Abstract
Huntington’s disease (HD) patients suffer from a progressive neurodegenerative disorder that inflicts both motor and non-motor symptoms. HD is caused by a CAG repeat expansion within the first exon of the huntingtin (HTT) gene that produces a polyglutamine repeat that leads to protein misfolding, soluble aggregates, and inclusion bodies detected throughout the body. Both clinical and preclinical research indicate that cardiovascular dysfunction should be considered a core symptom in at least a subset of HD patients. There is strong evidence for dysautonomia (dysfunctional autonomic nervous system, ANS) in HD patients that can be detected early in the disease progression. The temporal patterning of ANS function is controlled by the circadian timing system based in the anterior hypothalamus. Patients with neurodegenerative diseases including HD exhibit disrupted sleep/wake cycle and, in preclinical models, there is compelling evidence that the circadian timing system is compromised early in the disease process. Here we review data from preclinical models of HD that explore the intersection between disruption of circadian rhythms and dysautonomia. This work will lead to new therapeutic strategies and standards of care for HD and other neurodegenerative diseases.
Keywords: autonomic nervous system, circadian, cardiovascular disease, dysautonomia, Huntington’s disease, neurodegenerative disease, sleep
Introduction
Huntington’s Disease is a Genetically-determined Neurodegenerative Disease
Huntington’s disease (HD) patients suffer from a progressive neurodegenerative disorder that inflicts cognitive, psychiatric, and motor symptoms [1,2]. HD is caused by a CAG repeat expansion within the first exon of the huntingtin (HTT) gene which produces a polyglutamine repeat that leads to protein misfolding, soluble aggregates, and inclusion bodies detected [3,4]. The normal function of the protein (Huntingtin) is unknown; however, the mutated form leads to dysfunction of a broad range of cellular processes including cytoskeletal organization, metabolism, and transcriptional activities. Based on the broad distribution of the HTT, the mutation would be expected to produce symptoms throughout the body. Indeed, recent work suggests HD is a systemic illness affecting the entire body and data from animal studies suggests that core features of the disease can be modified by treatments that target tissues outside of the nervous system [5]. To further preclinical research, a large number of animal models of HD have been developed, each with strengths and weaknesses (see [6]). The work covered in this review was mostly conducted on the Q175, BACHD, R6/2, and R6/1 mouse lines.
Cardiovascular Dysfunction May Be Common in Huntington’s Disease
Cardiovascular (CV) events are a major cause of early death in the HD population and occur at a higher rate compared to the rest of the population [7,8]. Possible cardiomyopathies have not been well explored in HD patients. One recent study examined the electrocardiograms of over 500 early symptomatic HD patients and found evidence for low heart rate (bradycardia) and conduction abnormalities in a significant fraction of the patients [9]. Preclinical models of HD have found clear evidence for reduced contractility and cardiac output [10-15]. For example, a recent study of the cardiomyocytes of BACHD mice found evidence for increased calcium/calmodulin-dependent protein kinase II activity as well as structural abnormalities in the mitochondria [16]. Also, metabolic analysis of heart tissue from patients and mouse models found evidence for energy equilibrium imbalances in cardiac cells [17]. Finally, the cardiac-specific expression of polyQ repeats leads to CV dysfunction, suggesting that CV disease may be the result of local abnormalities [18,19].
In addition, there is strong evidence for dysautonomia (dysfunctional autonomic nervous system, ANS) in HD patients that can be detected early in the disease progression. The sympathetic nervous system appears to be most impacted during the very early stages of HD [20-22]. As the disease advances, the parasympathetic activity progressively decreases as well [22-24]. Therefore, both the clinical and preclinical research indicate that CV dysfunction should be considered a core symptom of at least a subset of HD patients [25].
Sleep and Circadian Dysfunction are an Integral Component of Huntington’s Disease Pathophysiology
Sleep disorders are prevalent in HD patients and have detrimental effects on the daily functioning and quality of life of patients and their caregivers [26,27]. The most common symptoms found in these studies include a delay in sleep onset, fragmented sleep during the night, and daytime sleepiness. Importantly, these disruptions in the sleep/wake cycle occur early in the disease progression and so could serve as a biomarker for HD as well as a target for interventions. The sleep/wake cycle is conceptualized as being driven by two, anatomically-distinct processes: a homeostatic sleep mechanism (process S) as well as by the circadian timing system (process C). To date, there is little evidence that HD impacts sleep homeostasis, but there is growing evidence for HD-driven disruption in circadian timing. Still, it is difficult to determine whether the disease alters the circadian timing system in humans, and animal models provide critical insights. Mouse models of HD also exhibit a progressive and rapid breakdown of the circadian rest/activity cycle that closely mimics the condition observed in human patients, typified by loss of consolidated sleep, increased activity during the rest phase, and more sleep during the active phase [11,28-30]. Importantly, the disruptions are seen under both light/dark (LD) cycle as well as the mice are held in constant darkness (DD). This latter step is critical to establish that the circadian system is compromised. Disorganized circadian timing leads to undesirable effects throughout the body [31], altering the function of key organ systems including heart, pancreas, liver, lungs, as well as the brain. Collectively this prior research supports the hypothesis that circadian dysfunction is an integral component of HD pathophysiology. We have been testing the hypothesis that dysfunction in the circadian system contributes to the CV disease in HD (Figure 1) and, in this review, we will summarize our progress.
Figure 1.
Huntington’s disease (HD) is traditionally considered a motor disease treated by neurologists. However, there is increasing evidence that HD patients and animal models exhibit cardiovascular (CV) symptoms including dysautonomia (dysfunctional autonomic nervous system, ANS). Dysautonomia has been well-characterized in PD, and we believe that it is also a core symptom of a subset of HD patients and may be usefully applied to segregate this patient population for tailored treatments. There is also a growing body of evidence that HD patients and animal models exhibit disruption in circadian timing although not in sleep homeostasis. In our work, we have been testing the hypothesis that dysfunction in the circadian system in HD contributes to the CV disease and, in this review, we will summarize our progress.
Topics
Baroreceptor Reflex is Blunted in Huntington’s Disease
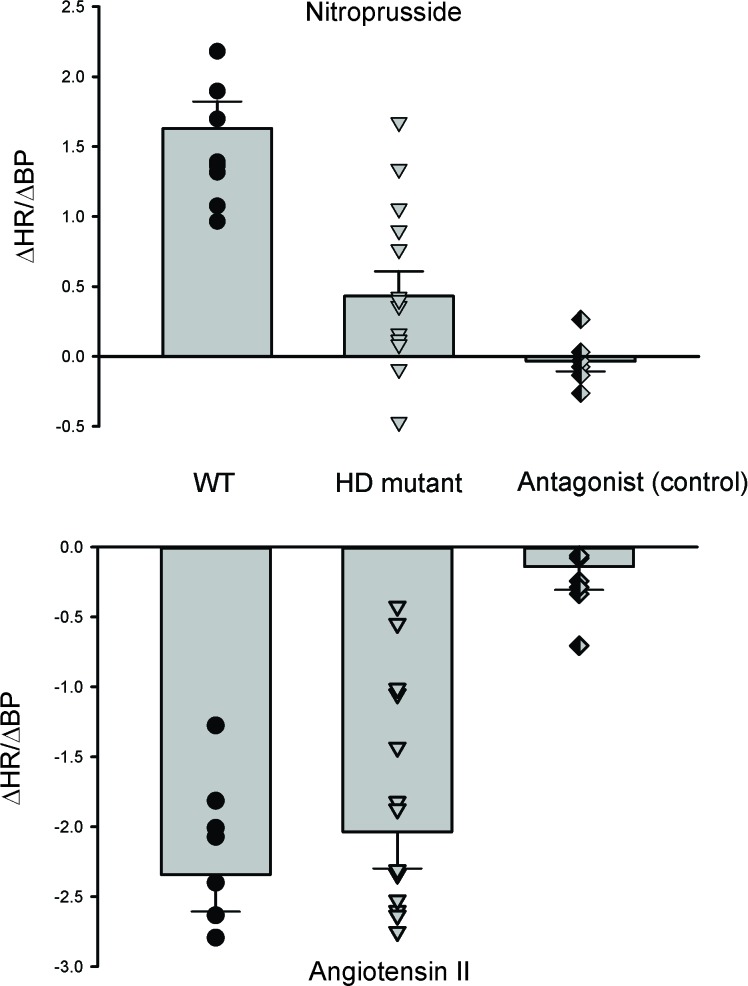
One of the classic physiological tests of the ANS function is the measurement of the baroreceptor reflex in which changes in blood pressure (BP) evoke alterations in the heart rate (HR). Baroreceptors are mechanoreceptors located in the carotid sinus and aortic arch that sense the pressure changes. Although they are sensitive to both increases and decreases in arterial pressure, their primary role is to respond to a sudden fall in arterial pressure. This sudden decrease is detected by baroreceptors and signals are sent to the medulla to elevate the sympathetic activity and reduce the parasympathetic activity. These changes lead to vasoconstriction and increased HR to bring back arterial pressure into the normal range. Recordings from the BACHD [32] as well as the Q175 [15] lines of HD mice indicate that the baroreceptor reflex is dramatically altered (Figure 2). The administration of Angiotensin II (ATII) or Nitroprusside (NP) leads to episodes of hypertension or hypotension, respectively. Changes in BP as a result of drug administration should elicit a compensatory response of HR via the baroreceptor reflex in order to normalize BP levels. The Q175 mutants showed a dramatically blunted response in HR to the transient hypotension induced by NP suggesting that the sympathetic branch has impaired. The BACHD mutants showed a blunted response to both ATII and NP indicating both sympathetic and parasympathetic arms were impacted. In both cases, we confirmed that there were no genotypic differences in the change in BP evoked by the injected drug as well as performed pharmacological controls to showing that appropriate receptor blockers prevented the change in HR.
