Alzheimer’s disease (AD) and the evolution of the “Amyloid Hypothesis”: The primary risk factor for dementia is aging, as the overwhelming majority of individuals who have the disease (~95%) are 65 years old or older, and the rate of development of AD doubles roughly every five years from that age, peaking at a nearly 50% population prevalence by the age of 85. The disease is progressive and irreversible, with an average time course of 8 to 10 years. Regardless of catastrophic forecasts for the next decades, its actual prevalence has huge family and social costs. The exact mechanisms leading to AD remain unknown, limiting the identification of effective disease-modifying therapies. The two principal neuropathological hallmarks of AD are extracellular β-amyloid (Aβ) peptide deposition (senile plaques, SPs) and intracellular neurofibrillary tangles, containing hyperphosphorylated tau protein. In 1999, with a pioneering work, Dale and Hardy (2016) opened the way to the “era” of the so-called “Amyloid Hypothesis”. It supports the concept that an imbalance between production and clearance of Aβ42 and related Aβ neurotoxic peptides may be the initiating factor in AD, with consequent accumulation and deposition of oligomeric or fibrillar forms of Aβ. Since then, many therapies have focused on the removal of extracellular Aβ (eAβ). All these have given good cognitive benefits on animal models, but, as far as we know, none of them allowed the recovery to the cognitive starting point in all respects. The predominant role that the Aβ has in the development of AD is now widely accepted. While eAβ has historically garnered the greatest attention, the intracellular Aβ (iAβ) is receiving increasing consideration for its pathophysiological contributions to AD (LaFerla et al., 2007). Similarly to Caccamo et al. (2010), we also consider our approach more efficacious on iAβ than on neurofibrillary tangle removal (Cassano et al., 2019). We therefore moved towards the latest version of the “Amyloid Hypothesis”, aiming to set up a fully and readily translational protocol.
After the introduction of the “Amyloid Hypothesis”, several therapeutic strategies have been attempted, mainly aimed at reducing the burden of eAβ. The Aβ immunotherapy has been the most promising approach to modify the course of AD. The goal of this pharmacological approach is the stimulation of eAβ clearance from the brain of AD patients via the administration of Aβ antigenic peptides (active vaccination) or anti-Aβ monoclonal antibodies (passive vaccination). Several active and passive anti-Aβ vaccines have undergone clinical investigation. Anti-Aβ monoclonal antibodies (e.g. bapineuzumab, solanezumab, crenezumab, gantenerumab, and aducanumab) have been developed and are under evaluation, but the clinical results of the initial studies were equivocal in terms of cognitive benefits. The on-going, large phase III trials will dispel concerns about their efficacy and safety. Other interesting studies targeted the ApoE, the lipoproteins that remove the eAβ from SPs, by the administration of anti-ApoE antibodies or bexarotene, a third generation agonist of a nuclear transcription factor called retinoid X receptor, approved by both Food and Drug Administration and European Medicines Agency to treat T-cell lymphoma of the skin. The primary end-point (the reduction of SPs) of the above cited immunotherapy and bexarotene studies conflicted with the evidence that most elderly people show a heavy load of eAβ without any sign of dementia (SantaCruz et al., 2012). This is why we found more convincing the pathogenic role of iAβ combined with a neuroinflammatory factor (Heneka et al., 2015).
The mammalian target of rapamycin (mTOR) at the crossroad between autophagy and immunology: mTOR is an ubiquitously expressed serine-threonine kinase and is formed by two protein complexes, mTORC1 and mTORC2. It integrates several intracellular and environmental cues to orchestrate major processes, such as cell growth, mitosis and metabolism. Rapamycin and its analogs, rapalogs, represent a large class of drugs that act by inhibiting the mTOR (mainly mTORC1) activity. Their immunosuppressive and anticancer effects were the first to be clinically exploited. Now, they are approved and used widely. The role of mTOR in immune system and tolerance has been extensively investigated, and the immunosuppressive effect is only one of the numerous immunoregulatory effects of mTOR and its inhibitors (Chapman and Chi, 2014).
In the last decade, the role of mTOR in inhibiting autophagy has been deeply investigated. Autophagy represents the natural, regulated mechanism of the cell that disassembles unnecessary or dysfunctional components and its impairment leads to the accumulation of protein aggregates (Kim and Guan, 2015). After oral administration, rapalogs demonstrated their efficacy in reducing neurological proteinopaties in animal models (Crino, 2016). Sun et al. (2014) suggest that, both in animal models and human AD brain, the hyperactivation of the PI3K/AKT/mTOR axis is closely associated with the reduced autophagy. On the one hand, mTOR inhibits unc-51-like kinase 1 complex, responsible for the autophagosome formation that triggers autophagy. On the other hand, it phosphorylates the transcription factor EB, thus down-regulating its nuclear translocation, which inhibits lysosomal biogenesis. This prevents its activity as activator of the expression of lysosome-formation promoting genes.
