Abstract
Circadian rhythms, or biological oscillations of approximately 24 hours, impact almost all aspects of our lives by regulating the sleep-wake cycle, hormone release, body temperature fluctuation, and timing of food consumption. The molecular machinery governing these rhythms is similar across organisms ranging from unicellular fungi to insects, rodents, and humans. Circadian entrainment, or temporal synchrony with one’s environment, is essential for survival. In mammals, the central circadian pacemaker is located in the suprachiasmatic nucleus (SCN) of the hypothalamus and mediates entrainment to environmental conditions. While the light:dark cycle is the primary environmental cue, arousal-inducing, non-photic signals such as food consumption, exercise, and social interaction are also potent synchronizers. Many of these stimuli enhance dopaminergic signaling suggesting that a cohesive circadian physiology depends on the relationship between circadian clocks and the neuronal circuits responsible for detecting salient events. Here, we review the inner workings of mammalian circadian entrainment, and describe the health consequences of circadian rhythm disruptions with an emphasis on dopamine signaling.
Keywords: Circadian rhythms, Dopamine, Photoentrainment, Circadian disorder, Jet-lag, Shift-work, Desynchrony
Introduction
Circadian rhythms regulate biological processes ranging from gene expression to behavior. The period, amplitude, phase, and waveform of these oscillations are governed by an internal clock that has evolved in a variety of organisms to anticipate events such as sunrise and sunset [1,2]. Proper phase alignment of the circadian pacemaker to environmental timing cues is critical for an organism’s well-being and survival. Darwinian pressures have changed for humans as many of the emergent stressors of modern society burden our ancient circadian physiology. Varying environmental conditions experienced during shift work or transmeridian travel create desynchrony between the time of day and the internal clocks [3]. Additionally, inappropriately timed light exposure from portable hand-held devices present a chronic source of circadian and sleep disruptions [4]. When prolonged, such misalignments result in higher incidences of mood disorders, obesity, cardiovascular disease, and cancer [5]. As such, pathologies associated with circadian dysfunction are increasing at an alarming rate, creating the pressing need to better understand the basis of circadian physiology in order to advance the practice of psychiatry, nutrition, and medicine [6,7].
While the endogenous circadian clock remains functional in constant conditions [8-10], it relies on environmental signals (Zeitgebers) to synchronize the organism’s physiology to daily external rhythms, such as the earth’s 24-hour light:dark (LD) cycle. As reviewed in [11], the synchronization of an organism’s internal rhythms to an external cycle, termed entrainment, requires the molecular clock machinery to align endogenous rhythms with the exogenous daily cycles. In mammals, entrainment by light, termed photoentrainment, is mediated by the light-activated neural circuits originating in the retina that project to the suprachiasmatic nucleus (SCN) through the retino-hypothalamic tract [12-14]. Non-image-forming, irradiance information is primarily transmitted to the SCN by the melanopsin-expressing intrinsically photosensitive retinal ganglion cells (ipRGCs) [15-18]. Located in the basal hypothalamus dorsal to the optic chiasm, the SCN orchestrates rhythms throughout the rest of the brain and body as described in the following reviews [19-21]. The diverse cellular components of the SCN are both necessary and sufficient for circadian rhythm maintenance as surgical ablation of this nucleus produces behavioral arrhythmia, while grafts of neonatal SCN into a SCN-ablated host restores rest-activity rhythms [22-24].
For proper adaptation to a dynamic world, this circadian timing system also requires the ability to anticipate salient events such as food or mate availability. While light is the primary entraining agent, arousal-inducing non-photic cues such as palatable foods, social interaction, and physical exercise also influence the phase of the SCN molecular clock [25,26]. Dopamine (DA), a neurotransmitter mostly known for its role in reward processing and motivation, is a significant modulator of the aforementioned behaviors that drive non-photic circadian entrainment [27,28]. Additionally, patients suffering psychiatric and neurodegenerative pathologies associated with DA signaling dysregulation such as depression, bipolar disorder, schizophrenia, drug addiction, and Parkinson’s disease are known to have perturbations of circadian rhythms [29-33]. As such, DA is emerging as an important regulator of central and peripheral circadian rhythms and has been reviewed in the following publications [34,35]. In this review, we focus on how DA-mediated neural circuits influence our daily rhythms and the attendant consequences of circadian misalignment on well-being, metabolism, and mental health.
Non-photic Entrainment
The regulation of circadian entrainment is accomplished through various neuropeptides and neurotransmitters such as: vasoactive intestinal peptide (VIP), arginine vasopressin (AVP), neuromedin S (NMS), glutamate, gamma aminobutyric acid (GABA), serotonin, Neuropeptide Y (NPY), and DA [36-40]. In addition to retina-dependent photoentrainment, light-independent neural circuits likewise directly influence SCN neurons to regulate circadian phase. The most prominent non-photic entrainment cues in mammals are behavioral arousal induced by sleep deprivation, animal handling, or exposure to a novel running wheel [41,42]. Serotonin and NPY are known to directly change SCN molecular rhythms and induce phase shifts of circadian activity during the subjective day, when the SCN is least sensitive to light and most sensitive to non-photic entrainment cues [43-46]. However, the precise mechanism of phase-resetting by behavioral arousal remains unknown. Below we discuss the potential involvement of dopamine signaling in mediating these behaviors and highlight several connections between changes in DA tone and circadian entrainment.
Dopamine Signaling and Circadian Rhythms
Dopamine, a monoamine neurotransmitter well known for its role in reward and motivation, is also important for the detection of salient events such as food or mate availability [28,47-49]. To facilitate a myriad of physiological and behavioral outputs, DA modulates neural activity through a group of G-protein coupled receptors distinguished by their cognate G-proteins—Gs-coupled (D1 and D5), and Gi-coupled receptors (D2, D3, D4)—that are expressed in anatomically distinct regions throughout the brain and body [50-52]. Importantly, DA signaling associated behaviors such as drug self-administration, food reward, and mating all fluctuate in the extent of their expression across the day:night cycle revealing an association with circadian regulation [34,53,54]. Having a well-coordinated neuronal communication between the dopaminergic and circadian systems is likely necessary for appropriately timed behavioral responses, adaptation to the environment, and survival.
