Keywords: nerve regeneration, neurodegeneration, Parkinson's disease, ginsenoside Rb1, neuroinflammation, lipopolysaccharide, dopaminergic neuron, microglia, nuclear factor kappa B, dopamine, tyrosine hydroxylase, substantia nigra, neural regeneration
Abstract
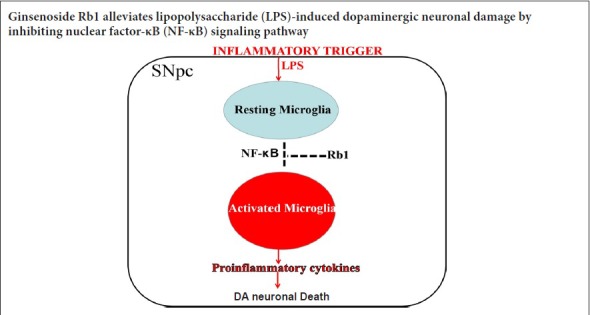
Accumulating studies suggest that neuroinflammation characterized by microglial overactivation plays a pivotal role in the pathogenesis of Parkinson’s disease. As such, inhibition of microglial overactivation might be a promising treatment strategy to delay the onset or slow the progression of Parkinson’s disease. Ginsenoside Rb1, the most active ingredient of ginseng, reportedly exerts neuroprotective effects by suppressing inflammation in vitro. The present study aimed to evaluate the neuroprotective and anti-inflammatory effects of ginsenoside Rb1 in a lipopolysaccharide-induced rat Parkinson’s disease model. Rats were divided into four groups. In the control group, sham-operated rats were intraperitoneally administered normal saline for 14 consecutive days. In the ginsenoside Rb1 group, ginsenoside Rb1 (20 mg/kg) was intraperitoneally injected for 14 consecutive days after sham surgery. In the lipopolysaccharide group, a single dose of lipopolysaccharide was unilaterally microinjected into the rat substantial nigra to establish the Parkinson’s disease model. Lipopolysaccharide-injected rats were treated with normal saline for 14 consecutive days. In the ginsenoside Rb1 + lipopolysaccharide group, lipopolysaccharide was unilaterally microinjected into the rat substantial nigra. Subsequently, ginsenoside Rb1 was intraperitoneally injected for 14 consecutive days. To investigate the therapeutic effects of ginsenoside Rb1, behavioral tests were performed on day 15 after lipopolysaccharide injection. We found that ginsenoside Rb1 treatment remarkably reduced apomorphine-induced rotations in lipopolysaccharide-treated rats compared with the lipopolysaccharide group. To investigate the neurotoxicity of lipopolysaccharide and potential protective effect of ginsenoside Rb1, contents of dopamine and its metabolites in the striatum were measured by high-performance liquid chromatography. Compared with the lipopolysaccharide group, ginsenoside Rb1 obviously attenuated the lipopolysaccharide-induced depletion of dopamine and its metabolites in the striatum. To further explore the neuroprotective effect of ginsenoside Rb1 against lipopolysaccharide-induced neurotoxicity, immunohistochemistry and western blot assay of tyrosine hydroxylase were performed to evaluate dopaminergic neuron degeneration in the substantial nigra par compacta. The results showed that lipopolysaccharide injection caused a large loss of tyrosine hydroxylase-immunoreactive neurons in the substantia nigra and a significant decrease in overall tyrosine hydroxylase expression. However, ginsenoside Rb1 noticeably reversed these changes. To investigate whether the neuroprotective effect of ginsenoside Rb1 was associated with inhibition of lipopolysaccharide-induced microglial activation, we examined expression of the microglia marker Iba-1. Our results confirmed that lipopolysaccharide injection induced a significant increase in Iba-1 expression in the substantia nigra; however, ginsenoside Rb1 effectively suppressed lipopolysaccharide-induced microglial overactivation. To elucidate the inhibitory mechanism of ginsenoside Rb1, we examined expression levels of inflammatory mediators (tumor necrosis factor-α, interleukin-1β, inducible nitric oxide synthase, and cyclooxygenase 2) and phosphorylation of nuclear factor kappa B signaling-related proteins (IκB, IKK) in the substantia nigra with enzyme-linked immunosorbent and western blot assays. Our results revealed that compared with the control group, phosphorylation and expression of inflammatory mediators IκB and IKK in the substantia nigra of lipopolysaccharide group rats were significantly increased; whereas, ginsenoside Rb1 obviously reduced lipopolysaccharide-induced changes on the lesioned side of the substantial nigra par compacta. These findings confirm that ginsenoside Rb1 can inhibit inflammation induced by lipopolysaccharide injection into the substantia nigra and protect dopaminergic neurons, which may be related to its inhibition of the nuclear factor kappa B signaling pathway. This study was approved by the Experimental Animal Ethics Committee of Shandong University of China in April 2016 (approval No. KYLL-2016-0148).
Chinese Library Classification No. R453; R364
Introduction
Parkinson’s disease (PD) is an age-related neurodegenerative disorder characterized by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc), a marked decrease of dopamine in the striatum, and the presence of Lewy bodies or Lewy neuritis in surviving neurons (Rizek et al., 2016; Zeng et al., 2018; Zhou et al., 2019). PD manifests clinically as motor disorders, such as bradykinesia, rigidity, postural instability and rest tremor, and non-motor features, such as olfactory deficits, sleep disturbance, and psychosis secondary to autonomic dysfunction. Although these clinical symptoms result from the gradual degeneration of dopaminergic neurons in SNpc, the precise mechanism of neuronal degeneration remains elusive. Several lines of evidence suggest that microglia-mediated neuroinflammation may contribute to nigrostriatal pathway degeneration and modulate the progression of PD (Hirsch and Hunot, 2009; Tansey and Goldberg, 2010; Wang et al., 2017; Gupta et al., 2018).
Microglia, the resident macrophages of the brain, have been recognized as primary actors in neuroinflammatory responses in the central nervous system. Overactivated microglia release various pro-inflammatory enzymes and/or cytotoxic cytokines that are deleterious to the surrounding neurons, resulting in a self-sustaining cycle of neuronal death (Wang et al., 2015; Le et al., 2016; Rai et al., 2017; Lee et al., 2019). Hence, anti-inflammatory treatment via inhibition of microglial release of these pro-inflammatory molecules may be a promising treatment strategy to delay the onset or slow the progression of PD.
Panax ginseng Meyer is a well-known medicinal herb that has been used as a tonic to improve well-being and alleviate fatigue in Asia, particularly China, for millennia. Numerous studies have indicated that the pharmacological actions of ginseng can be attributed to its bioactive ingredients, called ginsenosides, which have been shown to elicit beneficial therapeutic effects in several degenerative diseases (Song et al., 2017). Ginsenoside Rb1 (GRb1), the major component of ginseng, exhibits a wide range of neurotrophic and neuroprotective effects in the central nervous system. GRb1 can reportedly protect neurons from injuries induced by amyloid β-peptide (Chen et al., 2008) and α-synuclein (Ardah et al., 2015). Furthermore, Radad et al. (2004) and Hashimoto et al. (2012) reported that GRb1 could protect dopaminergic neurons from undergoing apoptosis after exposure to 1-methyl-4-phenylpyridinium-iodide. The results of these studies indicate that GRb1 may be a potent neuroprotectant for neurodegenerative disorders such as PD. Recently, several mechanistic studies demonstrated that the neuroprotective effects of GRb1 might be partly attributed to its anti-inflammatory effects. In vitro studies indicated that GRb1 alleviates lipopolysaccharide (LPS)-induced inflammatory reaction in N9 microglia (Ke et al., 2014), RAW267.4 macrophages (Smolinski and Pestka, 2003), EOC20 microglia (Beamer and Shepherd, 2012), and BV2 microglia (Lee et al., 2012). In an in vivo study, Lee et al. (2013) reported that GRb1 inhibited systemic LPS-induced microglial activation and expression of pro-inflammatory factors in both the cortex and hippocampus of mice. However, despite numerous studies, the modulatory effects of GRb1 on neuroinflammation still remain unclear (Yu et al., 2017), and no previous study has investigated the protective effect of GRb1 against inflammation in the nigrostriatal system in vivo.
