Keywords: nerve regeneration, stem cells, collagen, chitosan, scaffolds, traumatic brain injury, bone marrow mesenchymal stem cells, brain tissue engineering, neural regeneration
Abstract
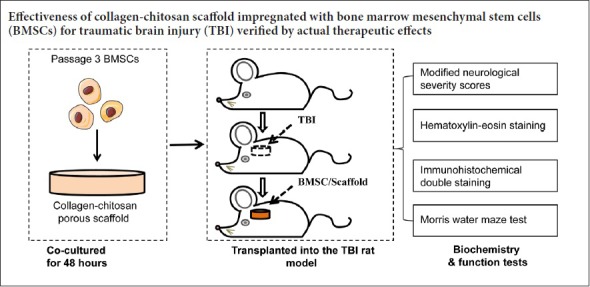
Combinations of biomaterials and cells can effectively target delivery of cells or other therapeutic factors to the brain to rebuild damaged nerve pathways after brain injury. Porous collagen-chitosan scaffolds were prepared by a freeze-drying method based on brain tissue engineering. The scaffolds were impregnated with rat bone marrow mesenchymal stem cells. A traumatic brain injury rat model was established using the 300 g weight free fall impact method. Bone marrow mesenchymal stem cells/collagen-chitosan scaffolds were implanted into the injured brain. Modified neurological severity scores were used to assess the recovery of neurological function. The Morris water maze was employed to determine spatial learning and memory abilities. Hematoxylin-eosin staining was performed to measure pathological changes in brain tissue. Immunohistochemistry was performed for vascular endothelial growth factor and for 5-bromo-2-deoxyuridine (BrdU)/neuron specific enolase and BrdU/glial fibrillary acidic protein. Our results demonstrated that the transplantation of bone marrow mesenchymal stem cells and collagen-chitosan scaffolds to traumatic brain injury rats remarkably reduced modified neurological severity scores, shortened the average latency of the Morris water maze, increased the number of platform crossings, diminished the degeneration of damaged brain tissue, and increased the positive reaction of vascular endothelial growth factor in the transplantation and surrounding areas. At 14 days after transplantation, increased BrdU/glial fibrillary acidic protein expression and decreased BrdU/neuron specific enolase expression were observed in bone marrow mesenchymal stem cells in the injured area. The therapeutic effect of bone marrow mesenchymal stem cells and collagen-chitosan scaffolds was superior to stereotactic injection of bone marrow mesenchymal stem cells alone. To test the biocompatibility and immunogenicity of bone marrow mesenchymal stem cells and collagen-chitosan scaffolds, immunosuppressive cyclosporine was intravenously injected 12 hours before transplantation and 1–5 days after transplantation. The above indicators were similar to those of rats treated with bone marrow mesenchymal stem cells and collagen-chitosan scaffolds only. These findings indicate that transplantation of bone marrow mesenchymal stem cells in a collagen-chitosan scaffold can promote the recovery of neuropathological injury in rats with traumatic brain injury. This approach has the potential to be developed as a treatment for traumatic brain injury in humans. All experimental procedures were approved by the Institutional Animal Investigation Committee of Capital Medical University, China (approval No. AEEI-2015-035) in December 2015.
Chinese Library Classification No. R452; R363; R741
Introduction
Traumatic brain injury (TBI) is a leading cause of mortality and morbidity. Following injury, microenvironmental signals released from areas of cell loss can induce neuroblasts to differentiate into oligodendrocyte progenitor cells, resulting in partial recovery of neurological function (Jones and Connor, 2017). However, these endogenous neural stem cells are unable to migrate effectively to areas of injury and to adequately differentiate into functional neurons. There are no efficient therapies for repairing TBI-related loss of cerebral parenchyma. To address this problem, stem/progenitor cells have been transplanted into injured adult brains to replace dead or injured tissues (Pettikiriarachchi et al., 2010). The transplantation of human fetal neural progenitor cells in a clinically adapted model after TBI improved the long-term functional outcome because of increased angiogenesis and reduced astrogliosis (Skardelly et al., 2011). Bone marrow mesenchymal stem cells (BMSCs), pluripotent adult stem-like cells that are capable of differentiating into neuron-like cells, can be easily obtained and rapidly amplified and raise few ethical issues or immunological rejection problems. Multiple hormonal and neurotrophic factors secreted by BMSCs promote the growth, differentiation and protection of endogenous neuronal precursor cells and thus exert positive effects on nerve damage repair and regeneration (Cantinieaux et al., 2013; Xia et al., 2013; Chen et al., 2015; Greer et al., 2017). During cell transplantation therapy for TBI, cell distribution and survival rate play vital roles in the overall recovery of neural functional (Guan et al., 2013). To promote effectively targeted delivery of cells or other therapeutic factors to the brain, biomaterials are being used in combination with cell therapies. This strategy is known as brain tissue engineering (Orive et al., 2009). Therefore, tissue engineering approaches are raising the possibility of enhancing brain repair and regeneration at an injury site to re-establish functionality at both the cellular and organ levels.
