Abstract
Stroke is the 5th leading cause of death in the United States and a leading cause of long-term disability. Ischemic strokes account for 87 percent of total stroke cases, yet the only FDA-approved treatments involve disruption of the blood clot to restore blood flow. New treatments aimed at saving or protecting neural tissue have largely failed in clinical trials and so new methodology or targets must be found. The occurrence of strokes significantly increases between 6 AM and 12 PM, implicating the circadian system in the onset of this debilitating brain injury. But it is not known whether or how the circadian system may regulate the response to and recovery from stroke. New strategies to identify treatments for stroke are beginning to look at cell types other than neurons as therapeutic targets, including astrocytes. In this review, we present links between the astrocyte circadian clock, the molecular response to stroke, and the damage caused by ischemia. We highlight aspects of astrocyte circadian function that could dictate new methodologies for stroke treatment, including the potential of chronotherapy.
Keywords: stroke, circadian, astrocyte, ischemia
Introduction
Stroke is a leading cause of long-term disability and is the 5th leading cause of death in the United States. In 2012, stroke was estimated to account for $33 billion in annual direct and indirect medical costs [1]. In its most recent report, the Centers for Disease Control reported an increase in the age-adjusted death rate from stroke between 2016 to 2017 [2], even though stroke mortality has decreased dramatically in the last decade. Despite improved survival, stroke patients suffer long-term cognitive and behavioral deficits due to a lack of therapeutics focused on neural recovery post-stroke. Currently, treatments for stroke are limited to thrombolytics (both chemical and mechanical) that aim to restore blood flow by lysis or surgical removal (thrombectomy) of the thrombus. The lack of blood flow, or ischemia, associated with stroke affects all cell-types of the brain with important physical (proximity to the infarct) and temporal (timing relative to stroke onset) components. These parameters must be considered when researching new stroke treatments. Potential new treatments for neural recovery have focused primarily on neurons, but none have had clinical success. More recently, greater recognition has been given to the important roles of other cell types in the brain, not just neurons, to recovery from brain injury [3]. In particular, astrocyte glial cells are emerging as attractive targets to identify new stroke treatments.
Astrocytes serve many roles in the brain, having connections to both the vasculature and neuronal synapses. Their functions are critical for neuronal maintenance and survival in both the normal and ischemic brain [4,5]. If new stroke treatments aim to target astrocyte function, this necessitates a greater understanding of astrocyte molecular control. Recent work has determined that many of the processes exhibited by astrocytes are rhythmic and display 24-hr, or circadian, oscillations [6,7]. The circadian system governs many aspects of physiology and behavior. The obvious behavioral oscillation governed in-part by the circadian system is the sleep-wake cycle. However, a number of metabolic and hormonal processes also oscillate and have peak times throughout the 24-hr day, including parts of the cardiovascular system that are responsible for stroke onset (Figure 1). While it has long been accepted that circadian physiology contributes to the increased frequency in stroke onset upon awakening and in the early morning hours [8-11], how the circadian system might regulate stroke recovery is unknown. In this review, we will link different aspects of stroke etiology and response with new molecular insights into the circadian regulation of neurobiology, focusing particularly on astrocytes. We hope that illuminating these links may provide new insight into stroke pathology and offer new avenues for novel stroke treatments aimed at long-term neural recovery.
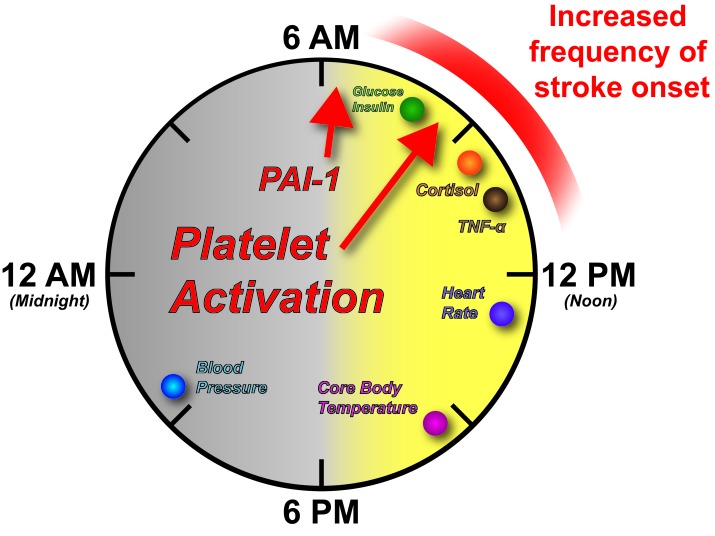
Figure 1.