Therefore, the present study was designed to investigate whether GRb1 attenuated dopaminergic neuronal damage via its anti-inflammatory actions in an LPS-induced neurotoxic rat model.
Materials and Methods
Animals
Forty-eight male Wistar rats aged 3 months and weighing 240–280 g were supplied by Shandong Experimental Animal Center, China (license number: SCXK (Lu) 2015002). Rats were housed under standard conditions of controlled temperature (22 ± 3°C) and light cycle (12-hour light and 12-hour dark) with free access to food and water. Rats were allowed to acclimate to their new surroundings for 1 week before experimental manipulations. All animal experimental procedures were approved by the Animal Ethics Committee of Shandong University, China in April 2016 (approval No. KYLL-2016-0148). All efforts were made to minimize the number of animals used and their suffering.
Stereotaxic surgery
Rats were intraperitoneally anesthetized with chloral hydrate (400 mg/kg) and placed on a stereotaxic frame (Standard Stereotaxic Frame, Stoelting, Wood Dale, IL, USA) to conform to the brain atlas of Paxinos and Watson (Paxinos and Watson, 2007). As previously described (Sharma et al., 2017), LPS (5.0 μg dissolved in 2 μL of 0.9% saline) was injected into the right side of the SNpc at a rate of 0.5 μL/min using the following stereotaxic coordinates (Paxinos and Watson, 2007): anteroposterior: −5.2 mm, mediolateral: 2 mm; dorsoventral: 7.9 mm. After each injection, the needle remained in position for an additional 5 minutes to prevent reflux of the toxin along the injection tract. Sham-operated rats were subjected to the same surgical procedures, except that 2 μL of normal saline, instead of LPS, was injected into the right SNpc (Sun et al., 2016). Rats with more than 200 revolving cycles in 30 minutes were successfully modeled.
Experimental groups and drug administration
GRb1 (purity > 99%) was purchased from Jilin University, Changchun, China. The chemical formula of Rb1 is C54H92O23 and its molecular weight is 1109.29. Purity of GRb1 powder was 99% as determined by reverse phase high-performance liquid chromatography (HPLC). GRb1 was dissolved in physiological saline (10 mg/mL) and administered intraperitoneally.
Forty-eight male Wistar rats were divided into the following four groups (12 rats per group): (1) Control group: sham-operated rats were intraperitoneally administered with normal saline (2 mL/kg per day) for 14 consecutive days; (2) GRb1 group: Same as control group except GRb1 (20 mg/kg, 10 mg/mL) was injected intraperitoneally; (3) LPS group followed by vehicle treatment: LPS-injected rats were treated as described in the control group; (4) GRb1 + LPS group: Same as LPS group except that GRb1 (20 mg/kg) was injected intraperitoneally. GRb1 dosage was chosen in accordance with previous reports (Zhu et al., 2012; Chen et al., 2015; Wang et al., 2018; Zhao et al., 2018) and preliminary experiments. After behavioral testing on day 15, rats were sacrificed and used for HPLC analysis, immunohistochemistry, enzyme-linked immunosorbent assay (ELISA), and western blot assay.
Rotational behavior analysis
Ten rats in each group were tested for rotational behavior after 14 days of treatment. The rotational behavior test was carried out as previously described (Hritcu and Ciobica, 2013; Bjorklund and Dunnett, 2019). Briefly, rats were placed in stainless steel bowls and allowed to adapt for 10 minutes to the testing environment on day 15. The rat chest was surrounded by a harness that connected to an automatic four-channel rotameter (Taishan Medica University, China). Next, rats were intraperitoneally injected with apomorphine (0.5 mg/kg) dissolved in normal saline. The rotational behavior test began at 5 minutes after injection and lasted for 30 minutes under minimal external stimuli. Rats were placed in a rotating container, and the number of rotations for each group of rats was counted for 30 minutes for statistical comparison.
HPLC
The brain was quickly obtained from 32 rats after behavioral testing. The striatum was stored at −80°C until subsequent HPLC detection. Contents of dopamine and its metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid in the rat striatum were determined by HPLC, as previously described (Liu et al., 2008). Briefly, the striatum was weighed and homogenized in 0.3 mL liquid A (0.4 M perchloric acid) and centrifuged at 12,000 r/min for 20 minutes at 4°C. In total, 240 μL of supernatant was collected and mixed with 120 μL liquid B, which contained 20 mM citromalic acid-potassium, 300 mM dipotassium phosphate, and 2 mM EDTA-2Na. Subsequently, the supernatant was evaluated with an HPLC system (Waters e2695, Milford, MA, USA) equipped with an Electrochemical Detector (Waters 2465).
Immunohistochemistry
After behavioral testing, 16 rats were anesthetized with chloral hydrate and transcardially perfused with 150 mL of normal saline and 350 mL of 4% paraformaldehyde. Brains were fixed and processed for immunostaining as previously described (Sun et al., 2016). Serial coronal sections (25 μm) were cut through the SNpc (from anteroposterior: −4.8 mm to anteroposterior: −6.2 mm) on a cryostat (Leica, Germany). Free-floating brain sections were pre-incubated in 5% bovine serum albumin containing 0.2% Triton X-100 for 30 minutes at 37°C. These sections were incubated overnight at 4°C with a rabbit polyclonal anti-tyrosine hydroxylase (TH) (1:2000) or mouse monoclonal ionized calcium-binding adaptor molecule-1 (1:200) antibody, both purchased from Millipore (Bedford, MA, USA). Afterwards, sections were incubated with goat anti-rabbit and goat anti-mouse IgG horseradish peroxidase-conjugated secondary antibodies (1:1000; Santa Cruz Biotechnology, Dallas, TX, USA) for 2 hours at room temperature, separately. Stained samples were visualized by diaminobenzidine, and six sections of each animal were counted by two individuals in a blind fashion using a microscope (Olympus, Tokyo, Japan). The survival rate of TH neurons was calculated as the number of TH neurons on the lesioned side relative to the number of TH neurons on the non-lesioned side.
ELISA
After behavioral testing, the brain was quickly obtained from 16 rats, and the substantia nigra was stored at −80°C until subsequent ELISA. Amounts of TNF-α and IL-1β in the rat substantia nigra were determined with ELISA Kits (R&D Systems, Minneapolis, MN, USA). Tissues were weighed and homogenized in ice-cold lysis buffer containing 1 mM phenylmethylsulfonyl fluoride according to the manufacturer’s instructions. The supernatant was collected and protein concentrations were measured using a PierceMT BCA protein assay (Thermo Scientific, Waltham, MA, USA). Sandwich ELISA was then performed in accordance with the manufacturer’s instructions. Sensitivity of the ELISA assay was 5 pg/mL for both TNF-α and IL-1β. The protein concentration was determined using a detergent-compatible protein assay with a bovine serum albumin standard.
