Abstract
Perinatal hypoxic-ischemic encephalopathy is a leading cause of neonatal death and disability. Therapeutic hypothermia significantly reduces death and major disability associated with hypoxic-ischemic encephalopathy; however, many infants still experience lifelong disabilities to movement, sensation and cognition. Clinical guidelines, based on strong clinical and preclinical evidence, recommend therapeutic hypothermia should be started within 6 hours of birth and continued for a period of 72 hours, with a target brain temperature of 33.5 ± 0.5°C for infants with moderate to severe hypoxic-ischemic encephalopathy. The clinical guidelines also recommend that infants be rewarmed at a rate of 0.5°C per hour, but this is not based on strong evidence. There are no randomized controlled trials investigating the optimal rate of rewarming after therapeutic hypothermia for infants with hypoxic-ischemic encephalopathy. Preclinical studies of rewarming are conflicting and results were confounded by treatment with sub-optimal durations of hypothermia. In this review, we evaluate the evidence for the optimal start time, duration and depth of hypothermia, and whether the rate of rewarming after treatment affects brain injury and neurological outcomes.
Keywords: hypoxia-ischemia, hypoxic-ischemic encephalopathy, therapeutic hypothermia, neuroprotection, therapeutic strategies, randomized controlled trials, animal models, fetal sheep, piglets
Introduction
Moderate to severe perinatal hypoxic-ischemic encephalopathy (HIE) affects 1–3 per 1000 live-term births (Lundgren et al., 2018). In most cases the pathogenesis involves profoundly reduced oxygenation (hypoxia) leading to secondary hypotension with reduced cerebral perfusion (ischemia, i.e., hypoxia-ischemia (HI)) (Dhillon et al., 2018). Moderate to severe acute HI can trigger evolving cell death that continues for days or even weeks later, offering the potential for delayed treatment. The only available treatment for moderate to severe HIE in term infants is therapeutic hypothermia, which significantly reduces death and severe disability in term and near-term infants (Jacobs et al., 2013). However, nearly half of infants with moderate to severe HIE treated with hypothermia still die or survive with disability despite intervention (Jacobs et al., 2013). Thus, it is very important to determine whether current hypothermia protocols can be optimized to further improve outcomes.
Pathophysiology of Hypoxic-Ischemic Encephalopathy
We now know that HIE is not a static injury, but rather represents an evolving cascade of brain injury that may continue for days to weeks after the initial insult (Wassink et al., 2018). During exposure to HI, which may be termed the primary phase of injury, reduced supply of oxygen and glucose leads to insufficient production of high energy phosphates such as adenosine triphosphate. In turn, this triggers anoxic depolarization with efflux of potassium and influx of sodium and calcium ions and water into the cells, leading to cell swelling; if sufficiently prolonged this can trigger acute cell lysis (Tan et al., 1996; Gunn et al., 1997; Wassink et al., 2014).
If oxygenation can be restored in time then cerebral oxidative metabolism, as measured by magnetic resonance spectroscopy and near infrared spectroscopy, may partially or even completely recover to control levels in a latent phase (Bennet et al., 2007; Bainbridge et al., 2014). After moderate to severe HI, despite normal brain oxygenation, this transient recovery is followed by delayed, progressive failure of oxidative metabolism, typically starting after approximately six hours (Lorek et al., 1994; Bennet et al., 2007; Bainbridge et al., 2014). This secondary deterioration is accompanied by delayed seizures, secondary cytotoxic edema (Williams et al., 1991), neuroinflammation (Mallard et al., 2018), and, ultimately, extensive programmed cell death (Northington et al., 2007). These events typically resolve over approximately 72 hours. Greater severity of secondary energy failure is correlated with worse outcomes, and the timing of this energy failure is closely associated with histological manifestation of brain damage (Roth et al., 1997). Interestingly, there is growing evidence that active pathological processes may continue for months after the initial insult, termed the tertiary phase of injury (Fleiss and Gressens, 2012). This phase is characterized by chronic neuroinflammation, epigenetic modification, withdrawal of trophic support and impaired connectivity (Fleiss and Gressens, 2012; Hassell et al., 2015).
The latent phase in the first six hours of life represents the key window of opportunity to start treatment with therapeutic hypothermia and prevent secondary programmed cell death and neuroinflammation (Gunn et al., 1999; Roelfsema et al., 2004; Seo et al., 2012). It is important to appreciate that in clinical practice exposure to HI is often not clearly defined, and injury may have been initiated and then evolved into the secondary phase before birth. Thus, in some cases even very early treatment after birth may correspond with relatively late in the window of opportunity or even completely miss the window for successful intervention altogether. Furthermore, more severe HI is associated with a shorter duration of the latent phase and hence length of window of opportunity, reducing the time before irreversible programmed cell death is initiated (Bainbridge et al., 2014).