The inhibition of mTORC1 is also likely to exert the inhibition of the immunomediated-AD. This aspect is currently investigated, but is far from being fully elucidated. Therefore, rapalogs seem to powerfully combine both a pro-autophagic and an immunomodulatory effect.
AD is an organ-specific disease: Being a central nervous system disease, with no systemic signs and symptoms, we reckoned that only a local (e.g. Ommaya reservoir-mediated) treatment could fully exploit both its pharmacological effects (Figure 1). Allowing high-dose (rapamycin and rapalogs have few neurological side effects), short-term local administration prevents the systemic immunosuppressive side effects that hampered the clinical translation of the systemic administration of rapalogs in AD. We tested this strategy on a triple transgenic mouse model of AD (3×Tg-AD) that well represents the human pathology. Since its first publication in 2003, we know that all pathological hallmarks and cognitive deficits of the 3×Tg-AD mouse are highly significant by 6 months of age. Some studies report even earlier cognitive deterioration. An evident mood alteration can be detected too, a characteristic that makes this model even closer to the human disease. At this age eAβ deposits seem negligible. Among mTOR inhibitors, everolimus has been developed to improve the pharmacokinetic characteristics of rapamycin, and has been extensively profiled in preclinical and clinical studies as an anticancer and immunosuppressive agent. We explored (Cassano et al., 2019) whether short-term, high-dose, and local treatment with everolimus, injected directly into the lateral ventricles, intracerebroventricularly by osmotic pumps, was able to modify AD-like pathology with low impact on peripheral organs. We first established the stability of everolimus in non-transgenic mice at 37°C in comparison with rapamycin, then evaluated its pharmacokinetics and pharmacodynamics profiles through either a single peripheral (intraperitoneal) or central (intracerebroventricular) route of administration. Everolimus showed higher stability than rapamycin at 37°C, and poorly crossed the blood-brain barrier both when entering the brain after intraperitoneal injection and leaving it after intracerebral infusion. Finally, 6-month-old (symptomatic phase) 3×Tg-AD mice were treated with continuous intracerebroventricular infusion of either vehicle or everolimus (0.167 μg/μL per day), Everolimus within the pump rapidly decayed at body temperature, even if slower than rapamycin, and became undetectable on day 12. We can thus argue that its concentration was pharmacologically effective only during the first few days of administration. Four weeks after the infusion, we tested the treatment efficacy by an integrated approach, including biochemical, immunohistochemical analyses and behavioural tests. The everolimus-induced mTOR inhibition reduced APP/Aβ, tau levels and recovered cognitive function and depressive-like phenotype to healthy levels. Therefore, the intracerebroventricular infusion of everolimus fully restored the AD-like phenotype rapidly when compared to previous peripheral routes of administration (Caccamo et al., 2010). Although the method is invasive, the entity, speed and duration of cognitive and mood recovery are astonishing and leave no room for doubts about its efficacy.
Figure 1.
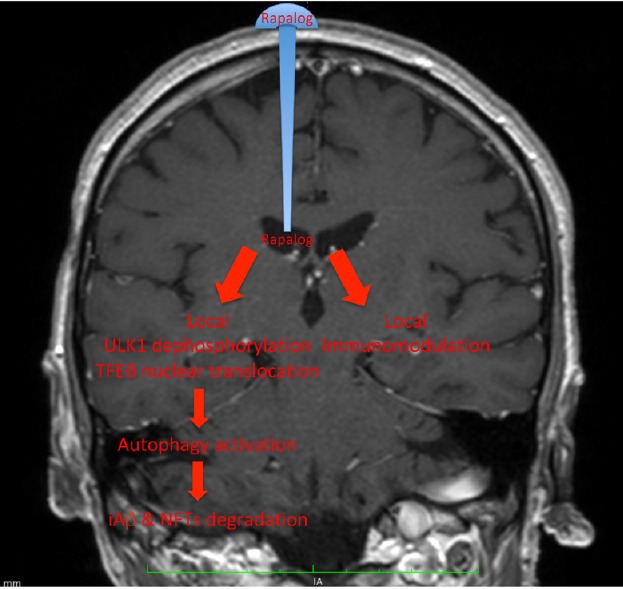
Schematic representation of the main local effect of rapalogs in Alzheimer’s disease following administration via Ommaya reservoir.
The systemic side effects (e.g. immunosuppression, glycaemic and lipid disturbances) would be avoided, due to poor drug concentration in peripheral blood.