Human circadian physiology. A variety of human physiologic processes are under circadian control. Peak times of different processes and clock outputs are shown as colored circles. Highlighted with red arrows are the peak times of Plasminogen Activator Inhibitor-1 (PAI-1) and markers of Platelet Activation. Note there is an increased frequency of stroke onset between 6 AM and 12 PM as indicated by the shaded red bar.
Stroke
The main types of stroke occur with varying frequency. Ischemic strokes account for 87 percent of total stroke occurrences, while 10 percent are intracerebral hemorrhage and 3 percent are subarachnoid hemorrhage [1]. This review is focused mainly on the mechanisms in play with ischemic strokes, due to their higher frequency of occurrence. Treatments for stroke are severely limited. Currently, tissue Plasminogen Activator (tPA) is the only FDA-approved pharmacological medication for the treatment of stroke, though thrombectomy now offers a surgical form of treatment that, when used alone or in combination with tPA, has significantly improved functional outcomes [1,12]. tPA acts as a thrombolytic, promoting the lysis of blood clots, or thrombi. However, tPA treatment post-stroke must be administered within a narrow window of time from the onset of stroke (less than 3-4.5 hours), in order to avoid the risk of hemorrhage [13-15]. Consequently, the number of patients eligible to receive the drug is relatively small, less than 5 percent of patients [16]. As previously mentioned, surgical methods relying on the mechanical disruption and removal of blood clots (thrombectomy) offer an additional treatment for clot removal and have extended the window of intervention, though they are still only available for a small proportion of patients [17,18]. But again, therapeutics designed to directly target neural tissue and reduce brain damage and promote recovery have not been identified. In general, the ischemic event created by the stroke sets in motion a secondary cascade of molecular pathways leading to death of more neural tissue and alterations in brain function. It is this neural disruption that leads to long-term disability in patients and its treatment is currently an unmet challenge for the medical community.
Stroke is a major financial burden with annual direct medical costs projected to triple between 2012 to 2030 from $71.6 billion to $184.1 billion [1]. This will be due in large part to the increase in number of people aged 65 to 79, who are at a higher risk for stroke. Annually, the largest percentage of strokes are first-time occurrences. However, stroke itself increases the risk of another stroke occurring. The recurrence rate of stroke was found to rise to 18.1 percent at 4 years after an initial stroke in a large U.S. cohort [1]. Risk factors for stroke include high blood pressure, Diabetes Mellitus, heart arrhythmias, high cholesterol, and nutrition, among others [1]. Many of these risk factors are associated with conditions other than stroke, such as obesity. With a worldwide increase in these risk factors, there is a high likelihood that the incidence of stroke will also experience a consequent increase. Mechanisms addressing neural recovery following stroke clearly need to be pursued, but the etiology of stroke should be considered not just as an isolated, acute event, but also as a neural injury with an important temporal component.
Circadian Physiology and Disease
The incidence of stroke onset increases in frequency upon awakening in humans between 6 AM and 12 PM. This correlation has been confirmed across a number of cohorts worldwide [8,9,11,19]. A variety of subtypes exist for both hemorrhagic and ischemic strokes, including intracerebral hemorrhagic stroke, large artery atherosclerotic stroke (AT), cardioembolic stroke (CE), lacunar stroke (LA), and cryptogenic stroke (CRY). The diurnal peak in stroke onset applies to both hemorrhagic and ischemic stroke, when considered broadly, and when considering subtypes [10,19]. Yet, some differences exist in the diurnal patterning between subtypes. It was observed that LA, CE, CRY, and hemorrhagic subtypes occur more frequently in the early portion of the morning. Atherosclerotic stroke onset occurred broadly throughout the morning [10]. While the majority of ischemic strokes occur upon awakening or throughout the morning, ischemic strokes with known onset during sleep have been reported to have greater severity and result in higher mortality [10,20]. The mechanism behind this is unclear, but an association with sleep-onset strokes and obesity has been found, confounding interpretation of cause-and-effect by other obesity-related conditions such as diabetes and sleep-apnea, which have known associations with stroke [1,20]. However, obesity can directly impact molecular circadian components in rodent models [21,22]. How this translates mechanistically to human stroke is an open question.