The bi-directional nature of this link has gradually been uncovered in the last few decades. DA synthesis, release, and signaling within the retina, olfactory bulb, ventral tegmental area, and striatum are all regulated in a circadian manner [55-58]. DA has been shown to directly alter clock gene expression within extra-SCN circadian oscillators [59-61]. Early studies in Xenopus revealed an important role for DA in the entrainment of retinal circadian rhythms whereby Per2 expression, a core molecular component of the circadian clock, is induced in response to both light and DA [59,62]. Similarly, activation or inhibition of the D1 dopamine receptor (Drd1) in the mammalian retina enhances or attenuates the extent of light induced phase shifts, respectively [60]. Additionally, D2 dopamine receptor (Drd2) null mice have significantly diminished suppression of wheel running activity by light [63]. Taken together these data support that DA signaling outside of the central pacemaker is an important mediator of circadian regulated behaviors.
Midbrain dopaminergic neurons of the ventral tegmental area (VTA) and substantia nigra (SN) are particularly relevant to DA-induced behavioral modification due to their involvement in locomotion, addiction, and reward recognition [64-66]. Additionally, the expression of circadian clock genes such as Per, Clock, and Bmal1 are found within both neuronal populations suggesting a molecular link to circadian regulation [54,67-69]. Selective manipulation of VTA neurons regulates sleep-wake states by promoting salience-induced arousal, enabling the regulation of ethologically relevant behaviors [70]. Lesioning the VTA of rats with 6-hydroxydopamine treatment has been shown to elongate circadian free-running period, alter the onset of drinking behavior, and decrease wheel running activity rhythms [71]. These changes in circadian behavior following alteration of the mesolimbic DA system further highlight the significant interaction between these two systems. Additionally, the striatum, a midbrain DA-neuron projection site important for learning, reward, and motor control, has been shown to exhibit rhythmic circadian clock gene expression [72,73]. Within this brain region, depletion of DA innervation and pharmacological inhibition of Drd2 signaling disrupts the expression profile of Per2, implicating a role for circadian regulators on reward driven processes [61,67]. This is further supported by the evidence that Per2 mutant mice exhibit heightened sensitivity to cocaine [53]. A complete understanding of how DA influences these extra-SCN oscillators will provide important insight into how substance abuse or neurogenerative disorders that impact the dopaminergic system are able to disrupt circadian rhythms. The link between these two systems is briefly described below and detailed in the following manuscripts [74-76].
When the SCN is compromised, nearly all circadian functions disappear [23]. However, non-photic stimuli such as restricted food access or chronic exposure to methamphetamine (MA) can restore rhythmic behavior in SCN lesioned animals. Interestingly, these SCN-independent pacemakers of unknown origin are modulated by the dopaminergic system, which likely mediates additional biological oscillations and their entrainment [77-80]. For instance, scheduled feeding during a restricted portion of the day produces increased locomotion prior to the availability of food, a behavior known as food anticipatory activity (FAA) [79,81,82]. FAA persists even after SCN ablation, suggesting the presence of an independent food entrainable oscillator (FEO) [41,83-85]. The dorsal striatum has been implicated as a mediator of FAA, while Drd1 null mice demonstrate reduced FAA implicating DA-Drd1 signaling as a modulator of this important anticipatory behavior of food availability [79,86].
Similar to anticipation of food reward, daily administration of MA, a DA enhancing psychostimulant, increases locomotor activity immediately preceding the time of injection [87]. Strikingly, arrhythmic SCN-lesioned animals regain circadian rhythmicity via a methamphetamine-sensitive circadian oscillator (MASCO) when presented with ad libitum access to MA in their drinking water [77,80]. Furthermore, a recently described dopaminergic ultradian oscillator (DUO) was found to produce aberrant patterns of arousal when DA tone was elevated through selective activation of the VTA [78]. While these extra-SCN oscillators can compensate for a compromised SCN-based clock, it is possible that DA signaling also directly influences the intact SCN to relay information about salient events such as food or mate availability.
Dopamine in the SCN
Almost 30 years ago, DA signaling within the embryonic SCN was first demonstrated to synchronize maternal-fetal circadian rhythms. Administration of dopaminergics to pregnant dams induced c-fos mRNAexpression, a marker for neural activity, within the fetal SCN, while periodic injections of a Drd1 agonist were shown to set the phase of the fetal biological clock [88,89]. These treatments fail to induce molecular changes within the SCN of the Drd1 null mice, confirming the importance of this Gs-coupled receptor in mediating the effects of DA on the circadian axis [90]. Despite persistent expression of SCN-Drd1 mRNA in adult rodents, baboons, and humans, administration of Drd1 agonist alone is not sufficient to induce c-fos mRNA expression within the SCN or induce behavioral phase shifts of free running animals after postnatal development [91-93]. Based on these findings, it was concluded that sensitivity to DA signaling within the SCN is transient and is lost after the development of the retinohypothalamic tract [91,94]. However, recent advances in mouse genetics, designer actuators, and viral vector technologies have enabled investigators to challenge that notion and develop a more complete understanding of how Drd1-mediated DA signaling directly modulates the central circadian clock throughout adulthood.
Drd1-expressing neurons represent approximately 60 percent of the cells within the SCN, including partial overlap with NMS, VIP, and AVP-expressing neurons [95]. Acute treatment of mouse SCN explants with the Drd1 agonist, SKF 38393, lengthens the free running period of circadian molecular rhythms, suggesting that DA signaling remains functional in the adult SCN [96]. Use of advanced genetic tools has recently identified the behavioral phase and period-resetting properties of Drd1-expressing neurons within the adult mammalian SCN [40,95,97]. Optogenetic stimulation of channelrhodopsin (ChR2)-expressing Drd1-SCN cells is sufficient to entrain free-running mice to the time of stimulus [97] in a similar manner to the entrainment capacity of a scheduled palatable snack [98-100]. This finding is significant because an entrainable circadian pacemaker requires resetting by which the intensity, duration, and phase of the applied stimulus determines the extent and direction of the behavioral phase change [101,102].
