Abstract
Neurogenesis is a persistent and essential feature of the adult mammalian hippocampus. Granular neurons generated from resident pools of stem or progenitor cells provide a mechanism for the formation and consolidation of new memories. Regulation of hippocampal neurogenesis is complex and multifaceted, and numerous signaling pathways converge to modulate cell proliferation, apoptosis, and clearance of cellular debris, as well as synaptic integration of newborn immature neurons. The expression of functional P2X7 receptors in the central nervous system has attracted much interest and the regulatory role of this purinergic receptor during adult neurogenesis has only recently begun to be explored. P2X7 receptors are exceptionally versatile: in their canonical role they act as adenosine triphosphate-gated calcium channels and facilitate calcium-signaling cascades exerting control over the cell via calcium-encoded sensory proteins and transcription factor activation. P2X7 also mediates transmembrane pore formation to regulate cytokine release and facilitate extracellular communication, and when persistently stimulated by high extracellular adenosine triphosphate levels large P2X7 pores form, which induce apoptotic cell death through cytosolic ion dysregulation. Lastly, as a scavenger receptor P2X7 directly facilitates phagocytosis of the cellular debris that arises during neurogenesis, as well as during some disease states. Understanding how P2X7 receptors regulate the physiology of stem and progenitor cells in the adult hippocampus is an important step towards developing useful therapeutic models for regenerative medicine. This review considers the relevant aspects of adult hippocampal neurogenesis and explores how P2X7 receptor activity may influence the molecular physiology of the hippocampus, and neural stem and progenitor cells.
Keywords: P2X7, P2X7R, adult neurogenesis, neural stem cells, neural progenitor cells, hippocampus, SGZ, calcium signaling, purinergic signaling
Adult Neurogenesis
Adult neurogenesis refers to the generation of new nervous tissue within the adult central nervous system (CNS), and infers the presence of a population of neural stem and progenitor cells. The term neural stem cells (NSCs) is loosely applied to a subset of primary progenitor cells that are defined as self-renewing and multipotent, able to give rise to all three primary cell types of the CNS: neurons, astrocytes, and oligodendrocytes. Since the discovery of NSCs in the adult mammalian brain, investigations have continued to uncover roles that adult NSCs play in a wide range of physiological events, from memory and olfaction to neurological disorders and age-related neurodegeneration (Götz and Huttner, 2005; Merkle and Alvarez-Buylla, 2006). Regenerative therapies, such as autologous stem cell transplantation and pharmacological manipulation of resident progenitor pools, have been areas of much interest (Trounson and McDonald, 2015) though the current understanding of molecular mechanisms involved is lacking. This is in part due to the sophisticated and intricate nature of regulatory pathways, many of which can elicit opposing outcomes depending on a variety of factors. Nevertheless, it is hoped that NSC therapies may soon provide treatment for neurological disease and injury.
Under normal physiological conditions, neurogenesis is primarily restricted to two discrete neurogenic niches: the subgranular zone (SGZ) of the hippocampal dentate gyrus and the subventricular zone (SVZ) of the anterior lateral ventricles (Götz and Huttner, 2005; Ming and Song, 2005), though presence of a progenitor population has also been reported in the basolateral amygdala (Jhaveri et al., 2018). The SVZ houses resident radial-glial-like NSC capable of producing transit amplifying cells, which differentiate into neuroblasts and migrate via the rostral migratory stream to the olfactory bulb (Ming and Song, 2011) and the striatum (Ernst et al., 2014). The SGZ of the hippocampus generates new granule neurons that play a crucial role in synaptic plasticity and the formation and consolidation of short-term memories (Kempermann and Gage, 2002; McEown and Treit, 2013; von Allmen et al., 2013).