Collagen-chitosan scaffolds are widely used in brain tissue engineering because of several favorable features, such as biodegradability, low antigenicity, good biocompatibility, and absence of pyrogenic effects (Ji et al., 2011; Moura et al., 2016; Fu et al., 2017a, b). Guan et al. (2013) loaded human mesenchymal stem cells into collagen scaffolds to treat TBI in rats, which facilitated initial engraftment and supported the survival of grafted cells after transplantation. Lu et al. (2007) implanted collagen scaffolds impregnated with human marrow stromal cells into the cortical lesion cavity of TBI rats, and showed that a stem cell-populated porous material can improve spatial learning and sensorimotor functions. As a natural amino polysaccharide with structural similarity to glycosaminoglycan, chitosan and its derivatives are superior supporting materials for tissue engineering applications because of their excellent biocompatibility, biodegradability and low immunogenicity (Prabaharan and Sivashankari, 2016). Chitosan served as an effective neuroprotector following acute spinal cord trauma (Chedly et al., 2017). Mo et al. (2010) implanted a chitosan scaffold into an injured adult rat hippocampus, which stimulated axons and cells to migrate into the damaged area and to rebuild the neural circuit, with subsequent improvement in the impaired cognitive function. A neuroprotective effect was also seen after collagen-chitosan scaffolds loaded with BMSCs were successfully prepared and transplanted into the ischemic area of an animal ischemic stroke model (Yan et al., 2015).
Previous analysis of collagen or chitosan-based scaffolds for brain tissue engineering applications has mainly focused on cytocompatibility or regional histochemical evaluation (Guan et al., 2013; Yan et al., 2015; Betancur et al., 2017). In this study, to explore an effective treatment method for TBI, collagen-chitosan scaffolds were prepared and impregnated with BMSCs. These were then implanted into a TBI rat model to test biocompatibility, immunogenicity and their ability to promote the regeneration of neurons and to improve functional outcomes.
Materials and Methods
BMSC culture, identification and labeling
Six specific-pathogen-free, healthy, 3-week-old male Wistar rats weighing 100 g were provided by the Environment Institute of the Academy of Military Medical Sciences, Chinese People’s Liberation Army, China [license number: SCXK (Jing) 2015-006]. All rats were used for BMSC isolation. All experimental procedures were approved by the Institutional Animal Investigation Committee of Capital Medical University, China (approval No. AEEI-2015-035) in December 2015 and performed in accordance with the National Institutes of Health (USA) guidelines for care and use of laboratory animals (NIH Publication No. 85-23, revised 1996). We also conformed to internationally accepted ethical standards. All rats were handled humanely and the experimental procedures were in accordance with our institutional guidelines and research ethics. Rats were euthanized by terminal anesthesia and BMSCs from whole bone marrow of all four limbs of each rat were collected and cultured using a complete bone marrow adherent method (Zhao et al., 2011). Flow cytometry was performed to purify passage 3 cells using CD29-FITC (fluorescein isothiocyanate) and CD45RA-PE (phycoerythrin) surface antigen detection. Forty-eight hours prior to cell transplantation, a final concentration of 10 μM of BrdU was added to the culture medium to label BMSCs. All reagents were provided by Sinopharm Chemical Reagent Co., Ltd., Beijing, China and all the chemicals were of analytical grade and were used as received unless otherwise noted. Deionized water was used throughout.
Scaffold preparation, porosity and degradation evaluation
Collagen-chitosan porous scaffolds were produced using a freeze-drying method (Kim et al., 2007). Briefly, 1 mL of a 2% collagen-chitosan and acetic acid solution was put into a well of a 24-well plate and incubated at 4°C for 6 hours and then at −25°C overnight. The scaffold was then dried in a freeze-drying machine at −75°C for 24 hours. Subsequently, 1 mL 0.1 M NaOH was added to each well, incubated for 20 minutes and the scaffold then dried again with the freeze-drying machine. Finally, the powder (or pellet) was immersed in 75% ethanol for 30 minutes and then rinsed thoroughly five times with phosphate-buffered saline. The following formula was used to measure its porosity: P = (V1−V3)/V × 100%; where ‘P’ is the porosity of the collagen-chitosan porous scaffold; ‘V1’ is the total volume of ethanol; ‘V3’ is the remaining ethanol volume after removing the collagen-chitosan porous scaffold; ‘V’ is the total volume of the scaffold. In a preliminary experiment, we performed a degradation test by immersing the scaffold into phosphate-buffered saline and transplanting it into a rat TBI model.
Scanning electron microscopy
The collagen-chitosan porous scaffolds were cut into small pieces, rinsed repeatedly with phosphate-buffered saline, fixed with 2.5% glutaraldehyde and then incubated in 1% of osmium tetroxide (OsO4) at 4°C. After gradient ethanol dehydration, scaffold pieces were dried using a critical point drying apparatus, and then coated with the gold ion sputtering method. Samples were observed and photographed using a scanning electron microscope (Hitachi, Tokyo, Japan).
Cell seeding before transplantation
Collagen-chitosan porous scaffolds with a volume size of 3.0 mm × 3.0 mm × 2.0 mm were prepared by the freeze-drying method (Zhang et al., 2013). After disinfection in alcohol, each scaffold was cultured with BrdU-labeled BMSCs at a cell concentration of 2 × 106/μL at 37°C in a 5% CO2, and saturated humidity incubator for 48 hours. The morphology of BMSCs adhered within scaffolds was observed by scanning electron microscopy.
Establishment of a TBI model and treatment with BMSC-impregnated collagen-chitosan scaffolds
Forty-four, 12-week-old, male, specific-pathogen-free Wistar rats, weighing approximately 250 g were used. Another cohort of animals (n = 8) allocated to groups A–D were sacrificed for immunohistochemistry. All rats were obtained from the Environment Institute of the Academy of Military Medical Sciences, Chinese People’s Liberation Army, China. The animals were housed (five per cage) with food and water available ad libitum and were maintained on a 12-hour light/dark cycle (lights on at 7:00 a.m.) in a controlled temperature (22°C) environment. The above conditions were maintained throughout the experiments.