Therapeutic Hypothermia – Current Protocols
Current clinical guidelines recommend that hypothermia must be started within the first six hours of life and maintained for 72 hours with a target brain temperature between 33–34°C in infants with moderate to severe HIE (Natarajan et al., 2018). Meta-analysis of multiple large pragmatic clinical trials suggests that therapeutic hypothermia is an effective treatment for moderate to severe HIE, with reduced composite score of death or severe disability at 18 months after treatment (relative risk = 0.81) (Edwards et al., 2010; Jacobs et al., 2013). Critically, long-term follow-up of the clinical trials suggests that the benefits associated with treatment with therapeutic hypothermia persist into middle childhood (Guillet et al., 2012; Shankaran et al., 2012; Azzopardi et al., 2014).
Despite the success of therapeutic hypothermia, treatment is partially protective. A meta-analysis of 11 randomized controlled trials comparing mild induced hypothermia to normothermia reported a number needed to treat of seven to eight (Jacobs et al., 2013). That is, to prevent one case of death or disability from HIE, seven or eight infants must be treated with therapeutic hypothermia.
When Should Hypothermia Be Started?
There is considerable evidence from animal studies that hypothermia must be started as early as possible within the latent phase for optimal benefit. This pattern is highly consistent with the finding of progressive mitochondrial failure during the latent phase demonstrated by magnetic resonance spectroscopy after moderate to severe HI in human infants (Azzopardi et al., 1989; Roth et al., 1997) and piglets (Thoresen et al., 1995).
In unanesthetized 21 day old rat pups, mild hypothermia (2 to 3°C decrease in brain temperature) for 72 hours after HI prevented cortical infarction, whereas cooling delayed until six hours after the insult had an intermediate, non-significant effect (Sirimanne et al., 1996). More recently, in seven day old rat pups, hypothermia was found to be notably protective after a “moderate” duration of HI (90 minutes) (Sabir et al., 2012), such that immediate induction of hypothermia after moderate HI significantly reduced the area of cortical infarction (P < 0.05), with a linear loss of effect with greater delay in starting cooling, at least up to 6 hours of delay. Similarly, in near-term fetal sheep, hypothermia started 90 minutes (Gunn et al., 1997) or 3 hours (Davidson et al., 2015) after global cerebral ischemia for a period of 72 hours was associated with reduced neuronal and oligodendroglial death, and significantly improved recovery of brain activity compared to normothermia animals. However, when treatment was delayed until 5.5 hours, secondary cytotoxic edema was prevented but there was only partial recovery of brain activity and partial protection of grey and white matter (Gunn et al., 1998). When hypothermia was delayed until 8.5 hours after HI, after the onset of seizures, no significant reduction in cell death or improvement in brain activity was seen (Gunn et al., 1999).
Although there is little systematic clinical evidence, some clinical studies support the importance of initiating hypothermia as early as possible. For example, in a cohort study of 80 term infants with moderate to severe HIE treated with hypothermia, infants in whom hypothermia was started less than three hours after birth had significantly higher median psychomotor development index at 18 months than infants in whom hypothermia was started between three to six hours after birth (Thoresen et al., 2013). Taken together, these studies suggest that hypothermia needs to be started within 6 hours of birth but that the earlier treatment can be started, the more likely it is to achieve improved outcomes.
How Long Should Hypothermia Be Continued For?
Broadly at least, the optimal duration of hypothermia seems to correspond with the duration of the secondary phase of seizures and cytotoxic events. Early studies using relatively short durations of hypothermia found that even brief delay after reperfusion was associated with loss of neuroprotection. For example, in a classic study, after three minutes of global ischemia in gerbils, hypothermia started one hour post-HI for a period of 12 hours reduced hippocampal CA1 injury, however neuroprotection was lost when ischemia was continued for five minutes (Colbourne and Corbett, 1994). Strikingly, extending the duration of hypothermia to 24 hours resulted in a 90% preservation of CA1 neurons after severe ischemia (Colbourne and Corbett, 1994). At six months after hypothermia treatment, CA1 neuronal survival was reduced to 70%, compared to 5% survival in non-treated animals, suggesting prolonged benefit after delayed but extended hypothermia (Colbourne and Corbett, 1995). These studies suggest that there is an interaction between the severity of HI, the delay in treatment onset and duration of treatment that helps to determine the overall efficacy of treatment.
In near-term fetal sheep, delayed but prolonged cooling for 72 hours consistently demonstrates a reduction in neuronal injury and restoration of brain activity to near-baseline levels (Gunn et al., 1997, 1998; Roelfsema et al., 2004). However, extending the duration of hypothermia to 120 hours started 3 hours after global ischemia was associated with a small but significant reduction in cortical and dentate gyral neuronal survival, and no greater microglial suppression compared to 72 hours of hypothermia (Davidson et al., 2015). In a follow-up study, partial protection of oligodendrocytes and myelin basic protein expression was seen after 120 hours of hypothermia, but this protocol was not associated with significant microglial suppression in the white matter (Davidson et al., 2016). Taken together, these data suggest that prolonging the duration of hypothermia is not beneficial and may be deleterious. The apparently worse histological outcomes may be related to over-suppression of neuronal activity and delayed restoration of the central nervous system microenvironment with longer cooling (Wassink et al., 2018).