Figure 2.
Baroreceptor reflex is disrupted in mouse models of HD. In the baroreceptor reflex, changes in BP are detected by the baroreceptors and evoke compensatory changes in ANS. The Q175 and BACHD mutants showed a dramatically blunted response in HR to the transient hypotension induced by nitroprusside (NP) suggesting that the sympathetic branch has impaired. The effect of NP was blocked by a beta-adrenergic receptor blocker (β-blocker) propranolol. The Q175 mutants did not show a significantly altered response to transient hypertension induced by angiotensin II. HD patients complain of dizziness and light-headedness upon standing, all symptoms of baroreceptor dysregulation resulting in orthostatic hypotension. Data from [15,32].
HD patients complain of dizziness and light-headedness upon standing, all symptoms of baroreceptor dysregulation resulting in orthostatic hypotension [20,33]. While not well documented in HD patients, orthostatic hypotension has been extensively examined in Parkinson’s disease (PD) [34]. In this neurodegenerative disease, the neurogenic hypotension impacts about 30 percent of the patients with dizziness or lightheadedness, fatigue when standing, and difficulty walking to be the most common symptoms [35,36]. In a significant number of patients, the hypotension is responsible for the falls which one of the main complications of PD that has a major impact on the quality of life. Patients with neurogenic orthostatic hypotension exhibit in enhanced reduction in systolic BP (40 vs. 20 mmHg) in response to a stressor and thus are vulnerable for falls due to failure to maintain BP [37]. Like the PD patients, we expect that a subset of HD patients will be vulnerable to neurogenic orthostatic hypotension.
Heart Rate Variability is Reduced in Huntington’s Disease
Heart rate variability (HRV) is a measure of variation in the beat-to-beat interval that reflects the dynamic balance of sympathetic and parasympathetic control of heart function. Traditionally, HRV is generally considered an indication of CV health and low HRV proposed as a predictor for CV disease and mortality [38,39]. More recently, HRV has become a popular index of cardiac autonomic control in the biobehavioral sciences due to its relationship with stress disorders and other illnesses [40]. In humans, it is typically measured for short intervals and is increasingly available in health records as a non-invasive measure of CV function. One of the most commonly used methods for HRV evaluation is power spectral density analysis in which high-frequency (HF) and low-frequency (LF) bands are extracted from the HRV signal, and the spectral power is calculated (e.g., [41]). Traditionally, the LF power is viewed as a measure of regulation by the sympathetic branch of the ANS, although it is more likely a general index of ANS function [42,43].
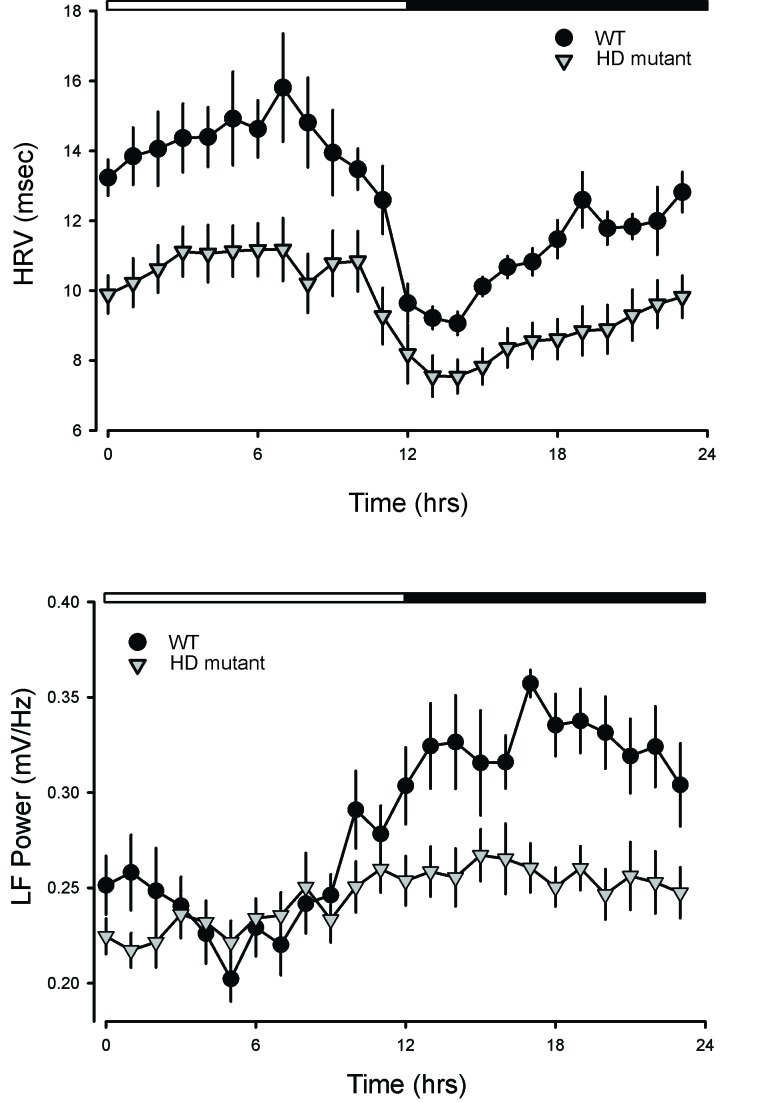
In our work, we measured HRV in WT and HD mutant mice over several days in both LD and DD conditions. In WT mice, HRV displayed a robust diurnal and circadian rhythm consistent with circadian regulation of the ANS (Figure 3). Both the LF and HF domains of the HRV exhibited robust daily rhythms. In the Q175 line [15], the HRV was low in young mutants, and this reduction was largest during the rest phase. The power in the LF domain was significantly reduced in the young mutants with the most prominent effects during the night. A similar reduction in HRV was previously observed in the BACHD line [11,14] as well as in the R6/1 model [44].
Figure 3.
HRV is a measure of variation in the beat-to-beat (R-R) interval that reflects the dynamic balance of sympathetic and parasympathetic control of heart function. Traditionally, HRV is an indication of CV health and low HRV proposed as a predictor for CV disease and mortality. One of the most commonly used methods for evaluation of HRV is power spectral density analysis in which high-frequency (HF) and low-frequency (LF) bands are extracted from the HRV signal, and the spectral power is calculated. Traditionally, the LF power is viewed as a measure of regulation by the sympathetic branch of the ANS, although it is more likely a general index of ANS function. We found robust daily and circadian rhythms in HRV and LP power in WT mice. As shown in the panels, HRV increased during sleep while LF power (sympathetic outflow) was higher when the mice were active. In the HD mutants (Q175), the HRV was low in young mutants and this reduction was largest during the rest phase. The power in the LF domain was significantly reduced in the young mutants with the biggest effects during the night. A similar reduction in HRV was previously observed in the BACHD line [11,14] as well as in the R6/1 model [44]. In HD patients, a similar decrease in HRV has also been reported during the presymptomatic and early stages of HD progression.