The rat TBI model was prepared according to the principle of Feeney’s free-fall combat injury (Blennow et al., 2012). Rats were intraperitoneally anesthetized with 10% chloral hydrate (0.33 mL/100 g) and then placed in a stereotactic frame. A 10 mm diameter craniotomy was performed adjacent to the sagittal suture, and midway between lambda and bregma. The endocranium was kept intact over the cortex. TBI was induced with the weight drop impact acceleration method using a controlled cortical impact device. A sterile metal plate was used to prevent skull fracture. After pushing the fascia aside, the steel was placed on the dura. The head was then placed in an appropriate position on a platform and a band was put under the lower jaw, which allowed head movement when the impact was created by the falling of a 300 g weight. The weight was dropped from a height of 1 meter onto the parietal bone.
Fifty-two rats were randomly and equally assigned to four groups (n = 13): Group A (TBI + immunosuppressor + BMSCs/scaffold), group B (TBI + BMSCs/scaffold), group C (TBI + BMSCs stereotactic injection), and group D (TBI model with scalp incision and skull window). For groups A and B, at 72 hours after TBI the cell/scaffold complexes were transplanted in situ into the damaged brain tissue area. Under aseptic conditions and general anesthesia with ketamine (40 mg/kg) and xylazine (8 mg/kg), a 1 cm incision was made along the midline of the scalp. The lesion cavity induced by TBI in the left hemisphere was exposed. A scaffold seeded with BMSCs was placed directly into the lesion cavity without removal of additional brain tissue, and subsequently covered by surgical foam (polyurethane foam). The incision was closed with 4-0 absorbable gut surgical sutures. In group C, the rats received a transplantation of a single stereotactic BMSC injection. Group A rats were given intravenous cyclosporin 12 hours prior to the transplantation and on days 1–5 after the transplantation. Simultaneously, rats of other groups were given intravenous injections of normal saline. The experimental process is illustrated in Figure 1.
Figure 1.
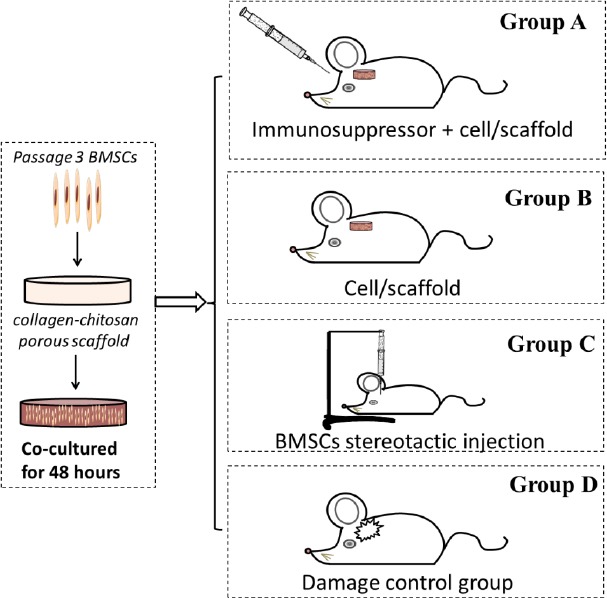
Illustration of the treatments given to the rats of four groups.
BMSCs: Bone marrow mesenchymal stem cells.
Behavioral and cognitive function tests
Modified neurological severity scores (mNSS) were used to assess neurological function of rats before TBI (day 0), and on day 1, 7, 14 and 35 after TBI (Mahmood et al., 2011). Briefly, the mNSS test is scaled from 0 to 18; a score of 0 indicates normal and 18 indicates a maximal deficit (Mahmood et al., 2011).
At 31–35 days after TBI, the Morris water maze test was performed, which comprised a navigation test and a spatial probe test. We recorded the time required for the rat to find the platform from entering the water (escape latency) and frequency to cross the original platform within 120 seconds. This test was used to determine the spatial learning and memory capabilities of each rat over 5 consecutive days.
Hematoxylin-eosin staining and immunohistochemistry
Rats were euthanized by excessive anesthesia and the whole brain was isolated on day 7, 14, 32 or 35 after TBI. Conventional paraffin sections were prepared and standard hematoxylin and eosin staining was performed. Immunohistochemical staining was performed for vascular endothelial growth factor (VEGF). Double immunohistochemistry was performed for 5-bromo-2-deoxyuridine (BrdU)/neuron specific enolase (NSE) and BrdU/glial fibrillary acidic protein (GFAP). For double staining, sections were incubated with sheep anti-BrdU antibody and mouse anti-GFAP antibody (1:200, CST, Shanghai, China) or mouse anti-NSE antibody (1:200), simultaneously, at 4°C overnight. Sections were then washed with phosphate-buffered saline and incubated with horseradish peroxidase-labeled rabbit anti-sheep IgG (1:100; Abcam, Shanghai, China) and sheep anti-mouse IgG. The results were observed under an optical microscope (Chongqing Optical Instrument Factory, Chongqing, China) and recorded.
Statistical analysis
The data were analyzed using SPSS 13.0 statistical software package (SPSS, Chicago, IL, USA) and are expressed as the mean ± SD. A paired t-test and one-way analysis of variance analysis were used for data analysis within and among groups. P < 0.05 was considered statistically significant.
Results
Identification and morphology of passage 3 BMSCs
Primary BMSCs were isolated from rat limbs and adherently cultured. The colony size was remarkably increased by the fifth day. The cells gradually expanded to a spindle-like, polygonal, irregular shape and eventually formed a visible colony. The proportion of mixed cells remained elevated until passage 3 when BMSCs started to develop a relatively uniform morphology and showed fibrous characteristics. The proportion of mixed cells subsequently diminished thereafter (Figure 2A). Passage 3 BMSCs were detected by flow cytometry with a CD29-positive rate of 99.97%, and a CD45-positive rate as low as 0.81%. This was taken into consideration to meet with the requirements for cell transplantation (Figure 2B).