Interestingly, adverse cardiovascular events such as stroke and myocardial infarction both show increased frequency of onset upon awakening and throughout the morning hours in humans [23]. This increase lies within the peak time of a number of physiological parameters (Figure 1). Behaviorally, this is when human activity begins to increase, with changes in posture from reclined (sleeping) to inclined during awakening and activity. Our heart rate begins to rise with activity, peaking around 1 PM and core body temperature peaks in late afternoon [24,25]. A number of metabolic and hormonal factors also peak in the early portion of the light phase with peaks in glucose, insulin and cortisol between 6 AM and 12 PM [25,26]. Importantly, several clotting factors display peaks in the vasculature during the peak frequency of stroke onset. These include factors for platelet activation and Plasminogen Activator Inhibitor-1 (PAI-1) (Figure 1) [27,28]. The inflammatory cytokine TNF-α responds to a bacterial (Lipopolysaccharide) challenge with peak expression within the 6 AM to 12 PM window of time as well [24]. The convergence of both peak metabolic and inflammatory physiology within the window of peak frequency for stroke onset begs the question of whether the circadian system also gates the response to stroke. Notably, circadian misalignment, which mimics shift-work, results in reversal of cortisol rhythms, increased blood pressure and creates a prediabetic state by altering glucose and insulin levels [29]. As shift work is associated with an increased risk for ischemic stroke [30], the molecular mechanisms behind circadian disruption should be explored in greater detail in the context of stroke. Similarly, the role the circadian system plays in regulating the response to and recovery from stroke should be explored. We will focus on the role of the circadian system in the ischemic stroke response and recovery in the following sections.
Ischemic Cascade – The Importance of Astrocytes
An ischemic stroke causes a local lack of oxygen and nutrients in the brain due to blockage of blood flow. This nutrient deprivation leads to a metabolic challenge for the different cell types of the brain. The blockage of blood flow during a stroke deprives brain cells not only of oxygen, but also a vital metabolite, glucose. Glucose is the primary energy source utilized by the cells of the brain to produce energy, in the form of ATP. When a clot forms and blocks a blood vessel in the brain, there is a local area of cell death termed the ischemic core (Figure 2) [31]. The cell death in this region is rapid, mainly due to the inability of the cells to produce ATP and due to a massive ionic imbalance produced by accumulation of intracellular sodium, chloride, and calcium (Figure 2). ATP production highlights another important role of astrocytes during ischemia. While neurons depend mainly on glucose import for ATP production, they can also metabolize lactate, shuttled from astrocytes, for ATP production [32,33]. In addition, neurons are more susceptible to acute reductions in glucose, while astrocytes have additional sources of energy, such as significant stores of glycogen, which they can metabolize into glucose for ATP production or lactate for shuttling to neurons [4]. We have also shown that astrocyte mitochondrial metabolism can be modulated to reduce brain infarcts and neuronal damage through purinergic receptor stimulation [34,35] or increased by limiting the capacity for fatty acid oxidation [36].
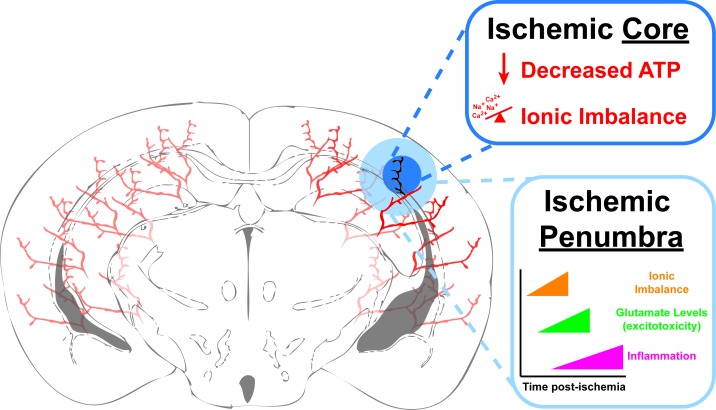
Figure 2.
Ischemic Stroke. Schematic diagram of an ischemic stroke in a coronal section of a murine brain. The blocked blood vessel is shown as black and its immediate vicinity is categorized as the Ischemic Core (dark blue). Within the core, cell death occurs rapidly after the ischemia due initially to decreased ATP production causing an improper ionic balance and osmotic pressure. This in turn leads to edema and depolarization, which in neurons causes massive release of glutamate and excitotoxicity due to opening of calcium (Ca2+) permeable NMDA channels. The area surrounding the Ischemic Core is categorized as the Ischemic Penumbra and is considered “at-risk” tissue that, as the time post-ischemia increases, is at-risk for cell death due to cell lysis form edema, excitotoxicity from excess extracellular glutamate and eventually, inflammation.
