCSD Academic Senate and R01 grant NICHD-36460 to MRG.
References
- Dumont M, Benhaberou-Brun D, Paquet J. Profile of 24-h light exposure and circadian phase of melatonin secretion in night workers. J Biol Rhythms. 2001. October;16(5):502–11. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Boulos Z, Terman M, Campbell SS, Dijk DJ, Lewy AJ. Light Treatment for Sleep Disorders: Consensus Report: VI. Shift Work. J Biol Rhythms. 1995;10:157–64. [DOI] [PubMed] [Google Scholar]
- Kerin A, Carbone J. Financial opportunities in extended hours operations: managing costs, ricks and liabilities. Lexington (MA): Circadian Technologies; 2003. [Google Scholar]
- Smith MR, Fogg LF, Eastman CI. Practical Interventions to Promote Circadian Adaptation to Permanent Night Shift Work: Study 4. J Biol Rhythms. 2009. April;24(2):161–72. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Johnson MP, Duffy JF, Brown EN, Ronda JM, Kronauer RE. Exposure to Bright Light and Darkness to Treat Physiologic Maladaptation to Night Work. N Engl J Med. 1990. May;322(18):1253–9. [DOI] [PubMed] [Google Scholar]
- Boivin DB, James FO. Circadian adaptation to night-shift work by judicious light and darkness exposure. J Biol Rhythms. 2002. December;17(6):556–67. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. Complete or partial circadian re-entrainment improves performance, alertness, and mood during night-shift work. Sleep. 2004. September;27(6):1077–87. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. Combinations of Bright Light, Scheduled Dark, Sunglasses, and Melatonin to Facilitate Circadian Entrainment to Night Shift Work. J Biol Rhythms. 2003. December;18(6):513–23. [DOI] [PubMed] [Google Scholar]
- Jewett ME, Kronauer RE, Czeisler CA. Light-induced suppression of endogenous circadian amplitude in humans. Nature. 1991. March;350(6313):59–62. [DOI] [PubMed] [Google Scholar]
- Harrison EM, Gorman MR. Changing the waveform of circadian rhythms: considerations for shift-work. Front Neurol. 2012. May;(May):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr TA. Seasonal photoperiodic responses of the human circadian system In: Takahashi JS, Turek FW, Moore RY. Circadian Clocks. Kluwer; 2001. pp. 715–44. [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents - V. Pacemaker structure: A clock for all seasons. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1976;106(3):333–55. [Google Scholar]
- Glickman GL, Harrison EM, Elliott JA, Gorman MR. Increased photic sensitivity for phase resetting but not melatonin suppression in Siberian hamsters under short photoperiods. Horm Behav. 2014;65(3):301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman G, Webb IC, Elliott JA, Baltazar RM, Reale ME, Lehman MN, et al. Photic sensitivity for circadian response to light varies with photoperiod. J Biol Rhythms. 2012;27(4):308–18. [DOI] [PubMed] [Google Scholar]
- Elliott JA. Circadian rhythms, entrainment and photoperiodism in the Syrian hamster. In: BK Follett, Follett DE. Biological Clocks in Seasonal Reproductive Cycles. J. Wright and Sons; 1981. [Google Scholar]
- Goldman BD, Elliott JA. Processing of environmental information in vertebrates. In: Stetson MH, editor. Proceedings in Life Sciences. 1988. p. 203–18.
