Traumatic brain injury (TBI), an unmet need: TBI is an alteration in brain function caused by an external force with evidence of brain pathology. It could be from a bump, blow, blast or jolt to the head including penetrating the cranium. TBI is a public health concern worldwide due to its economic impact. Most TBIs are survivable, do not need hospitalization but may influence productivity. A smaller percentage of TBI due to falls or penetrating TBI (PTBI) needs hospitalization and accounts for largest fraction of TBI care costs. PTBI especially that involving firearm injury is an increasingly serious issue. In the United States, PTBI is an issue both in the military and civilian context costing more than $70–75 billion annually. PTBI has become increasingly survivable including previously lethal midline crossing of projectile due to brain trauma foundation guidelines as well as timely neurosurgical intervention. The extent of recovery is proportional to initial damage; injuries limited to single hemisphere stabilize earlier than those crossing the midline do. However, currently the consequence of surviving a PTBI is most likely to be permanent disability. Rate of disability has not changed over the past 5 decades. Almost 3.2 million Americans live with neurobehavioral disability i.e., chronic cognitive and functional impairment requiring support from their families and the State, with lifetime costs of millions of dollars per patient. The TBI lesion is dynamic with continued brain atrophy, which correlates with persistent neurological deficit and overall social outcome. Observations of post-TBI tissue loss by pathologists were confirmed by longitudinal imaging studies in living TBI survivors. Progressive volume loss was coincident with persistent neuroinflammation thought to be due to chronic microglial activation (Smith, 2013; Lee et al., 2018). In a study of veterans living with TBI spanning 4 decades, loss of tissue following PTBI was approximately 56 mL. Magnification of the primary injury via secondary mechanism underlies such volume loss and consequent disability. The PTBI penumbra (tissue surrounding the PTBI injury core) is render vulnerable by pyroptosis of chronically activated microglia. Continued pyroptosis of microglia and adjacent cells facilitate the lesion expansion into otherwise intact remote regions (Figure 1A & B). Spontaneous recovery in TBI generally takes place within the first 3 months after injury and is mainly due to neural plasticity but not endogenous reparative neurogenesis. Success in restoring an injured brain with current therapies is limited by inability of central nervous system to regenerate spontaneously. Hence, current medical treatments albeit unsuccessfully, have sought to (i) prevent neuroinflammation driven deterioration and (ii) replace lost cells. The historical failure of acute neuroprotective trials has led to alternate approaches i.e., recruit endogenous neural stem cells (NSCs) or replace via transplantation of exogenous NSCs with a goal to rebuild circuitry. Both preclinical and clinical attempts to boost endogenous NSCs have failed to repair injured brains. In addition, the concept of recruiting endogenous NSCs to repair injured brain developed in rodents may have limited scope in humans given the differences in neurogenesis rate and recruitment distances (Kassi et al., 2018). Could precise stereotactic placement of NSCs help address the unmet need and repair an injured brain?
Figure 1.
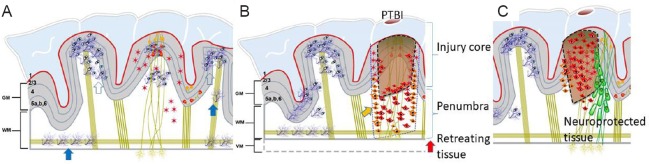
Schematic of an intact gyrencephalic brain.
The picture shows the gyri, sulci, six layers of cortical neurons (red triangles with yellow nuclei) in gray matter (GM) and neuronal density dependent projections into white matter (WM) with normal astrocytes (red stars), highly ramified resting (filled blue arrow) and primed microglia (open blue arrow) in (A). After penetrating traumatic brain injury (PTBI), represented by a cortical tissue penetration the penumbra surrounding the injury core is replete with proinflammatory-activated microglia (orange arrow). Activated microglial pyroptosis (secondary damage) in penumbra causing cortical thinning (red arrow) and manifest as ventriculomegaly (VM). Persistent axonal debris and chronic microglial activation mediate toxic inflammation fueling tissue loss years after injury (B). Stereotaxic transplantation of human neural stem cells (green shapes) in penumbra would provide a two-in-one solution, a source of cells to replace lost cell types, resolve chronic microglial activation preventing further tissue loss (C).
Human NSC (hNSC) transplantation studies in preclinical TBI: Transplantation of exogenous rodent NSCs not only replaces lost neurons but also boosts endogenous neurogenesis, angiogenesis ameliorating ischemia/TBI induced cognitive deficits (Ryu et al., 2016; Kassi et al., 2018). Lack of robust durable engraftment of human NSC in rodent TBI models failed to provide rational for clinical trials aimed at assessing cell therapy potential in human TBI. Recently amelioration of cognitive deficits via sub-acute transplantation of clinical trial grade hNSCs in immunosuppressed rats as well as athymic rats has been reported (Haus et al., 2016; Spurlock et al., 2017). In other TBI and central nervous system disease models, NSC transplants mediated increased phagocytosis, decreased gliosis, and disrupted toxic inflammatory signaling (Kassi et al., 2018). Another secondary mechanism known to exacerbate TBI i.e., posttraumatic epilepsy, hNSC transplantation mitigated it too. In a temporal lobe epilepsy model 6 months post-transplantation, hNSC derived neurons conferred both neuroprotection and antiepileptogenic effects via synaptic connections with host (Upadhya et al., 2019). hNSCs transplant studies in other central nervous system regions with longer survival periods (9–18 months) in primates and rodents replaced lost neurons, oligodendrocytes and astrocytes depending on transplant location recapitulating neural development (Lu et al., 2012; Rosenzweig et al., 2018; Lien et al., 2019).