Both neurogenic zones follow a similar process of neurogenesis beginning with the asymmetrical division of an adult NSC to a daughter stem cell (self-renewal) and a highly proliferative progenitor referred to as a transit amplifying cell (type C cell) in the SVZ or an intermediate progenitor cell (IPC, or type 2 cell) in the dentate gyrus (Song et al., 2002; Kempermann et al., 2004). These neural progenitor cells (NPCs) symmetrically divide in an expansion phase before differentiating into more fate-restricted neuroblasts. Neuroblasts continue to proliferate as they migrate out of the niche to their target zones, where they differentiate into postmitotic immature neurons. The immature neurons integrate into the existing neural network by extending axons and increasing their connectivity, gradually obtaining the physiological characteristics of the local mature neurons (Götz and Huttner, 2005; Ming and Song, 2011). This process is summarised in Figure 1. Amplification of relatively large numbers of new neural progenitors correlates to the physiological requirements of the CNS. Of the newly formed neuroblasts, only a subset will go on to form immature neurons that integrate into the neural circuitry of the target; the remainder undergo programmed cell death (PCD; Southwell et al., 2012). The nomenclature used to define each cell type is relative as cells do not progress in discrete stages, but rather undergo gradual maturation with an ever-increasing neuronal phenotype. Thus, there is an overlap of some protein markers used to identify cell type, and the use of these delineation stages is a convenience utilized primarily for analytical purposes (Kempermann et al., 2004; Ehninger and Kempermann, 2008; Li et al., 2009).
Figure 1.
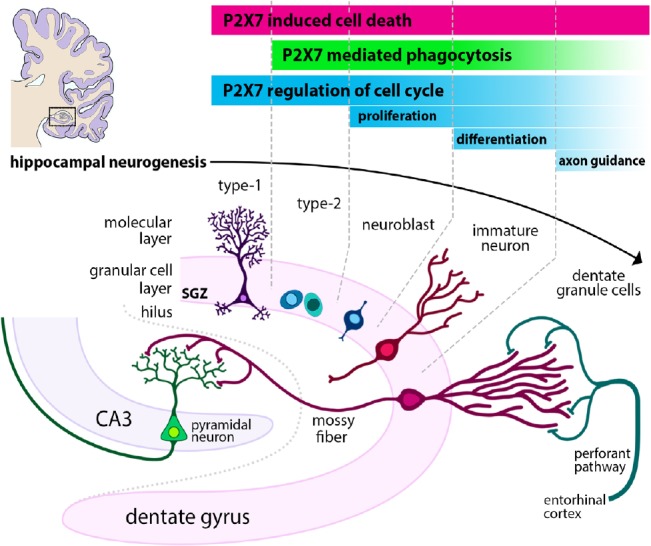
P2X7 receptors can influence the hippocampal neurogenic niche.
Hippocampal neurogenesis proceeds in stages, beginning with type 1 NSCs which asymmetrically divide in the subgranular zone (SGZ), before differentiating to become proliferative type 2 (intermediate progenitor) cells. In the third stage, type 3 neuroblasts migrate to the granule cell zone. In the final stages of neurogenesis, immature neurons extend axons called mossy fibers to the CA3 before being fully integrated in existing synaptic transmission pathways. P2X7 receptors can regulate multiple aspects of this process, most notably by inducing cell death via pore formation. Cellular debris may be cleared by neural progenitors and neuroblasts via P2X7-mediated phagocytosis, in addition to phagocytosis performed by resident microglia, which may also utilize P2X7. The receptor also has potential roles in the regulation of proliferation, differentiation and axonal extension.
During adult neurogenesis, cellular maturation is both continuous and heterogeneous, unlike the orchestrated waves of cell proliferation and maturation observed during embryonic development. Niche derived growth factors, and other signaling molecules provide support and maintenance of ongoing neurogenesis, ensuring the physiological conditions in the niches remain permissible to continued neuron production. Intrinsic regulation of transcription factors and cell cycle regulators has been demonstrated to play a major role in maintaining the homeostatic environment of the neurogenic niches (Ming and Song, 2005; Goritz and Frisén, 2012). External stimuli, such as physical exercise, environmental enrichment, natural aging, mental illness, chronic pain and pathological states induced by seizure or stroke are also strongly correlated with altered rates of neurogenesis, particularly in the hippocampus (Kempermann et al., 2002; Steiner et al., 2008; Zheng et al., 2017).
