The American psychologist William James once wrote that nervous tissue seems to be endowed with a “very extraordinary” degree of plasticity. To be plastic means to be capable of being molded, receiving shape, or being made to assume a desired form. This can be achieved consequential to changes in the internal and/or external environment. Being plastic, neurons are highly able to amend their structural and functional output, through alteration of their morphology, activation of intracellular signaling cascades, regulation of synaptic density and neurotransmitter release, or a combination of these events. This can all be accomplished without compromising the stability and integrity of the entire neuronal network. Neuronal plasticity can be affected by many factors. For instance, the behaviour of the organism can influence neuronal plasticity, where dendritic spine density increases following learning. And, exposure to biochemicals in the environment can result in changes to neurotransmitter release and dendritic spine shape. From an altruistic standpoint, the question arises as to whether plasticity-modulating compounds can be harnessed for therapeutic purposes.
Supplementation of the dietary micronutrient choline was recently demonstrated to ameliorate the pathological phenotype of the neurodevelopmental disorder Rett syndrome (RTT) (Chin et al., 2016, 2018). This was found to be attributable to mechanisms related to neuronal plasticity – choline supplementation enhanced the morphology of neurons, rescued defects in synaptic transmission, and improved the behavioural deficits of a RTT mouse model. Because of the multiple biological functions that choline subserves, it can be hypothesized that the modulation of neuronal plasticity by choline be due to one or more of its physiological roles. In support of this, modulation of neuronal plasticity events by choline has been described in studies on fetal brain development, where choline supplementation was found to be correlated with altered distribution, morphology, and electrophysiological properties of neurons (Zeisel and Niculescu, 2006). Prenatal choline availability has also been shown to modulate expression of genes in the hippocampus and cerebral cortex (Mellot et al., 2007). In addition, memory decline in old age has been reported to be associated with choline supplementation (Blusztajn et al., 2017).
Choline is a quaternary amine that is important for the normal functioning of all cells. Dietary intake of choline is essential; choline and choline-containing esters are present in many foods, such as eggs, beef, liver, and cruciferous vegetables. Choline is present in tissues predominantly as phosphatidylcholine and sphingomyelin. Other metabolites of choline, such as acetylcholine, lysophophatidylcholine, phosphocholine, glycerophophocholine, and betaine, make up a smaller proportion of the total choline pool in cells. Choline and its metabolites are important for maintaining the structural integrity of cell membranes, methyl-metabolism, cholinergic transmission, and transmembrane signaling. All these different forms of choline and their mechanisms of actions have the potential to be harnessed for the restoration of wild-type phenotype to RTT neurons.
Earlier studies (Nag and Berger-Sweeney, 2007; Ricceri et al., 2011) focused on the use of choline for the treatment of RTT from the perspective of its role as a precursor of the neurotransmitter acetylcholine. The cholinergic system modulates cognitive and motor functions, and these functions are observed to be deficient in RTT. Thus, it was postulated that supplementation of choline would improve RTT deficits through the cholinergic system. Indeed, choline supplementation to RTT mouse models improved motor deficits and increased choline acetyltransferase activity (Nag and Berger-Sweeney, 2007; Ricceri et al., 2011). However, given the lapse of time between supplementation and behavioural testing, it seems improbable that the effects of increased acetylcholine production at the time of supplementation would last long enough to still be evident at the time of testing. This can be further expounded by evidence that choline supplementation to a RTT mouse model was able to alleviate behaviours which are not known to be directly linked to cholinergic function, such as anxiety-like and diminished social preference behaviors (Chin et al., 2018). Hence, it is possible that restoration of cholinergic function in RTT by choline may not be the only and main mechanism in play.