Western blot assay
After behavioral testing, the brain was quickly obtained from 16 rats, and the substantia nigra was stored at −80°C until subsequent western blot assay.
Substantia nigra tissues were lysed in RIPA buffer (Beyotime, Haimen, China) supplemented with 1% phenylmethyl sulfonylfluoride. After centrifugation at 12,000 r/min for 15 minutes at 4°C, supernatants were collected and protein concentrations in the supernatants were tested using a Pierce BCA protein assay kit. In total, 30 μg of protein was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transblotted onto polyvinylidene fluoride membranes (Immobilin-P, Millipore, Bedford, MA, USA). Membranes were blocked with 5% bovine serum albumin in Tris-buffered saline containing 0.1% Tween for 1 hour at room temperature, washed with Tris-buffered saline with 0.1% Tween three times for 10 minutes, then incubated separately with primary antibodies for TH (1:2000; rabbit polyclonal antibody), Iba-1 (1:500; mouse monoclonal antibody), COX-2 (1:1000; rabbit polyclonal antibody), inducible nitric oxide synthase (iNOS) (1:2000; rabbit polyclonal antibody), IκB (1:1000; rabbit polyclonal antibody), phospho-IκB (1:1000; rabbit polyclonal antibody), IKK and phospho-IKK (1:1000; rabbit polyclonal antibody), and β-actin (1:5000) overnight at 4°C. Primary antibodies against phospho-IκB, IκB, phospho-IKK, IKK, COX-2, iNOS, β-actin (Hertfordshire, England), TH and Iba-1 (Bedford) were supplied by Cell Signaling Biotechnology, Beverly, MA, USA. Membranes were washed with Tris-buffered saline containing 0.1% Tween three times for 15 minutes each, and incubated with rabbit or goat secondary horseradish peroxidase-linked antibodies (Santa Cruz Biotechnology) for 2 hours at room temperature. Blots were detected with enhanced chemiluminescence reagent (Millipore) and visualized with an imaging system (UVP Biospectrum 810, USA). Density of the target protein and β-actin was measured using ImageJ software (NIH, Bethesda, MD, USA), and their ratio was used as the relative optical density.
Statistical analysis
Data are expressed as the mean ± SEM. Statistical analysis was performed using GraphPad Prism 5.0 software (GraphPad Software Inc., San Diego, CA, USA). Statistical analysis was carried out using one-way analysis of variance followed by Student-Newman-Keuls post hoc test. A value of P < 0.05 was considered statistically significant.
Results
Quantitative analysis of experimental animals
A total of 48 rats were selected for this experiment. Three died of infection during the experiment, and were supplemented with new ones. A total of 48 animals entered final analysis. Behavioral testing was performed on all animals, and 10 out of each group (n = 12) were randomly selected for analysis (n = 10). After behavioral testing, 8 rats from each group were selected to isolate the striatum and substantia nigra. The striatum was used for HPLC analysis (n = 8); half of the substantia nigra was used for ELISA (n = 4), and the other half was used for immunoblot detection (n = 4). In addition, four rats from each group were perfused with paraformaldehyde and fixed for immunohistochemistry (n = 4).
GRb1 administration ameliorates apomorphine-induced behavioral impairments
Apomorphine-induced rotation to the lesioned side is the most commonly used quantitative index of dopaminergic neuronal damage in animal models of PD (Alzoubi et al., 2018; Miyanishi et al., 2019). To investigate the therapeutic effects of GRb1, behavioral tests were performed on day 15 after LPS injection. The results showed that treatment with apomorphine, an indirect dopamine receptor agonist, elicited rotational behavior towards the lesioned side in LPS-induced PD model rats. Compared with rats lesioned with LPS alone, GRb1 treatment remarkably attenuated apomorphine-induced rotation (Figure 1). These data suggest that GRb1 had beneficial effects on motor dysfunction in LPS-induced PD model rats.
Figure 1.
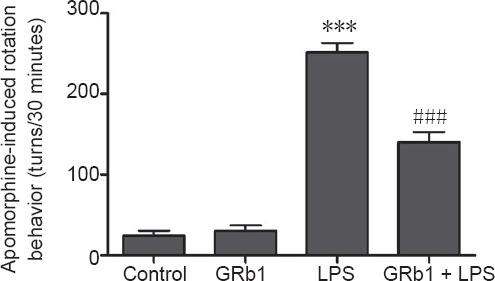
GRb1 treatment improves apomorphine-induced rotation behavior.
Rats were pretreated with GRb1 (20 mg/kg per day) or vehicle 3 days before LPS (5.0 μg) injection and subsequently for 14 days after LPS injection. Apomorphine-induced rotational behavior was tested and total turns were counted for 30 minutes after intraperitoneal injection with apomorphine (0.5 mg/kg). ***P < 0.001, vs. control group; ###P < 0.001, vs. LPS group. Data are expressed as the mean ± SEM (n = 10; one-way analysis of variance followed by Student-Newman-Keuls post hoc test). LPS: Lipopolysaccharide; GRb1: ginsenoside Rb1.
GRb1 administration attenuates depletion of dopamine and its metabolites DOPAC and homovanillic acid in the striatum induced by intranigral injection of LPS
Reduction of dopamine and its metabolites DOPAC and homovanillic acid in the striatum is believed to be a neurochemistry hallmark of LPS neurotoxicity. Therefore, contents of dopamine and its metabolites in the striatum were measured using HPLC. As shown in Figure 2, contents of dopamine, DOPAC, and homovanillic acid on the LPS-injected side were noticeably decreased compared with the uninjected side or control animals. After treatment with GRb1 (20 mg/kg per day) for 14 days, LPS-induced depletion of dopamine and its metabolites in the striatum was obviously attenuated. No changes in the contents of dopamine or its metabolites were found on the unlesioned side of the striatum in all groups. These results indicated that GRb1 effectively increased levels of dopamine neurotransmitter in PD rats induced by LPS through intranigral injection.
Figure 2.
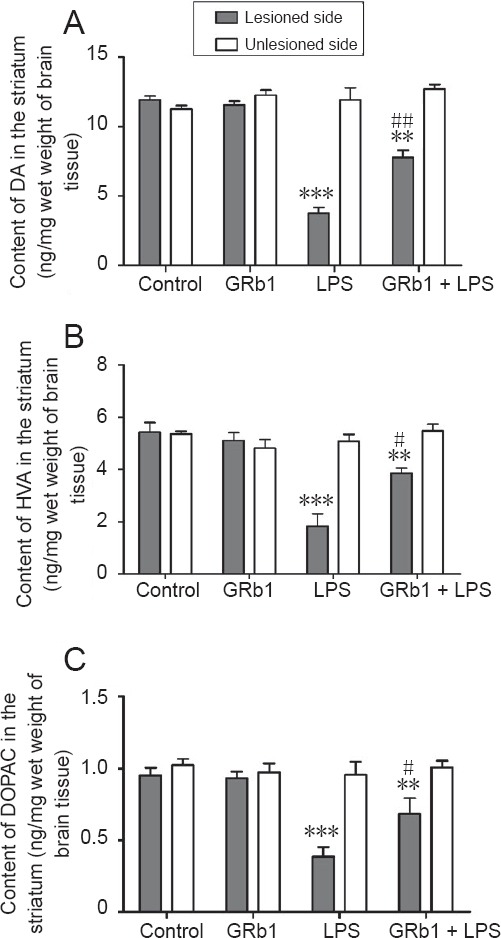
Effect of GRb1 on contents of DA and its metabolites on the lesioned side of the striatum.