Figure 2.
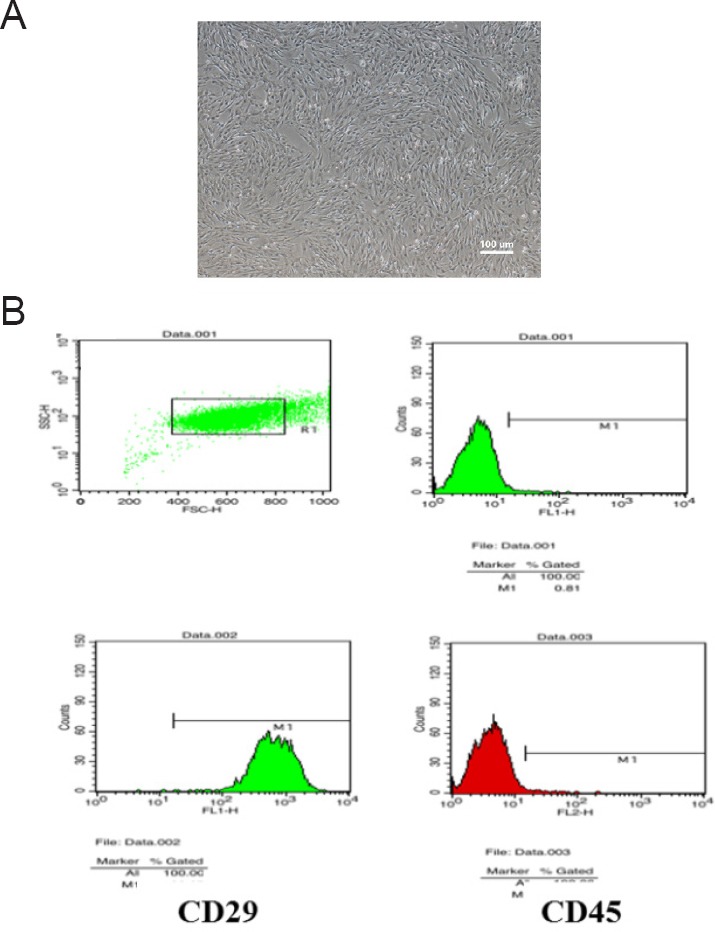
Morphology and identification of passage 3 BMSCs.
(A) Morphology of passage 3 BMSCs (light microscopy, original magnification, 40×). (B) Flow cytometry identification of surface antigen CD29 (99.97%) and CD45 (0.81%) on passage 3 BMSCs. BMSCs: Bone marrow mesenchymal stem cells.
Compatibility of BMSCs with the collagen-chitosan porous scaffold in three-dimensional co-culture
The porosity of the collagen-chitosan scaffold was measured by the ethanol alteration method, which revealed 90% porosity. The scaffold gradually degraded within 1 month in degradation tests in vitro and in vivo, which provided the necessary microenvironment for BMSCs to grow and migrate (Figure 3). Scanning electron microscopy revealed relatively uniformly sized pores in scaffold cross sections, and the holes were well interconnected. The pore size was estimated at 80–200 μm, with an average size of 140 μm, which matched the size of BMSCs. Scanning electron microscopy showed that BMSCs and porous collagen-chitosan scaffolds were successfully co-cultured in vitro for 48 hours. Thereafter, BMSCs spread out on the surface and within the holes of the scaffold. BMSCs in spindle form secreted an extracellular matrix to adhere to the scaffold and they extended their pseudopodia into the material, indicating good cytocompatibility of the scaffold (Figure 4). BMSCs therefore grew well in the three-dimensional culture conditions.
Figure 3.
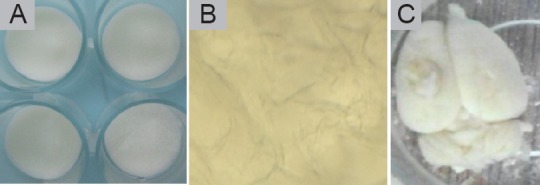
Tissue compatibility of collagen-chitosan scaffolds.
(A) General shape of collagen-chitosan porous scaffolds. (B) After 1 month, the scaffold was immersed in phosphate-buffered saline, degraded in vitro and the porous structure was visible under a microscope. (C) The scaffold was transplanted into a rat brain after traumatic brain injury. The scaffold was fused with brain tissue, but there was still a general structure.
Figure 4.
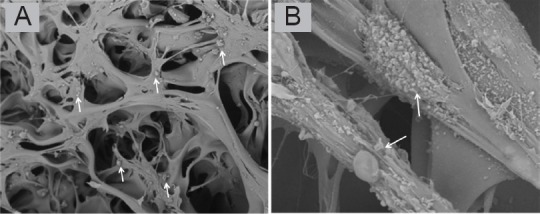
Scanning electron microscopy images of collagen-chitosan porous scaffold impregnated with bone marrow mesenchymal stem cells after co-culture for 48 hours.
Bone marrow mesenchymal stem cells are indicated by arrows. Original magnification, 300× (A), 5000× (B).
Neurological function of TBI rats after transplantation of BMSC-impregnated collagen-chitosan porous scaffold
mNSS results
There were no significant changes in mNSS for rats in groups A, B, C and D on days 1 and 7 (P > 0.05). In comparison with the model control group (group D), the mNSS of rats in the experimental groups was significantly declined on day 14. There were no significant differences in mNSS scores between group A and group B (P > 0.05), but when all groups were considered, groups C and D exhibited significant differences (P < 0.05). mNSS scores were significantly different between groups C and D (P < 0.01; Figure 5).
Figure 5.