There are a number of ways in which choline can potentially modulate neuronal plasticity (Figure 1). The overall oxidative breakdown of choline to carbon dioxide produces electron-carriers such as NADH, which can be utilized by a neuron’s mitochondria to neutralize the reactive oxygen species that they produce in the electron transport chain. Neurons have a large number of mitochondria to cope with their high energy demands, and thus, choline can have an important role in neuronal health by mitigating reactive oxygen species damage. Indeed, choline deficiency has been shown to impair brain mitochondrial function, increase rate of protein oxidation, and thereby affect motor and learning behaviours in rats (Pacelli et al., 2010). Besides relieving oxidative stress, the first oxidative product of choline, betaine, is necessary for the formation of S-adenosylmethionine, a major methyl group donor. Methylation is an important epigenetic modulator involved in regulating the transcription and repression of many genes. Choline supplementation has been shown to induce the expression of important cellular signaling proteins and transcription factors, such as Bdnf, CaMKI, Creb, Gababr, Igf2, and Vegf (Mellot et al., 2007; Blusztajn et al., 2017). Incidentally, the expression levels of some of these genes and their isoforms (e.g. Bdnf, CamKI, and Vegf) have been reported to be regulated by their methylation statuses (Mellén et al., 2012). Donation of methyl groups via choline supplementation could potentially be an initiating event for the activation of genes involved in cytoskeletal remodeling or synaptic transmission, hence leading to alterations in neuronal morphology and neurotransmission. However, this effect may be temporally regulated. There is a higher demand for cellular components during the prenatal development stage, with presumably a corresponding increase in methylation events and demand for methyl group donors for purposes of epigenetic regulation of gene expression. In contrast to observations from earlier studies conducted at prenatal stages, methylation may not be the most important pathway during the postnatal stage, as blocking DNA methylation through pharmacological inhibition of choline oxidization to betaine did not prevent choline’s rescue of RTT neuronal morphology (Chin et al., 2018).
Figure 1.
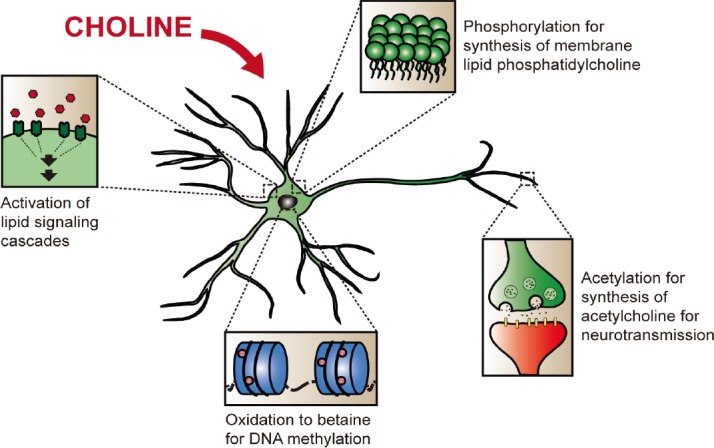
The main physiological roles of choline in that may modulate plasticity in neuronal cells.
Choline can be phosphorylated as part of the synthesis pathway of phosphatidylcholine, for the synthesis of neuronal membranes. It can also be acetylated to form acetylcholine for use in neurotransmission. Oxidation of choline gives betaine, an intermediate in the production of labile methyl groups. Choline and its metabolites can further participate in cellular lipid signaling mechanisms.
Another important metabolite of choline is phosphatidylcholine, one of the most abundant phospholipid in eukaryotic cell membranes. This particular metabolism pathway of choline is crucial for the building of neuronal membranes, which is needed for neurite extension and remodeling of synaptic connections (Chin et al., 2018). Since much of the function of a neuron is attributable to its morphology which aids the shaping, filtering, and isolating of individual synaptic responses at their site of generation, a compound with the capability of modulating neuronal morphology, such as choline, can greatly influence neurotransmission.
Besides its use as a building block of membranes, phospholipid metabolites of choline can have other bio-signaling functions. This role is important given that the levels of choline metabolites are known to be altered when choline is supplemented to neuronal cells. Cellular phospholipids are generally bioactive, participating in many cellular signaling mechanisms (Fernandis and Wenk, 2007). Some phospholipids such as lysophophatidic acid, a downstream metabolite of phosphatidylcholine, can control short-term tuning of synaptic signaling, with implications on information processing at cellular and network levels. Another example is glycerophosphocholine, an important organic osmolyte in cells. For neurons, their high level of ion flux makes them vulnerable to osmolarity insult. Hence, maintenance of the osmolyte pool in a neuron is crucial for its health. The phospholipid pool in a neuron is very dynamic – there is constant synthesis and degradation. This turnover can be a driver for vesicular trafficking, thereby regulating neurotransmitter secretion and recycling at neuronal synapses. This can further be facilitated by lipid-rich microdomains on the neuronal membrane, which are thought to provide anchoring points for channel and signaling proteins (Simons and Ikonen, 1997). Despite the many important roles choline-containing phospholipids are described to play in a neuron, it remains unclear whether the supplemented choline restored deficits of particular lipid metabolites in the cellular lipid pool, or if it boosted the levels of key metabolites that may have greater bioactivity. Thus, it would be intriguing to identify the main choline metabolites responsible for the improvement in neuronal function.