Neurogenesis in the Adult Hippocampus
In humans, neurogenesis within the dentate gyrus continues throughout adulthood, generating granule cells thought crucial for memory formation. Spalding and colleagues examined the generation rate of granule cells using 14C concentrations in genomic DNA and estimated that 700 new neurons are added to the hippocampus each day, with only a modest decline with age (Spalding et al., 2013). Adult-born neurons display enhanced synaptic plasticity for a limited period, and have a key role in pattern separation and cognitive adaptability, allowing a relatively small number of granule cells to have a significant influence on circuitry and function in the hippocampus (Ge et al., 2007). These new neurons represent a minority of the progenitor cells produced in the adult hippocampus; a large number of the newly generated cells undergo PCD, and overall there remains a net loss of neuron numbers within the hippocampus.
Neurogenesis is vital for hippocampal function, in particular learning and memory formation, and the role of newly formed granule neurons in pattern separation is thought to be a key mechanism in this process (Clelland et al., 2009; Sahay et al., 2011). Pattern separation processes neural inputs into distinct outputs, allowing memories to be stored without overlap or interference. Neurogenesis also modulates fear learning and supports the association between events and predictive cues (Seo et al., 2015). Newly generated granule neurons have differing characteristics from mature neurons; they possess increased intrinsic excitability with higher potentiation amplitude and a lower induction threshold (Schmidt-Hieber et al., 2004; Ge et al., 2007). This results in enhanced synaptic plasticity and preferential activation of new granule neurons and suggests they are major mediators of hippocampal synaptic plasticity (Yau et al., 2015).
While basal neurogenesis rates are thought to be genetic, the process is heavily regulated by both physiological and pathological stimuli. Mental disorders, such as stress, depression, schizophrenia and substance addiction have all been correlated with a decrease in hippocampal size and structural integrity, possibly due to decreased neurogenesis (Eisch et al., 2000; Videbech and Ravnkilde, 2004; Kang et al., 2016; Schoenfeld et al., 2017). Alternatively, exercise, environmental enrichment and use of antidepressants have shown to promote hippocampal neurogenesis (Santarelli et al., 2003; van Praag, 2008; Ruitenberg et al., 2017; Sun et al., 2017). Mice and rats provided with an enriched environment and/or voluntary exercise consistently show increased rates of neurogenesis (Nokia et al., 2016; Zang et al., 2017) and display improved performance in learning and short term memory when assessed by the water maze task (Bruel-Jungerman et al., 2005; Iso et al., 2007). In human studies, a meta-analysis of over 700 participants in 14 studies did not find a significant change in overall hippocampal volume. However, aerobic exercise significantly increased left hippocampal volume (Firth et al., 2018). The ability of the hippocampus to modulate neurogenesis in response to stimuli highlights the potential of therapies, including exercise and diet modulation, for the treatment of disorders ranging from stress and depression to complex neurological diseases and cognitive decline (Hueston et al., 2017; Ma et al., 2017).
Regulation of Hippocampal Neurogenesis
Adult hippocampal neurogenesis is a complex and multi-faceted process, and many mechanisms are involved in regulating NPCs and the changes they undergo during neurogenesis. Growth factors and neurotrophins are key regulators of adult neurogenesis and facilitate proliferation, migration, transcription factor regulation and maturation processes (Oliveira et al., 2013). These pathways converge with cell death mechanisms to tightly regulate cell numbers. Calcium and purinergic signaling also contribute significantly to cell communication, proliferation, differentiation, migration and PCD in adult progenitor populations in the hippocampus, and indeed in the entire CNS (Abbracchio et al., 2009; Ulrich et al., 2012; Burnstock, 2016).
Calcium signaling
There are few signaling molecules as versatile as the calcium ion, and through specific modulation of internal stores and membrane channels, fluctuations in cytoplasmic calcium concentrations form complex signaling events. In this way, a single ion can exert a wide influence over a large number of biological processes occurring in adult NSCs and NPCs, including excitability, synaptic transmission, gene expression, proliferation, differentiation and apoptosis (Tonelli et al., 2012). The regulation of internal concentrations is crucial and as cytosolic calcium concentrations are significantly lower than those in the extracellular fluid, cells expend a large amount of energy to tightly control cytosolic concentrations through numerous adenosine triphosphate (ATP) driven pumps, binding buffer proteins and channels. The cell is able to control cytosolic calcium in a manner that generates distinct downstream signaling events that are eventually translated into biological changes through decoder proteins (Berridge et al., 2003; Tonelli et al., 2012).