After LPS (5.0 μg) injection, rats were treated with GRb1 (20 mg/kg) or vehicle for 2 weeks. (A–C) Contents of (A) DA, (B) HVA and (C) DOPAC in the striatum as detected by high-performance liquid chromatography (ng/mg wet weight of brain tissue). **P < 0.01, ***P < 0.001, vs. control group; #P < 0.05, ##P < 0.01, vs. LPS group (lesioned side). Data are expressed as the mean ± SEM (n = 8; one-way analysis of variance followed by Student-Newman-Keuls post hoc test). DA: Dopamine; DOPAC: 3,4-dihydroxyphenylacetic acid; HVA: homovanillic acid; GRb1: ginsenoside Rb1; LPS: lipopolysaccharide.
Protective effect of GRb1 on LPS-induced dopaminergic neuronal deficits
To further explore the neuroprotective effect of GRb1 against LPS-induced neurotoxicity, immunohistochemistry and western blot assay of TH were performed to evaluate dopaminergic degeneration in the substantia nigra. As shown in Figure 3, severe nigrastriatal lesions with a marked loss of TH-immunoreactive neurons and their dendrites were observed in rats receiving vehicle treatment after intranigral LPS injection. Only 33.6% of TH-immunoreactive neurons in the SNpc on the LPS-injected side survived compared with the non-injected side. In contrast, GRb1 treatment remarkably increased the survival of TH neurons, such that the survival ratio was 57.8% on the LPS-injected side. Western blot assay results revealed that LPS injection markedly decreased TH protein levels on the lesioned side compared with the control group. In the GRb1 group, expression of TH was remarkably increased compared with the PD group. The above results show that GRb1 can alleviate damage to dopaminergic neurons induced by intranigral LPS injection.
Figure 3.
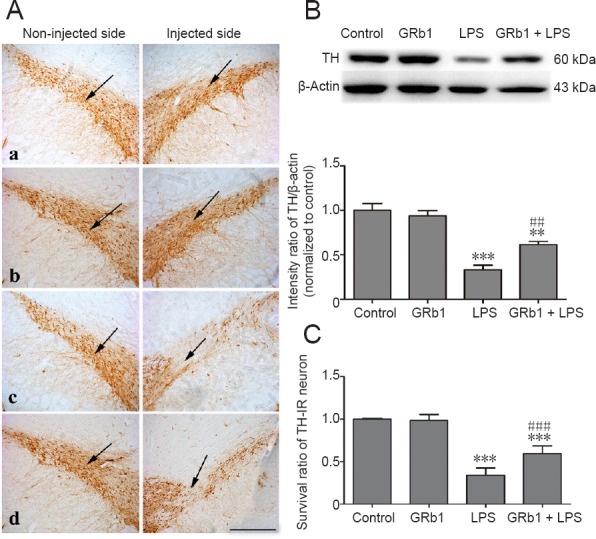
Effect of GRb1 on TH-IR neurons and TH expression in the substantia nigra pars compacta of LPS-induced Parkinson’s disease model rats.
Normal saline or 5 μg LPS was unilaterally injected into the right substantia nigra pars compacta of rats. After 2 weeks of treatment with vehicle or GRb1 (20 mg/kg), rats were sacrificed and then coronal sections passing through the substantia nigra were processed for TH immunostaining. Sections were photographed using an Olympus BX51 microscope. (A) Representative microphotographs of TH-positive neurons on unlesioned and lesioned sides of the substantia nigra in (a) control, (b) GRb1, (c) LPS, and (d) GRb1 + LPS groups (immunohistochemical staining, original magnification, 100×). Scale bar: 200 μm. (B) Immunoblotting analysis of TH expression on the lesioned side of substantia nigra. (C) Quantitative analysis of TH-positive neuron survival in the substantia nigra pars compacta (lesioned side vs. unlesioned side). **P < 0.01, ***P < 0.001, vs. control group, ##P < 0.01, ###P < 0.001, vs. LPS group. Data are expressed as the mean ± SEM (n = 4; one-way analysis of variance followed by Student-Newman-Keuls post hoc test). The arrow point represents dopaminergic neurons in the compact part of the substantia nigra. TH: Tyrosine hydroxylase; TH-IR: TH-immunoreactive; LPS: lipopolysaccharide; GRb1: ginsenoside Rb1.
GRb1 treatment inhibits LPS-induced microglial activation in the substantia nigra
Compelling evidence indicates that microglial overactivation is a key factor in the loss of nigral dopaminergic neurons in PD. To determine whether GRb1 has a neuroprotective effect via regulation on microglial inflammation, Iba-1 expression (a marker of microglial activation) in the substantia nigra was examined by immunohistochemistry and immunoblotting. As shown in Figure 4, Iba-1-immunoreactive cells were ramified resting microglia with two or three fine processes in the SNpc of sham-operated rats. After LPS injection, activated amoeboid microglia were readily identifiable throughout the SNpc. However, treatment with GRb1 for 14 days markedly reduced activated microglia/macrophage cells and LPS-induced elevations in Iba-1 expression levels. These results confirmed that GRb1 was capable of suppressing neuroinflammation in the LPS-infused substantia nigra.
Figure 4.
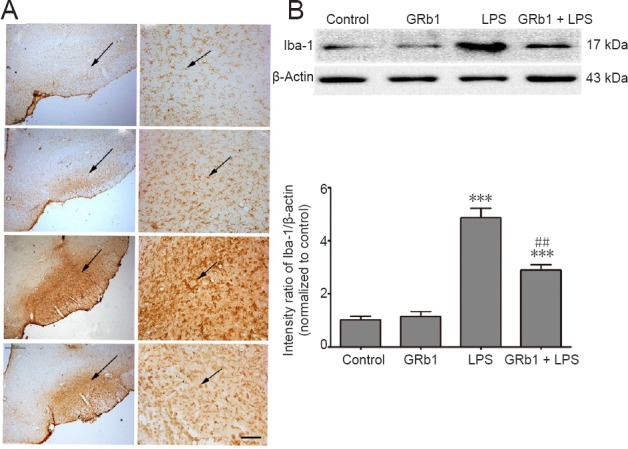
GRb1 treatment suppresses microglial activation and downregulates Iba-1 expression in the substantia nigra.
(A) Representative microphotographs of Iba-1 immunoreactive microglial cells (arrows) on the lesioned side of SNpc in (a) control, (b) GRb1 (20 mg/kg), (c) LPS (5.0 μg), and (d) GRb1 (20 mg/kg) + LPS (5.0 μg) groups (immunohistochemical staining). Original magnification, 100× in the left column and 200× in the right column. Scale bar: 30 μm. (B) Immunoblotting analysis of Iba-1 expression on the lesioned side of substantia nigra. ***P < 0.001, vs. control group; ##P < 0.01, vs. LPS group. Data are expressed as the mean ± SEM (n = 4; one-way analysis of variance followed by Student-Newman-Keuls post hoc test). The arrow represents microglia in the compact part of the substantia nigra. Iba-1: Ionized calcium binding adapter molecule 1; LPS: lipopolysaccharide; GRb1: ginsenoside Rb1.