Consistent with this, a randomized clinical trial that included a subgroup investigating the efficacy of 120 hours of hypothermia was discontinued part-way through after futility analysis indicated a less than 2% probability of significantly improving neurological outcomes compared to the standard 72 hours duration (Shankaran et al., 2014). Subsequent neurodevelopmental follow-up confirmed that there was no significant reduction in death or disability (Shankaran et al., 2017).
Conversely, hypothermia for only 48 hours after global ischemia in near-term fetal sheep was associated with a progressive deterioration of brain activity after rewarming, with impaired neuronal survival and myelination, and increased microglial activation compared to 72 hours of hypothermia as shown in Figure 1. These findings strongly suggest a role for re-activation of deleterious inflammatory processes associated with premature rewarming after therapeutic hypothermia (Davidson et al., 2018b).
Figure 1.
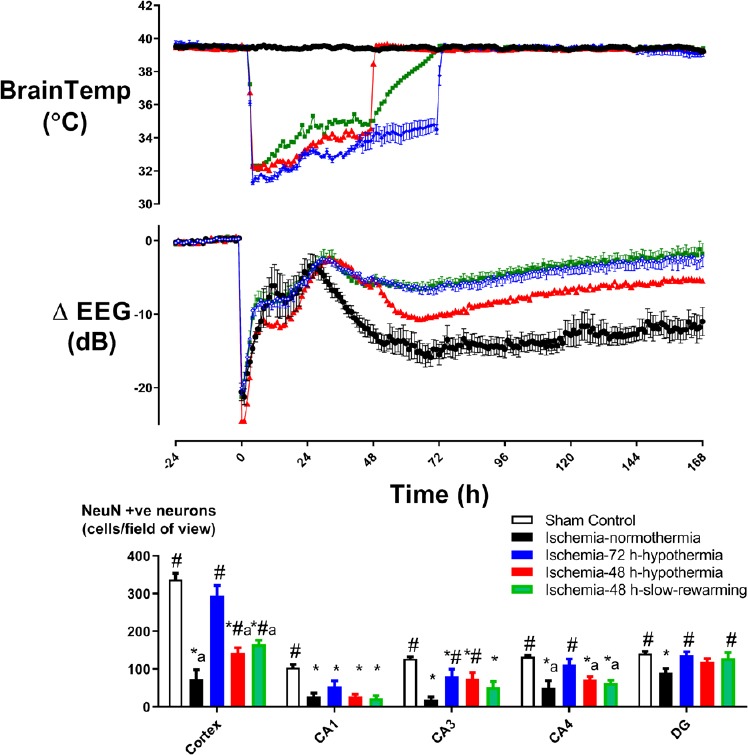
The effect of duration of hypothermia and rate of rewarming on electrophysiological and histological recovery from cerebral ischemia in near-term fetal sheep.
Top: Changes in electroencephalography (EEG) power (db) and brain temperature (°C), before, during and after 30 minutes of global cerebral ischemia (time zero) in the ischemia-normothermia (n = 8), ischemia-48 hours (h)-hypothermia (n = 8), ischemia-48 h-hypothermia-slow-rewarming (n = 7) and ischemia-72 h-hypothermia (n = 8) groups. Bottom: Neuronal survival seven days after 30 minutes of global cerebral ischemia in the sham control (n = 9), ischemia-normothermia (n = 8), ischemia-48 h-hypothermia (n = 8), ischemia-48 h-hypothermia-slow-rewarming (n = 7) and ischemia-72 h-hypothermia (n =8) groups. *P < 0.05, vs. sham control; #P < 0.05, vs. ischemia-normothermia; aP < 0.05, vs. ischemia-72 h-hypothermia. Data were derived from Davidson et al. (2018a).
Together, pre-clinical studies of hypothermia demonstrate that hypothermia should be started in the latent phase and continued for 72 hours to adequately suppress programmed cell death and inflammation until normal homeostasis can return, and so provide optimal neuroprotection.
How Much Should We Cool?
The temperature to which infants are cooled during therapeutic hypothermia is also important in determining neuroprotection. In near-term fetal sheep, a reduction in brain temperature from 39.5°C to ~34°C was sufficient to achieve neuroprotection after global ischemia, however, no further improvement was achieved with deeper cooling (Gunn et al., 1997). In full-term piglets, reducing core temperature by 3.5°C for 12 hours or 5°C from 2–26 hours after HI prevented significant secondary metabolic failure and improved neuronal survival after HI (Thoresen et al., 1995; Alonso-Alconada et al., 2015). Interestingly, reducing core temperature by 8.5°C was associated with impaired neuroprotection in thalamic and striatal regions, and has been associated with adverse systemic effects such as alterations to blood pH, glucose handling and cardiac arrest in neonatal piglets after HI (Kerenyi et al., 2012; Alonso-Alconada et al., 2015).
Similarly, the clinical trial investigating longer duration of cooling also investigated deeper cooling of HIE infants to 32°C (Shankaran et al., 2014). In this trial, the probability of detecting a statistically significant benefit for longer or deeper cooling, or both, was less than 2% (Shankaran et al., 2014, 2017). Collectively, hypothermia started within six hours of birth, continued for 72 hours to a depth of 33.5°C appears to provide optimal neuroprotection.