Calcium holds influence over such a large number of mechanisms due to the diversity of its signaling events in terms of frequency, amplitude and spatiotemporal patterning. These are initiated by either global waves or localised spikes in cytosolic calcium concentration and are controlled by finely-tuned co-ordination between channels and compartmentalization mechanisms, such as the endoplasmic reticulum (Berridge, 1997). Receiver proteins decode the calcium signals and alter the biological activity accordingly, through activation of transcription factors or other secondary signaling proteins (Smedler and Uhlen, 2014).
Purinergic signaling
Purinergic signaling is mediated by purine and pyrimidine nucleotides, such as ATP, adenosine diphosphate, uridine triphosphate and uridine diphosphate, and is an important modulator of cellular activity, having roles in signal transduction and cell-cell communications. The capacity of ATP to act as a neurotransmitter was identified in the 1970s, and since then the role of purinergic receptors in neural tissue has attracted much interest (Burnstock, 1972). Purinergic receptors mediate the effects of purines via three distinct receptor classes; P1, P2Y and P2X receptors. P1 receptors are metabotropic G protein-coupled receptors activated by adenosine. P2 receptors respond to the binding of extracellular purinergic molecules, and are divided into P2X and P2Y subfamilies. P2X receptors 1 through 7 are ionotropic cation channels activated by extracellular ATP and allow for the passage of Ca2+, K+ and Na+ ions (Ulrich et al., 2012). P2Y receptors, on the other hand, are G protein-coupled receptors and may be activated by ATP, as well as its derivatives adenosine diphosphate, uridine triphosphate and uridine diphosphate. Most P2Y receptors appear to signal via PLC, leading to an increase in IP3 concentrations and the release of calcium from the endoplasmic reticulum (Grimm et al., 2010). Thus both P2X and P2Y signaling can cause influx of calcium ions to the cytosol and initiate downstream signaling cascades to regulate transcription factor activation, cell cycle events, differentiation, migration and cell death in nervous tissues (Abbracchio et al., 2009).
In neurotransmission, purinergic signaling is involved in neuron-glial interactions, and P2X receptors can regulate rapid synaptic signal transmission and synaptic plasticity. Purines are also involved in control of learning, memory, feeding and sleep behaviour, as well as pathophysiologies, neurodegenerative and neuropsychiatric disorders (Burnstock, 2013). P2X receptors are known to play a critical role in pathological processes, such as thrombosis, inflammation and neuropathic pain (North, 2002; Kaczmarek-Hajek et al., 2012). P2X7 in particular has been heavily implicated in inflammatory responses, thereby providing possible therapeutic applications for endogenous tissue repair and stimulation of neurogenesis in cases of neurodegenerative diseases (Glaser et al., 2012; Ulrich et al., 2012).
P2X7 Receptors
P2X7 receptors share the least homology (35–40%) with other P2X receptors and have a number of important physiological functions that distinguish it from the others in its family (North, 2002; Sperlagh et al., 2006). They contain two hydrophobic regions that transverse the plasma membrane, with both the N- and C-terminus located intracellularly. The P2X7 receptor forms a homo-trimeric structure, with the bulk of the protein on the external membrane surface (Jiang et al., 2013), and appears to be the only P2X subunit unable to form a heteromeric complex (North, 2002). Endogenous ATP has a relatively low potency against P2X7 receptors, generally requiring concentrations 100 µM and above, compared to other P2X receptors, which are activated by ATP in the low micromolar range (Virginio et al., 1999). As an agonist, 2′,3′-O-(4-benzoyl-benzoyl) adenosine 5′-triphosphate (BzATP) is approximately 10 to 30 times more potent than ATP, and although it is not specific to P2X7 receptors, its higher affinity to P2X7 makes it a useful tool for examining the pharmacological activity of P2X7 receptors (Bianchi et al., 1999).