In HD patients, a similar decrease in HRV has also been reported during the presymptomatic and early stages of HD progression [20-23]. Prior studies reported HRV deficits during the Valsalva maneuver, hand-grip test, and the head up tilt test in HD patients [22,24,44-46]. As far as we know, the spectral power analysis has not been carried out on data from patients. In human subjects, HRV changes with daily cycle and with sleep state (e.g., [47]) but we do not have this data from the HD patients. Thus, both the preclinical and clinical work is consistent with a disruption in the sympathetic branch early in the disease progression that is reflected as a decrease in HRV.
Abnormal Diurnal and Circadian Rhythms in Heart Rate Observed in Huntington’s Disease
In humans and other animals, HR varies dramatically with acute physical demands, i.e., higher HR when we are physically active while low HR when sedentary. In addition to these acute changes, there are also robust 24-hr circadian rhythms in the heart and vasculature to prepare the CV system for higher output during the day in humans. Interestingly, these natural rhythms may increase the risk for vulnerable individuals and daily rhythms in the symptoms of CV disease peak in the morning hours (e.g., [48]).
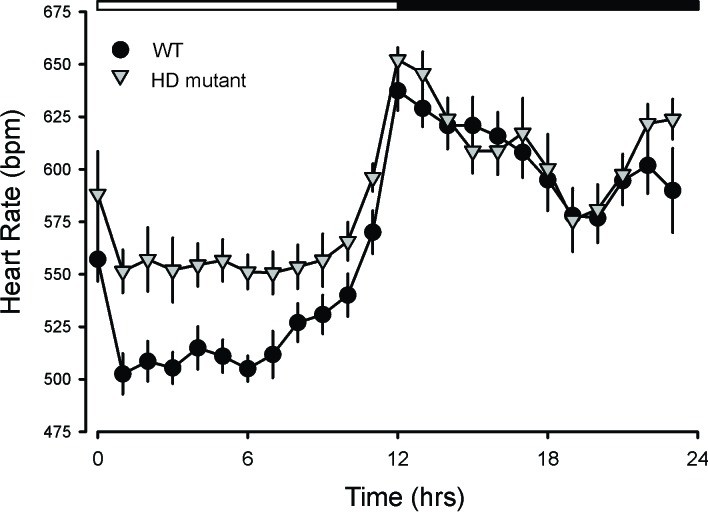
In nocturnal mice, diurnal and circadian of CV output peak in the night. While influenced by activity levels, the rhythms occur even when measured in windows of time when the mice were inactive. As measured by telemetry, diurnal and circadian rhythms in HR are disrupted in the BACHD, Q175, R6/1 lines of mice [11,14,15,44]. For example, the Q175 exhibited highly pronounced tachycardia during their normal sleep time, with high HR and reduced amplitude in the HR rhythm (Figure 4). Besides, the normal strong correlation between activity and HR, which is mediated by the ANS, was dramatically reduced in the mutants. These changes suggest that ANS disruption occurs early in disease progression in the Q175 line. In agreement, we also saw evidence in the BACHD of the circadian system failing to lower BP during sleep [32]. The HR rhythm in R6/1 mice are disrupted with the mutants showing a higher HR than WT littermates as young adults but ultimately exhibiting low HR [44]. In two other models (R6/2 and HdhQ150 lines), the HR was significantly reduced in symptomatic mice [49]. Therefore, overlapping data from several studies using different mouse models all found evidence for disrupted diurnal or circadian rhythms of HR.
Figure 4.
HR varies dramatically with a daily cycle in which HR is high during the active part of the sleep/wake cycle. As measured by telemetry, HD mutants (BACHD and Q175) exhibited highly pronounced tachycardia during their normal sleep time, with high HR and reduced amplitude in the HR rhythm. These changes suggest that ANS disruption occurs early in disease progression in these mutant lines. In agreement, we also saw evidence in the BACHD of the circadian system failing to lower blood pressure (BP) during sleep [32]. The HR rhythm is also disrupted in R6/1 [44], R6/2 and HdhQ150 lines [49]. Therefore, overlapping data from several studies using different mouse models all found evidence for disrupted diurnal or circadian rhythms of HR. We have not seen evidence that this has been examined in HD patients, but the pre-clinical data supports 24-hr monitoring in patients.
As far as we know, the question of whether the rhythms in HR and BP are disrupted have not been examined in HD patients. The preclinical data suggest that the disruptions are most likely to occur at the beginning of the sleep and can be difficult to diagnose. Office visits are unlikely to coincide with the expression of hypertension. This type of “masked” or nocturnal hypertension is associated with poor clinical outcomes as the symptoms are likely to be untreated and patients frequently develop organ damage prior to transitioning to sustained hypertension which can be detected [50,51]. Based on the preclinical data, HD patients should be strong candidates for 24-hr BP monitoring which is the best method for uncovering this type of hypertension.
Possible Mechanisms Underlying the Dysautonomia
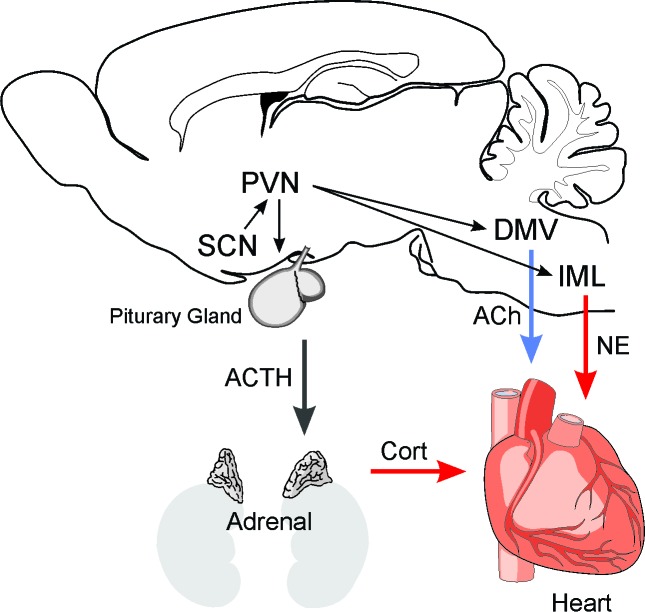
The circuits involved in the generation of rhythms in HR and autonomic function are relatively well defined. The central circadian clock in the suprachiasmatic nucleus (SCN) orchestrates the peripheral clocks via ANS [52-56]. Complete separation between pre-sympathetic and pre-parasympathetic neurons starts from the SCN (Figure 5) [56,57]. These separate pre-sympathetic and pre-parasympathetic neurons project to the paraventricular nucleus (PVN) and its separated pre-autonomic neurons have projections to either the preganglionic sympathetic neurons in the intermediolateral (IML) column of the spinal cord or the preganglionic neurons of the dorsal motor nucleus of the vagus (DMV). There are axon collaterals of the pre-sympathetic PVN neurons in the nucleus tractus solitarius (NTS). Particularly, the SCN utilizes completely separated sympathetic and parasympathetic neurons to convey the circadian information to the periphery including the heart, liver, and adrenal gland. Various neuroendocrine and autonomic neurons are located in the PVN where appropriate hormonal and autonomic responses are coordinated [56,57].
Figure 5.
The circuits involved in the generation of rhythms in HR and autonomic function are relatively well defined. The central circadian clock in the suprachiasmatic nucleus (SCN) orchestrates the peripheral clocks via ANS. It appears that separate populations of SCN neurons project to the paraventricular nucleus (PVN) and innervate pre-sympathetic and pre-parasympathetic cell populations. These separate pre-sympathetic and pre-parasympathetic neurons project to either the preganglionic sympathetic neurons in the intermediolateral (IML) column of the spinal cord or the preganglionic neurons of the dorsal motor nucleus of the vagus (DMV). There are axon collaterals of the pre-sympathetic PVN neurons in the nucleus tractus solitarius (NTS). Both pathways innervate the heart to regulate HR via release of acetylcholine (ACh) and norepinephrine (NE). Also, the SCN regulates the HPA axis and the secretion of cortisol. The glucocorticoid receptors are also potent regulators of CV function. In HD patients, there is evidence for the degeneration of neurons in the SCN as well as the brain stem. There is also evidence for the strong expression of p62 immunopositive protein aggregates in axons of brainstem fiber tracts including the vagal nerve and the NTS [70].