Does the Rate of Rewarming Affect Neuroprotection?
The clinical trials of hypothermia rewarmed neonates at a rate of 0.5°C/h until they were within a temperature range between 36.5–37.5°C (Gluckman et al., 2005; Shankaran et al., 2005; Azzopardi et al., 2009); this approach was based on case reports suggesting that rapid rewarming might lead to cardiovascular instability (Thoresen and Whitelaw, 2000). Moreover, a clinical case report suggested that rewarming at 0.5°C per hour was associated with rebound seizure activity in a neonate treated for moderate to severe HIE (Battin et al., 2004). A more recent case report described a neonate who began having electrographic seizures for the first time during rewarming, which continued in the hours after rewarming (Kendall et al., 2012). The seizures stopped within 30 minutes of re-cooling the neonate to 33.5°C without anticonvulsant medication. Similarly, in early studies of hypothermia after HI in near-term fetal sheep, rewarming spontaneously over approximately 30 minutes was associated with a small increase in seizure activity compared to normothermic animals. However the protocol was still associated with markedly improved recovery of brain activity and neuronal survival (Gerrits et al., 2005). These studies suggest that rapid rewarming may be associated with rebound seizure activity but the effect of rate of rewarming on neuroprotection is unclear.
Potentially, the pro-inflammatory release of complement and cell adhesion molecules that is attenuated by hypothermia could be re-activated after rewarming. Thus, it has been suggested that rapid rewarming could more rapidly re-activate inflammatory responses suppressed during hypothermia (Scaravilli et al., 2012). Alternatively, rapid rewarming could lead to reversal of hypothermic suppression of oxidative stress and excitotoxin release; however these data relate to cooling during HI (Nakashima and Todd, 1996; Hashimoto et al., 2003). Limited evidence as discussed next, suggests that a slower rate of rewarming after therapeutic hypothermia may be associated with improved neuroprotection. However, it is critical to note that these studies of the rate of rewarming are largely confounded by suboptimally short durations of hypothermia, which as described above, are an important determinant of the extent of neuroprotection. That is to say, if a sub-optimal duration of hypothermia is tested, then subsequent slower rewarming after hypothermia also inevitably extends the total duration of hypothermia closer to an optimal duration than fast rewarming.
In 3–5 day old piglets exposed to systemic hypoxia and endotracheal asphyxia, rewarming at a rate of 4°C/h from 18 hours of hypothermia was associated with an increased expression of cleaved caspase-3 in cortical homogenates compared to animals rewarmed at 0.5°C/h (Wang et al., 2015). However, the cell type in which apoptosis was upregulated was not discernible due to use of homogenate-based assays. Further, the highly sub-optimal duration of hypothermia in the control group receiving rapid rewarming may have contributed to apoptosis from reduced suppression of injury. Thus, the reduced apoptosis in the group rewarmed more slowly may not have been related to the rate of rewarming per se but rather to the greater total duration of hypothermia.
In traumatic brain injury in adult rats, rapid rewarming over 15 minutes after one hour of hypothermia was associated with increased axonal injury and impaired cerebrovascular responsiveness to vasoactive agents and injury expansion, whereas rewarming over 90 minutes was associated with improved axonal recovery (Suehiro and Povlishock, 2001; Ueda et al., 2004; Povlishock and Wei, 2009). Further, in adult gerbils, rapid rewarming over 30 minutes after two hours of hypothermia was associated with a mismatch between cerebral circulation and metabolism, evidenced by an increase in cerebral glutamate and lactate washout (Nakamura et al., 2003). However, the applicability of these findings to neonatal brain injury and moderate to severe HIE is unknown.
Effects of rapid rewarming may not be limited to the brain. A mismatch between total body oxygen demand and oxygen delivery, termed rewarming shock, has been reported after deep hypothermia for cardiovascular surgery (Scaravilli et al., 2012). Rewarming shock has been associated with decline in blood pH, respiratory inadequacy, bradycardia and hypotension in human adults (Bigelow, 1959). In adult rats, rapid rewarming at approximately 4.5°C/h is associated with greater earlier alterations to heart rate and cardiac output (Eshel et al., 2002), consistent with empiric concerns of cardiovascular destabilization after rapid rewarming in some human infants (Thoresen and Whitelaw, 2000).
By contrast, studies using longer durations of hypothermia have not reported any cardiovascular instability during rapid rewarming (Davidson et al., 2015, 2016). Of interest, a recent study in near-term fetal sheep reported that slow rewarming over 24 hours, after 48 hours of hypothermia that was started three hours after global cerebral ischemia, prevented deterioration of electroencephalography (EEG) activity compared to rapid rewarming at 48 hours. However, recovery of EEG activity was comparable with that after rapid rewarming from 72 hours of hypothermia as shown in Figure 1 (Davidson et al., 2018a). Of concern, neuronal survival remained significantly less than when hypothermia was continued for 72 hours. Together, these findings suggest that overall improvements to neuronal survival and EEG recovery were mediated by the longer duration of hypothermia rather than the rate of rewarming per se. Future studies should investigate the long-term impact of rewarming on neuroprotection after 72 hours of hypothermia.