The cells around the ischemic core are also at-risk for cell death and this at-risk region is called the ischemic penumbra (Figure 2). The penumbra is the area where new stroke treatments are aimed to help the cells regain or maintain their normal functions, and to limit damage and cell death produced by the ischemic stroke. Both within the core and penumbra, cells are disrupted through a series of molecular mechanisms [3,31,37-40]. Loss of cellular ATP disrupts the ionic balance of both neurons and astrocytes leading to membrane depolarization. This results in an osmotic imbalance that causes cellular edema and an increased release of glutamate from neurons. The increased extracellular glutamate, in turn, causes persistent excitotoxicity, and a massive influx of calcium into cells leading to necrotic cell death. Astrocytes play a significant role in regulating ionic balance near neuronal synapses by taking up glutamate and regulating sodium and hydrogen ion levels. If ATP production can continue via alternate energy stores in astrocytes, they could continue to uptake excitotoxic glutamate from synapses during transient ischemia [4]. Recently, knockout of the Na+/H+ exchanger NHE1, specifically in astrocytes, has proven beneficial in a rodent model of transient ischemia by reducing infarct size and improving blood flow within the ischemic hemisphere [41].
In the hours to days that follow the initial ischemia, there is a systemic inflammatory response that aims to recruit inflammatory cells to the site of injury. Astrocytes become activated and proliferate in a process termed reactive astrogliosis [42]. Astrocytes can also secrete cytokines which can be detrimental to neurons and contribute to cell death [43]. Activated astrocytes form a glial scar around the site of injury in attempt to limit its spread [44]. While activated astrocytes display a wide range of transcriptional heterogeneity ranging from neurotoxic to neurotrophic [45,46], astrocyte reactivity is key to mediating infarct volume and ionic buffering [47]. Indeed, the astrocyte transcriptome in response to ischemia is being mined for potential new stroke therapeutic targets [48].
As can be seen, neurons need astrocytes to survive and astrocytes play a key role in the neural response to ischemia, consequently, neuroprotective treatments for stroke should consider the neuron-astrocyte interactions and functioning of the astrocytes. Astrocytes have multiple unique functions and the importance of the circadian system in regulating these functions has recently been described. Their rhythmic functions imply the circadian system, and/or the astrocyte circadian machinery specifically, as a new potential target for stroke therapeutics.
The Circadian System in Astrocytes
The functional circadian system in astrocytes was first described in 2005 [49]. The core circadian mechanism responsible for rhythm generation and maintenance has grown in complexity and has been reviewed in detail elsewhere [50]. Briefly, the circadian system consists of interlocked transcription/translation feedback loops. The core protein transcription factors CLOCK and BMAL1 heterodimerize and bind to E-Box regulatory elements of the Period (Per1/2/3) and Cryptochrome (Cry1/2) genes, activating their transcription. PER and CRY proteins accumulate in the cytoplasm where, together with Casein kinase 1δ (CK1δ) and CK1ε, they translocate back into the nucleus and disrupt CLOCK and BMAL1, thus shutting off their own transcription. Another loop also exists involving the REV-ERBα/β and RORα/β/γ proteins, which regulate the rhythmicity of BMAL1. The master circadian pacemaker is located within cells of the suprachiasmatic nucleus (SCN) of the hypothalamus in mammals [51]. However, this molecular time-keeping machinery exists in nearly every cell type of the body and governs the timing of most cellular processes and outputs. CLOCK and BMAL1 don’t just regulate transcription of core clock genes. They also have binding sites on thousands of other genes, leading to rhythmic transcription of clock-controlled genes as well [52]. When the astrocyte clock was first described, astrocyte-enriched primary cells were cultured from either Per1:luciferase transgenic rats or Per2:luciferase knockin mice. Realtime recording of luciferase oscillations in vitro revealed the primary astrocytes to have self-sustaining circadian rhythmicity that could entrain and phase shift to different stimuli, including diffusible signals from the co-cultured SCN slices [49].
Subsequent studies looked at circadian signals secreted by astrocytes that may signal to surrounding neurons within the brain and, specifically, within the SCN. Extracellular ATP was found to oscillate with circadian rhythmicity in cultured astrocytes and in rats in vivo [53]. This was later found to be clock-dependent as astrocytes from mice harboring mutations in core clock genes lost their circadian release of ATP [54]. Indeed, older studies found rhythmic ATP accumulation within numerous brain regions, with the most robust amplitude seen within the SCN [55]. In ischemic strokes, one of the key factors leading to neuronal cell death is decreased ATP production. However, ATP released near synapses is rapidly converted to adenosine, which can be neuroprotective by reducing excitotoxicity [4,56]. Because astrocytes possess alternate fuel sources for ATP production, ischemic insults that occur at a time when ATP release or accumulation is at its circadian peak may result in lower cell damage or death.