HD is a neurodegenerative disease so the most obvious cause of dysautonomia is the loss of cell populations in the hypothalamus or brain stem. We examined possible anatomical changes within the SCN of the BACHD mouse at 3 months of age just as the motor symptoms can first be measured. We found that the male, but not the female, SCN was smaller than WT controls. There were no differences in peptide expression (arginine vasopressin, AVP; vasoactive intestinal peptide, VIP) within the SCN with genotype or sex. In the more severely impacted R6/2 mice, the SCN shows decreased expression of VIP and its receptor, VPAC2 [58]. VIP plays a key role in synchronizing cell populations within the SCN and its reduction would be expected to disrupt the population rhythms in neural activity from this structure. The SCN sends projections out to regulate the temporal patterns of activity in several major arousal centers including the orexin expressing cell population in the lateral hypothalamus. The expression levels of orexin are reduced in HD models (e.g., [59,60]). Changes in the expression of brain-derived neurotrophic factor (BDNF) have also been observed. Expression of BDNF is normally high in the brainstem where this neuromodulator plays a crucial for regulating HR [61-63]. The levels of BDNF are known to be reduced in the brainstem of mutant mice and resorting BDNF level can ameliorate HD pathology and prolong the lifespan in HD mice [64]. Importantly, restoring BDNF levels also returned the HR of the HD mice to the control levels [65]. Therefore, in the mouse models, HD alters the expression of peptides and neuromodulators in the brain regions involved in the neural regulation of CV function without necessarily causing a significant loss of hypothalamic neurons.
In HD patients, there is evidence that the disease causes similar changes in expression as well as degeneration in the brain regions involved in the central nervous system (CNS) regulation of CV function. In the hypothalamus, there is evidence for the reduction in the expression of orexin and AVP in the brains of the HD patients [66,67]. In addition, the levels of BDNF and its receptors (TrkB) are reduced in HD patients [68], and the HD aggregates directly interfere with the transcription of BDNF [69]. Finally, there is evidence for degeneration in the brainstem nuclei in HD patients including loss of neurons in the substantia nigra, pontine nuclei, reticulotegmental nucleus of the pons, superior and inferior olives [70]. This study also reported the strong expression of p62 immunopositive protein aggregates in axons of brainstem fiber tracts including the vagal nerve and the nucleus of the solitary tract. These structures are centrally involved in the CNS regulation of the CV system. Thus, by the end of life, HD certainly impacts the brain regions responsible for the CNS regulation of CV function and establishes a possible structural cause for this dysfunction.
Altered Suprachiasmatic Nucleus-driven Physiology Output in Huntington’s Disease
The circadian system is composed of cell-autonomous clock gene expression rhythms that are synchronized and adaptively phase aligned in tissues throughout the body by a rhythmic output from the SCN [71]. Individual SCN neurons express rhythms in spontaneous firing rate, with higher firing rates observed during the day and low rates at night [72,73]. The BACHD and Q175 mouse models of HD exhibit decreased electrical activity in the SCN during the day [11,74]. This decrease in daytime firing in the SCN was not seen in the R6/2 model [75] although firing rate deficits were seen in an SCN-driven output in the orexin neurons [60]. Using electrophysiological techniques, we found that SCN neural activity rhythms were lost early in the disease progression and were accompanied by loss of the normal daily variation in resting membrane potential in the mutant SCN neurons [74]. The low neural activity could be transiently reversed by direct current injection thus demonstrating that the neurons have the capacity to discharge at WT levels. Exploring the potassium currents known to regulate the electrical activity of SCN neurons, our most striking finding was that these cells in the mutants exhibited an enhancement in the large-conductance calcium- and voltage-activated potassium (BK) currents. The expression of the pore-forming subunit (Kcnma1) of the BK channel was higher in the mutant SCN. These findings demonstrate that SCN neurons of both BACHD and Q175 HD models exhibit early pathophysiology and that dysregulation of BK current may be responsible.
Circadian Molecular Feedback Loop May Be Impacted by Huntington’s Disease
The rhythms in neural activity in the SCN are driven by cell autonomous molecular feedback loops. At a molecular level, circadian rhythms are generated by the intracellular transcriptional/translational feedback loop, driving daily oscillations with a period of approximately 24-hrs in the expression of core clock proteins. CLOCK (Circadian Locomotor Output Cycles Kaput) and BMAL1 (Brain and muscle aryl-hydrocarbon receptor nuclear translocator-like 1) the positive components of the clock bind to E-box sequences and drive the expression of the negative elements period (Per) and cryptochrome (Cry) which can inhibit their own transcription by repressing the CLOCK/BMAL1 heterodimer. Once the levels of PER and CRY are decreased, the new cycle of transcription/translation is started by CLOCK-BMAL1. This feedback loop constitutes a 24-hr period of the internal circadian rhythms. The circadian nuclear receptor, Rev-erbα and a retinoic acid-related orphan receptor (ROR) regulate the Bmal1 expression via activation and repression, respectively. Post-translational modifications are crucial for regulating the clock. Casein kinase 1 (CK1) phosphorylate PER and CRY which is vital for the circadian cycle length. The expression of other clock-controlled genes and output genes is modulated by the CLOCK/BMAL1 heterodimer, which involves many physiological functions [76]. We found that the circadian rhythms in PER2-driven bioluminescence were not altered in the SCN in the BACHD [77]; however, deficits in gene expression rhythms were found in the SCN of the more severely impacted R6/2 model [28]. Thus, the evidence that the molecular circadian clockwork is disrupted is so far mixed, and more work is required to identify at what stage in the disease progression these disruptions occur.
These molecular rhythms are not just expressed in the SCN and work done in the R6/2 model clearly demonstrates that circadian rhythms of clock-driven genes that are critical metabolic outputs in the liver are abolished in vivo [78]. This deficiency is accompanied by arrhythmic expression of the clock genes Cry1 and Dbp, and a phase-advanced Per2 cycle. There is overwhelming evidence that circadian clock genes including Bmal1, Clock, Rev-erbα, Per 1, Per 2, Cry1, and Cry2 are rhythmically expressed in cardiomyocytes [79,80]. Functionally this timing system has a major impact on the expression of the genes involved in cardiac metabolism and electrical activity display circadian expression. For example, the metabolic genes, pyruvate dehydrogenase kinase 4 (pdk4) and uncoupling protein 3 (ucp3), are known to be regulated by peroxisome proliferator-activated receptor alpha (PPARα). PPARα displays a robust rhythmic expression in the heart and is also shown to modulate Bmal1 by directly binding to the Bmal1 promoter [81]. Daily oscillations of the expression in Pdk4 and ucp3 are associated with oscillations in the clock gene expression with the peak during the active phase. Glucose levels are known to exhibit diurnal rhythms. Glucose transporters 1 and 4 (Glut1,4) have a peak in the expression level at the same time, suggesting increased glucose transport and utilization at the same time with diurnal variations. Potassium channels, Kv1.5 and Kv4.2, as well as calcium transients show rhythmicity in protein levels [82].
There is still much work to do to understand how the molecular clock controlling transcription interacts with the physiology of CV function. The general assumption is that the temporal pattern of transcription favors ATP production and energy utilization during the active phase while allowing remodeling and repair to dominate during rest. One particularly interesting case study involves the rhythmically expressed kruppel-like factor 15 (KLF15). Depletion of KLF15 in cardiomyocytes leads to a disorganized circadian behavior despite an intact core clock [83]. KLF15 transcriptionally controls the rhythmic expression of Kv channel-interacting protein 2, a critical subunit required for generating a transient potassium current. Deficiency or excess of KLF15 causes loss of rhythmic QT variation, abnormal repolarization and enhanced susceptibility to ventricular arrhythmias [84]. We do not know whether the HD mutation impacts KLF15 or other critical rhythmic outputs. We consider this lack of knowledge a major hole in the literature as HD-driven alterations in circadian output could be an important clinical symptom of the disease.