To date, no clinical trial has investigated the rate of rewarming after the optimal start time, duration and depth of therapeutic hypothermia for moderate to severe HIE. Given that most of the animal studies of slow rewarming were confounded by use of sub-optimal durations or depths of hypothermia, it is difficult to conclude whether apparent improvements were truly due to the rate of rewarming, or merely represent prolongation of sub-optimal treatment. It may be that slow rewarming could allow a more gradual recovery or adjustment of the central nervous system microenvironment after metabolic suppression and injury. However, there is concern that extending the duration of hypothermia past the seemingly optimal 72 hours duration may be associated with poorer cell survival in some brain regions (Davidson et al., 2015, 2016).
It should also be noted that there is no consensus between studies on what constitutes a “rapid” or “slow” rate of rewarming, which in pre-clinical models largely depends on the species used, their baseline temperature and target cooling depth. Future animal studies should systematically evaluate the effects of different rates of rewarming, to find that which may strike a balance between optimal neuroprotection whilst minimizing cardiovascular and systemic destabilization.
Conclusions & Future Directions
As reviewed above, there is now compelling evidence that therapeutic hypothermia for moderate to severe HIE started within 6 hours after birth, for a period of 72 hours to a target brain temperature of 33.5 ± 0.5°C significantly reduces neonatal mortality and major morbidity. Deviation from this evidence-based protocol compromises neurological recovery and worsens outcomes in large animal models of HIE. By contrast, the available large animal evidence suggests that the rate of rewarming after therapeutic hypothermia does not appear to be a critical variable, and is much less important than simply providing a sufficient duration of hypothermia. The intriguing differences in the effect of very slow rewarming on EEG recovery compared with neuronal survival suggests that further studies of rewarming in animal models after an optimal hypothermia protocol may be of value. Pragmatically, it is most likely that substantial further improvements in outcome will come from systematic studies of adding other neuroprotective interventions to hypothermic neuroprotection, as recently reviewed (Davidson et al., 2018c). In current clinical practice, the focus should be on finding ways to initiate hypothermia as soon as possible after birth for infants with HIE.
Additional file: Open peer review report 1 (98.7KB, pdf) .
Footnotes
Conflicts of interest: All authors disclose no conflicts of interest.
Financial support: The work was supported by The Health Research Council of New Zealand (grant No. 16/003, 17/601) and the Marsden Fund (grant No. 17-UOA232). JOD holds a Sir Charles Hercus Fellowship from the Health Research Council of New Zealand (grant No. 16/003).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Nobuyuki Ishibashi, Children’s National Health System, USA.
Funding: The work was supported by The Health Research Council of New Zealand (grant No. 16/003, 17/601) and the Marsden Fund (grant No. 17-UOA232). JOD holds a Sir Charles Hercus Fellowship from the Health Research Council of New Zealand (grant No. 16/003).
P-Reviewer: Ishibashi N; C-Editors: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Alonso-Alconada D, Broad KD, Bainbridge A, Chandrasekaran M, Faulkner SD, Kerenyi A, Hassell J, Rocha-Ferreira E, Hristova M, Fleiss B, Bennett K, Kelen D, Cady E, Gressens P, Golay X, Robertson NJ. Brain cell death is reduced with cooling by 3.5 degrees C to 5 degrees C but increased with cooling by 8.5 degrees C in a piglet asphyxia model. Stroke. 2015;46:275–278. doi: 10.1161/STROKEAHA.114.007330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azzopardi D, Wyatt JS, Cady EB, Delpy DT, Baudin J, Stewart AL, Hope PL, Hamilton PA, Reynolds EO. Prognosis of newborn infants with hypoxic-ischemic brain injury assessed by phosphorus magnetic resonance spectroscopy. Pediatr Res. 1989;25:445–451. doi: 10.1203/00006450-198905000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Azzopardi D, Strohm B, Marlow N, Brocklehurst P, Deierl A, Eddama O, Goodwin J, Halliday HL, Juszczak E, Kapellou O, Levene M, Linsell L, Omar O, Thoresen M, Tusor N, Whitelaw A, Edwards AD TOBY Study Group. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med. 2014;371:140–149. doi: 10.1056/NEJMoa1315788. [DOI] [PubMed] [Google Scholar]