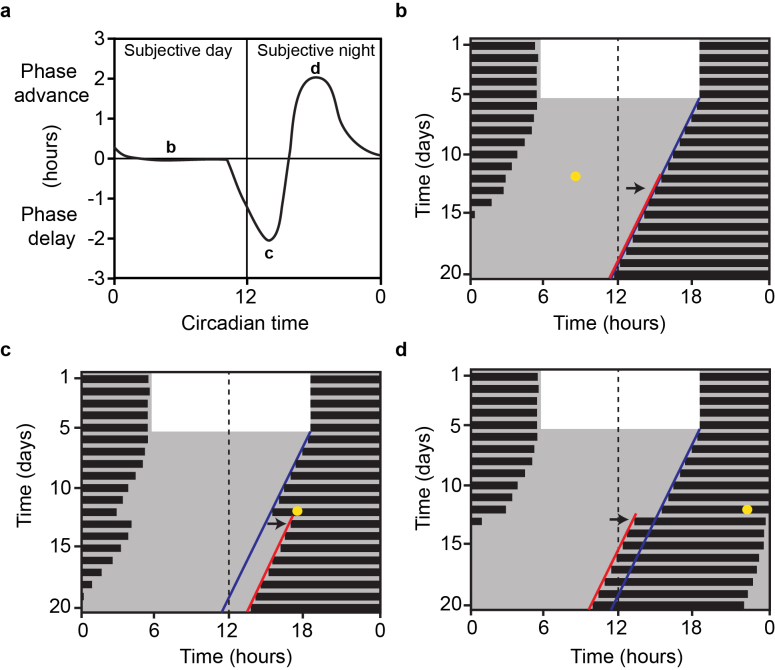
Phase sensitivity of resetting is best summarized by a phase-response curve (PRC), which plots the amplitude of phase change against the circadian phase when the phase shifting stimulus was provided. For instance, the photic PRC is achieved by administering light pulses to free running animals at distinct time points across the circadian day (Figure 1a). The circadian day is defined as one activity-rest cycle, and by convention, the onset of activity is denoted as circadian time 12 (CT 12). Nocturnal animals exposed to a light pulse during the subjective day, known as the “dead zone”, experience no physiological change within the SCN or in wheel running activity in the subsequent days (Figure 1b). However, a light pulse in the early subjective night (CT 14) produces a phase delay in the onset of locomotor activity (Figure 1c), while a pulse at CT 18 results in a phase advance (Figure 1b). Interestingly, animals exposed to non-photic cues such as restricted availability of food and behavioral arousal, exhibit a similar but antiphasic PRC, with large phase advances occurring during the subjective day [103-105]. Surprisingly, chemogenetic activation of Drd1-SCN neurons mimics the behavioral phase shift to photic stimuli [40], suggesting that Drd1-expressing SCN neurons are able to influence photic sensitivity of the central circadian pacemaker.
Figure 1.
Phase response curve of circadian rhythms to light. a. Illustration of the photic PRC in mice. By convention, phase advances are recorded as positive values and phase delays as negative. Plot of wheel running actograms representing the locomotor response of to a brief light pulse during the b., subjective day, c., early subjective night (inducing a phase delay), and d., late subjective night (inducing a phase advance). Black bars represent wheel running activity; yellow dots indicate time of light pulses in DD; dark blue lines represent an extended regression line derived by activity onsets prior to the light pulse; red lines follow actual onset of activity after the light pulse. The duration of phase shift is quantified as the horizontal difference between the two regression lines on the day after the light pulse marked by the black arrows [40].
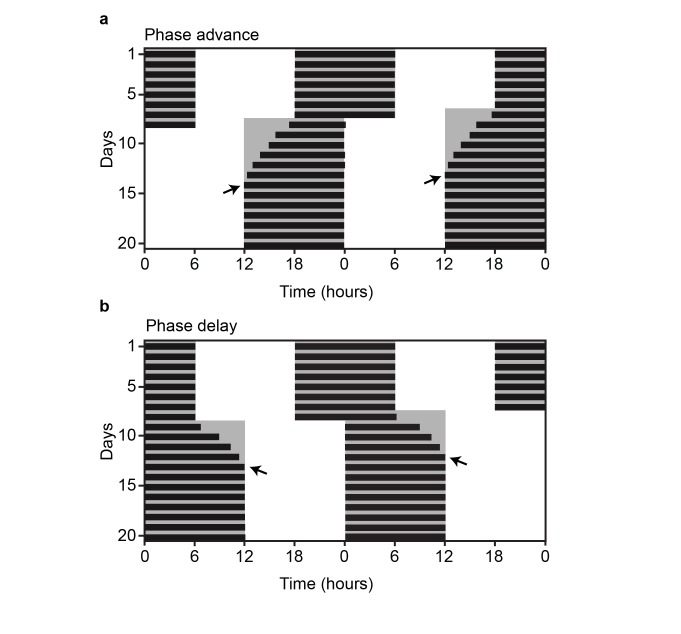
An additional way to evaluate responsiveness to changes in environmental lighting conditions is through a shift in the LD cycle similar to what one would experience when jet-lagged. Transmeridian travel across several time zones creates a rapid change in environmental conditions leading to the general malaise and compromised daytime function associated with jet-lag disorder [3]. Jet-lag primarily is a consequence of imposed internal desynchrony within the SCN resulting from an incongruence between the phase of the endogenous circadian pacemaker and the local time. Reducing the duration of this desynchrony is a paramount concern for shift-workers who are constantly exposed to irregular work and sleep schedules, increasing their susceptibility to cardiovascular disease, ulcers, depression, and obesity [3,106]. As such, considerable effort has been placed into understanding the mechanism of circadian resynchronization in response to abrupt changes in environmental lighting conditions [40,107-109]. Jet-lag is simulated in the laboratory by advancing the LD cycle (Figure 2a: simulating eastward travel) or delaying it (Figure 2b: simulating westward travel). Resynchronization of wheel running activity to these shifts occurs gradually with incremental phase changes (transients) each day until a stable phase of entrainment has been achieved. Manipulations of the LD cycle paired with analysis of activity rhythms have been used to reveal the factors that influence the rate of entrainment. In rodents, introduction of a novel running wheel, exposure to sexually receptive partners, or elevated DA tone have all resulted in accelerated circadian photoentrainment, demonstrating that arousal-inducing stimuli influence the rate of circadian resynchrony [40,110,111].
Figure 2.
Jet-lag paradigms. Representative double-plotted actograms of an a. advance and b., delay of the LD cycle by 6 hours. Black arrows indicate the day of entrainment.
Most strikingly, Drd1-null mice exhibit a significantly diminished rate of photoentrainment to advances and delays of the LD cycle (Wild-type: ~ 6.5 days vs Drd1-KO: ~8.5 days) [40]. A normal photoentrainment rate is fully restored in these Drd1-null mice through viral-vector-mediated rescue of Drd1 expression selectively within the SCN (SCN-Rescue: ~ 6.5 days; Figure 3a-c). Tracing studies suggest a direct connection from the VTA to the SCN, consistent with previous findings that electrolytic lesion of midbrain dopaminergic neurons results in a 40 percent reduction of DA levels within the SCN [40,112]. Chemogenetic activation of the VTA-DA neuron population is sufficient to accelerate the rate of photoentrainment in response to a 6-hour advance in the LD cycle [40]. From these recent discoveries, it is evident that Drd1 signaling within the SCN remains functional through adulthood and that appropriately timed elevation of DA tone aids in the synchronization of endogenous rhythms to the environmental time cues. Because of the VTA’s established role in reward, this direct neuronal connection could prove to be a major source of circadian rhythmicity disruptions associated with substance abuse, mood disorders, and neurodegenerative diseases. Further research based on these findings could provide the insight needed to develop effective therapeutic strategies to facilitate entrainment, thereby effectively treating disorders exacerbated by circadian desynchrony to environmental timing cues.