GRb1 inhibits LPS-induced release of inflammatory mediators in the substantia nigra
To further analyze whether the neuroprotective and anti-inflammatory effects of GRb1 are associated with inhibited release of inflammatory mediators, expression levels of TNF-α, IL-1β, COX-2, and iNOS were examined using ELISA and western blot assay, respectively. As shown in Figure 5, intranigral LPS injection surprisingly upregulated TNF-α, IL-1β, iNOS, and COX-2 expression levels. Compared with vehicle-treated rats, systemic administration of GRb1 markedly inhibited the upregulation of pro-inflammatory mediators induced by intranigral LPS injection.
Figure 5.
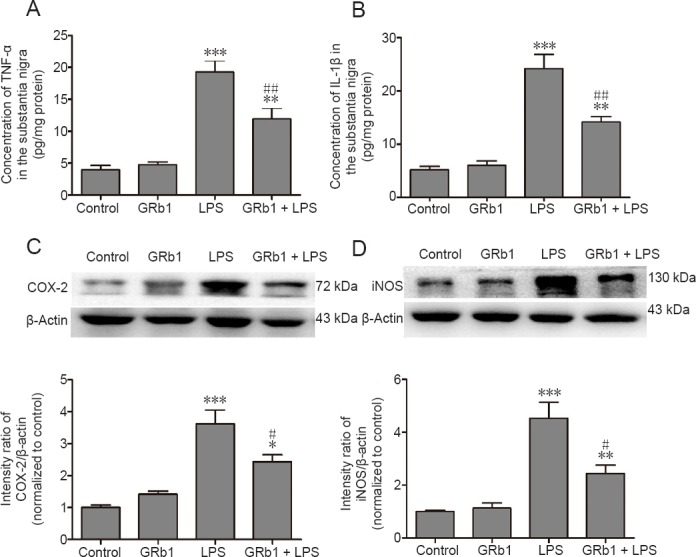
Inhibitory effect of GRb1 on LPS-induced release of TNF-α, IL-1β, COX-2, and iNOS in the substantia nigra on the lesioned side.
After LPS injection, rats were treated with GRb1 or normal saline for 14 days. Contents of TNF-α (A) and IL-1β (B) in lesioned substantia nigra were detected using ELISA kits. Expression of COX-2 (C) and iNOS (D) were detected by western blot assay. Band intensities were measured with ImageJ software. Ratios of intensities of COX-2 and iNOS to β-actin were calculated. Data are expressed as the mean ± SEM (n = 4; one-way analysis of variance followed by Student-Newman-Keuls post hoc test). *P < 0.05, **P < 0.01, ***P < 0.001, vs. control group; #P < 0.05, ##P < 0.01, vs. LPS group (lesioned side). ELISA: Enzyme-linked immunosorbent assay; TNF-α: tumor necrosis factor-α; IL-1β: interleukin-1β; iNOS: inducible nitric oxide synthase; COX-2: cyclooxygenase 2; LPS: lipopolysaccharide; GRb1: ginsenoside Rb1.
GRb1 downregulates LPS-induced activation of the NF-κB signaling pathway
The NF-κB pathway is known to be a major mediator of inflammation via the control of pro-inflammatory cytokine synthesis in microglial overactivation. To explicate the anti-inflammatory mechanism of GRb1 in LPS-lesioned substantia nigra, expression of IκB and IKK and their phosphorylation levels in the substantia nigra were examined by western blot assay. Our data clearly showed that GRb1 treatment significant reduced IκB and IKK phosphorylation compared with LPS-injected rats (Figure 6), indicating that GRb1 suppresses LPS-induced microglial activation via NF-κB signaling.
Figure 6.
Inhibitory effect of GRb1 on LPS-induced activation of NF-κB signaling in the rat substantia nigra.
(A, B) The lesioned side of the substantia nigra was removed from sacrificed rats to detect phosphorylated or total IκB (A) and phosphorylated IKK or total IKK (B). Expression of phosphorylated and total protein was monitored on the same membrane after stripping and reprobing with specific antibodies. Band intensities were measured with ImageJ software. Ratios of intensities of phosphorylated IκB and IKK to total IκB and IKK were calculated. *P < 0.05, **P < 0.01, ***P < 0.001, vs. control group; #P < 0.05, ##P < 0.01, vs. LPS group (lesioned side). Data are expressed as the mean ± SEM (n = 4; one-way analysis of variance followed by Student-Newman-Keuls post hoc test). GRb1: Ginsenoside Rb1; LPS, lipopolysaccharide; NF-κB: nuclear factor κB; IκB: inhibitor protein of κB; IKK: IκB-kinase complex.
Discussion
Accumulating studies have confirmed the neuroprotective effects of GRb1 on neurodegenerative diseases in the central nervous system in vivo and in vitro (Liu et al., 2013; Ahmed et al., 2016). However, the precise mechanisms underlying neuroprotection at the molecular and cellular levels remain elusive. In the present study, our results clearly demonstrated that GRb1 exerted protective effects on dopaminergic neurons by suppressing neuroinflammation characterized by microglial overactivation in an in vivo rat model induced by single intranigral LPS injection. Further investigation showed that the inhibitory effect of GRb1 on neurogliocytes was mediated by the downregulation of pro-inflammatory mediators corresponding to suppression of the NF-κB signaling pathway. These data further suggest the potential of GRb1 as a prospective therapy for PD.
Recent studies have demonstrated that neuroinflammation induced by microglial activation plays a pivotal role in the pathogenesis of PD (Nolan et al., 2013; Roussakis and Piccini, 2018; Tansey and Romero-Ramos, 2019). Microglial overactivation contributes to progressive dopaminergic neuron death through the production of pro-inflammatory cytokines. As such, inhibition of overactivated microglia might be beneficial to retard or reverse the development of neuroinflammatory diseases. Therefore, anti-inflammatory strategies have attracted increasing attention for their potential to inhibit microglial activation and subsequently prevent further deterioration of dopaminergic neurons in the substantia nigra (Tonges et al., 2018; Zhang et al., 2018a). A growing body of evidence indicates that PD models induced by LPS may offer a good in vivo model for studying the selective effects of inflammatory reactions on the dopaminergic system (Castano et al., 1998; Herrera et al., 2000; Machado et al., 2011). As the major component of the cell wall of gram-negative bacteria, LPS can act on Toll-like receptor 4 on the surface of microglia to activate a series of inflammatory signaling pathway elements, as well as the synthesis and release of downstream inflammatory mediators (Woller et al., 2016). Simultaneously, LPS had no direct effect on neurons because of a lack of Toll-like receptor 4, which makes it suitable to study inflammation-mediated neuronal loss (Erny et al., 2015). Therefore, in the current study, a single dose of LPS was microinjected into the rat substantia nigra to establish an inflammation-induced PD model with the aim of identifying potential therapeutic agents.