- 4.Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, Kapellou O, Levene M, Marlow N, Porter E, Thoresen M, Whitelaw A, Brocklehurst P. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–1358. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 5.Bainbridge A, Tachtsidis I, Faulkner SD, Price D, Zhu T, Baer E, Broad KD, Thomas DL, Cady EB, Robertson NJ, Golay X. Brain mitochondrial oxidative metabolism during and after cerebral hypoxia-ischemia studied by simultaneous phosphorus magnetic-resonance and broadband near-infrared spectroscopy. Neuroimage. 2014;102:173–183. doi: 10.1016/j.neuroimage.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battin MR, Bennet L, Gunn AJ. Rebound seizures during rewarming. Pediatrics. 2004;114:1369. doi: 10.1542/peds.2004-1695. [DOI] [PubMed] [Google Scholar]
- 7.Bennet L, Roelfsema V, Dean JM, Wassink G, Power GG, Jensen EC, Gunn AJ. Regulation of cytochrome oxidase redox state during umbilical cord occlusion in preterm fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1569–1576. doi: 10.1152/ajpregu.00743.2006. [DOI] [PubMed] [Google Scholar]
- 8.Bigelow WC. Methods for inducing hypothermia and rewarming. Ann N Y Acad Sci. 1959;80:522–532. doi: 10.1111/j.1749-6632.1959.tb49229.x. [DOI] [PubMed] [Google Scholar]
- 9.Colbourne F, Corbett D. Delayed and prolonged post-ischemic hypothermia is neuroprotective in the gerbil. Brain Res. 1994;654:265–272. doi: 10.1016/0006-8993(94)90488-x. [DOI] [PubMed] [Google Scholar]
- 10.Colbourne F, Corbett D. Delayed postischemic hypothermia: a six month survival study using behavioral and histological assessments of neuroprotection. J Neurosci. 1995;15:7250–7260. doi: 10.1523/JNEUROSCI.15-11-07250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson JO, Wassink G, Yuill CA, Zhang FG, Bennet L, Gunn AJ. How long is too long for cerebral cooling after ischemia in fetal sheep? J Cereb Blood Flow Metab. 2015;35:751–758. doi: 10.1038/jcbfm.2014.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson JO, Yuill CA, Zhang FG, Wassink G, Bennet L, Gunn AJ. Extending the duration of hypothermia does not further improve white matter protection after ischemia in term-equivalent fetal sheep. Sci Rep. 2016;6:25178. doi: 10.1038/srep25178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson JO, Wassink G, Draghi V, Dhillon SK, Bennet L, Gunn AJ. Limited benefit of slow rewarming after cerebral hypothermia for global cerebral ischemia in near-term fetal sheep. J Cereb Blood Flow Metab. 2018a doi: 10.1177/0271678X18791631. doi: 10.1177/0271678X18791631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidson JO, Draghi V, Whitham S, Dhillon SK, Wassink G, Bennet L, Gunn AJ. How long is sufficient for optimal neuroprotection with cerebral cooling after ischemia in fetal sheep? J Cereb Blood Flow Metab. 2018b;38:1047–1059. doi: 10.1177/0271678X17707671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson JO, Dean JM, Fraser M, Wassink G, Andelius TC, Dhillon SK, Bennet L, Gunn AJ. Perinatal brain injury: mechanisms and therapeutic approaches. Front Biosci (Landmark Ed) 2018c;23:2204–2226. doi: 10.2741/4700. [DOI] [PubMed] [Google Scholar]
- 16.Dhillon SK, Lear CA, Galinsky R, Wassink G, Davidson JO, Juul S, Robertson NJ, Gunn AJ, Bennet L. The fetus at the tipping point: modifying the outcome of fetal asphyxia. J Physiol. 2018;596:5571–5592. doi: 10.1113/JP274949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, Strohm B, Thoresen M, Whitelaw A, Azzopardi D. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010;340:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eshel G, Reisler G, Berkovitch M, Shapira S, Grauer E, Barr J. Comparison of fast versus slow rewarming following acute moderate hypothermia in rats. Paediatr Anaesth. 2002;12:235–242. doi: 10.1046/j.1460-9592.2002.00801.x. [DOI] [PubMed] [Google Scholar]
- 19.Fleiss B, Gressens P. Tertiary mechanisms of brain damage: a new hope for treatment of cerebral palsy? Lancet Neurol. 2012;11:556–566. doi: 10.1016/S1474-4422(12)70058-3. [DOI] [PubMed] [Google Scholar]
- 20.Gerrits LC, Battin MR, Bennet L, Gonzalez H, Gunn AJ. Epileptiform activity during rewarming from moderate cerebral hypothermia in the near-term fetal sheep. Pediatr Res. 2005;57:342–346. doi: 10.1203/01.PDR.0000150801.61188.5F. [DOI] [PubMed] [Google Scholar]
- 21.Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 22.Guillet R, Edwards AD, Thoresen M, Ferriero DM, Gluckman PD, Whitelaw A, Gunn AJ. Seven- to eight-year follow-up of the CoolCap trial of head cooling for neonatal encephalopathy. Pediatr Res. 2012;71:205–209. doi: 10.1038/pr.2011.30. [DOI] [PubMed] [Google Scholar]
- 23.Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest. 1997;99:248–256. doi: 10.1172/JCI119153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunn AJ, Gunn TR, Gunning MI, Williams CE, Gluckman PD. Neuroprotection with prolonged head cooling started before postischemic seizures in fetal sheep. Pediatrics. 1998;102:1098–1106. doi: 10.1542/peds.102.5.1098. [DOI] [PubMed] [Google Scholar]