Astrocytes also play key roles in regulating the release and uptake of glutamate. Recent reports have demonstrated that extracellular glutamate displays circadian accumulation and is out-of-phase with neuronal calcium, but in-phase with astrocytic calcium [6]. This implies that astrocytes mediate the rhythmic release of glutamate, yet older studies report that rhythmic uptake of glutamate by astrocytes, while modulated by the clock, does not display overt circadian rhythmicity [57]. Again, if we look at the disturbances following ischemia, one of the potent forms of cell death is mediated by excitotoxicity from excess release and accumulation of glutamate. Peaks in extracellular glutamate occur in the mid/late biological day in mice, which might make this a window of time where ischemia would produce more damage or cell death as compared with other circadian phases. With disruption of clock genes associated with decreased glutamate uptake by astrocytes [57], this could have implications for stroke severity in shift-workers. While associations of stroke occurrence and shift-work have been made, these molecular findings might imply that shift-workers could also suffer from greater stroke severity. More work in this area is clearly needed.
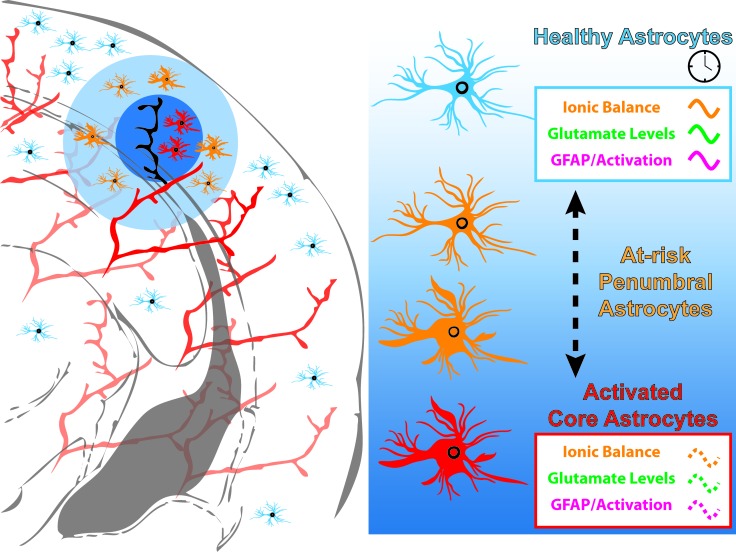
Astrocytes can buffer oxidative stress by scavenging reactive oxygen species via glutathione metabolism [43]. Astrocyte activation occurs both within the ischemic core and the penumbra following stroke. This activation leads to a markedly altered gene transcription profile within astrocytes that can either be neurotoxic or neuroprotective, depending on the extent and type of injury [45,46]. Interestingly, disruption of the core clock machinery within astrocytes by Bmal1 deletion causes astrocyte activation, yet produces a transcriptional profile unique from being either neurotoxic or neuroprotective [7]. However, there was significant dysregulation of transcripts encoding glutathione metabolism intermediates. Bolstering glutathione metabolism partially rescued the astrocytic activation in this astrocyte-clock mutant model. Significantly, wildtype primary neurons co-cultured with primary astrocytes derived from astrocyte-clock mutants displayed greater toxicity when challenged with oxidative stress in vitro. This would imply that behavioral circadian disruptions (e.g. jet-lag) that preceded a neural injury such as stroke, could elicit a different response than if the circadian system was functioning normally. Or, effects of ischemia on the core clock within cells of the ischemic core and penumbra may be different, thus affecting regional differences in astrocyte activation (Figure 3).
Figure 3.
Astrocyte processes are under circadian regulation. The majority of astrocytic processes display varying levels of circadian oscillation. Extracellular glutamate oscillates in-phase with astrocyte intracellular Ca2+ levels. Glial Fibrillary Acidic Protein (GFAP), a marker for activated astrocytes, shows modest circadian oscillation in the cortex of mice. Astrocytes are critical for maintenance of healthy neurons post-stroke and their response to the ischemia of stroke may vary, depending on the time of day the stroke occurs. On the other hand, stroke may disrupt the normal cycling of astrocytic processes and also contribute to the spread of cell damage from the lesion core out to the penumbra as the at-risk penumbral astrocytes switch over to a more activated state.
Conclusions and Perspectives
The circadian regulation of processes throughout the body, including cell types of the brain, necessitates studying its role in disease. Stroke is a leading cause of death within the United States and worldwide, yet current clinical treatments only address the acute blockage of blood flow and not the progressive neural injury that leads to long-term disability or death. In this review, we have highlighted the well-established temporal increase in stroke onset frequency upon awakening between 6 AM and 12 PM. We have deepened the associations between stroke and the circadian system by linking newly reported molecular functions of astrocytes governed by the astrocyte clock. This implicates the circadian system generally, and perhaps specifically within astrocytes, in also mediating the response to stroke and neural recovery after ischemia. Recently, several of these astrocyte processes were found to be under circadian control, including glutamatergic signaling, ionic balance, and astrocyte activation [6,7] (Figure 3).