- Puchalski W, Lynch GR. Characterization of circadian function in Djungarian hamsters insensitive to short day photoperiod. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1988. February;162(3):309–16. [DOI] [PubMed] [Google Scholar]
- Evans J, Elliott J, Gorman M. Photoperiod differentially modulates photic and nonphotic phase response curves of hamsters. Am J Physiol Regul Integr Comp Physiol. 2004;286(3):R539–46. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Elliott J, Takamura T. The Circadian Component in Photoperiodic Induction. Ciba Found Symp. 1984;104:26–41. [Google Scholar]
- de la Iglesia HO, Meyer J. Carpino a, Schwartz WJ. Antiphase oscillation of the left and right suprachiasmatic nuclei. Science. 2000;290(5492):799–801. [DOI] [PubMed] [Google Scholar]
- Campuzano A, Vilaplana J, Cambras T, Díez-Noguera A. Dissociation of the rat motor activity rhythm under T cycles shorter than 24 hours. Physiol Behav. 1998. January;63(2):171–6. [DOI] [PubMed] [Google Scholar]
- Vilaplana J, Cambras T, Campuzano A, Díez-Noguera A. Simultaneous manifestation of free-running and entrained rhythms in the rat motor activity explained by a multioscillatory system. Chronobiol Int. 1997. January;14(1):9–18. [DOI] [PubMed] [Google Scholar]
- De La Iglesia HO, Cambras T, Schwartz WJ, Díez-Noguera A. Forced desynchronization of dual circadian oscillators within the rat suprachiasmatic nucleus. Curr Biol. 2004;14(9):796–800. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Elliott JA. Entrainment of 2 subjective nights by daily light:dark:light:dark cycles in 3 rodent species. J Biol Rhythms. 2003;18(6):502–12. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Elliott JA, Evans JA. Plasticity of hamster circadian entrainment patterns depends on light intensity. Chronobiol Int. 2003;20(2):233–48. [DOI] [PubMed] [Google Scholar]
- Yan L, Silver R, Gorman M. Reorganization of suprachiasmatic nucleus networks under 24-h LDLD conditions. J Biol Rhythms. 2010;25(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Naito E, Nakao N, Tei H, Yoshimura T, Ebihara S. Bimodal clock gene expression in mouse suprachiasmatic nucleus and peripheral tissues under a 7-hour light and 5-hour dark schedule. J Biol Rhythms. 2007;22:58–68. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Harrison EM, Sun J, May D, Ng A, Welsh DK, et al. Circadian rhythm bifurcation induces flexible phase resetting by reducing circadian amplitude [Epub ahead of print] Eur J Neurosci. 2018: 10.1111/ejn.14086 [DOI] [PubMed] [Google Scholar]
- Raiewski EE, Elliott JA, Evans JA, Glickman GL, Gorman MR. Twice daily melatonin peaks in Siberian but not Syrian hamsters under 24 h Light:Dark:Light:Dark cycles. Chronobiol Int. 2012;29(9):1206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal SL, Vakili MM, Evans JA, Elliott JA, Gorman MR. Influence of photoperiod and running wheel access on the entrainment of split circadian rhythms in hamsters. BMC Neurosci. 2005:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman MR, Lee TM. Daily novel wheel running reorganizes and splits hamster circadian activity rhythms. J Biol Rhythms. 2001;16(6):541–51. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Steele NA. Phase angle difference alters coupling relations of functionally distinct circadian oscillators revealed by rhythm splitting. J Biol Rhythms. 2006;21(3):195–205. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Yellon SM, Lee TM. Temporal reorganization of the suprachiasmatic nuclei in hamsters with split circadian rhythms. J Biol Rhythms. 2001;16(6):552–63. [DOI] [PubMed] [Google Scholar]
- Harrison EM, Carmack SA, Block CL, Sun J, Anagnostaras SG, Gorman MR. Circadian waveform bifurcation, but not phase-shifting, leaves cued fear memory intact. Physiol Behav. 2017;169:106–13. [DOI] [PubMed] [Google Scholar]
- Harrison EM, Gorman MR. Rapid Adjustment of Circadian Clocks to Simulated Travel to Time Zones across the Globe. J Biol Rhythms. 2015;30(6):557–62. [DOI] [PubMed] [Google Scholar]
- Harrison EM, Walbeek TJ, Sun J, Johnson J, Poonawala Q, Gorman MR. Extraordinary behavioral entrainment following circadian rhythm bifurcation in mice. Sci Rep. 2016:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walbeek TJ, Gorman MR. Simple Lighting Manipulations Facilitate Behavioral Entrainment of Mice to 18-h Days. J Biol Rhythms. 2017;32(4):309–22. [DOI] [PubMed] [Google Scholar]