Panax ginseng has been extensively used in Traditional Chinese Medicine or as a dietary supplement. Many studies have shown that GRb1, the major bioactive component of ginseng, possesses a variety of biological activities such as anti-inflammatory and neuroprotective effects (Beamer and Shepherd, 2012; Xu et al., 2019). It has been shown to alleviate many central nervous system disorders both in vitro and in vivo (Ahmed et al., 2016; Zhu et al., 2018). In the present study, GRb1 (20 mg/kg) treatment remarkably ameliorated apomorphine-induced turning behavior compared with the LPS group. Further experiments showed that GRb1 attenuated LPS-induced deficits of dopamine content on the lesioned side of the striatum and reversed LPS-induced loss of TH-immunoreactive neurons in the SNpc. Western blot assay results also demonstrated that LPS-induced decreases in TH protein expression on the lesioned side of the substantia nigra could be restored by GRb1 treatment. No significant changes were observed on the unlesioned side of the substantia nigra or striatum. Our results are in agreement with previous reports, further suggesting that GRb1 conferred the protective effect on dopamine neurons in vivo (Ardah et al., 2015; Zhang et al., 2018b). However, little is known about potential mechanisms and anti-inflammatory roles of GRb1 in the nigrostriatal system. Several lines of evidence indicate that the neuroprotective activity of GRb1 involves its anti-inflammatory function (Ke et al., 2014). Zhu et al. (2012) showed that suppression of local inflammation contributed to the neuroprotection elicited by GRb1 in cerebral ischemia rats. A recent study demonstrated that GRb1 alleviated cognitive impairment by inhibiting neuroinflammation in the hippocampus (Wang et al., 2011) and cerebral cortex (Miao et al., 2017). The present study further suggested that GRb1 inhibits microglial overactivation and the release of pro-inflammatory factors that are paramount in the generation of an inflammatory response, and may be responsible for the degeneration of dopaminergic neurons in the substantia nigra following LPS infusion. These data are the first to suggest that GRb1 exerts protective effects on mesencephalic dopaminergic neurons by inhibiting microglial inflammation in an LPS-induced PD model.
NF-κB is located in the cytoplasm as an inactive complex bound to its inhibitory factor IκB. Once activated, NF-κB dissociates from IκB and translocates into the nucleus to induce target gene transcription. It has been well documented that the NF-κB signaling pathway, the most important modulator of pro-inflammatory gene expression, is involved in LPS-induced microglial activation (Sun et al., 2016). GRb1 attenuated pro-inflammatory mediator expression by inactivating NF-κB in LPS-stimulated RAW264.7 cells (Park et al., 2005; Rhule et al., 2006). In the present study, the anti-inflammatory effect of GRb1 on LPS-induced NF-κB activation was determined by western blot assay. Our results showed that IκB phosphorylation levels were markedly increased by LPS stimulation in the substantia nigra, but were effectively reversed by GRb1 treatment. Expression of IKK, a central component of the signaling cascade that controls NF-κB-dependent gene transcription, was also inhibited by GRb1. These results clearly suggest that NF-κB signaling is involved in the anti-inflammatory effect of GRb1 against LPS-induced microglial activation in the SNpc. In addition, some studies have reported that the anti-neuroinflammatory effects of GRb1 may be related to protection of blood-brain barrier and estrogen receptor activation (Cho et al., 2004; Chen et al., 2015). Therefore, the detailed mechanism of the anti-inflammatory effect of GRb1 still needs to be verified in vivo and in vitro.
Collectively, the above results indicate that GRb1 can prevent LPS-induced neuroinflammation and dopaminergic neuron loss in the nigrostriatal system. Regulation of local inflammation, as demonstrated by inhibition of microglial overactivation and a reduction of pro-inflammatory factors via inactivation of NF-κB phosphorylation, may be potential mechanisms by which GRb1 elicits neuroprotection. Thus, the very low toxicity and multifactorial pharmacological activities of GRb1 make it a potential therapeutic to prevent or delay the neuroinflammatory progression of PD.
Taken together, previous studies on GRb1 in PD mainly focused on neuroprotective aspects, such as antioxidant properties (Liu et al., 2018) and scavenging of oxygen free radicals (Liu et al., 2013; Fernandez-Moriano et al., 2017); whereas, our research focused on inhibiting microglial inflammation. This study only observed the effect of GRb1 on the NF-kB inflammatory pathway in PD models rats, but did not observe changes of other inflammatory pathways. There are many inflammatory-related factors involved in PD. As such, in subsequent experiments, we will continue to observe the effects of GRb1 on various inflammatory pathways, as well as interacting effects occurring between different pathways.
Additional file: Open peer review report 1 (104.4KB, pdf) .
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This work was supported by the Medical and Health Technology Development Plan of Shandong Province of China, No. 2011HD009 (to AHW); the Chinese Medicine Science and Technology Development Plan Project of Shandong Province of China, No. 2017-163 (to AHW); the Natural Science Foundation of Shandong Province of China, No. ZR2016HP23 (to AHW); the Science and Technology Development Plan Project of Taian City of China, No. 2017NS0151 (to XCS). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement: All experimental procedures and protocols were approved by the Experimental Animal Ethics Committee of Shandong University of China in April 2016 (approval No. KYLL-2016-0148). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No 85-23, revised 1985).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Xavier d’Anglemont de Tassigny, Hospital Universitario Virgen del Rocio, Spain; Manju Bhaskar, National Institute of Neurological Disorders and Stroke, USA.
Funding: This work was supported by the Medical and Health Technology Development Plan of Shandong Province of China, No. 2011HD009 (to AHW); the Chinese Medicine Science and Technology Development Plan Project of Shandong Province of China, No. 2017-163 (to AHW); the Natural Science Foundation of Shandong Province of China, No. ZR2016HP23 (to AHW); the Science and Technology Development Plan Project of Taian City of China, No. 2017NS0151 (to XCS).