Most of the earlier studies (Nag and Berger-Sweeney, 2007; Ricceri et al., 2011) on the relationship between choline and neuronal plasticity have focused on the micronutrient’s role in cholinergic neurotransmission. To this end, it is ventured that the role of choline as a precursor of phospholipid metabolites may be a more relevant mechanism in modulating neuronal plasticity, as a means of ameliorating RTT pathophenotypes. The current global nutraceutical market is worth approximately USD$200 billion. Given that choline supplementation is generally safe and well-tolerated, a foresighted strategy could include the use of choline as a non-invasive and inexpensive means to modulate neuronal plasticity for the improvement of cognitive function, or in the treatment of neurological disorders. As yet, the jury is still out as to whether choline supplementation has a significant and detectable effect on cognitive function in humans – many clinical trials are ambivalent in their results on the effect of choline supplementation on cognitive function (Parnetti et al., 2007). It remains hypothetical the myriad ways in which choline can effect functional changes via modulating neuronal plasticity, until validation by future studies.
Additional file: Open peer review report 1 (97.4KB, pdf) .
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Giacinto Bagetta, University of Calabria, Italy.
P-Reviewer: Bagetta G; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Blusztajn JK, Slack BE, Mellott TJ. Neuroprotective actions of dietary choline. Nutrients. 2017 doi: 10.3390/nu9080815. doi: 10.3390/nu9080815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chin EW, Lim WM, Ma D, Rosales FJ, Goh ELK. Choline rescues behavioural deficits in a mouse model of Rett syndrome by modulating neuronal plasticity. Mol Neurobiol. 2018 doi: 10.1007/s12035-018-1345-9. doi: 10.1007/s12035-018-1345-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chin EW, Marcy G, Yoon SI, Ma D, Rosales FJ, Augustine GJ, Goh EL. Choline ameliorates disease phenotypes in human iPSC models of Rett syndrome. Neuromolecular Med. 2016;18:364–377. doi: 10.1007/s12017-016-8421-y. [DOI] [PubMed] [Google Scholar]
- 4.Fernandis AZ and Wenk MR. Membrane lipids as signaling molecules. Curr Opin Lipidol. 2007;18:121–128. doi: 10.1097/MOL.0b013e328082e4d5. [DOI] [PubMed] [Google Scholar]
- 5.Mellén M, Ayata P, Dewell S, Kriaucionis S, Heintz N. MeCP2 binds to 5hmC enriched within active genes and accessible chromatin in the nervous system. Cell. 2012;151:1417–1430. doi: 10.1016/j.cell.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mellott TJ, Williams CL, Meck WH, Blusztajn JK. Prenatal choline supplementation advances hippocampal development and enhances MAPK and CREB activation. FASEB J. 2004;18:545–547. doi: 10.1096/fj.03-0877fje. [DOI] [PubMed] [Google Scholar]
- 7.Nag N, Berger-Sweeney JE. Postnatal dietary choline supplementation alters behaviour in a mouse model of Rett syndrome. Neurobiol Dis. 2007;26:473–480. doi: 10.1016/j.nbd.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Pacelli C, Coluccia A, Grattagliano I, Cocco T, Petrosillo G, Paradies G, De Nitto E, Massaro A, Persichella M, Borracci P, Portincasa P, Carratù MR. Dietary choline deprivation impairs rat brain mitochondrial function and behavioral phenotype. J Nutr. 2010;140:1072–1079. doi: 10.3945/jn.109.116673. [DOI] [PubMed] [Google Scholar]
- 9.Parnetti L, Mignini F, Tomassoni D, Traini E, Amenta F. Cholinergic precursors in the treatment of cognitive impairment of vascular origin: ineffective approaches or need for re-evaluation? J Neurol Sci. 2007;257:264–269. doi: 10.1016/j.jns.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 10.Ricceri L, de Filippis B, Fuso A, Laviola G. Cholinergic hypofunction in MeCP2-308 mice: beneficial neurobehavioural effects of neonatal choline supplementation. Behav Brain Res. 2011;221:623–629. doi: 10.1016/j.bbr.2011.03.051. [DOI] [PubMed] [Google Scholar]
- 11.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 12.Zeisel SH, Niculescu MD. Perinatal choline influences brain structure and function. Nutr Rev. 2006;64:197–203. doi: 10.1111/j.1753-4887.2006.tb00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