- Evans JA, Elliott JA, Gorman MR. Dim nighttime illumination accelerates adjustment to timezone travel in an animal model. Vol. 19. Curr Biol. 2009:R156–7. [DOI] [PubMed] [Google Scholar]
- Chiesa JJ, Anglès-Pujolràs M, Díez-Noguera A, Cambras T. History-Dependent Changes in Entrainment of the Activity Rhythm in the Syrian Hamster (Mesocricetus auratus). J Biol Rhythms. 2006. February;21(1):45–57. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Kendall M, Elliott JA. Scotopic illumination enhances entrainment of circadian rhythms to lengthening light:dark cycles. J Biol Rhythms. 2005;20(1):38–48. [DOI] [PubMed] [Google Scholar]
- Evans JA, Elliott JA, Gorman MR. Dynamic interactions between coupled oscillators within the hamster circadian pacemaker. Behav Neurosci. 2010;124(1):87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CH, Elliott JA, Foster R. Entrainment of circadian programs. Chronobiol Int. 2003;20:741–74. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents - IV. Entrainment: pacemaker as clock. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1976;106(3):291–331. [Google Scholar]
- Burgess HJ, Eastman CI. Short nights attenuate light-induced circadian phase advances in humans. J Clin Endocrinol Metab. 2005;90(8):4437–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Eastman CI. Short nights reduce light-induced circadian phase delays in humans. Sleep. 2006;29:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschoff J, Pohl H. Phase relations between a circadian rhythm and its zeitgeber within the range of entrainment. Naturwissenschaften. 1978;65:80–4. [DOI] [PubMed] [Google Scholar]
- Bruce VG. Environmental Entrainment of Circadian Rhythms. Cold Spring Harb Symp Quant Biol. 1960;25:29–48. [DOI] [PubMed] [Google Scholar]
- Boulos Z, Macchi MM, Terman M. Twilights widen the range of photic entrainment in hamsters. J Biol Rhythms. 2002;17:353–63. [DOI] [PubMed] [Google Scholar]
- Elliott JA. Circadian rhythms and photoperiodic time measurement in mammals. Fed Proc. 1976;35:2339–46. [PubMed] [Google Scholar]
- Usui S, Takahashi Y, Okazaki T. Range of entrainment of rat circadian rhythms to sinusoidal light-intensity cycles. Am J Physiol Integr Comp Physiol. 2000;278:R1148–56. [DOI] [PubMed] [Google Scholar]
- Casiraghi LP, Oda GA, Chiesa JJ, Friesen WO, Golombek DA. Forced desynchronization of activity rhythms in a model of chronic jet lag in mice. J Biol Rhythms. 2012;27(1):59–69. [DOI] [PubMed] [Google Scholar]
- Chiesa JJ, Angles-Pujolras M, Diez-Noguera A, Cambras T. Activity rhythm of golden hamster (Mesocricetus auratus) can be entrained to a 19-h light-dark cycle. Am J Physiol Integr Comp Physiol. 2005;289(4):R998–1005. [DOI] [PubMed] [Google Scholar]
- Chiesa JJ, Díez-Noguera A, Cambras T. Effects of transient and continuous wheel running activity on the upper and lower limits of entrainment to light-dark cycles in female hamsters. Chronobiol Int. 2007;24(2):215–34. [DOI] [PubMed] [Google Scholar]
- Redlin U, Mrosovsky N. Masking of locomotor activity in hamsters. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1999;184:429–37. [DOI] [PubMed] [Google Scholar]
- Li X, Gilbert J, Davis FC. Disruption of masking by hypothalamic lesions in Syrian hamsters. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:23–30. [DOI] [PubMed] [Google Scholar]
- Aschoff J. Freerunning and entrained circadian rhythms In: Aschoff J. Handbook of Behavioral Neurobiology Biological Rhythms. New York: Plenum Press; 1981. pp. 81–93. [Google Scholar]
- Cambras T, Chiesa J, Araujo J, Díez-Noguera A. Effects of photoperiod on rat motor activity rhythm at the lower limit of entrainment. J Biol Rhythms. 2004;19:216–25. [DOI] [PubMed] [Google Scholar]
- Gorman MR. Exotic photoperiods induce and entrain split circadian activity rhythms in hamsters. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2001;187(10):793–800. [DOI] [PubMed] [Google Scholar]
- Agostino PV, Plano SA, Golombek DA. Sildenafil accelerates reentrainment of circadian rhythms after advancing light schedules. Proc Natl Acad Sci USA. 2007;104:9834–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler EJ, Sprouse J, Harrington ME. NAN-190 potentiates the circadian response to light and speeds re-entrainment to advanced light cycles. Neuroscience. 2008;154:1187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]