P-Reviewer: Deusen AV; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Qiu Y, Song LP; T-Editor: Jia Y
References
- 1.Ahmed T, Raza SH, Maryam A, Setzer WN, Braidy N, Nabavi SF, de Oliveira MR, Nabavi SM. Ginsenoside Rb1 as a neuroprotective agent: a review. Brain Res Bull. 2016;125:30–43. doi: 10.1016/j.brainresbull.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Alzoubi KH, Mokhemer E, Abuirmeileh AN. Beneficial effect of etazolate on depression-like behavior and, learning, and memory impairment in a model of Parkinson’s disease. Behav Brain Res. 2018;350:109–115. doi: 10.1016/j.bbr.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Ardah MT, Paleologou KE, Lv G, Menon SA, Abul Khair SB, Lu JH, Safieh-Garabedian B, Al-Hayani AA, Eliezer D, Li M, El-Agnaf OM. Ginsenoside Rb1 inhibits fibrillation and toxicity of alpha-synuclein and disaggregates preformed fibrils. Neurobiol Dis. 2015;74:89–101. doi: 10.1016/j.nbd.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beamer CA, Shepherd DM. Inhibition of TLR ligand- and interferon gamma-induced murine microglial activation by Panax notoginseng. J Neuroimmune Pharmacol. 2012;7:465–476. doi: 10.1007/s11481-011-9333-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjorklund A, Dunnett SB. The amphetamine induced rotation test: a re-assessment of its use as a tool to monitor motor impairment and functional recovery in rodent models of Parkinson’s disease. J Parkinsons Dis. 2019;9:17–29. doi: 10.3233/JPD-181525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castano A, Herrera AJ, Cano J, Machado A. Lipopolysaccharide intranigral injection induces inflammatory reaction and damage in nigrostriatal dopaminergic system. J Neurochem. 1998;70:1584–1592. doi: 10.1046/j.1471-4159.1998.70041584.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen W, Guo Y, Yang W, Zheng P, Zeng J, Tong W. Protective effect of ginsenoside Rb1 on integrity of blood-brain barrier following cerebral ischemia. Exp Brain Res. 2015;233:2823–2831. doi: 10.1007/s00221-015-4352-3. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Huang T, Zhang J, Song J, Chen L, Zhu Y. Involvement of calpain and p25 of CDK5 pathway in ginsenoside Rb1’s attenuation of beta-amyloid peptide 25-35-induced tau hyperphosphorylation in cortical neurons. Brain Res. 2008;1200:99–106. doi: 10.1016/j.brainres.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 9.Cho J, Park W, Lee S, Ahn W, Lee Y. Ginsenoside-Rb1 from Panax ginseng C.A. Meyer activates estrogen receptor-alpha and -beta, independent of ligand binding. J Clin Endocrinol Metab. 2004;89:3510–3515. doi: 10.1210/jc.2003-031823. [DOI] [PubMed] [Google Scholar]
- 10.Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V, Utermohlen O, Chun E, Garrett WS, McCoy KD, Diefenbach A, Staeheli P, Stecher B, Amit I, Prinz M. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez-Moriano C, Gonzalez-Burgos E, Iglesias I, Lozano R, Gomez-Serranillos MP. Evaluation of the adaptogenic potential exerted by ginsenosides Rb1 and Rg1 against oxidative stress-mediated neurotoxicity in an in vitro neuronal model. PLoS One. 2017;12:e0182933. doi: 10.1371/journal.pone.0182933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta N, Shyamasundar S, Patnala R, Karthikeyan A, Arumugam TV, Ling EA, Dheen ST. Recent progress in therapeutic strategies for microglia-mediated neuroinflammation in neuropathologies. Expert Opin Ther Targets. 2018;22:765–781. doi: 10.1080/14728222.2018.1515917. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto R, Yu J, Koizumi H, Ouchi Y, Okabe T. Ginsenoside Rb1 prevents MPP(+)-induced apoptosis in PC12 cells by stimulating estrogen receptors with consequent activation of ERK1/2, akt and inhibition of SAPK/JNK, p38 MAPK. Evid Based Complement Alternat Med. 2012;2012:693717. doi: 10.1155/2012/693717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrera AJ, Castano A, Venero JL, Cano J, Machado A. The single intranigral injection of LPS as a new model for studying the selective effects of inflammatory reactions on dopaminergic system. Neurobiol Dis. 2000;7:429–447. doi: 10.1006/nbdi.2000.0289. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 16.Hritcu L, Ciobica A. Intranigral lipopolysaccharide administration induced behavioral deficits and oxidative stress damage in laboratory rats: relevance for Parkinson’s disease. Behav Brain Res. 2013;253:25–31. doi: 10.1016/j.bbr.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Inoue M, Oishi N, Fukuyama H. A MRI template of rat brain matched to the Paxinos and Watson atlas sixth edition. Neurosci Res. 2010;68:E444. [Google Scholar]
- 18.Ke L, Guo W, Xu J, Zhang G, Wang W, Huang W. Ginsenoside Rb1 attenuates activated microglia-induced neuronal damage. Neural Regen Res. 2014;9:252–259. doi: 10.4103/1673-5374.128217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le W, Wu J, Tang Y. Protective microglia and their regulation in parkinson’s disease. Front Mol Neurosci. 2016;9:89. doi: 10.3389/fnmol.2016.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JS, Song JH, Sohn NW, Shin JW. Inhibitory effects of ginsenoside Rb1 on neuroinflammation following systemic lipopolysaccharide treatment in mice. Phytother Res. 2013;27:1270–1276. doi: 10.1002/ptr.4852. [DOI] [PubMed] [Google Scholar]
- 21.Lee KW, Jung SY, Choi SM, Yang EJ. Effects of ginsenoside Re on LPS-induced inflammatory mediators in BV2 microglial cells. BMC Complement Altern Med. 2012;12:196. doi: 10.1186/1472-6882-12-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee Y, Lee S, Chang SC, Lee J. Significant roles of neuroinflammation in Parkinson’s disease: therapeutic targets for PD prevention. Arch Pharm Res. 2019 doi: 10.1007/s12272-019-01133-0. doi: 10.1007/s.12272-019-01133-0. [DOI] [PubMed] [Google Scholar]
- 23.Liu D, Zhang H, Gu W, Liu Y, Zhang M. Neuroprotective effects of ginsenoside Rb1 on high glucose-induced neurotoxicity in primary cultured rat hippocampal neurons. PLoS One. 2013;8:e79399. doi: 10.1371/journal.pone.0079399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu LX, Chen WF, Xie JX, Wong MS. Neuroprotective effects of genistein on dopaminergic neurons in the mice model of Parkinson’s disease. Neurosci Res. 2008;60:156–161. doi: 10.1016/j.neures.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Gu X, Yu M, Zi Y, Yu H, Wang Y, Xie Y, Xiang L. Effects of ginsenoside Rb1 on oxidative stress injury in rat spinal cords by regulating the eNOS/Nrf2/HO-1 signaling pathway. Exp Ther Med. 2018;16:1079–1086. doi: 10.3892/etm.2018.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machado A, Herrera AJ, Venero JL, Santiago M, de Pablos RM, Villaran RF, Espinosa-Oliva AM, Arguelles S, Sarmiento M, Delgado-Cortes MJ, Maurino R, Cano J. Inflammatory animal model for Parkinson’s disease: the intranigral injection of lps induced the inflammatory process along with the selective degeneration of nigrostriatal dopaminergic neurons. ISRN neurol. 2011;2011:476158. doi: 10.5402/2011/476158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miao HH, Zhang Y, Ding GN, Hong FX, Dong P, Tian M. Ginsenoside Rb1 attenuates isoflurane/surgery-induced cognitive dysfunction via inhibiting neuroinflammation and oxidative stress. Biomed Environ Sci. 2017;30:363–372. doi: 10.3967/bes2017.047. [DOI] [PubMed] [Google Scholar]