Figure 3.
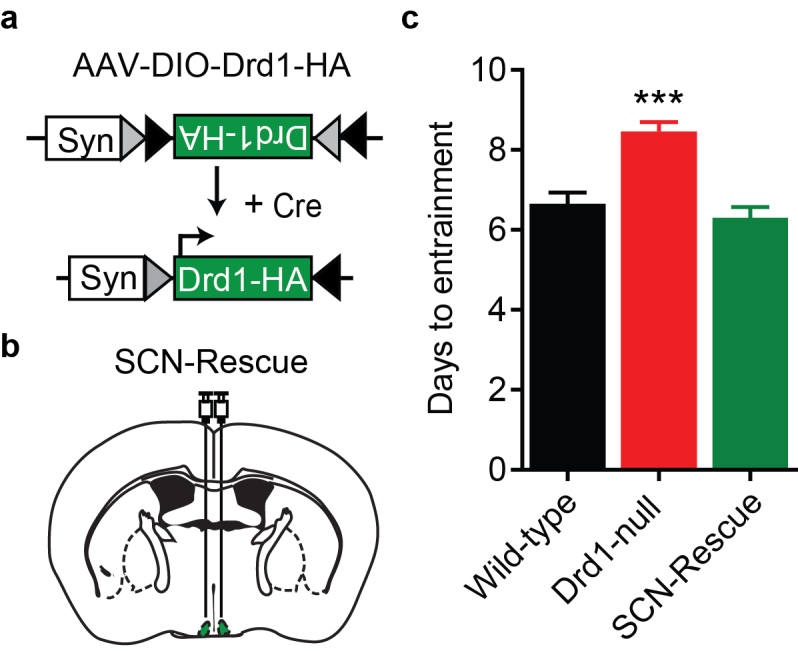
Drd1 expression in the SCN accelerates photoentrainment. a. Schematic representation of the Cre-dependent AAV-DIO-Drd1-HA construct used to re-express Drd1 expression within the SCN. b. Diagram of bilateral stereotaxic delivery of Drd1 re-expression virus to the SCN. c. Group analysis of days to entrain following a six-hour advance in the LD cycle; F (2,62) = 19.42; P< 0.0001; One-way ANOVA with Bonferroni post hoc comparison; n= 13-26/group; ***p < 0.0001.Data are represented as mean ± SEM. Reprinted from [40] with permission from Elsevier.
Consequences of Aberrant Entrainment Conditions
For humans, the advent of electricity and artificial lights has disrupted the sun’s role in entraining circadian rhythms, resulting in serious health consequences including a range of metabolic disorders that have been detailed in the following review [113]. Even brief exposure to dim light at night can lead to significant weight gain and metabolic disruption [114]. Interestingly, a genetic mutation of the circadian core gene Clock, results in elevated DA signaling, dampened feeding rhythms, and metabolic disease in mice, suggesting an important role for circadian rhythms in energy regulation [115]. Along these lines, access to high-fat, palatable food also disrupts the timing of food intake and lengthens the period of free-running activity and temperature rhythms in mice [116,117]. This alteration of circadian timing suggests a connection between energy dense food intake and the circadian pacemaker. Recently, circadian peak of dopaminergic activity in and around the SCN has been found to be a modulator of metabolism in rats [118]. Consequently, consumption of hypercaloric diets impairs adjustment to photic resetting and reduces light mediated c-fos mRNA induction within the SCN [119]. Additionally, regularly timed daily access to a palatable snack (chocolate pellet) entrains behavioral rhythms in constant darkness, reduces light-induced phase shifts, increases DA content in the forebrain, and increases c-fos mRNA expression within DA neurons of the midbrain [120]. These important findings uncover an underappreciated relationship between disrupted circadian rhythms and the dysregulation of the DA signaling. Future work must address how aberrant lighting conditions and rewarding foods impact the SCN, the consequence of this interaction, and how to reduce its negative impact.
Circadian Disruption in Addiction, Mood Disorders, and Parkinson’s Disease
Based on the recent studies linking DA signaling to the circadian clock, and perturbations of circadian genes with drug addiction, it is critical to evaluate the connection between circadian rhythm disturbances and the abuse of addictive substances [121]. DA enhancing drugs such as cocaine or methamphetamine negatively impact circadian entrainment and sleep [122,123]. Cocaine abuse in pregnant females is particularly detrimental to the proper function of the fetal SCN as exposure to cocaine during gestation results in prolonged disruption of photoentrainment after birth [124]. Clock mutant mice show overall hyperactivity, exaggerated locomotion in a novel environment, and high levels of sensitization to cocaine after repeated exposures [67]. These micealso exhibit a greater degree of place preference conditioning with low doses of cocaine, suggesting an elevated reward response to the drug. It is plausible that circadian genes directly regulate dopaminergic circuitry permitting circadian disruptions to alter the true value of a reward and the motivation for addictive substances [53,67,121,125,126].
In addition to drug addiction, several mood disorders and neurodegenerative diseases that involve alterations in DA neurotransmission are accompanied by increased disruptions in circadian rhythms [32,127]. Major depressive disorder (MDD) is commonly associated with sleep abnormalities and a reduction in the amplitude of daily oscillations of body temperature, cortisol, and melatonin rhythms [128]. Bipolar disorder (BD), is characterized by alternating episodes of mania and depression which result in significant sleep and circadian disruptions. Elevated DA contributes to manic episodes in BD and may be a factor in the entrainment disruption. Circadian disorganization is also observed in Parkinson’s disease (PD), a neurodegenerative disorder where loss of nigrostriatal dopaminergic neurons results in tremors, impaired balance, depression, and deterioration of the sleep-wake cycle [74,75,129]. PD patients demonstrate a reduction in nighttime sleep quality, alertness, and cognitive performance which can all be attributed to alterations in circadian entrainment [74]. In a mouse model of PD, in which progressive degeneration of midbrain DA neurons is chemically induced, rest/activity patterns show a gradual decline in amplitude and stability [130]. Further evaluation of how neurodegeneration of dopaminergic neurons influences circadian rhythms may provide novel diagnostic tools to detect PD earlier in its progression than currently possible. Additionally, improving the circadian rhythmicity of high-risk patients (i.e. through regulation of lighting conditions and feeding times), may help to alleviate negative consequences of sleep disturbances and inappropriately timed bouts of wakefulness associated with the disease.