Circadian clock disruption specifically within astrocytes is beginning to reveal significant alterations in astrogliosis and the response to oxidative stress. These models could be explored in the context of stroke by examining stroke severity and long-term behavioral and circuit-level recovery. A study examining whole-body Bmal1 knockout (Bmal1-/-) and stroke severity found no difference between wildtype and Bmal1-/- littermates using Rose Bengal Photothrombotic Stroke (RBPS) [58]. However, the RBPS model, while inducing focal ischemia, does not replicate all aspects of human stroke [18,59]. Testing these mice in models that mimic other aspects of stroke would be beneficial, as well as using astrocyte-specific clock mutants. Other models of ischemia such as those testing myocardial infarction have shown clear alterations with clock mutants, though different models testing permanent [60] or transient [61] occlusion give different results regarding specific time-of-day responses.
It has been widely reported that essentially all potential new treatments for stroke, specifically addressing the need for limiting neural damage, have failed in clinical trials. Reasons for this could include limitations of cell-type specificity, dosing, or as we have highlighted in this review, circadian time. Different cell types of the brain must be considered with new treatments and, among them, astrocytes play a key role in maintaining neuronal integrity and health. With the emerging evidence for circadian control of astrocyte function and the response to injury, treatments should consider that their molecular targets may be oscillating and may require different doses at different times of day. Importantly, one should consider that patients who are shift-workers or have recently experienced jet-lag may have compromised circadian oscillations and thus necessitate different treatments. A recent search for novel therapeutic stroke targets using activated astrocyte transcriptomics revealed a highly downregulated cluster of genes associated with circadian entrainment [48]. Future studies should examine how, specifically, clock machinery in all cell types is altered by ischemia. If the molecular clock is altered, recent pharmacological interventions that boost clock amplitude [62] or modulate clock gene expression [63] could provide therapeutic value in stroke recovery. Additionally, non-pharmacological interventions that enhance clock function, such as temporal food restriction [64,65], reduce many of the risk factors for stroke such as obesity and diabetes, and mimic forms of calorie restriction or fasting that have been associated with decreased stroke severity [66,67]. How these metabolic paradigms impact astrocyte function could help identify specific molecular therapeutic targets for stroke recovery. Taken together, treatment based on circadian time, or chronotherapy, could offer much needed insight into novel stroke therapeutics.
Acknowledgments
JJS was supported in-part by a T32 Training Grant (Award # 4T32HL007446-35) and a IRACDA Grant (K12GM111726).
Glossary
- tPA
tissue Plasminogen Activator
- PAI-1
Plasminogen Activator Inhibitor-1
- TNF-α
Tumor Necrosis Factor-α
- ATP
adenosine triphosphate
- SCN
suprachiasmatic nucleus
- AT
large artery atherosclerotic stroke
- CE
cardioembolic stroke
- LA
lacunar stroke
- CRY
cryptogenic stroke
Author Contributions
JJS reviewed the literature, wrote the manuscript, and designed the figures. JDL edited the manuscript.
References
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation. 2016;133(4):e38–360. [DOI] [PubMed] [Google Scholar]
- Murphy SL, Xu J, Kochanek KD, Arias E. Mortality in the united states, 2017. NCHS Data Brief. 2018;(328):1–8. [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67(2):181–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Brady JD, Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nat Neurosci. 2007;10(11):1377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Oberheim N, Cotrina ML, Nedergaard M. Astrocytes and ischemic injury. Stroke. 2009;40(3 Suppl):S8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancaccio M, Patton AP, Chesham JE, Maywood ES, Hastings MH. Astrocytes control circadian timekeeping in the suprachiasmatic nucleus via glutamatergic signaling. Neuron. 2017;93(6):1420-35 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lananna BV, Nadarajah CJ, Izumo M, Cedeno MR, Xiong DD, Dimitry J, et al. Cell-autonomous regulation of astrocyte activation by the circadian clock protein bmal1. Cell Rep. 2018;25(1):1-9 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argentino C, Toni D, Rasura M, Violi F, Sacchetti ML, Allegretta A, et al. Circadian variation in the frequency of ischemic stroke. Stroke. 1990;21(3):387–9. [DOI] [PubMed] [Google Scholar]
- Muller JE. Circadian variation in cardiovascular events. Am J Hypertens. 1999;12(2 Pt 2):35S–42S. [DOI] [PubMed] [Google Scholar]