Genetic or Environmental Disruption of the Circadian Timing System Leads to Cardiovascular Symptoms
Regardless of the specific cause, prior work has shown that the genetic or environmental disruption in circadian rhythms can result in CV symptoms. For example, mice held in a 20-hr (LD10:10) cycle exhibit worse cardiac damage in response to high BP compared to controls held on a normal 24-hr cycle (LD 12:12) [85]. These lighting conditions alter rhythms in clock gene expression in both the heart and brain. Furthermore, “tau” mutant hamsters have a mutation in CK1 which results in a short endogenous cycle length of approximately 22-hrs [86] and, when they are held under a 24-hr LD cycle, these mutant hamsters die early with cardiomyopathy including fibrosis [86]. However, when the mutant hamsters are housed in a 22-hr LD cycle or are made arrhythmic by the destruction of their circadian clock (SCN), the hamsters are protected from developing cardiac dysfunction [86]. These results suggest that discord between the internal circadian clock (22 hrs) and external environment (24 hrs) can trigger cardiac disease. To provide a final example, Bmal1-knock out (KO) mice exhibit arrhythmic circadian behavior, age-related dilated cardiomyopathy, and shortened life span [87-90]. These symptoms are also seen in the cardiomyocyte-specific Bmal1 KO mice [91]. To understand the role of the circadian clock within the cardiomyocyte on myocardial biology, a cardiomyocyte-specific Clock mutant (CCM) mouse model was assessed [92,93]. CCM mice show decreased diurnal rhythms in HR, reduced cardiac efficiency, and bradycardia [88]. The cardiac hypertrophic markers are elevated in CCM mice [93]. These data suggest an essential role of cardiomyocyte circadian clock in CV function and highlight the possibility that at least some of the CV symptoms seen in HD could be the consequence of the well-established circadian dysfunction.
Finally, sleep disruption and work schedules that disrupt the circadian system are also risk factors for CV disease in humans [48]. Epidemiological studies show that approximately 40 percent of the population in North America do not get the recommended amount of sleep, and the reduced sleep duration is associated with a high risk of cardiac disease [94]. In the laboratory, placing healthy adults in an inverted sleep/wake and meal cycle for three days is sufficient to increase their BP and levels of the inflammatory markers [95]. A short 2 hr disruption of the sleep/wake can increase resting HR and cortisol levels in healthy subjects [96]. The sleep and rhythms disruption due to work schedule is associated with the adverse endocrine and CV profiles in subjects, putting them at increased CV risk [96]. In a study performed on healthy male shift workers, 24-hr electrocardiogram recordings are used to investigate the effect of the work schedule on the cardiac autonomic control [97]. Results show a reduction in the cardiac sympathetic modulation based on the HRV analysis, suggesting an increased risk for CV events [97]. Therefore, the data from humans is consistent with our assertion that the sleep and circadian dysfunction seen in HD could contribute to the CV symptoms seen in the patients.
Summary and Outlook
There are several lines of evidence for dysautonomia in HD. First, HD patients complain of dizziness and light-headedness upon standing, all symptoms of baroreceptor dysregulation resulting in orthostatic hypotension [21,33]. Work in animal models has confirmed dysfunction in the baroreceptor reflex [15,32]. Second, HD patients [20-23] as well as mouse models [11,14,15,44] show reduced HRV. This variation in heart rate is a well-established marker of cardiac autonomic control and a reduction in HRV is associated with poor CV outcomes. Third, pathological analysis of HD patients has shown degeneration and intranuclear neuronal inclusions throughout the brainstem including regions known to be centrally involved in the autonomic regulation of the CV system [70]. We do not yet know whether these regions are also impacted in animal models of HD. There is evidence to suggest that the postganglionic sympathetic and intrinsic neurons in the heart may exhibit reduced functionality in mouse models of HD [49]. The disruption of the autonomic system is a well-established consequence of PD [34] and, collectively, this data argues that dysautonomia should also be considered in HD as well.
The body of evidence presented in this review is consistent with the hypothesis that the disruption of the SCN circuit contributes to the autonomic dysfunction seen in HD. If correct, this hypothesis has a number of predictions. First, the normal diurnal and circadian rhythms in HR and BP driven by the ANS will be disrupted in HD. To date, the disrupted rhythms in HR, BP, and baroreceptor reflex from data in HD animal models and patients support this hypothesis. Second, removing the mutant HTT from the heart may not be sufficient to rescue cardiac function. There is evidence of direct effects of mutant HTT in the heart (reviewed in [25]) but we would emphasize that the dysautonomia is likely to be the result of dysfunction in the CNS circuit controlling autonomic function. This prediction is clinically important as clinical trials with mutant HTT lowering agents are ongoing and understanding the therapeutic targets are important for the success of these trials. Third, treatment with drugs focusing on dysautonomia should be targeted at specific times of the daily cycle. For example, beta-adrenergic receptor blockers may produce the most substantial benefits when taken before bed. Fourth, cardiac function shows a robust diurnal variation including rhythms in HR and BP. This body of preclinical data described in this review suggests that monitoring of the CV system in HD patients should start at an early age so therapeutic interventions can be employed to slow the progression of these pathological processes and prevent early death. Most importantly, our data suggest that this early screening must include observations during the usual sleep hours of the day, as early anomalies may go undetected at the times of day that patients would usually interact with clinicians. Finally, we predict that treatments or lifestyle changes that improve the circadian timing system will reduce the autonomic dysfunction in HD.
Acknowledgments
We would like to acknowledge the prior intellectual contributions of Dr. Analyne Schroeder and Ms. Tamara Cutler. Work discussed in this review was funded by CHDI Foundation.
Glossary
- ATII
Angiotensin II
- AVP
arginine vasopressin
- ANS
autonomic nervous system
- BP
blood pressure
- BMAL1
Brain and muscle aryl-hydrocarbon receptor nuclear translocator-like 1
- BDNF
brain-derived neurotrophic factor
- CCM
cardiomyocyte-specific Clock mutant
- CV
cardiovascular
- CNS
central nervous system
- CLOCK
Circadian Locomotor Output Cycles Kaput
- Cry
cryptochrome
- DD
constant darkness
- DMV
dorsal motor nucleus of the vagus
- HR
heart rate
- HF
high-frequency
- HD
Huntington’s disease
- Htt
huntingtin
- IML
intermediolateral
- BK
large-conductance calcium- and voltage-activated potassium currents
- KLF15
kruppel-like factor 15
- LD
light/dark
- LF
low-frequency
- NP
nitroprusside
- NTS
nucleus tractus solitarius
- PVN
paraventricular nucleus
- Per
period
- PPARα
peroxisome proliferator activated receptor alpha
- pdk4
pyruvate dehydrogenase kinase 4
- ROR
retinoic acid-related orphan receptor
- SCN
suprachiasmatic nucleus
- ucp3
uncoupling protein 3
- VIP
vasoactive intestinal peptide
Author Contributions
SP wrote the first draft; CSC finalized text and generated figures.