hNSCs in TBI: Autologous NSC transplantation in human TBI provides the evidence for feasibility but not sufficient to launch a clinical trial. The absence of NSC-TBI clinical trial in the 50 ongoing clinical trials listed by California Institute for Regenerative Medicine is a glaring reminder of the gap in knowledge (Kassi et al., 2018). The only human cell therapies for TBI in clinical trials are NCT02416492, NCT02525432, both mesenchymal stem cells (MSC). In rodents, the mechanism of action for MSCs is anti-apoptotic (Zhang et al., 2019) and recruitment of endogenous stem cells/immature neurons to TBI lesion ‘biobridge’. MSCs cannot function as agents of cell replacement as they do not differentiate into neural cell types nor engraft. Therapeutic role of ‘MSC transplant induced’ endogenous cell recruitment to TBI lesion and its relevance to humans remains to be determined. Human neural stem cell based therapy not yet available for TBI has precedent in stroke. An immortalized hNSC line derived CTX0E03 from first trimester fetal brain tissue (of unspecified age) has progressed to multiple stroke clinical trials with encouraging results despite low engraftment and persistence of cells only up to 5 weeks post-transplantation (Sinden et al., 2017).
Conclusions: The preclinical studies support the notion that hNSC therapy can address multiple TBI pathologies. Transplanted hNSCs could (i) be an enduring source of cells to replace lost neural cells, recapitulating neural development in transplantation site dependent manner, (ii) confer neuroprotection via immunomodulation in sub-acute phase (Figure 1C), (iii) mitigate vulnerability to injury induced posttraumatic epilepsy. Prior to use of the approach in clinic, mechanistic insights with gyrencephalic TBI models and a safety trial in TBI patients will be necessary.
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Haus DL, Lopez-Velazquez L, Gold EM, Cunningham KM, Perez H, Anderson AJ, Cummings BJ. Transplantation of human neural stem cells restores cognition in an immunodeficient rodent model of traumatic brain injury. Exp Neurol. 2016;281:1–16. doi: 10.1016/j.expneurol.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Kassi AAY, Mahavadi AK, Clavijo A, Caliz D, Lee SW, Ahmed AI, Yokobori S, Hu Z, Spurlock MS, Wasserman JM, Rivera KN, Nodal S, Powell HR, Di L, Torres R, Leung LY, Rubiano AM, Bullock RM, Gajavelli S. Enduring neuroprotective effect of subacute neural stem cell transplantation after penetrating TBI. Front Neurol. 2018;9:1097. doi: 10.3389/fneur.2018.01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SW, Gajavelli S, Spurlock MS, Andreoni C, de Rivero Vaccari JP, Bullock MR, Keane RW, Dietrich WD. Microglial inflammasome activation in penetrating ballistic-like brain injury. J Neurotrauma. 2018;35:1681–1693. doi: 10.1089/neu.2017.5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lien BV, Tuszynski MH, Lu P. Astrocytes migrate from human neural stem cell grafts and functionally integrate into the injured rat spinal cord. Exp Neurol. 2019;314:46–57. doi: 10.1016/j.expneurol.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, Conner JM, Marsala M, Tuszynski MH. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenzweig ES, Brock JH, Lu P, Kumamaru H, Salegio EA, Kadoya K, Weber JL, Liang JJ, Moseanko R, Hawbecker S, Huie JR, Havton LA, Nout-Lomas YS, Ferguson AR, Beattie MS, Bresnahan JC, Tuszynski MH. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat Med. 2018;24:484–490. doi: 10.1038/nm.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryu S, Lee SH, Kim SU, Yoon BW. Human neural stem cells promote proliferation of endogenous neural stem cells and enhance angiogenesis in ischemic rat brain. Neural Regen Res. 2016;11:298–304. doi: 10.4103/1673-5374.177739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinden JD, Hicks C, Stroemer P, Vishnubhatla I, Corteling R. Human neural stem cell therapy for chronic ischemic stroke: charting progress from laboratory to patients. Stem Cells Dev. 2017;26:933–947. doi: 10.1089/scd.2017.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith C. Review: the long-term consequences of microglial activation following acute traumatic brain injury. Neuropathol Appl Neurobiol. 2013;39:35–44. doi: 10.1111/nan.12006. [DOI] [PubMed] [Google Scholar]
- 10.Spurlock MS, Ahmed AI, Rivera KN, Yokobori S, Lee SW, Sam PN, Shear DA, Hefferan MP, Hazel TG, Johe KK, Gajavelli S, Tortella FC, Bullock RM. Amelioration of penetrating ballistic-like brain injury induced cognitive deficits after neuronal differentiation of transplanted human neural stem cells. J Neurotrauma. 2017;34:1981–1995. doi: 10.1089/neu.2016.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Upadhya D, Hattiangady B, Castro OW, Shuai B, Kodali M, Attaluri S, Bates A, Dong Y, Zhang SC, Prockop DJ, Shetty AK. Human induced pluripotent stem cell-derived MGE cell grafting after status epilepticus attenuates chronic epilepsy and comorbidities via synaptic integration. Proc Natl Acad Sci U S A. 2019;116:287–296. doi: 10.1073/pnas.1814185115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Yu S, Tuazon JP, Lee JY, Corey S, Kvederis L, Kingsbury C, Kaneko Y, Borlongan CV. Neuroprotective effects of human bone marrow mesenchymal stem cells against cerebral ischemia are mediated in part by an anti-apoptotic mechanism. Neural Regen Res. 2019;14:597–604. doi: 10.4103/1673-5374.247464. [DOI] [PMC free article] [PubMed] [Google Scholar]