The P2X7 receptor was first described as the P2Z receptor, or the ‘cell death’ receptor, as its activation with high concentrations of ATP results in the opening of a pore that allows macromolecule exchange leading to cell death (Surprenant et al., 1996). They were first detected in the immune system on antigen presenting immune cells, where they are rapidly activated in response to inflammatory stimuli in immune cells, releasing pro-inflammatory mediators, including cytokines, such as interleukin (IL)-1β and tumour necrosis factor, from the cytosol as part of the host defence reactions (North, 2002; Tsukimoto et al., 2006). Activation of transcription factors is thought to be one method utilised by P2X7 receptors to affect downstream responses. In macrophages, stimulation of P2X7 with ATP resulted in activation of nuclear factor of activated T cells 1 and 2 (Ferrari et al., 1999), as well as nuclear factor κB (NFκB) (Ferrari et al., 1997). In neurons of the hippocampus, purinergic activation of NFκB through P2X7 receptors have been of interest due to its implications in the pathophysiology of neurological damage or trauma (Kim et al., 2013).
P2X7 receptors have since been identified in an ever-increasing number of cell types, from bone and muscle to neural and stem cell lineages, and have a number of non-immune functions, with distinct responses depending on exposure time and concentration. Brief activation results in cation influx for the purposes of neurotransmitter and signal transduction (Papp et al., 2004), while prolonged activation results in the formation of a large transmembrane pore permeable to molecules up to 900 Da. This leads to cytoskeletal rearrangement, transmembrane pore formation, and potentially apoptosis and/or necrosis (Delarasse et al., 2009). The latter has significant implications in pathophysiological events, where P2X7 receptors are rapidly activated in response to inflammatory stimuli, releasing pro-inflammatory mediators, such as IL-1β and tumour necrosis factor (Sperlagh and Illes, 2014). In the absence of ATP, P2X7 receptors have been demonstrated to facilitate phagocytosis in both the immune system and the nervous system (Wiley and Gu, 2012; Lovelace et al., 2015; Leeson et al., 2018). The diverse functions of P2X7 receptors and how they may regulate hippocampal neurogenesis are the focus of this review, and are summarised in Figure 1 and depicted in greater detail in Figure 2.
Figure 2.

Distinct signaling pathways of P2X7 receptors.
P2X7 receptors have at least three distinct roles in adult hippocampal neural progenitor cells, these being phagocytosis, transmembrane pore formation, and cation signaling. In the absence of adenosine triphosphate (ATP), the C terminus of P2X7 receptors interacts with the heavy chain of non-muscle myosin IIa (NMM IIa), and can facilitate phagocytosis by actin rearrangement. This interaction with NMM IIa dissociates in the presence of ATP. When extracellular ATP concentrations are high (in the millimolar range) P2X7 receptors form a large transmembrane pore which causes cell death by macromolecule flux and cytosolic calcium overload. In the presence of lower concentrations of ATP, a cation channel opens, allowing calcium into the cell. Calcium is a powerful and versatile signaling molecule and influx via P2X7 can lead to release of pro-inflammatory molecules (interleukin (IL)-1β and IL-6), as well as regulation of cell cycle events. Calcium induced calcium release from the endoplasmic reticulum may contribute to cytoplasmic calcium oscillations, which are translated by decoder proteins to effect biological changes. One mechanism of regulation is by calcium dependent transcription factors, such as nuclear factor κB (NFκB) and nuclear factor of activated T cells (NFATs), which can become activated following P2X7 signaling. P2X7 receptors have also been shown to act via protein kinase C (PKC) regulation, though more investigations are required to confirm the signaling mechanism involved in the regulation of proliferation and differentiation. CAMKII: Ca2+/calmodulin-dependent protein kinase II; MAPK: mitogen-activated protein kinases; IP3R: inositol 1,4,5-trisphosphate receptor.