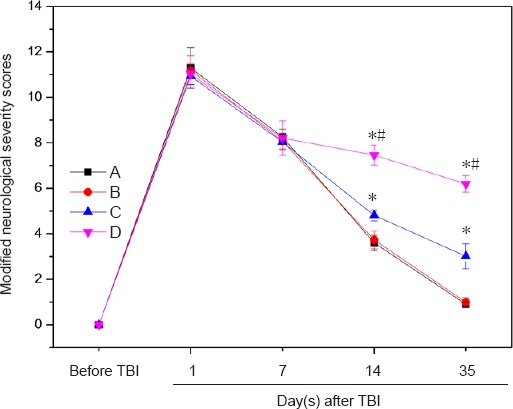
Effect of BMSC-impregnated collagen-chitosan porous scaffold transplantation on neurological function of TBI rats.
Group A: TBI + immunosuppressor + BMSCs/scaffold; group B: TBI + BMSCs/scaffold; group C: TBI + BMSCs stereotactic injection; group D: TBI only. Data are expressed as the mean ± SD (n = 10 rats in each group at each time point). *P < 0.05, vs. group A/B; #P < 0.01, vs. group C (paired t-test and one-way analysis of variance). TBI: Traumatic brain injury; BMSCs: bone marrow mesenchymal stem cells.
Morris water maze test results
The average latency to escape in groups A and B was less than that in groups C and D (P > 0.05), while that of group C was less than that of group D (P > 0.05). Moreover, no significant differences were found between groups A and B (Figure 6A).
Figure 6.
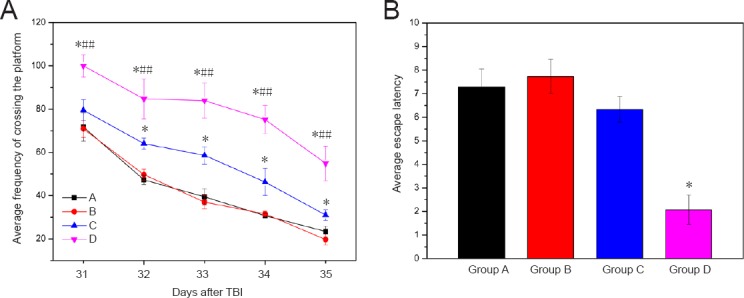
Morris water maze test of TBI rats transplanted with BMSC-impregnated collagen-chitosan porous scaffold.
(A) Average frequency of crossing the platform; (B) average escape latency. Group A: TBI + immunosuppressor + BMSCs/scaffold; group B: TBI + BMSCs/scaffold; group C: TBI + BMSCs stereotactic injection; group D: TBI only. Data are expressed as the mean ± SD (n = 10 rats in each group at each time point). *P < 0.05, vs. group A/B; ##P < 0.01, vs. group C (paired t-test and one-way analysis of variance). TBI: Traumatic brain injury; BMSCs: bone marrow mesenchymal stem cells.
Compared with group D (the control model group), the average escape latencies of rats in the other groups were rapidly reduced from day 31 to day 33 after TBI, and slowly reduced from day 34 to day 35. The average escape latency was equivalent between groups A and B (P > 0.05; Figure 6). The average escape latency was significantly different between groups C and D (P < 0.05). The average frequency of crossing the platform was not significantly different between groups A and B (P = 0.139), but was significantly different between groups C and D (P < 0.01; Figure 6B).
Morphology and markers in TBI rat brains after transplantation of BMSC-impregnated collagen-chitosan porous scaffold
Hematoxylin-eosin staining
Routine hematoxylin-eosin staining was used to observe the brain tissues of each group. More degenerative cells were observed in the hippocampus, cortex and striatum on the lesion side, which looked like “shrunken pink cells” and “black contracted cells”, in group D compared with groups A, B and C. Degenerated cells became more numerous with time in all groups (Figure 7).
Figure 7.
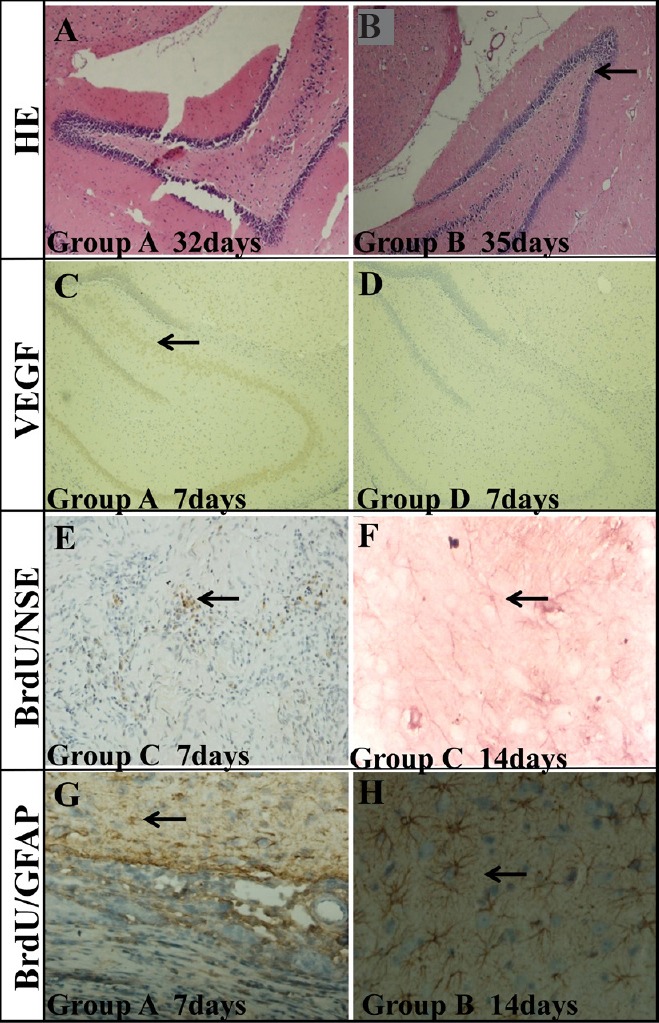
Histochemical analysis of the hippocampus of TBI rats transplanted with BMSC-impregnated collagen-chitosan porous scaffold.