References
- Kuljis D, Schroeder AM, Kudo T, Loh DH, Willison DL, Colwell CS. Sleep and circadian dysfunction in neurodegenerative disorders: insights from a mouse model of Huntington’s disease. Minerva Pneumol. 2012;51:93–106. [PMC free article] [PubMed] [Google Scholar]
- Bates GP, Dorsey R, Gusella JF, Hayden MR, Kay C, Leavitt BR, et al. Huntington disease. Nat Rev Dis Primers. 2015;1(1):15005 10.1038/nrdp.2015.5 [DOI] [PubMed] [Google Scholar]
- Ciammola A, Sassone J, Alberti L, Meola G, Mancinelli E, Russo MA, et al. Increased apoptosis, huntingtin inclusions and altered differentiation in muscle cell cultures from Huntington’s disease subjects. Cell Death Differ. 2006;13(12):2068–78. 10.1038/sj.cdd.4401967 [DOI] [PubMed] [Google Scholar]
- Saft C, Zange J, Andrich J, Müller K, Lindenberg K, Landwehrmeyer B, et al. Mitochondrial impairment in patients and asymptomatic mutation carriers of Huntington’s disease. Mov Disord. 2005;20(6):674–9. 10.1002/mds.20373 [DOI] [PubMed] [Google Scholar]
- Carroll JB, Bates GP, Steffan J, Saft C, Tabrizi SJ. Treating the whole body in Huntington’s disease. Lancet Neurol. 2015. November;14(11):1135–42. 10.1016/S1474-4422(15)00177-5 [DOI] [PubMed] [Google Scholar]
- Pouladi MA, Morton AJ, Hayden MR. Choosing an animal model for the study of Huntington’s disease. Nat Rev Neurosci. 2013;14(10):708–21. 10.1038/nrn3570 [DOI] [PubMed] [Google Scholar]
- Sørensen SA, Fenger K. Causes of death in patients with Huntington’s disease and in unaffected first degree relatives. J Med Genet. 1992;29(12):911–4. 10.1136/jmg.29.12.911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abildtrup M, Shattock M. Cardiac Dysautonomia in Huntington’s Disease. J Huntingtons Dis. 2013;2:251–61. 10.3233/JHD-130054 [DOI] [PubMed] [Google Scholar]
- Stephen CD, Hung J, Schifitto G, Hersch SM, Rosas HD. Electrocardiogram Abnormalities Suggest Aberrant Cardiac Conduction in Huntington’s Disease. Mov Disord Clin Pract (Hoboken). 2018;5(3):306–11. 10.1002/mdc3.12596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonincontri G, Wood NI, Puttick SG, Ward AO, Carpenter TA, Sawiak SJ, et al. Right ventricular dysfunction in the R6/2 transgenic mouse model of Huntington’s disease is unmasked by dobutamine. J Huntingtons Dis. 2014;3:25–32. 10.3233/JHD-130083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Schroeder A, Loh DH, Kuljis D, Jordan MC, Roos KP, et al. Dysfunctions in circadian behavior and physiology in mouse models of Huntington’s disease. Exp Neurol. 2011;228(1):80–90. 10.1016/j.expneurol.2010.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihm MJ, Amann DM, Schanbacher BL, Altschuld RA, Bauer JA, Hoyt KR. Cardiac dysfunction in the R6/2 mouse model of Huntington’s disease. Neurobiol Dis. 2007;25(2):297–308. 10.1016/j.nbd.2006.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood NI, Sawiak SJ, Buonincontri G, Niu Y, Kane AD, Carpenter TA, et al. Direct evidence of progressive cardiac dysfunction in a transgenic mouse model of Huntington’s disease. J Huntingtons Dis. 2012;1:57–64. 10.3233/JHD-2012-120004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder AM, Wang HB, Park S, Jordan MC, Gao F, Coppola G, et al. Cardiac Dysfunction in the BACHD Mouse Model of Huntington’s Disease. Sadoshima J, editor. PLOS ONE. 2016;11: e0147269. doi: 10.1371/journal.pone.0147269 [DOI] [PMC free article] [PubMed]
- Cutler TS, Park S, Loh DH, Jordan MC, Yokota T, Roos KP, et al. Neurocardiovascular deficits in the Q175 mouse model of Huntington’s disease. Physiol Rep. 2017;5(11):e13289 10.14814/phy2.13289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joviano-Santos JV, Santos-Miranda A, Botelho AF, de Jesus IC, Andrade JN, de Oliveira Barreto T, et al. Increased oxidative stress and CaMKII activity contribute to electro-mechanical defects in cardiomyocytes from a murine model of Huntington’s disease. FEBS J. 2019;286(1):110–23. 10.1111/febs.14706 [DOI] [PubMed] [Google Scholar]
- Toczek M, Zielonka D, Zukowska P, Marcinkowski JT, Slominska E, Isalan M, et al. An impaired metabolism of nucleotides underpins a novel mechanism of cardiac remodeling leading to Huntington’s disease related cardiomyopathy. Biochim Biophys Acta. 2016. November;1862(11):2147–57. 10.1016/j.bbadis.2016.08.019 [DOI] [PubMed] [Google Scholar]
- Melkani GC, Trujillo AS, Ramos R, Bodmer R, Bernstein SI, Ocorr K. Huntington’s disease induced cardiac amyloidosis is reversed by modulating protein folding and oxidative stress pathways in the Drosophila heart. PLoS Genet. 2013;9(12):e1004024 10.1371/journal.pgen.1004024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattison JS, Sanbe A, Maloyan A, Osinska H, Klevitsky R, Robbins J. Cardiomyocyte Expression of a Polyglutamine Preamyloid Oligomer Causes Heart Failure. Circulation. 2008;117(21):2743–51. 10.1161/CIRCULATIONAHA.107.750232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobal J, Meglic B, Mesec A, Peterlin B. Early sympathetic hyperactivity in Huntington’s disease. Eur J Neurol. 2004;11(12):842–8. 10.1111/j.1468-1331.2004.00894.x [DOI] [PubMed] [Google Scholar]
- Kobal J, Melik Z, Cankar K, Bajrovic FF, Meglic B, Peterlin B, et al. Autonomic dysfunction in presymptomatic and early symptomatic Huntington’s disease. Acta Neurol Scand. 2010;121(6):392–9. 10.1111/j.1600-0404.2009.01251.x [DOI] [PubMed] [Google Scholar]
- Bär KJ, Boettger MK, Andrich J, Epplen JT, Fischer F, Cordes J, et al. Cardiovagal modulation upon postural change is altered in Huntington’s disease. Eur J Neurol. 2008;15(8):869–71. 10.1111/j.1468-1331.2008.02173.x [DOI] [PubMed] [Google Scholar]
- Andrich J, Schmitz T, Saft C, Postert T, Kraus P, Epplen JT, et al. Autonomic nervous system function in Huntington’s disease. J Neurol Neurosurg Psychiatry. 2002;72(6):726–31. 10.1136/jnnp.72.6.726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma KR, Romano JG, Ayyar DR, Rotta FT, Facca A, Sanchez-Ramos J. Sympathetic skin response and heart rate variability in patients with Huntington disease. Arch Neurol. 1999;56(10):1248–52. 10.1001/archneur.56.10.1248 [DOI] [PubMed] [Google Scholar]
- Critchley BJ, Isalan M, Mielcarek M. Neuro-Cardio Mechanisms in Huntington’s Disease and Other Neurodegenerative Disorders. Front Physiol. 2018;9:559 10.3389/fphys.2018.00559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AO, Rogers L, Pilsworth S, McAllister CJ, Shneerson JM, Morton AJ, et al. Asymptomatic sleep abnormalities are a common early feature in patients with Huntington’s disease. Curr Neurol Neurosci Rep. 2011;11(2):211–7. 10.1007/s11910-010-0163-x [DOI] [PubMed] [Google Scholar]
- Morton AJ. Circadian and sleep disorder in Huntington’s disease. Exp Neurol. 2013;243:34–44. 10.1016/j.expneurol.2012.10.014 [DOI] [PubMed] [Google Scholar]
- Morton AJ, Wood NI, Hastings MH, Hurelbrink C, Barker RA, Maywood ES. Disintegration of the sleep-wake cycle and circadian timing in Huntington’s disease. J Neurosci. 2005;25(1):157–63. 10.1523/JNEUROSCI.3842-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh DH, Kudo T, Truong D, Wu Y, Colwell CS. The Q175 mouse model of Huntington’s disease shows gene dosage- and age-related decline in circadian rhythms of activity and sleep. PLoS One. 2013;8(7):e69993 10.1371/journal.pone.0069993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuljis DA, Gad L, Loh DH, MacDowell Kaswan Z, Hitchcock ON, Ghiani CA, et al. Sex Differences in Circadian Dysfunction in the BACHD Mouse Model of Huntington’s Disease. PLoS One. 2016;11(2):e0147583 10.1371/journal.pone.0147583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS, Matveyenko AV. Timing Is Everything: Implications for Metabolic Consequences of Sleep Restriction. Diabetes. 2014;63(6):1826–8. 10.2337/db14-0283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder AM, Loh DH, Jordan MC, Roos KP, Colwell CS. Baroreceptor reflex dysfunction in the BACHD mouse model of Huntington’s disease. PLoS Curr. 2011;3:RRN1266 10.1371/currents.RRN1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz NA, Anguelova GV, Marinus J, Van Dijk JG, Roos RA. Autonomic symptoms in patients and pre-manifest mutation carriers of Huntington’s disease: autonomic symptoms in Huntington’s disease. Eur J Neurol. 2010;17(8):1068–74. 10.1111/j.1468-1331.2010.02973.x [DOI] [PubMed] [Google Scholar]