In general, circadian disruption is known to occur during the natural aging process. The elderly are susceptible to reduced amplitude in rest-activity cycles, body temperature, hormone levels, SCN firing rate, and they experience fragmented patterns of sleep [131]. Chronobiological treatments in the elderly, using light and physical exercise have shown promising benefits which boost the circadian rhythm amplitude when provided during the correct phase of the circadian cycle [132]. Further elucidation of the reciprocal relationship between aberrant DA signaling and circadian disruptions may aide in the development of novel chronotherapeutic strategies for psychiatric or some neurodegenerative disorders.
Conclusions and Outlook
Circadian rhythms perform a vital role in orchestrating all aspects of physiology to ensure that rest and active states are properly aligned with the solar day. However, obligatory schedules of modern society disrupt natural oscillations of biological clocks. Disturbing these rhythms increases the likelihood of metabolic, mental and physical disorders, thereby increasing the burden on healthcare around the globe. A challenge for researchers and clinicians is to elucidate the precise mechanisms of circadian rhythm disruptions and how to reduce their negative impact on well-being.
While significant advances have been made in the field of circadian biology, pressing issues remain. For instance, the processing of light information from the retina to the SCN has been well characterized, however, the mechanism of how the SCN communicates with the rest of the brain and body is less understood as reviewed in [133]. A mechanistic understanding of how the SCN integrates and relays photic and non-photic information to generate high amplitude biological rhythms is necessary to understand how daily physiological and metabolic rhythms deteriorate under certain conditions [134]. Furthermore, it is still unclear whether restoring the SCN oscillation amplitude would be enough to alleviate pathologies associated with circadian misalignment. In addition, maladaptive changes in the dopaminergic system underlie many neurological diseases such as depression, bipolar disease, and Parkinson’s disease, which share symptoms of circadian and sleep disruption. Thus, a mechanistic understanding of how dopamine signaling coordinates with the circadian system to govern daily physiological and behavioral functions will provide novel therapeutic avenues for these disorders. The integration of information learned from translational animal experiments into human clinical studies will be the next critical step toward identifying treatment plans to effectively alleviate symptoms of circadian rhythm disorders.
Acknowledgments
We would like to thank Ignacio Provencio and Aarti M. Purohit, for helpful comments on the manuscript and NIH National Institute of General Medicinal Sciences (NIGMS) R01GM11937 (A.D.G.) and UVA Brain Institute 2018 Seed Funding Award (A.D.G.) for grant support.
Glossary
- AAV
Adeno-associated virus
- AVP
Arginine vasopressin
- ChR2
Channelrhodopsin
- Cre
Cre recombinase
- CT
Circadian time
- DA
Dopamine
- DD
Constant darkness
- DIO
Double inverted open reading frame
- Drd1
D1 dopamine receptor
- Drd2
D2 dopamine receptor
- FEO
Food entrainable oscillator
- GABA
Gamma-Aminobutyric Acid
- HFD
High-fat diet
- ipRGC
intrinsically photosensitive retinal ganglion cell
- KO
Knockout
- LD
Light:dark cycle
- LD
Light-dark
- MA
Methamphetamine
- MASCO
Methamphetamine entrainable oscillator
- NMS
Neuromedin S
- NPY
Neuropeptide Y
- PACAP
Pituitary adenylate cyclase-activating polypeptide
- SCN
Suprachiasmatic nucleus
- VIP
Vasoactive intestinal peptide
- VTA
Ventral tegmental area
- ZT
Zeitgeber time
References
- Aschoff J. Exogenous and endogenous components in circadian rhythms. Cold Spring Harb Symp Quant Biol. 1960;25:11–28. [DOI] [PubMed] [Google Scholar]
- Panda S, Hogenesch JB, Kay SA. Circadian rhythms from flies to human. Nature. 2002;417(6886):329–35. [DOI] [PubMed] [Google Scholar]
- Zee PC, Goldstein CA. Treatment of shift work disorder and jet lag. Curr Treat Options Neurol. 2010;12(5):396–411. [DOI] [PubMed] [Google Scholar]
- Chang AM, Aeschbach D, Duffy JF, Czeisler CA. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci USA. 2015;112(4):1232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AC, Bechtold DA. The cost of circadian desynchrony: Evidence, insights and open questions. BioEssays. 2015;37(7):777–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolensky MH, Hermida RC, Reinberg A, Sackett-Lundeen L, Portaluppi F. Circadian disruption: new clinical perspective of disease pathology and basis for chronotherapeutic intervention. Chronobiol Int. 2016;33(8):1101–19. [DOI] [PubMed] [Google Scholar]
- Stevens RG, Blask DE, Brainard GC, Hansen J, Lockley SW, Provencio I, et al. Meeting report: the role of environmental lighting and circadian disruption in cancer and other diseases. Environ Health Perspect. 2007;115(9):1357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14(4):697–706. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog ED, Takahashi JS, Block GD. Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nat Neurosci. 1998;1(8):708–13. [DOI] [PubMed] [Google Scholar]
- Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev. 2010;90(3):1063–102. [DOI] [PubMed] [Google Scholar]
- Moga MM, Moore RY. Organization of neural inputs to the suprachiasmatic nucleus in the rat. J Comp Neurol. 1997;389(3):508–34. [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070–3. [DOI] [PubMed] [Google Scholar]
- Morin LP, Blanchard JH, Provencio I. Retinal ganglion cell projections to the hamster suprachiasmatic nucleus, intergeniculate leaflet, and visual midbrain: bifurcation and melanopsin immunoreactivity. J Comp Neurol. 2003;465(3):401–16. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4(12):1165. [DOI] [PubMed] [Google Scholar]
- Provencio I, Rollag MD, Castrucci AM. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature. 2002;415(6871):493. [DOI] [PubMed] [Google Scholar]
- Rollag MD, Berson DM, Provencio I. Melanopsin, ganglion-cell photoreceptors, and mammalian photoentrainment. J Biol Rhythms. 2003;18(3):227–34. [DOI] [PubMed] [Google Scholar]
- Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453(7191):102–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht U. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron. 2012;74(2):246–60. [DOI] [PubMed] [Google Scholar]
- Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton AP, Hastings MH. The suprachiasmatic nucleus. Curr Biol. 2018;28(15):R816–22. [DOI] [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42(1):201–6. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA. 1972;69(6):1583–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247(4945):975–8. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Reebs SG, Honrado GI, Salmon PA. Behavioural entrainment of circadian rhythms. Experientia. 1989;45(8):696–702. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Duffield GE, Ebling FJ, Kidd A, Maywood ES, Schurov I. Non-photic signalling in the suprachiasmatic nucleus. Biol Cell. 1997;89(8):495–503. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96(4):651–6. [DOI] [PubMed] [Google Scholar]
- Maywood ES, O’Neill J, Wong GK, Reddy AB, Hastings MH. Circadian timing in health and disease. Prog Brain Res. 2006;153:253–69. [DOI] [PubMed] [Google Scholar]
- Van Veen MM, Kooij JJ, Boonstra AM, Gordijn MC, Van Someren EJ. Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol Psychiatry. 2010;67(11):1091–6. [DOI] [PubMed] [Google Scholar]
- Wilson S, Argyropoulos S. Sleep in schizophrenia: time for closer attention. Br J Psychiatry. 2012;200(4):273–4. [DOI] [PubMed] [Google Scholar]
- Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. 2010;11(8):589–99. [DOI] [PubMed] [Google Scholar]
- Zanini MA, Castro J, Cunha GR, Asevedo E, Pan PM, Bittencourt L, et al. Abnormalities in sleep patterns in individuals at risk for psychosis and bipolar disorder. Schizophr Res. 2015 [DOI] [PubMed] [Google Scholar]
- Mendoza J, Challet E. Circadian insights into dopamine mechanisms. Neuroscience. 2014;282C:230–42. [DOI] [PubMed] [Google Scholar]
- Korshunov KS, Blakemore LJ, Trombley PQ. Dopamine: A Modulator of Circadian Rhythms in the Central Nervous System. Front Cell Neurosci. 2017;11:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8(4):476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S, Harang R, Meeker K, Granados-Fuentes D, Tsai CA, Mazuski C, et al. A neuropeptide speeds circadian entrainment by reducing intercellular synchrony. Proc Natl Acad Sci USA. 2013;110(46):E4355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram CD, Ciobanu R, Coculescu IL, Tanasescu R, Coculescu M, Mihai R. Vasopressin neurotransmission and the control of circadian rhythms in the suprachiasmatic nucleus. Prog Brain Res. 1998;119:351–64. [DOI] [PubMed] [Google Scholar]
- Lee IT, Chang AS, Manandhar M, Shan Y, Fan J, Izumo M, et al. Neuromedin s-producing neurons act as essential pacemakers in the suprachiasmatic nucleus to couple clock neurons and dictate circadian rhythms. Neuron. 2015;85(5):1086–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo RM, Purohit AM, Zhang Q, Zweifel LS, Guler AD. Direct Midbrain Dopamine Input to the Suprachiasmatic Nucleus Accelerates Circadian Entrainment. Curr Biol. 2017;27(16):2465-75 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE, Antle MC. Entrainment of circadian clocks in mammals by arousal and food. Essays Biochem. 2011;49(1):119–36. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Duffield GE, Smith EJ, Maywood ES, Ebling FJ. Entrainment of the circadian system of mammals by nonphotic cues. Chronobiol Int. 1998;15(5):425–45. [DOI] [PubMed] [Google Scholar]
- Glass JD, DiNardo LA, Ehlen JC. Dorsal raphe nuclear stimulation of SCN serotonin release and circadian phase-resetting. Brain Res. 2000;859(2):224–32. [DOI] [PubMed] [Google Scholar]
- Harrington ME, Rusak B. Photic responses of geniculo-hypothalamic tract neurons in the Syrian hamster. Vis Neurosci. 1989;2(4):367–75. [DOI] [PubMed] [Google Scholar]
- Johnson RF, Smale L, Moore RY, Morin LP. Lateral geniculate lesions block circadian phase-shift responses to a benzodiazepine. Proc Natl Acad Sci USA. 1988;85(14):5301–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrosovsky N. A non-photic gateway to the circadian clock of hamsters. Ciba Found Symp. 1995;183:154–67. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5(6):483–94. [DOI] [PubMed] [Google Scholar]
- Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006;361(1471):1149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80(1):1–27. [DOI] [PubMed] [Google Scholar]
- Seeman P. Brain dopamine receptors. Pharmacol Rev. 1980;32(3):229–313. [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78(1):189–225. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63(1):182–217. [DOI] [PubMed] [Google Scholar]
- Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci USA. 2002;99(13):9026–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb IC, Baltazar RM, Wang X, Pitchers KK, Coolen LM, Lehman MN. Diurnal variations in natural and drug reward, mesolimbic tyrosine hydroxylase, and clock gene expression in the male rat. J Biol Rhythms. 2009;24(6):465–76. [DOI] [PubMed] [Google Scholar]
- Doyle SE, McIvor WE, Menaker M. Circadian rhythmicity in dopamine content of mammalian retina: role of the photoreceptors. J Neurochem. 2002;83(1):211–9. [DOI] [PubMed] [Google Scholar]
- Corthell JT, Stathopoulos AM, Watson CC, Bertram R, Trombley PQ. Olfactory bulb monoamine concentrations vary with time of day. Neuroscience. 2013;247:234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Lee EJ, Yun S, Choe HK, Park SB, Son HJ, et al. Impact of circadian nuclear receptor REV-ERBalpha on midbrain dopamine production and mood regulation. Cell. 2014;157(4):858–68. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Espana RA, Locke JL, Konstantopoulos JK, Rose JH, Chen R, et al. Dopamine transporters govern diurnal variation in extracellular dopamine tone. Proc Natl Acad Sci USA. 2014;111(26):E2751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Resetting the circadian clock in cultured Xenopus eyecups: regulation of retinal melatonin rhythms by light and D2 dopamine receptors. J Neurosci. 1991;11(10):2959–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan GX, Allen GC, Yamazaki S, McMahon DG. An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA. PLoS Biol. 2008;6(10):e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood S, Cassidy P, Cossette MP, Weigl Y, Verwey M, Robinson B, et al. Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in the rat dorsal striatum via daily activation of D2 dopamine receptors. J Neurosci. 2010;30(42):14046–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosini G, Pozdeyev N, Sakamoto K, Iuvone PM. The circadian clock system in the mammalian retina. BioEssays. 2008;30(7):624–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi M, Yujnovsky I, Hirayama J, Malerba M, Tirotta E, Sassone-Corsi P, et al. Impaired light masking in dopamine D2 receptor-null mice. Nat Neurosci. 2006;9(6):732–4. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7(2):191–7. [DOI] [PubMed] [Google Scholar]