(A, B) HE staining in the hippocampus on the lesion side of TBI rats at 32 days (group A) (A) and 35 days (group B) (B) after BMSC transplantation. The degeneration of cells (arrow) was decreased remarkably with time. (C) Positive VEGF staining (arrow) in the hippocampus of TBI rats 7 days after BMSC transplantation (group A). (D) No VEGF staining was found in the hippocampus of group D. (E) BrdU/NSE double staining (arrow) in the damaged area 7 days after BMSC transplantation (group C). (F) BrdU/NSE double staining (arrow) in the damaged area 14 days after BMSC transplantation (group C). (G) BrdU/GFAP double staining (arrow) in the damaged area 7 days after BMSC transplantation (group A). (H) BrdU/GFAP double staining (arrow) in the damaged area 14 days after BMSC transplantation (group B). Group A: TBI + immunosuppressor + BMSCs/scaffold; group B: TBI + BMSCs/scaffold; group C: TBI + BMSCs stereotactic injection; group D: TBI only. TBI: Traumatic brain injury; BMSCs: bone marrow mesenchymal stem cells; HE: hematoxylin-eosin; VEGF: vascular endothelial growth factor; NSE: neuron specific enolase; GFAP: glial fibrillary acidic protein. Original magnifications: 100× in A–D, 40× in E and G, and 200× in F and H.
VEGF immunopositivity
As shown in Figure 7, there was significantly greater VEGF staining at the ischemic transplantation area and its surroundings (hippocampal dentate gyrus and subventricular zone) in groups A and B than in group C. No VEGF staining was seen in the above mentioned areas in group D.
Co-immunopositivity of BrdU/NSE and BrdU/GFAP
The double immunohistochemical staining on day 32 after transplantation showed that the growth and differentiation of the BrdU-labeled BMSCs in the brain occurred at different times following transplantation (Figure 7). BrdU-positive BMSCs were numerous in the damage area, and eventually also numerous in the periphery of the damaged area after transplantation. Some of the differentiated neuron-like cells had already migrated to a maximum distance of 2 mm along the periphery of the infracted area and even into normal brain tissues. They appeared circular and accumulating. NSE and GFAP were not expressed in BrdU-labeled BMSCs in the cortex and striatum after a short period following transplantation. On day 7, a few GFAP-positive and no NSE-positive BrdU-labeled BMSCs were observed; on day 14, more GFAP-positive and a few NSE-positive BrdU-labeled BMSCs were seen.
Discussion
In this study, transplantation therapy was conducted 72 hours after TBI with the purpose of reducing cell damage from cytokines or toxins, such as oxygen free radicals and inflammatory cytokines, which are produced within 24 hours of the injury. Another reason for doing this therapy 24 hours after TBI is that the damaged area was more clearly defined at this time, allowing a better traumatic repair (Blennow et al., 2012). The feasibility of brain tissue engineering using BMSCs seeded in a collagen-chitosan scaffold was verified by this study. When transplanted into the brain, the scaffold provided a viable microstructure for BMSC proliferation and differentiation. Scanning electron microscopy, hematoxylin-eosin staining and immunohistochemical staining indicated that the cell/scaffold transplantation complex and the normal brain tissue around the damaged region integrated well (Maclean et al., 2018).
The use of an immunosuppressive agent (cyclosporin injection) before or after transplantation produced no differences between groups A and B. These results indicate that the BMSC-impregnated collagen-chitosan scaffold showed low immunogenicity and good biocompatibility, which did not invoke the host’s immune response. Cyclosporin had no therapeutic effect on group A or B; however, cyclosporin has been investigated as a possible neuroprotective agent for the treatment of TBI with promising results in animal experiments. Cyclosporin blocks the formation of the mitochondrial permeability transition pore, which causes much of the damage associated with head injury and neurodegenerative diseases (Kilbaugh et al., 2011). The application of cyclosporin should be investigated for its potential selective neuroprotective effects, but currently its dose-dependency characteristics remain unknown. Recent studies indicate that gap junction-associated connexin 43 plays an important role in cell cycle regulation and in the function of neural stem/progenitor cells following TBI (Greer et al., 2017).
After transplanting the cell/scaffold complex into the injured cerebral cortex, hematoxylin-eosin staining and double immunohistochemical staining indicated that the seeded BMSCs in the transplantation zone had extended into the brain tissue of the host by day 35. BMSCs survived and eventually differentiated into neurons or glial cells, even though the collagen-chitosan scaffold had partially degraded at the transplantation zone. In group C, relatively few BMSCs could be injected intracranially, and the lesion core rapidly changed into a cavity that lacks a structural substrate to adequately support the inoculated cells. Compared with group D (the control TBI model), neuronal necrosis in the damaged region was dramatically decreased in the complex transplantation groups (group A or B), while number of strongly positive NSE and GFAP and VEGF cells was elevated. TBI causes neurological function deficits leading to cognitive impairment and learning dysfunction; therefore, we examined spatial learning and memory abilities of the test rats using the Morris water maze test and mNSS. The results illustrated that after TBI, mNSS scores gradually decreased in each group. A remarkable effect was detected in the BMSC intracranial injection groups compared with controls. Compared with the pure BMSCs intracranial injection groups and the control model group, neurological deficits and cognitive impairment were improved when the cell/scaffold complex was introduced.