- Ripamonti L, Riva R, Maioli F, Zenesini C, Procaccianti G. Daily variation in the occurrence of different subtypes of stroke. Stroke Res Treat. 2017;2017:9091250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott WJ. Circadian variation in the timing of stroke onset: A meta-analysis. Stroke. 1998;29(5):992–6. [DOI] [PubMed] [Google Scholar]
- Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-pa vs. T-pa alone in stroke. N Engl J Med. 2015;372(24):2285–95. [DOI] [PubMed] [Google Scholar]
- Brott TG, Haley EC, Jr, Levy DE, Barsan W, Broderick J, Sheppard GL, et al. Urgent therapy for stroke. Part i. Pilot study of tissue plasminogen activator administered within 90 minutes. Stroke. 1992;23(5):632–40. [DOI] [PubMed] [Google Scholar]
- Haley EC, Jr, Levy DE, Brott TG, Sheppard GL, Wong MC, Kongable GL, et al. Urgent therapy for stroke. Part ii. Pilot study of tissue plasminogen activator administered 91-180 minutes from onset. Stroke. 1992;23(5):641–5. [DOI] [PubMed] [Google Scholar]
- Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581–7. [DOI] [PubMed] [Google Scholar]
- Fang MC, Cutler DM, Rosen AB. Trends in thrombolytic use for ischemic stroke in the united states. J Hosp Med. 2010;5(7):406–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia NH, Leyden JM, Newbury J, Jannes J, Kleinig TJ. Determining the number of ischemic strokes potentially eligible for endovascular thrombectomy: A population-based study. Stroke. 2016;47(5):1377–80. [DOI] [PubMed] [Google Scholar]
- McCabe C, Arroja MM, Reid E, Macrae IM. Animal models of ischaemic stroke and characterisation of the ischaemic penumbra. Neuropharmacology. 2018;134(Pt B):169-77. [DOI] [PubMed] [Google Scholar]
- Chaturvedi S, Adams HP, Jr, Woolson RF. Circadian variation in ischemic stroke subtypes. Stroke. 1999;30(9):1792–5. [DOI] [PubMed] [Google Scholar]
- Jimenez-Conde J, Ois A, Rodriguez-Campello A, Gomis M, Roquer J. Does sleep protect against ischemic stroke? Less frequent ischemic strokes but more severe ones. J Neurol. 2007;254(6):782–8. [DOI] [PubMed] [Google Scholar]
- Eckel-Mahan KL, Patel VR, de Mateo S, Orozco-Solis R, Ceglia NJ, Sahar S, et al. Reprogramming of the circadian clock by nutritional challenge. Cell. 2013;155(7):1464–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6(5):414–21. [DOI] [PubMed] [Google Scholar]
- Thosar SS, Butler MP, Shea SA. Role of the circadian system in cardiovascular disease. J Clin Invest. 2018;128(6):2157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman SA, Castanon-Cervantes O, Scheer FA, Shea SA, Czeisler CA, Davidson AJ, et al. Endogenous circadian regulation of pro-inflammatory cytokines and chemokines in the presence of bacterial lipopolysaccharide in humans. Brain Behav Immun. 2015;47:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea SA, Hilton MF, Hu K, Scheer FA. Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circ Res. 2011;108(8):980–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J Clin Endocrinol Metab. 2005;90(5):2537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FA, Michelson AD, Frelinger AL, 3rd, Evoniuk H, Kelly EE, McCarthy M, et al. The human endogenous circadian system causes greatest platelet activation during the biological morning independent of behaviors. PLoS One. 2011;6(9):e24549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FA, Shea SA. Human circadian system causes a morning peak in prothrombotic plasminogen activator inhibitor-1 (pai-1) independent of the sleep/wake cycle. Blood. 2014;123(4):590–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106(11):4453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas MV, Garg AX, Iansavichus AV, Costella J, Donner A, Laugsand LE, et al. Shift work and vascular events: systematic review and meta-analysis. BMJ. 2012;345:e4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17(7):796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14(6):724–38. [DOI] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27(2):219–49. [DOI] [PubMed] [Google Scholar]
- Zheng W, Watts LT, Holstein DM, Prajapati SI, Keller C, Grass EH, et al. Purinergic receptor stimulation reduces cytotoxic edema and brain infarcts in mouse induced by photothrombosis by energizing glial mitochondria. PLoS One. 2010;5(12):e14401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Talley Watts L, Holstein DM, Wewer J, Lechleiter JD. P2y1r-initiated, ip3r-dependent stimulation of astrocyte mitochondrial metabolism reduces and partially reverses ischemic neuronal damage in mouse. J Cereb Blood Flow Metab. 2013;33(4):600–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayre NL, Sifuentes M, Holstein D, Cheng SY, Zhu X, Lechleiter JD. Stimulation of astrocyte fatty acid oxidation by thyroid hormone is protective against ischemic stroke-induced damage. J Cereb Blood Flow Metab. 2017;37(2):514–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George PM, Steinberg GK. Novel stroke therapeutics: unraveling stroke pathophysiology and its impact on clinical treatments. Neuron. 2015;87(2):297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47(2):122–9. [DOI] [PubMed] [Google Scholar]