- 25.Gunn AJ, Bennet L, Gunning MI, Gluckman PD, Gunn TR. Cerebral hypothermia is not neuroprotective when started after postischemic seizures in fetal sheep. Pediatr Res. 1999;46:274–280. doi: 10.1203/00006450-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto T, Yonetani M, Nakamura H. Selective brain hypothermia protects against hypoxic-ischemic injury in newborn rats by reducing hydroxyl radical production. Kobe J Med Sci. 2003;49:83–91. [PubMed] [Google Scholar]
- 27.Hassell KJ, Ezzati M, Alonso-Alconada D, Hausenloy DJ, Robertson NJ. New horizons for newborn brain protection: enhancing endogenous neuroprotection. Arch Dis Child Fetal Neonatal Ed. 2015;100:F541–552. doi: 10.1136/archdischild-2014-306284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;1:CD003311. doi: 10.1002/14651858.CD003311.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kendall GS, Mathieson S, Meek J, Rennie JM. Recooling for rebound seizures after rewarming in neonatal encephalopathy. Pediatrics. 2012;130:e451–455. doi: 10.1542/peds.2011-3496. [DOI] [PubMed] [Google Scholar]
- 30.Kerenyi A, Kelen D, Faulkner SD, Bainbridge A, Chandrasekaran M, Cady EB, Golay X, Robertson NJ. Systemic effects of whole-body cooling to 35 degrees C, 33.5 degrees C, and 30 degrees C in a piglet model of perinatal asphyxia: implications for therapeutic hypothermia. Pediatr Res. 2012;71:573–582. doi: 10.1038/pr.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lorek A, Takei Y, Cady EB, Wyatt JS, Penrice J, Edwards AD, Peebles D, Wylezinska M, Owen-Reece H, Kirkbride V. Delayed (“secondary”) cerebral energy failure after acute hypoxia- ischemia in the newborn piglet: continuous 48-hour studies by phosphorus magnetic resonance spectroscopy. Pediatr Res. 1994;36:699–706. doi: 10.1203/00006450-199412000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Lundgren C, Brudin L, Wanby AS, Blomberg M. Ante- and intrapartum risk factors for neonatal hypoxic ischemic encephalopathy. J Matern Fetal Neonatal Med. 2018;31:1595–1601. doi: 10.1080/14767058.2017.1321628. [DOI] [PubMed] [Google Scholar]
- 33.Mallard C, Tremblay ME, Vexler ZS. Microglia and neonatal brain injury. Neuroscience. 2018 doi: 10.1016/j.neuroscience.2018.01.023. doi: 10.1016/j.neuroscience.2018.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura T, Miyamoto O, Sumitani K, Negi T, Itano T, Nagao S. Do rapid systemic changes of brain temperature have an influence on the brain? Acta Neurochir (Wien) 2003;145:301–307. doi: 10.1007/s00701-002-1065-8. [DOI] [PubMed] [Google Scholar]
- 35.Nakashima K, Todd MM. Effects of hypothermia on the rate of excitatory amino acid release after ischemic depolarization. Stroke. 1996;27:913–918. doi: 10.1161/01.str.27.5.913. [DOI] [PubMed] [Google Scholar]
- 36.Natarajan G, Laptook A, Shankaran S. Therapeutic hypothermia: How can we optimize this therapy to further improve outcomes? Clin Perinatol. 2018;45:241–255. doi: 10.1016/j.clp.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Northington FJ, Zelaya ME, O’Riordan DP, Blomgren K, Flock DL, Hagberg H, Ferriero DM, Martin LJ. Failure to complete apoptosis following neonatal hypoxia-ischemia manifests as “continuum” phenotype of cell death and occurs with multiple manifestations of mitochondrial dysfunction in rodent forebrain. Neuroscience. 2007;149:822–833. doi: 10.1016/j.neuroscience.2007.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Povlishock JT, Wei EP. Posthypothermic rewarming considerations following traumatic brain injury. J Neurotrauma. 2009;26:333–340. doi: 10.1089/neu.2008.0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roelfsema V, Bennet L, George S, Wu D, Guan J, Veerman M, Gunn AJ. The window of opportunity for cerebral hypothermia and white matter injury after cerebral ischemia in near-term fetal sheep. J Cereb Blood Flow Metab. 2004;24:877–886. doi: 10.1097/01.WCB.0000123904.17746.92. [DOI] [PubMed] [Google Scholar]
- 40.Roth SC, Baudin J, Cady E, Johal K, Townsend JP, Wyatt JS, Reynolds EO, Stewart AL. Relation of deranged neonatal cerebral oxidative metabolism with neurodevelopmental outcome and head circumference at 4 years. Dev Med Child Neurol. 1997;39:718–725. doi: 10.1111/j.1469-8749.1997.tb07372.x. [DOI] [PubMed] [Google Scholar]
- 41.Sabir H, Scull-Brown E, Liu X, Thoresen M. Immediate hypothermia is not neuroprotective after severe hypoxia-ischemia and is deleterious when delayed by 12 hours in neonatal rats. Stroke. 2012;43:3364–3370. doi: 10.1161/STROKEAHA.112.674481. [DOI] [PubMed] [Google Scholar]