By exploiting a brain tissue engineering technique, three-dimensionally cultured BMSCs accelerated the functional recovery of rats after TBI (Xu et al., 2018). The possible mechanisms of neuroprotection and cognition improvement are that (1) BMSCs can differentiate into neurons and glial cells under specific conditions with vigorous regenerative behaviors. In response to brain damage, BMSCs partially reconstruct the damaged local brain circuits and compensate for the damaged brain cells. (2) BMSCs can alleviate secondary neurological damage after TBI by secreting BDNF, which promotes the restoration of neurological function and improves the local microenvironment following brain damage. (3) Following TBI, BMSCs can stimulate macrophage and endogenous anti-inflammatory cytokine production, which play cardinal roles in immune regulation and promote homeostasis. (4) The collagen-chitosan scaffold forms an effective and delicate substrate for synaptic connections between the transplantation complex and the other neurons of the central nervous system.
As a pilot study, this research has successfully verified the effectiveness of a chitosan-collagen scaffold and BMSCs for brain repair by examining actual therapeutic effects, such as the recovery of neurological, behavioral and cognitive functions. The occurrence of an inflammatory response (Maclean et al., 2018) and cerebral edema following TBI are important factors that contribute to the evolution of brain injury following initial trauma (Winkler et al., 2016).
This study has several limitations. This research was based on rats; therefore, further study is needed before our conclusions can be extended to humans. Moreover, further research should be carried out to control degeneration of the scaffold, as well as to explore related factors and the mechanism by which proliferation, migration, and differentiation occur. We will conduct comprehensive evaluations using a neural conduction pathway tracing method to unveil the underlying mechanisms for neural protection and regeneration.
Additional file: Open peer review report 1 (103.7KB, pdf) .
Footnotes
Conflicts of interest: The authors declare no competing interests.
Financial support: This study was financially supported by the Postdoctoral Research Foundation of Beijing of China, No. 2017-ZZ-120 (to FY); the Natural Science Foundation of Beijing of China, No. 2164073 (to ML); the Beijing Municipal Administration of Hospitals’ Youth Plan of China, No. QML20180804 (to ML). The funders had no roles in the study design, conduction of experiment, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement: The study was approved by the Institutional Animal Investigation Committee of Capital Medical University, China (approval No. AEEI-2015-035) in December 2015, and performed in accordance with the National Institutes of Health (USA) guidelines for care and use of laboratory animals.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Jianxun Ding, the First Hospital of Jilin University, China; Alavala Matta Reddy, Adikavi Nannaya University, India.
Funding: This study was financially supported by the Postdoctoral Research Foundation of Beijing of China, No. 2017-ZZ-120 (to FY); the Natural Science Foundation of Beijing of China, No. 2164073 (to ML); the Beijing Municipal Administration of Hospitals’ Youth Plan of China, No. QML20180804 (to ML).
P-Reviewers: Ding J, Reddy AM; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Allen J, Haase R, Qiu Y, Song LP; T-Editor: Liu XL
References
- 1.Betancur MI, Mason HD, Alvarado-Velez M, Holmes PV, Bellamkonda RV, Karumbaiah L. Chondroitin sulfate glycosaminoglycan matrices promote neural stem cell maintenance and neuroprotection post-traumatic brain injury. ACS Biomater Sci Eng. 2017;3:420–430. doi: 10.1021/acsbiomaterials.6b00805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blennow K, Hardy J, Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron. 2012;76:886–899. doi: 10.1016/j.neuron.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 3.Cantinieaux D, Quertainmont R, Blacher S, Rossi L, Wanet T, Noel A, Brook G, Schoenen J, Franzen R. Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: an original strategy to avoid cell transplantation. PLoS One. 2013;8:e69515. doi: 10.1371/journal.pone.0069515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chedly J, Soares S, Montembault A, von Boxberg Y, Veron-Ravaille M, Mouffle C, Benassy MN, Taxi J, David L, Nothias F. Physical chitosan microhydrogels as scaffolds for spinal cord injury restoration and axon regeneration. Biomaterials. 2017;138:91–107. doi: 10.1016/j.biomaterials.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Chen SL, Chen ZG, Dai HL, Ding JX, Guo JS, Han N, Jiang BG, Jiang HJ, Li J, Li SP, Li WJ, Jing, Liu Y, Ma JX, Peng J, Shen YD, Sun GW, Tang PF, Wang GH, Wang XH, Xiang LB, et al. Repair, protection and regeneration of peripheral nerve injury. Neural Regen Res. 2015;10:1777–1798. doi: 10.4103/1673-5374.170301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu F, Qin Z, Xu C, Chen XY, Li RX, Wang LN, Peng DW, Sun HT, Tu Y, Chen C, Zhang S, Zhao ML, Li XH. Magnetic resonance imaging-three-dimensional printing technology fabricates customized scaffolds for brain tissue engineering. Neural Regen Res. 2017a;12:614–622. doi: 10.4103/1673-5374.205101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu F, Qin Z, Li XH, Chen C, Wang LN, Xu C, Tu Y, Zhang S. Degradation rate of collagen-chitosan composite scaffold implanted into different rat tissues. Zhongguo Zuzhi Gongcheng Yanjiu. 2017b;21:864–870. [Google Scholar]