- 28.Miyanishi K, Choudhury ME, Watanabe M, Kubo M, Nomoto M, Yano H, Tanaka J. Behavioral tests predicting striatal dopamine level in a rat hemi-Parkinson’s disease model. Neurochem Int. 2019;122:38–46. doi: 10.1016/j.neuint.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Nolan YM, Sullivan AM, Toulouse A. Parkinson’s disease in the nuclear age of neuroinflammation. Trends Mol Med. 2013;19:187–196. doi: 10.1016/j.molmed.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Park EK, Shin YW, Lee HU, Kim SS, Lee YC, Lee BY, Kim DH. Inhibitory effect of ginsenoside Rb1 and compound K on NO and prostaglandin E2 biosyntheses of RAW264.7 cells induced by lipopolysaccharide. Biol Pharm Bull. 2005;28:652–656. doi: 10.1248/bpb.28.652. [DOI] [PubMed] [Google Scholar]
- 31.Paxinos G, Watson C. New York: Academic Press, USA; 1997. The rat brain in stereotaxic coordinates. [Google Scholar]
- 32.Radad K, Gille G, Moldzio R, Saito H, Ishige K, Rausch WD. Ginsenosides Rb1 and Rg1 effects on survival and neurite growth of MPP+-affected mesencephalic dopaminergic cells. J Neural Transm. 2004;111:37–45. doi: 10.1007/s00702-003-0063-1. [DOI] [PubMed] [Google Scholar]
- 33.Rai SN, Birla H, Zahra W, Singh SS, Singh SP. Immunomodulation of Parkinson’s disease using Mucuna pruriens (Mp) J Chem Neuroanat. 2017;85:27–35. doi: 10.1016/j.jchemneu.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Rhule A, Navarro S, Smith JR, Shepherd DM. Panax notoginseng attenuates LPS-induced pro-inflammatory mediators in RAW264.7 cells. J Ethnopharmacol. 2006;106:121–128. doi: 10.1016/j.jep.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Rizek P, Kumar N, Jog MS. An update on the diagnosis and treatment of Parkinson disease. CMAJ. 2016;188:1157–1165. doi: 10.1503/cmaj.151179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roussakis AA, Piccini P. Molecular imaging of neuroinflammation in idiopathic parkinson’s disease. Int Rev Neurobiol. 2018;141:347–363. doi: 10.1016/bs.irn.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Sharma N, Sharma S, Nehru B. Curcumin protects dopaminergic neurons against inflammation-mediated damage and improves motor dysfunction induced by single intranigral lipopolysaccharide injection. Inflammopharmacology. 2017;25:351–368. doi: 10.1007/s10787-017-0346-z. [DOI] [PubMed] [Google Scholar]
- 38.Smolinski AT, Pestka JJ. Modulation of lipopolysaccharide-induced proinflammatory cytokine production in vitro and in vivo by the herbal constituents apigenin (chamomile), ginsenoside Rb(1) (ginseng) and parthenolide (feverfew) Food Chemical Toxicol. 2003;41:1381–1390. doi: 10.1016/s0278-6915(03)00146-7. [DOI] [PubMed] [Google Scholar]
- 39.Song L, Xu MB, Zhou XL, Zhang DP, Zhang SL, Zheng GQ. A preclinical systematic review of ginsenoside-rg1 in experimental parkinson’s disease. Oxid Med Cell Longev. 2017;2017:2163053. doi: 10.1155/2017/2163053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun XC, Ren XF, Chen L, Gao XQ, Xie JX, Chen WF. Glucocorticoid receptor is involved in the neuroprotective effect of ginsenoside Rg1 against inflammation-induced dopaminergic neuronal degeneration in substantia nigra. J Steroid biochem Mol Biol. 2016;155:94–103. doi: 10.1016/j.jsbmb.2015.09.040. [DOI] [PubMed] [Google Scholar]
- 41.Tansey MG, Goldberg MS. Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis. 2010;37:510–518. doi: 10.1016/j.nbd.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tansey MG, Romero-Ramos M. Immune system responses in Parkinson’s disease: Early and dynamic. Eur J Neurosci. 2019;49:364–383. doi: 10.1111/ejn.14290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tonges L, Metzdorf J, Zella S. Parkinson’s disease and neuroinflammation-cellular pathology, mechanisms and therapeutic options. Fortschr Neurol Psychiatr. 2018;86:S10–S20. doi: 10.1055/s-0044-101608. [DOI] [PubMed] [Google Scholar]
- 44.Wang GQ, Li DD, Huang C, Lu DS, Zhang C, Zhou SY, Liu J, Zhang F. Icariin reduces dopaminergic neuronal loss and microglia-mediated inflammation in vivo and in vitro. Front Mol Neurosci. 2017;10:441. doi: 10.3389/fnmol.2017.00441. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Wang Q, Liu Y, Zhou J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl Neurodegener. 2015;4:19. doi: 10.1186/s40035-015-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Liu J, Zhang Z, Bi P, Qi Z, Zhang C. Anti-neuroinflammation effect of ginsenoside Rbl in a rat model of Alzheimer disease. Neurosci Lett. 2011;487:70–72. doi: 10.1016/j.neulet.2010.09.076. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Li Y, Yang W, Gao S, Lin J, Wang T, Zhou K, Hu H. Ginsenoside Rb1 inhibit apoptosis in rat model of Alzheimer’s disease induced by Abeta1-40. Am J Transl Res. 2018;10:796–805. [PMC free article] [PubMed] [Google Scholar]
- 48.Woller SA, Ravula SB, Tucci FC, Beaton G, Corr M, Isseroff RR, Soulika AM, Chigbrow M, Eddinger KA, Yaksh TL. Systemic TAK-242 prevents intrathecal LPS evoked hyperalgesia in male, but not female mice and prevents delayed allodynia following intraplantar formalin in both male and female mice: the role of TLR4 in the evolution of a persistent pain state. Brain Behav immun. 2016;56:271–280. doi: 10.1016/j.bbi.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu M, Chen Q, Fan R, Wang J, Li Y. Anti-inflammation effect of small molecule oligopeptides prepared from Panax ginseng C. A. Meyer in rats. Molecules. 2019;24:E858. doi: 10.3390/molecules24050858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu S, Zhou X, Li F, Xu C, Zheng F, Li J, Zhao H, Dai Y, Liu S, Feng Y. Microbial transformation of ginsenoside Rb1, Re and Rg1 and its contribution to the improved anti-inflammatory activity of ginseng. Sci Rep. 2017;7:138. doi: 10.1038/s41598-017-00262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeng XS, Geng WS, Jia JJ. Neurotoxin-induced animal models of Parkinson disease: pathogenic mechanism and assessment. ASN Neuro. 2018 doi: 10.1177/1759091418777438. doi: 10.1177/1759091418777438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Feng S, Nie K, Li Y, Gao Y, Gan R, Wang L, Li B, Sun X, Wang L, Zhang Y. TREM2 modulates microglia phenotypes in the neuroinflammation of Parkinson’s disease. Biochem Biophys Res Commun. 2018a;499:797–802. doi: 10.1016/j.bbrc.2018.03.226. [DOI] [PubMed] [Google Scholar]
- 53.Zhang YL, Liu Y, Kang XP, Dou CY, Zhuo RG, Huang SQ, Peng L, Wen L. Ginsenoside Rb1 confers neuroprotection via promotion of glutamate transporters in a mouse model of Parkinson’s disease. Neuropharmacology. 2018b;131:223–237. doi: 10.1016/j.neuropharm.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 54.Zhao D, Zhang M, Yuan H, Meng C, Zhang B, Wu H. Ginsenoside Rb1 protects against spinal cord ischemia-reperfusion injury in rats by downregulating the Bax/Bcl-2 ratio and caspase-3 and p-Ask-1 levels. Exp Mol Pathol. 2018;105:229–235. doi: 10.1016/j.yexmp.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 55.Zhou P, Xie W, Sun Y, Dai Z, Li G, Sun G, Sun X. Ginsenoside Rb1 and mitochondria: a short review of the literature. Mol Cell Probes. 2019;43:1–5. doi: 10.1016/j.mcp.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 56.Zhu J, Jiang Y, Wu L, Lu T, Xu G, Liu X. Suppression of local inflammation contributes to the neuroprotective effect of ginsenoside Rb1 in rats with cerebral ischemia. Neuroscience. 2012;202:342–351. doi: 10.1016/j.neuroscience.2011.11.070. [DOI] [PubMed] [Google Scholar]
- 57.Zhu JD, Wang JJ, Zhang XH, Yu Y, Kang ZS. Panax ginseng extract attenuates neuronal injury and cognitive deficits in rats with vascular dementia induced by chronic cerebral hypoperfusion. Neural Regen Res. 2018;13:664–672. doi: 10.4103/1673-5374.230292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