- Morris G, Nevet A, Arkadir D, Vaadia E, Bergman H. Midbrain dopamine neurons encode decisions for future action. Nat Neurosci. 2006;9(8):1057–63. [DOI] [PubMed] [Google Scholar]
- Roeper J. Dissecting the diversity of midbrain dopamine neurons. Trends Neurosci. 2013;36(6):336–42. [DOI] [PubMed] [Google Scholar]
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci USA. 2005;102(26):9377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampp G, Albrecht U. The circadian clock and mood-related behavior. Commun Integr Biol. 2008;1(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, et al. Regulation of monoamine oxidase A by circadian-clock components implies clock influence on mood. Curr Biol. 2008;18(9):678–83. [DOI] [PubMed] [Google Scholar]
- Eban-Rothschild A, Rothschild G, Giardino WJ, Jones JR, de Lecea L. VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat Neurosci. 2016;19(10):1356–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe Y, Nishino H. Circadian rhythm of drinking and running-wheel activity in rats with 6-hydroxydopamine lesions of the ventral tegmental area. Brain Res. 2001;899(1-2):187–92. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27(31):8161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbesi M, Yildiz S, Dirim Arslan A, Sharma R, Manev H, Uz T. Dopamine receptor-mediated regulation of neuronal “clock” gene expression. Neuroscience. 2009;158(2):537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videnovic A, Golombek D. Circadian and sleep disorders in Parkinson’s disease. Exp Neurol. 2013;243:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fifel K, DeBoer T. The central clock in patients with Parkinson disease. JAMA Neurol. 2014;71(11):1455–6. [DOI] [PubMed] [Google Scholar]
- Logan RW, McClung CA. Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat Rev Neurosci. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma K, Honma S. Effects of methamphetamine on development of circadian rhythms in rats. Brain Dev. 1986;8(4):397–401. [DOI] [PubMed] [Google Scholar]
- Blum ID, Zhu L, Moquin L, Kokoeva MV, Gratton A, Giros B, et al. A highly tunable dopaminergic oscillator generates ultradian rhythms of behavioral arousal. eLife. 2014:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo CM, Darvas M, Oviatt M, Chang CH, Michalik M, Huddy TF, et al. Dopamine receptor 1 neurons in the dorsal striatum regulate food anticipatory circadian activity rhythms in mice. eLife. 2014;3:e03781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataroglu O, Davidson AJ, Benvenuto LJ, Menaker M. The methamphetamine-sensitive circadian oscillator (MASCO) in mice. J Biol Rhythms. 2006;21(3):185–94. [DOI] [PubMed] [Google Scholar]
- Boulos Z, Terman M. Food availability and daily biological rhythms. Neurosci Biobehav Rev. 1980;4(2):119–31. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci Biobehav Rev. 1994;18(2):171–95. [DOI] [PubMed] [Google Scholar]
- Krieger DT, Hauser H, Krey LC. Suprachiasmatic nuclear lesions do not abolish food-shifted circadian adrenal and temperature rhythmicity. Science. 1977;197(4301):398–9. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Swann JM, Sisk CL. Anticipation of 24-hr feeding schedules in rats with lesions of the suprachiasmatic nucleus. Behav Neural Biol. 1979;25(3):346–63. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Neurobiology of food anticipatory circadian rhythms. Physiol Behav. 2011;104(4):535–45. [DOI] [PubMed] [Google Scholar]
- Liu YY, Liu TY, Qu WM, Hong ZY, Urade Y, Huang ZL. Dopamine is involved in food-anticipatory activity in mice. J Biol Rhythms. 2012;27(5):398–409. [DOI] [PubMed] [Google Scholar]
- Mohawk JA, Pezuk P, Menaker M. Methamphetamine and dopamine receptor D1 regulate entrainment of murine circadian oscillators. PLoS One. 2013;8(4):e62463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver DR, Rivkees SA, Reppert SM. D1-dopamine receptors activate c-fos expression in the fetal suprachiasmatic nuclei. Proc Natl Acad Sci USA. 1992;89(19):9201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan N, Weaver DR, Reppert SM, Davis FC. Entrainment of the fetal hamster circadian pacemaker by prenatal injections of the dopamine agonist SKF 38393. J Neurosci. 1994;14(9):5393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender M, Drago J, Rivkees SA. D1 receptors mediate dopamine action in the fetal suprachiasmatic nuclei: studies of mice with targeted deletion of the D1 dopamine receptor gene. Brain Res Mol Brain Res. 1997;49(1-2):271–7. [DOI] [PubMed] [Google Scholar]
- Weaver DR, Reppert SM. Definition of the developmental transition from dopaminergic to photic regulation of c-fos gene expression in the rat suprachiasmatic nucleus. Brain Res Mol Brain Res. 1995;33(1):136–48. [DOI] [PubMed] [Google Scholar]
- Grosse J, Davis FC. Transient entrainment of a circadian pacemaker during development by dopaminergic activation in Syrian hamsters. Brain Res Bull. 1999;48(2):185–94. [DOI] [PubMed] [Google Scholar]
- Rivkees SA, Lachowicz JE. Functional D1 and D5 dopamine receptors are expressed in the suprachiasmatic, supraoptic, and paraventricular nuclei of primates. Synapse. 1997;26(1):1–10. [DOI] [PubMed] [Google Scholar]
- Weaver DR, Roca AL, Reppert SM. c-fos and jun-B mRNAs are transiently expressed in fetal rodent suprachiasmatic nucleus following dopaminergic stimulation. Brain Res Dev Brain Res. 1995;85(2):293–7. [DOI] [PubMed] [Google Scholar]
- Smyllie NJ, Chesham JE, Hamnett R, Maywood ES, Hastings MH. Temporally chimeric mice reveal flexibility of circadian period-setting in the suprachiasmatic nucleus. Proc Natl Acad Sci USA. 2016;113(13):3657–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf D, Joiner WJ, McCarthy MJ, Kiessling S, Barandas R, Young JW, et al. The mood stabilizer valproic acid opposes the effects of dopamine on circadian rhythms. Neuropharmacology. 2016;107:262–70. [DOI] [PubMed] [Google Scholar]
- Jones JR, Tackenberg MC, McMahon DG. Manipulating circadian clock neuron firing rate resets molecular circadian rhythms and behavior. Nat Neurosci. 2015;18(3):373–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar C, Salgado R, Rodriguez K, Blancas Vazquez AS, Angeles-Castellanos M, Buijs RM. Scheduled meals and scheduled palatable snacks synchronize circadian rhythms: consequences for ingestive behavior. Physiol Behav. 2011;104(4):555–61. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Angeles-Castellanos M, Escobar C. A daily palatable meal without food deprivation entrains the suprachiasmatic nucleus of rats. Eur J Neurosci. 2005;22(11):2855–62. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Angeles-Castellanos M, Escobar C. Entrainment by a palatable meal induces food-anticipatory activity and c-Fos expression in reward-related areas of the brain. Neuroscience. 2005;133(1):293–303. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, DeCoursey PJ, Bauman L, Menaker M. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature. 1984;308(5955):186–8. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Gooley JJ. Sleep and circadian rhythms in humans. Cold Spring Harb Symp Quant Biol. 2007;72:579–97. [DOI] [PubMed] [Google Scholar]