- 8.Greer K, Chen J, Brickler T, Gourdie R, Theus MH. Modulation of gap junction-associated Cx43 in neural stem/progenitor cells following traumatic brain injury. Brain Res Bull. 2017;134:38–46. doi: 10.1016/j.brainresbull.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan J, Zhu Z, Zhao RC, Xiao Z, Wu C, Han Q, Chen L, Tong W, Zhang J, Han Q, Gao J, Feng M, Bao X, Dai J, Wang R. Transplantation of human mesenchymal stem cells loaded on collagen scaffolds for the treatment of traumatic brain injury in rats. Biomaterials. 2013;34:5937–5946. doi: 10.1016/j.biomaterials.2013.04.047. [DOI] [PubMed] [Google Scholar]
- 10.Ji C, Khademhosseini A, Dehghani F. Enhancing cell penetration and proliferation in chitosan hydrogels for tissue engineering applications. Biomaterials. 2011;32:9719–9729. doi: 10.1016/j.biomaterials.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Jones KS, Connor B. Endogenous Brain Repair: Overriding intrinsic lineage determinates through injury-induced micro-environmental signals. Neurogenesis (Austin) 2017;4:1–5. doi: 10.1080/23262133.2017.1297881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilbaugh TJ, Bhandare S, Lorom DH, Saraswati M, Robertson CL, Margulies SS. Cyclosporin A preserves mitochondrial function after traumatic brain injury in the immature rat and piglet. J Neurotrauma. 2011;28:763–774. doi: 10.1089/neu.2010.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim CH, Lee SJ, Choi YJ, Yun SH, Son Y. Protocol for Freeze-Drying Method. A Manual for Biomaterials/Scaffold Fabrication Technology. 2007:91–99. World Scientific. [Google Scholar]
- 14.Lu D, Mahmood A, Qu C, Hong X, Kaplan D, Chopp M. Collagen scaffolds populated with human marrow stromal cells reduce lesion volume and improve functional outcome after traumatic brain injury. Neurosurgery. 2007;61:596–603. doi: 10.1227/01.NEU.0000290908.38438.B2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maclean FL, Horne MK, Williams RJ, Nisbet DR. Review: Biomaterial systems to resolve brain inflammation after traumatic injury. APL Bioeng. 2018;2:021502. doi: 10.1063/1.5023709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahmood A, Qu C, Ning R, Wu H, Goussev A, Xiong Y, Irtenkauf S, Li Y, Chopp M. Treatment of TBI with collagen scaffolds and human marrow stromal cells increases the expression of tissue plasminogen activator. J Neurotrauma. 2011;28:1199–1207. doi: 10.1089/neu.2010.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mo L, Yang Z, Zhang A, Li X. The repair of the injured adult rat hippocampus with NT-3-chitosan carriers. Biomaterials. 2010;31:2184–2192. doi: 10.1016/j.biomaterials.2009.11.078. [DOI] [PubMed] [Google Scholar]
- 18.Moura D, Mano JF, Paiva MC, Alves NM. Chitosan nanocomposites based on distinct inorganic fillers for biomedical applications. Sci Technol Adv Mater. 2016;17:626–643. doi: 10.1080/14686996.2016.1229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orive G, Anitua E, Pedraz JL, Emerich DF. Biomaterials for promoting brain protection, repair and regeneration. Nat Rev Neurosci. 2009;10:682–692. doi: 10.1038/nrn2685. [DOI] [PubMed] [Google Scholar]
- 20.Pettikiriarachchi JTS, Parish CL, Shoichet MS, Forsythe JS, Nisbet DR. Biomaterials for brain tissue engineering. Aust J Chem. 2010;63:1143–1154. [Google Scholar]
- 21.Prabaharan M, Sivashankari PR. Prospects of bioactive chitosan-based scaffolds in tissue engineering and regenerative medicine. In: Dutta PK, editor. Chitin and Chitosan For Regenerative Medicine. New Delhi: Springer India; 2016. pp. 41–59. [Google Scholar]
- 22.Skardelly M, Gaber K, Burdack S, Scheidt F, Hilbig H, Boltze J, Forschler A, Schwarz S, Schwarz J, Meixensberger J, Schuhmann MU. Long-term benefit of human fetal neuronal progenitor cell transplantation in a clinically adapted model after traumatic brain injury. J Neurotrauma. 2011;28:401–414. doi: 10.1089/neu.2010.1526. [DOI] [PubMed] [Google Scholar]
- 23.Winkler EA, Minter D, Yue JK, Manley GT. Cerebral edema in traumatic brain injury: pathophysiology and prospective therapeutic targets. Neurosurg Clin N Am. 2016;27:473–488. doi: 10.1016/j.nec.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Xia J, Luo M, Ni N, Chen J, Hu Y, Deng Y, Ji J, Zhou J, Fan X, Gu P. Bone marrow mesenchymal stem cells stimulate proliferation and neuronal differentiation of retinal progenitor cells. PLoS One. 2013;8:e76157. doi: 10.1371/journal.pone.0076157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Z, Wang W, Ren Y, Zhang W, Fang P, Huang L, Wang X, Shi P. Regeneration of cortical tissue from brain injury by implantation of defined molecular gradient of semaphorin 3A. Biomaterials. 2018;157:125–135. doi: 10.1016/j.biomaterials.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Yan F, Yue W, Zhang YL, Mao GC, Gao K, Zuo ZX, Zhang YJ, Lu H. Chitosan-collagen porous scaffold and bone marrow mesenchymal stem cell transplantation for ischemic stroke. Neural Regen Res. 2015;10:1421–1426. doi: 10.4103/1673-5374.163466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Q, Lu H, Kawazoe N, Chen G. Preparation of collagen scaffolds with controlled pore structures and improved mechanical property for cartilage tissue engineering. J Bioact Compat Pol. 2013;28:426–438. [Google Scholar]
- 28.Zhao L, Feng ZH, Jiao SX, Li NN. Culture condition and biological characteristics of rat bone marrow mesenchymal stem cells by using the whole bone marrow adherence method. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2011;15:5923–5927. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