P2X7 Receptors in the Adult Hippocampus
An early report based on in situ hybridization studies in the adult brain suggested that P2X7 receptor mRNA was restricted to the ependymal layer of the third ventricle and in activated microglia (Collo et al., 1997). P2X7 receptors were thus believed to be absent from neurons until later studies indicated their presence. Using in situ hybridization and electron microscopy, P2X7 receptors were localised to the excitatory terminals in the CA1, CA3 and the dentate gyrus, and implicated in the regulation of γ-aminobutyric acid (GABA) and glutamatergic signaling (Sperlagh et al., 2002). P2X7 receptor involvement in modulating GABA and glutamate release in the hippocampus has been further confirmed by immunohistochemistry and located mostly to pre-synaptic nerve terminals (Atkinson et al., 2004) and by glutamate and GABA release and uptake experiments in mice lacking the P2X7 receptor (Papp et al., 2004). Further, application of P2X7 agonists decreased GABA and glutamate uptake in nerve terminals by disrupting sodium gradients, and this was rescuable by P2X7 inhibition (Barros-Barbosa et al., 2015). Despite this evidence, some disagreement regarding neuronal expression of P2X7 receptors persists as is addressed in detail in recent dual perspective publications in the Journal of Neuroscience (Illes et al., 2017; Miras-Portugal et al., 2017).
Unfortunately, the abundance of literature dedicated to P2X7 receptor functions in the hippocampus does not currently extend to research involving P2X7 receptors in NPCs. So far, P2X7 has been reported at embryonic stages in the SVZ (embryo (E)15.5), and the SGZ (E18.5 and postnatal (P)4), with P2X7 receptor mRNA expressed in terminally differentiated neural cells (Tsao et al., 2013). The receptor has also been identified in embryonic progenitor cells derived from the striatum (Delarasse et al., 2009) and the developing human telencephalon (Lovelace et al., 2015) and in adult neural progenitors of the SVZ (Messemer et al., 2013). We recently reported the presence of P2X7 in adult hippocampal NPCs derived from mice and demonstrated that these receptors were functional as calcium channels, were capable of pore formation and in the absence of agonist stimulation could facilitate phagocytosis (Leeson et al., 2018). These findings confirm those by Hogg et al. (2004), who reported the application of ATP and BzATP evoked inward current and depolarisation, as well as transient cytosolic calcium increases in NPCs derived from the hippocampus of adult rats. Further, the authors demonstrate positive P2X7 immunochemistry in undifferentiated and differentiated NPCs (Hogg et al., 2004). In juvenile mouse hippocampal slice preparations, ATP and BzATP induced membrane currents in NPCs, granule cells, and astrocytes, but not GABAergic or glutamatergic interneurons of the hilus. These NPC currents were inhibited by the presence of P2X7 inhibitor A438079, and were not observed in slice preparations from P2X7 deficient mice (Rozmer et al., 2017).
P2X7 Regulation of Proliferation and Differentiation
P2X7 receptor activity has been shown to regulate proliferation and differentiation pathways, by promoting cell survival and proliferation, while inhibition may result in differentiation and axon growth. As P2X7 receptors are generally associated with cell death pathways, this presents an interesting and somewhat conflicting role for the receptor (Tang and Illes, 2017). Using mouse embryonic stem cells, Ulrich and colleagues recently observed accelerated cell cycle entry following BzATP application, and that inhibition of P2X7 by pharmacological antagonists increased differentiation, measured by the number of cells expressing markers of early neuronal phenotypes (Glaser et al., 2014). They also demonstrated that levels of P2X7 receptor mRNA and protein decrease with increasing differentiation, and suggest P2X7 receptors have a role in proliferation and differentiation. Contrary to this data, another study found P2X7 receptor activation caused a decrease in proliferation and enhanced the expression of neural markers in embryonic NPCs; this neuronal differentiation was regulated by the protein kinase C extracellular signal-regulated kinases 1/2 signaling pathway (Tsao et al., 2013). Our recent findings also support this conclusion; in adult hippocampal NPCs application of ATP and BzATP was found to reduce proliferation rates via P2X7 without inducing cell death, and this coincided with a small yet significant increase in the percentage of doublecortin positive cells (Leeson et al., 2018). This followed P2X7 receptor facilitated calcium influx, and subsequent cytosolic calcium oscillations contributed to by calcium-induced calcium release from the endoplasmic reticulum (unpublished findings). These observations lend themselves to the theory that activation of P2X7 receptors following an ischemic or cell death event may result in a decrease in proliferation of NPCs as they are pushed further towards a state of maturation, potentially assisting in the replacement of lost neurons.