- Hastings M, Maywood ES. Circadian clocks in the mammalian brain. BioEssays. 2000;22(1):23–31. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Dwyer SM. Circadian phase shifting: relationships between photic and nonphotic phase-response curves. Physiol Behav. 2001;73(1-2):175–83. [DOI] [PubMed] [Google Scholar]
- Challet E, Caldelas I, Graff C, Pevet P. Synchronization of the molecular clockwork by light- and food-related cues in mammals. Biol Chem. 2003;384(5):711–9. [DOI] [PubMed] [Google Scholar]
- James SM, Honn KA, Gaddameedhi S, Van Dongen HP. Shift Work: Disrupted Circadian Rhythms and Sleep-Implications for Health and Well-Being. Curr Sleep Med Rep. 2017;3(2):104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano M, Adachi A, Nakahama K, Nakamura T, Tamada M, Meyer-Bernstein E, et al. An abrupt shift in the day/night cycle causes desynchrony in the mammalian circadian center. J Neurosci. 2003;23(14):6141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilorz V, Cunningham PS, Jackson A, West AC, Wager TT, Loudon AS, et al. A novel mechanism controlling resetting speed of the circadian clock to environmental stimuli. Curr Biol. 2014;24(7):766–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamovich Y, Ladeuix B, Golik M, Koeners MP, Asher G. Rhythmic Oxygen Levels Reset Circadian Clocks through HIF1alpha. Cell Metab. 2017;25(1):93–101. [DOI] [PubMed] [Google Scholar]
- Yamanaka Y, Honma S, Honma K. Scheduled exposures to a novel environment with a running-wheel differentially accelerate re-entrainment of mice peripheral clocks to new light-dark cycles. Genes Cells. 2008;13(5):497–507. [DOI] [PubMed] [Google Scholar]
- Bobrzynska KJ, Mrosovsky N. Phase shifting by novelty-induced running: activity dose-response curves at different circadian times. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1998;182(2):251–8. [DOI] [PubMed] [Google Scholar]
- Kizer JS, Palkovits M, Brownstein MJ. The projections of the A8, A9 and A10 dopaminergic cell bodies: evidence for a nigral-hypothalamic-median eminence dopaminergic pathway. Brain Res. 1976;108(2):363–70. [DOI] [PubMed] [Google Scholar]
- Arble DM, Ramsey KM, Bass J, Turek FW. Circadian disruption and metabolic disease: findings from animal models. Best Pract Res Clin Endocrinol Metab. 2010;24(5):785–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, et al. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci USA. 2010;107(43):18664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308(5724):1043–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6(5):414–21. [DOI] [PubMed] [Google Scholar]
- Blancas-Velazquez A, Mendoza J, Garcia AN, la Fleur SE. Diet-Induced Obesity and Circadian Disruption of Feeding Behavior. Front Neurosci. 2017;11:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Zhang Y, Ezrokhi M, Li Y, Tsai TH, Cincotta AH. Circadian peak dopaminergic activity response at the biological clock pacemaker (suprachiasmatic nucleus) area mediates the metabolic responsiveness to a high-fat diet. J Neuroendocrinol. 2018;30(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza J, Pevet P, Challet E. High-fat feeding alters the clock synchronization to light. J Physiol. 2008;586(24):5901–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza J, Clesse D, Pevet P, Challet E. Food-reward signalling in the suprachiasmatic clock. J Neurochem. 2010;112(6):1489–99. [DOI] [PubMed] [Google Scholar]
- Logan RW, Williams WP, 3rd, McClung CA. Circadian rhythms and addiction: mechanistic insights and future directions. Behav Neurosci. 2014;128(3):387–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA. Circadian rhythms, the mesolimbic dopaminergic circuit, and drug addiction. ScientificWorldJournal. 2007;7:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosobud AE, Gillman AG, Leffel JK, 2nd, Pecoraro NC, Rebec GV, Timberlake W. Drugs of abuse can entrain circadian rhythms. ScientificWorldJournal. 2007;7:203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SA, Rowe SA, Krupa M, Kennaway DJ. Prenatal exposure to the dopamine agonist SKF-38393 disrupts the timing of the initial response of the suprachiasmatic nucleus to light. Brain Res. 2000;858(2):284–9. [DOI] [PubMed] [Google Scholar]
- Sleipness EP, Sorg BA, Jansen HT. Contribution of the suprachiasmatic nucleus to day:night variation in cocaine-seeking behavior. Physiol Behav. 2007;91(5):523–30. [DOI] [PubMed] [Google Scholar]
- Sleipness EP, Sorg BA, Jansen HT. Diurnal differences in dopamine transporter and tyrosine hydroxylase levels in rat brain: dependence on the suprachiasmatic nucleus. Brain Res. 2007;1129(1):34–42. [DOI] [PubMed] [Google Scholar]
- Hasler BP, Smith LJ, Cousins JC, Bootzin RR. Circadian rhythms, sleep, and substance abuse. Sleep Med Rev. 2012;16(1):67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadnie CA, McClung CA. Circadian Rhythm Disturbances in Mood Disorders: Insights into the Role of the Suprachiasmatic Nucleus. Neural Plast. 2017;2017:1504507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen DP, Vuono R, Nawarathna U, Fisher K, Shneerson JM, Reddy AB, et al. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol. 2014;71(5):589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fifel K, Cooper HM. Loss of dopamine disrupts circadian rhythms in a mouse model of Parkinson’s disease. Neurobiol Dis. 2014;71:359–69. [DOI] [PubMed] [Google Scholar]
- Nakamura TJ, Nakamura W, Yamazaki S, Kudo T, Cutler T, Colwell CS, et al. Age-related decline in circadian output. J Neurosci. 2011;31(28):10201–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Someren EJ, Kessler A, Mirmiran M, Swaab DF. Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biol Psychiatry. 1997;41(9):955–63. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Yi CX, Cailotto C, la Fleur SE, Fliers E, Buijs RM. Mammalian clock output mechanisms. Essays Biochem. 2011;49(1):137–51. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Scheer FA, Perreau-Lenz S, La Fleur SE, Yi CX, Fliers E, et al. Circadian disruption and SCN control of energy metabolism. FEBS Lett. 2011;585(10):1412–26. [DOI] [PMC free article] [PubMed] [Google Scholar]