Intrathecal infusion through Ommaya reservoirs are routinely performed in several clinical conditions, mainly (i) meningoencephalites supported by strains resistant to antibiotics that cross the blood-brain barrier, but sensitive to others that can only reach the target if intracerebrally delivered, or (ii) brain tumours sensitive to systemically-administrated intolerable drug concentrations. In expert hands, the risk of infections (the most-feared complication), is insignificant. A review (Peyrl et al., 2014), refers about thousands of intracerebroventricular infusions, which have never caused the patient’s death. In the cited paper the duration of the antibiotic or anticancer therapy was rather long, sometimes chronic. In our case, while we do not think that the therapy could aspire to the amazing speed observed in the mouse, we can certainly hope in a limited duration of the treatment. Currently, the alternative is the higher or lower speed of the inexorable progression of dementia. We believe that in the face of this perspective, no dementing person would fear the invasiveness of a 15 minutes surgery. The availability of nanoparticles that can carry the drug specifically and exclusively into the central nervous system is a promising option. However, the specificity to central nervous system is not yet satisfactory. An important ratio of nanoparticles, even if intravenously administered, is captured by the pulmonary and hepatic filters, with much less predictable consequences on treatment duration than those conceivable with local intracerebroventricular administration. The duration of the benefit we observed was quite long (over four times the administration time). Even if the experiment was not designed to appraise this aspect, we did not notice any decay from the beginning to the end of the assessment phase, while in untreated 3×Tg-AD mice the downhill progression was relentlessly rapid. We believe that the intracerebroventricular and high-dose everolimus daily administration might be effective to treat prodromal AD (Petersen, 2018), through a brief and potentially cyclic administration regimen, with short-term outcomes and a low impact on peripheral organs. The same therapy, with short intervals between treatments, might be applicable to early-onset AD, which the 3×Tg-AD mouse mimics rigorously. The cyclic treatment would avert the previously reported mTOR escape from rapalogs-mediated inhibition (Kurdi et al., 2016).
We achieved the early full recovery of an already established cognitive decay. While the result has been obtained many times in the past, in different experimental settings and animal models, it was always by long and systemic administration, leading to severe immunosuppressive and systemic side effects, which our protocol promises to prevent.
In conclusion, (i) the human maximal tolerated dose, (ii) the treatment duration, and (iii) the interval between two administration cycles (if more than one is necessary) are unpredictable. These aspects claim for an urgent clinical assessment on prodromal AD (Petersen, 2018).
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Li JY; T-Editor: Liu XL
References
- 1.Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and tau: effects on cognitive impairments. J Biol Chem. 2010;285:13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cassano T, Magini A, Giovagnoli S, Polchi A, Calcagnini S, Pace L, Lavecchia MA4, Scuderi C, Bronzuoli MR, Ruggeri L, Gentileschi MP, Romano A, Gaetani S, De Marco F, Emiliani C, Dolcetta D. Early intrathecal infusion of everolimus restores cognitive function and mood in a murine model of Alzheimer’s disease. Exp Neurol. 2019;311:88–105. doi: 10.1016/j.expneurol.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Chapman NM, Chi H. mTOR signaling, Tregs and immune modulation. Immunotherapy. 2014;6:1295–1311. doi: 10.2217/imt.14.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crino PB. The mTOR signalling cascade: paving new roads to cure neurological disease. Nat Rev Neurol. 2016;12:379–392. doi: 10.1038/nrneurol.2016.81. [DOI] [PubMed] [Google Scholar]
- 5.Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015;125:25–32. doi: 10.1172/JCI73939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, et al. Neuroinflammation in Alzheimer’s Disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurdi A, De Doncker M, Leloup A, Neels H, Timmermans JP, Lemmens K, Apers S, De Meyer GRY, Martinet W. Continuous administration of the mTORC1 inhibitor everolimus induces tolerance and decreases autophagy in mice. Br J Pharmacol. 2016;173:3359–3371. doi: 10.1111/bph.13626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 9.Peyrl A, Chocholous M, Azizi AA, Czech T, Dorfer C, Mitteregger D, Gojo J, Minichmayr E, Slavc I. Safety of Ommaya reservoirs in children with brain tumors: a 20-year experience with 5472 intraventricular drug administrations in 98 patients. J Neurooncol. 2014;120:139–145. doi: 10.1007/s11060-014-1531-1. [DOI] [PubMed] [Google Scholar]
- 10.Petersen RC. How early can we diagnose Alzheimer disease (and is it sufficient)? The 2017 Wartenberg lecture. Neurology. 2018;91:395–402. doi: 10.1212/WNL.0000000000006088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SantaCruz KS, Sonnen JA, Pezhouh MK, Desrosiers MF, Nelson PT, Tyas SL. Alzheimer disease pathology in subjects without dementia in two studies of aging: the nun study and the adult changes in thought study. J Neuropathol Exp Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31822e8ae9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]