- 42.Scaravilli V, Bonacina D, Citerio G. Rewarming: facts and myths from the systemic perspective. Crit Care. 2012;16:A25. [Google Scholar]
- 43.Seo JW, Kim JH, Seo M, Han HS, Park J, Suk K. Time-dependent effects of hypothermia on microglial activation and migration. J Neuroinflammation. 2012;9:164. doi: 10.1186/1742-2094-9-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 45.Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, Gustafson KE, Leach TM, Green C, Bara R, Petrie Huitema CM, Ehrenkranz RA, Tyson JE, Das A, Hammond J, Peralta-Carcelen M, Evans PW, Heyne RJ, Wilson-Costello DE, Vaucher YE, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366:2085–2092. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shankaran S, Laptook AR, Pappas A, McDonald SA, Das A, Tyson JE, Poindexter BB, Schibler K, Bell EF, Heyne RJ, Pedroza C, Bara R, Van Meurs KP, Grisby C, Huitema CM, Garg M, Ehrenkranz RA, Shepherd EG, Chalak LF, Hamrick SE, et al. Effect of depth and duration of cooling on deaths in the NICU among neonates with hypoxic ischemic encephalopathy: a randomized clinical trial. JAMA. 2014;312:2629–2639. doi: 10.1001/jama.2014.16058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shankaran S, Laptook AR, Pappas A, McDonald SA, Das A, Tyson JE, Poindexter BB, Schibler K, Bell EF, Heyne RJ, Pedroza C, Bara R, Van Meurs KP, Huitema CMP, Grisby C, Devaskar U, Ehrenkranz RA, Harmon HM, Chalak LF, DeMauro SB, et al. Effect of depth and duration of cooling on death or disability at age 18 months among neonates with hypoxic-ischemic encephalopathy: a randomized clinical trial. JAMA. 2017;318:57–67. doi: 10.1001/jama.2017.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sirimanne ES, Blumberg RM, Bossano D, Gunning M, Edwards AD, Gluckman PD, Williams CE. The effect of prolonged modification of cerebral temperature on outcome after hypoxic-ischemic brain injury in the infant rat. Pediatr Res. 1996;39:591–597. doi: 10.1203/00006450-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Suehiro E, Povlishock JT. Exacerbation of traumatically induced axonal injury by rapid posthypothermic rewarming and attenuation of axonal change by cyclosporin A. J Neurosurg. 2001;94:493–498. doi: 10.3171/jns.2001.94.3.0493. [DOI] [PubMed] [Google Scholar]
- 50.Tan WK, Williams CE, During MJ, Mallard CE, Gunning MI, Gunn AJ, Gluckman PD. Accumulation of cytotoxins during the development of seizures and edema after hypoxic-ischemic injury in late gestation fetal sheep. Pediatr Res. 1996;39:791–797. doi: 10.1203/00006450-199605000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Thoresen M, Whitelaw A. Cardiovascular changes during mild therapeutic hypothermia and rewarming in infants with hypoxic-ischaemic encephalopathy. Pediatrics. 2000;106:92–99. doi: 10.1542/peds.106.1.92. [DOI] [PubMed] [Google Scholar]
- 52.Thoresen M, Penrice J, Lorek A, Cady EB, Wylezinska M, Kirkbride V, Cooper CE, Brown GC, Edwards AD, Wyatt JS. Mild hypothermia after severe transient hypoxia-ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatr Res. 1995;37:667–670. doi: 10.1203/00006450-199505000-00019. [DOI] [PubMed] [Google Scholar]
- 53.Thoresen M, Tooley J, Liu X, Jary S, Fleming P, Luyt K, Jain A, Cairns P, Harding D, Sabir H. Time is brain: starting therapeutic hypothermia within three hours after birth improves motor outcome in asphyxiated newborns. Neonatology. 2013;104:228–233. doi: 10.1159/000353948. [DOI] [PubMed] [Google Scholar]
- 54.Ueda Y, Suehiro E, Wei EP, Kontos HA, Povlishock JT. Uncomplicated rapid posthypothermic rewarming alters cerebrovascular responsiveness. Stroke. 2004;35:601–606. doi: 10.1161/01.STR.0000113693.56783.73. [DOI] [PubMed] [Google Scholar]
- 55.Wang B, Armstrong JS, Lee JH, Bhalala U, Kulikowicz E, Zhang H, Reyes M, Moy N, Spicer D, Zhu J, Yang ZJ, Koehler RC, Martin LJ, Lee JK. Rewarming from therapeutic hypothermia induces cortical neuron apoptosis in a swine model of neonatal hypoxic-ischemic encephalopathy. J Cereb Blood Flow Metab. 2015;35:781–793. doi: 10.1038/jcbfm.2014.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wassink G, Gunn ER, Drury PP, Bennet L, Gunn AJ. The mechanisms and treatment of asphyxial encephalopathy. Front Neurosci. 2014;8:40. doi: 10.3389/fnins.2014.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wassink G, Davidson JO, Lear CA, Juul SE, Northington F, Bennet L, Gunn AJ. A working model for hypothermic neuroprotection. J Physiol. 2018;596:5641–5654. doi: 10.1113/JP274928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams CE, Gunn A, Gluckman PD. Time course of intracellular edema and epileptiform activity following prenatal cerebral ischemia in sheep. Stroke. 1991;22:516–521. doi: 10.1161/01.str.22.4.516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