- Goldstein DS. Dysautonomia in Parkinson disease. Compr Physiol. 2014;4:805–26. 10.1002/cphy.c130026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnolo A, Zibetti M, Merola A, Canova D, Sarchioto M, Montanaro E, et al. Cardiovascular autonomic neuropathy and falls in Parkinson disease: a prospective cohort study. J Neurol. 2018;•••: 10.1007/s00415-018-9104-4 [DOI] [PubMed] [Google Scholar]
- Claassen DO, Adler CH, Hewitt LA, Gibbons C. Characterization of the symptoms of neurogenic orthostatic hypotension and their impact from a survey of patients and caregivers. BMC Neurol. 2018;18(1):125 10.1186/s12883-018-1129-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcliffe-Kaufmann L, Kaufmann H, Palma JA, Shibao CA, Biaggioni I, Peltier AC, et al. Orthostatic heart rate changes in patients with autonomic failure caused by neurodegenerative synucleinopathies. Ann Neurol. 2018;83(3):522–31. 10.1002/ana.25170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141(2):122–31. 10.1016/j.ijcard.2009.09.543 [DOI] [PubMed] [Google Scholar]
- Wulsin LR, Horn PS, Perry JL, Massaro JM, D’Agostino RB. Autonomic Imbalance as a Predictor of Metabolic Risks, Cardiovascular Disease, Diabetes, and Mortality. J Clin Endocrinol Metab. 2015;100(6):2443–8. 10.1210/jc.2015-1748 [DOI] [PubMed] [Google Scholar]
- Kemp AH, Koenig J, Thayer JF. From psychological moments to mortality: A multidisciplinary synthesis on heart rate variability spanning the continuum of time. Neurosci Biobehav Rev. 2017;83:547–67. 10.1016/j.neubiorev.2017.09.006 [DOI] [PubMed] [Google Scholar]
- Shaffer F, McCraty R, Zerr CL. A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability. Front Psychol. 2014;5:1040 10.3389/fpsyg.2014.01040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr RL. Interpretation of normalized spectral heart rate variability indices in sleep research: a critical review. Sleep. 2007;30(7):913–9. 10.1093/sleep/30.7.913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathers JA. Everything Hertz: methodological issues in short-term frequency-domain HRV. Front Physiol. 2014;5:177 10.3389/fphys.2014.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiriazis H, Jennings NL, Davern P, Lambert G, Su Y, Pang T, et al. Neurocardiac dysregulation and neurogenic arrhythmias in a transgenic mouse model of Huntington’s disease: neurocardiac phenotype in Huntington’s disease mice. J Physiol. 2012;590(22):5845–60. 10.1113/jphysiol.2012.238113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff MJ, Gross M. Vasoregulatory activity in patients with Huntington’s chorea. J Neurol Sci. 1974;21(1):33–8. 10.1016/0022-510X(74)90103-8 [DOI] [PubMed] [Google Scholar]
- Den Heijer JC, Bollen WL, Reulen JP, van Dijk JG, Kramer CG, Roos RA, et al. Autonomic nervous function in Huntington’s disease. Arch Neurol. 1988;45(3):309–12. 10.1001/archneur.1988.00520270087025 [DOI] [PubMed] [Google Scholar]
- Boudreau P, Yeh WH, Dumont GA, Boivin DB. Circadian variation of heart rate variability across sleep stages. Sleep (Basel). 2013;36(12):1919–28. 10.5665/sleep.3230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CJ, Yang JN, Scheer FA. The impact of the circadian timing system on cardiovascular and metabolic function. Prog Brain Res. 2012;199:337–58. 10.1016/B978-0-444-59427-3.00019-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielcarek M, Inuabasi L, Bondulich MK, Muller T, Osborne GF, Franklin SA, et al. Dysfunction of the CNS-heart axis in mouse models of Huntington’s disease. PLoS Genet. 2014;10(8):e1004550 10.1371/journal.pgen.1004550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin SS, O’Brien E, Staessen JA. Masked hypertension: understanding its complexity. Eur Heart J. 2017;38:1112–8. 10.1093/eurheartj/ehw502 [DOI] [PubMed] [Google Scholar]
- Bowles NP, Thosar SS, Herzig MX, Shea SA. Chronotherapy for Hypertension. Curr Hypertens Rep. 2018;20(11):97 10.1007/s11906-018-0897-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader N, Chrousos GP, Kino T. Interactions of the Circadian CLOCK System and the HPA Axis. Trends Endocrinol Metab. 2010;21(5):277–86. 10.1016/j.tem.2009.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaides NC, Charmandari E, Chrousos GP, Kino T. Circadian endocrine rhythms: the hypothalamic–pituitary–adrenal axis and its actions. Ann N Y Acad Sci. 2014;1318(1):71–80. 10.1111/nyas.12464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9(10):764–75. 10.1038/nrg2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs RM. Chapter 1 - The autonomic nervous system: a balancing act. In: Buijs RM, Swaab DF. Handbook of Clinical Neurology. Elsevier; 2013. pp. 1–11. doi: 10.1016/B978-0-444-53491-0.00001-8 [DOI] [PubMed] [Google Scholar]
- Buijs RM, la Fleur SE, Wortel J, Van Heyningen C, Zuiddam L, Mettenleiter TC, et al. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J Comp Neurol. 2003;464(1):36–48. 10.1002/cne.10765 [DOI] [PubMed] [Google Scholar]
- Buijs RM, Escobar C, Swaab DF. Chapter 15 - The circadian system and the balance of the autonomic nervous system. In: Buijs RM, Swaab DF. Handbook of Clinical Neurology. Elsevier; 2013. pp. 173–191. doi: 10.1016/B978-0-444-53491-0.00015-8 [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J, Popovic N, Georg B, Brundin P, Hannibal J. Decreased VIP and VPAC2 receptor expression in the biological clock of the R6/2 Huntington’s disease mouse. J Mol Neurosci. 2007;31:139–48. [DOI] [PubMed] [Google Scholar]
- Kotliarova S, Jana NR, Sakamoto N, Kurosawa M, Miyazaki H, Nekooki M, et al. Decreased expression of hypothalamic neuropeptides in Huntington disease transgenic mice with expanded polyglutamine-EGFP fluorescent aggregates. J Neurochem. 2005;93(3):641–53. 10.1111/j.1471-4159.2005.03035.x [DOI] [PubMed] [Google Scholar]
- Williams RH, Morton AJ, Burdakov D. Paradoxical function of orexin/hypocretin circuits in a mouse model of Huntington’s disease. Neurobiol Dis. 2011;42(3):438–45. 10.1016/j.nbd.2011.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CG, Hasser EM, Kunze DL, Katz DM, Kline DD. Endogenous Brain Derived Neurotrophic Factor in the Nucleus Tractus Solitarius Tonically Regulates Synaptic and Autonomic Function. J Neurosci. 2011;31(34):12318–29. 10.1523/JNEUROSCI.0746-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan R, Weigand LA, Bateman R, Griffioen K, Mendelowitz D, Mattson MP. Evidence that BDNF regulates heart rate by a mechanism involving increased brainstem parasympathetic neuron excitability. J Neurochem. 2014;129(4):573–80. 10.1111/jnc.12656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhou XF. Injection of brain-derived neurotrophic factor in the rostral ventrolateral medulla increases arterial blood pressure in anaesthetized rats. Neuroscience. 2002;112(4):967–75. 10.1016/S0306-4522(02)00085-4 [DOI] [PubMed] [Google Scholar]
- Duan W, Guo Z, Jiang H, Ware M, Mattson MP. Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor. Endocrinology. 2003;144(6):2446–53. 10.1210/en.2002-0113 [DOI] [PubMed] [Google Scholar]