- Zhao LR, Willing A. Enhancing endogenous capacity to repair a stroke-damaged brain: an evolving field for stroke research. Prog Neurobiol. 2018;163-164:5–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79(4):1431–568. [DOI] [PubMed] [Google Scholar]
- Begum G, Song S, Wang S, Zhao H, Bhuiyan MI, Li E, et al. Selective knockout of astrocytic na(+) /h(+) exchanger isoform 1 reduces astrogliosis, bbb damage, infarction, and improves neurological function after ischemic stroke. Glia. 2018;66(1):126–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims NR, Yew WP. Reactive astrogliosis in stroke: contributions of astrocytes to recovery of neurological function. Neurochem Int. 2017;107:88–103. [DOI] [PubMed] [Google Scholar]
- Liu Z, Chopp M. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog Neurobiol. 2016;144:103–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV. Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci. 2015;16(5):249–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, et al. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32(18):6391–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, et al. Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab. 2008;28(3):468–81. [DOI] [PubMed] [Google Scholar]
- Rakers C, Schleif M, Blank N, Matuskova H, Ulas T, Handler K, et al. Stroke target identification guided by astrocyte transcriptome analysis. Glia. 2019;67(4):619–33. [DOI] [PubMed] [Google Scholar]
- Prolo LM, Takahashi JS, Herzog ED. Circadian rhythm generation and entrainment in astrocytes. J Neurosci. 2005;25(2):404–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017;18(3):164–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohawk JA, Takahashi JS. Cell autonomy and synchrony of suprachiasmatic nucleus circadian oscillators. Trends Neurosci. 2011;34(7):349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338(6105):349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womac AD, Burkeen JF, Neuendorff N, Earnest DJ, Zoran MJ. Circadian rhythms of extracellular atp accumulation in suprachiasmatic nucleus cells and cultured astrocytes. Eur J Neurosci. 2009;30(5):869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marpegan L, Swanstrom AE, Chung K, Simon T, Haydon PG, Khan SK, et al. Circadian regulation of atp release in astrocytes. J Neurosci. 2011;31(23):8342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Ishida Y, Inouye S. Circadian rhythms of adenosine triphosphate contents in the suprachiasmatic nucleus, anterior hypothalamic area and caudate putamen of the rat—negative correlation with electrical activity. Brain Res. 1994;664(1-2):237–40. [DOI] [PubMed] [Google Scholar]
- Wei CJ, Li W, Chen JF. Normal and abnormal functions of adenosine receptors in the central nervous system revealed by genetic knockout studies. Biochim Biophys Acta. 2011;1808(5):1358–79. [DOI] [PubMed] [Google Scholar]
- Beaule C, Swanstrom A, Leone MJ, Herzog ED. Circadian modulation of gene expression, but not glutamate uptake, in mouse and rat cortical astrocytes. PLoS One. 2009;4(10):e7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembach A, Stahr A, Ali AA, Ingenwerth M, von Gall C. Sex-dependent effects of bmal1-deficiency on mouse cerebral cortex infarction in response to photothrombotic stroke. Int J Mol Sci. 2018;19(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST. Rodent models of focal stroke: Size, mechanism, and purpose. NeuroRx. 2005;2(3):396–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Tang D, Liu N, Xiong W, Huang H, Li Y, et al. Reciprocal regulation between the circadian clock and hypoxia signaling at the genome level in mammals. Cell Metab. 2017;25(1):73–85. [DOI] [PubMed] [Google Scholar]
- Durgan DJ, Pulinilkunnil T, Villegas-Montoya C, Garvey ME, Frangogiannis NG, Michael LH, et al. Short communication: Ischemia/reperfusion tolerance is time-of-day-dependent: Mediation by the cardiomyocyte circadian clock. Circ Res. 2010;106(3):546–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Nohara K, Park N, Park YS, Guillory B, Zhao Z, et al. The small molecule nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metab. 2016;23(4):610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin P, Dimitry JM, Sheehan PW, Lananna BV, Guo C, Robinette ML, et al. Circadian clock protein rev-erbalpha regulates neuroinflammation. Proc Natl Acad Sci USA. 2019;116(11):5102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15(6):848–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20(6):991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ZF, Mattson MP. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J Neurosci Res. 1999;57(6):830–9. [PubMed] [Google Scholar]
- Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann Neurol. 2010;67(1):41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]