- Griffioen KJ, Wan R, Brown TR, Okun E, Camandola S, Mughal MR, et al. Aberrant heart rate and brainstem brain-derived neurotrophic factor (BDNF) signaling in a mouse model of Huntington’s disease. Neurobiol Aging. 2012;33(7):1481.e1–5. 10.1016/j.neurobiolaging.2011.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabery S, Murphy K, Schultz K, Loy CT, McCusker E, Kirik D, et al. Changes in key hypothalamic neuropeptide populations in Huntington disease revealed by neuropathological analyses. Acta Neuropathol. 2010;120(6):777–88. 10.1007/s00401-010-0742-6 [DOI] [PubMed] [Google Scholar]
- van Wamelen DJ, Aziz NA, Roos RA, Swaab DF. Roos R a. C, Swaab DF. Hypothalamic alterations in Huntington’s disease patients: comparison with genetic rodent models. J Neuroendocrinol. 2014;26(11):761–75. 10.1111/jne.12190 [DOI] [PubMed] [Google Scholar]
- Zuccato C, Marullo M, Conforti P, MacDonald ME, Tartari M, Cattaneo E. Systematic assessment of BDNF and its receptor levels in human cortices affected by Huntington’s disease. Brain Pathol. 2008;18(2):225–38. 10.1111/j.1750-3639.2007.00111.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, Goffredo D, Conti L, et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science. 2001;293(5529):493–8. 10.1126/science.1059581 [DOI] [PubMed] [Google Scholar]
- Rüb U, Hentschel M, Stratmann K, Brunt E, Heinsen H, Seidel K, et al. Huntington’s disease (HD): degeneration of select nuclei and widespread occurrence of neuronal nuclear and axonal inclusions in the brainstem. Brain Pathol. 2014;24(3):247–60. 10.1111/bpa.12115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohawk JA, Green CB, Takahashi JS. Central and Peripheral Circadian Clocks in Mammals. Annu Rev Neurosci. 2012;35(1):445–62. 10.1146/annurev-neuro-060909-153128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AB, Angelo N, Huettner JE, Herzog ED. Intrinsic, nondeterministic circadian rhythm generation in identified mammalian neurons. Proc Natl Acad Sci USA. 2009;106(38):16493–8. 10.1073/pnas.0902768106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS. Linking neural activity and molecular oscillations in the SCN. Nat Rev Neurosci. 2011;12(10):553–69. 10.1038/nrn3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuljis D, Kudo T, Tahara Y, Ghiani CA, Colwell CS. Pathophysiology in the suprachiasmatic nucleus in mouse models of Huntington’s disease. J Neurosci Res. 2018;96(12):1862–75. 10.1002/jnr.24320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallier PN, Maywood ES, Zheng Z, Chesham JE, Inyushkin AN, Dyball R, et al. Pharmacological imposition of sleep slows cognitive decline and reverses dysregulation of circadian gene expression in a transgenic mouse model of Huntington’s disease. J Neurosci. 2007;27(29):7869–78. 10.1523/JNEUROSCI.0649-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder AM, Colwell CS. How to fix a broken clock. Trends Pharmacol Sci. 2013;34(11):605–19. 10.1016/j.tips.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker DS, Loh DH, Wang HB, Tahara Y, Kuljis D, Cutler T, et al. Circadian-based Treatment Strategy Effective in the BACHD Mouse Model of Huntington’s Disease. J Biol Rhythms. 2018;33(5):535–54. 10.1177/0748730418790401 [DOI] [PubMed] [Google Scholar]
- Maywood ES, Fraenkel E, McAllister CJ, Wood N, Reddy AB, Hastings MH, et al. Disruption of peripheral circadian timekeeping in a mouse model of Huntington’s disease and its restoration by temporally scheduled feeding. J Neurosci. 2010;30(30):10199–204. 10.1523/JNEUROSCI.1694-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ME, Razeghi P, Taegtmeyer H. Clock genes in the heart: characterization and attenuation with hypertrophy. Circ Res. 2001;88(11):1142–50. 10.1161/hh1101.091190 [DOI] [PubMed] [Google Scholar]
- Durgan DJ, Hotze MA, Tomlin TM, Egbejimi O, Graveleau C, Abel ED, et al. The intrinsic circadian clock within the cardiomyocyte. Am J Physiol Heart Circ Physiol. 2005;289(4):H1530–41. 10.1152/ajpheart.00406.2005 [DOI] [PubMed] [Google Scholar]
- Canaple L, Rambaud J, Dkhissi-Benyahya O, Rayet B, Tan NS, Michalik L, et al. Reciprocal regulation of brain and muscle Arnt-like protein 1 and peroxisome proliferator-activated receptor alpha defines a novel positive feedback loop in the rodent liver circadian clock. Mol Endocrinol. 2006;20(8):1715–27. 10.1210/me.2006-0052 [DOI] [PubMed] [Google Scholar]
- Martino TA, Young ME. Influence of the cardiomyocyte circadian clock on cardiac physiology and pathophysiology. J Biol Rhythms. 2015;30(3):183–205. 10.1177/0748730415575246 [DOI] [PubMed] [Google Scholar]
- Zhang L, Prosdocimo DA, Bai X, Fu C, Zhang R, Campbell F, et al. KLF15 Establishes the Landscape of Diurnal Expression in the Heart. Cell Rep. 2015;13(11):2368–75. 10.1016/j.celrep.2015.11.038 [DOI] [PubMed] [Google Scholar]
- Jeyaraj D, Haldar SM, Wan X, McCauley MD, Ripperger JA, Hu K, et al. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature. 2012;483(7387):96–9. 10.1038/nature10852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino TA, Tata N, Belsham DD, Chalmers J, Straume M, Lee P, et al. Disturbed diurnal rhythm alters gene expression and exacerbates cardiovascular disease with rescue by resynchronization. Hypertension. 2007;49(5):1104–13. 10.1161/HYPERTENSIONAHA.106.083568 [DOI] [PubMed] [Google Scholar]
- Martino TA, Oudit GY, Herzenberg AM, Tata N, Koletar MM, Kabir GM, et al. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294(5):R1675–83. 10.1152/ajpregu.00829.2007 [DOI] [PubMed] [Google Scholar]
- Bunger MK, Walisser JA, Sullivan R, Manley PA, Moran SM, Kalscheur VL, et al. Progressive arthropathy in mice with a targeted disruption of the Mop3/Bmal-1 locus. Genesis. 2005;41(3):122–32. 10.1002/gene.20102 [DOI] [PubMed] [Google Scholar]
- Laposky A, Easton A, Dugovic C, Walisser J, Bradfield C, Turek F. Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep. 2005;28(4):395–409. 10.1093/sleep/28.4.395 [DOI] [PubMed] [Google Scholar]
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006;20(14):1868–73. 10.1101/gad.1432206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefta M, Campbell KS, Feng HZ, Jin JP, Esser KA. Development of dilated cardiomyopathy in Bmal1-deficient mice. Am J Physiol Heart Circ Physiol. 2012;303(4):H475–85. 10.1152/ajpheart.00238.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ME, Brewer RA, Peliciari-Garcia RA, Collins HE, He L, Birky TL, et al. Cardiomyocyte-specific BMAL1 plays critical roles in metabolism, signaling, and maintenance of contractile function of the heart. J Biol Rhythms. 2014;29(4):257–76. 10.1177/0748730414543141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray MS, Shaw CA, Moore MW, Garcia RA, Zanquetta MM, Durgan DJ, et al. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am J Physiol Heart Circ Physiol. 2008;294(2):H1036–47. 10.1152/ajpheart.01291.2007 [DOI] [PubMed] [Google Scholar]
- Durgan DJ, Tsai JY, Grenett MH, Pat BM, Ratcliffe WF, Villegas-Montoya C, et al. Evidence Suggesting that the Cardiomyocyte Circadian Clock Modulates Responsiveness of the Heart to Hypertrophic Stimuli in Mice. Chronobiol Int. 2011;28(3):187–203. 10.3109/07420528.2010.550406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, et al. A Prospective Study of Sleep Duration and Coronary Heart Disease in Women. Arch Intern Med. 2003;163(2):205–9. 10.1001/archinte.163.2.205 [DOI] [PubMed] [Google Scholar]
- Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci USA. 2016;113(10):E1402–11. 10.1073/pnas.1516953113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutters F, Lemmens SG, Adam TC, Bremmer MA, Elders PJ, Nijpels G, et al. Is social jetlag associated with an adverse endocrine, behavioral, and cardiovascular risk profile? J Biol Rhythms. 2014;29(5):377–83. 10.1177/0748730414550199 [DOI] [PubMed] [Google Scholar]
- Raffaello F, Franca B, Simona P, Mauro T, Paolo S, Alberto M. Modifications of Cardiac Autonomic Profile Associated With a Shift Schedule of Work. Circulation. 2000;102(16):1912–6. 10.1161/01.CIR.102.16.1912 [DOI] [PubMed] [Google Scholar]