One of the most important aspects of neurogenesis is the migration of the axon’s growth cone towards its target. By responding to positive and negative stimuli, the growth cone is able to establish correct neuronal circuits. Exposure of cultured hippocampal neurons to ATP inhibited axonal growth via P2X7 receptor-mediated calcium transients. In this study, inhibition or silencing of P2X7 receptors generated growth cone extension and longer and more branched axons (Diaz-Hernandez et al., 2008). This effect was also observed when the neuron culture was treated with alkaline phosphatase (Diez-Zaera et al., 2011). Neurite outgrowth was also reported in neuroblastoma cells in response to P2X7 inhibition (Wu et al., 2009). Together, these studies demonstrate the ambiguous roles P2X7 receptors can play, and further investigation of P2X7 receptor involvement in cell cycle control, proliferation and differentiation are required.
Cell Death and Phagocytosis During Adult Neurogenesis
Neurogenesis is marked by an overproduction of progenitor cells, which are selected for differentiation and integration into neuronal networks depending on the requirements of the brain. PCD is essential for controlling cell numbers in proliferative stages and for synaptic pruning and removal of immature neurons that fail to correctly integrate into the existing cytoarchitecture (Ryu et al., 2016). In the SGZ of the adult rat dentate gyrus, an estimated 9000 progenitor cells are produced each day (Cameron and McKay, 2001). Of these newly generated progenitor cells, around 50% will undergo PCD at a steady rate during the first four weeks (Dayer et al., 2003). Young neurons surviving 4 weeks make up about 6% of the total population of granule neurons in the hippocampus (West et al., 1991; Kempermann et al., 1998; Cameron and McKay, 2001). By five months most of these neurons have matured and become incorporated into existing circuitry where they function together with the rest of the granule cells formed during development (Dayer et al., 2003).
Sierra and colleagues observed that the majority of adult hippocampal neuroblasts underwent apoptosis one to four days after their initial division and that microglia rapidly phagocytosed the dead cells (Sierra et al., 2010). Apoptotic bodies are cleared in just a few hours, meaning the total amount of cell death is difficult to estimate, and not necessarily reflected by the number of pyknotic or TUNEL positive nuclei observed (Chung and Yu, 2013). In these situations, the apoptotic cells are progenitor cells still in the proliferation stages and have not yet matured into neurons capable of producing an axon to connect with a target, suggesting this cell death is target independent and is regulated by cell autonomous signals or region-specific signals.
Clearance of cell corpses following PCD is essential for maintaining homeostasis in the neurogenic niche, and dysregulation in phagocytosis results in the build-up of cellular debris leading to brain dysfunction (Fuchs and Steller, 2011). Microglia appear in the cerebrum during the second trimester of human gestation and once present act as the principal phagocyte of the CNS, playing a vital role in the maintenance of the adult hippocampus (Rezaie and Male, 1999; Sierra et al., 2010). Microglia play an important role in the removal of apoptotic neurons and also aid in the pruning of synapses during development (Kettenmann et al., 2011). The role of microglia goes beyond the phagocytosis of debris; they also have crucial roles in the reorganisation and repair of neural structures (Neumann et al., 2009). Resting microglia monitor their environment for changes in homeostasis caused by infection, injury or altered neuronal activity, which result in the release of microglial-activating and pro-inflammatory signals from the affected cells, including chemo-attractants, ATP, chemokines and growth factors. These ‘find me’ and ‘eat me’ signals are recognised by receptors on the surface of the microglia. In response, microglia are activated and migrate to the affected area, producing further pro-inflammatory mediators as they migrate (Wake et al., 2009; Kettenmann et al., 2011). Scavenger receptors on the surface of microglia play an important role in the initiation of phagocytosis. For example, P2X7 receptors can act as scavenger receptors in monocytes and macrophages in the absence of ATP (Wiley and Gu, 2012), and in primary microglial cultures, phagocytosis was also facilitated by P2X7 receptors (Fang et al., 2009).
Until recently, microglia were assumed to be solely responsible for clearance of apoptotic debris, though it has now been revealed that adult NPCs are capable of phagocytosing fluorescent beads (Leeson et al., 2018), as well as other apoptotic progenitors (Lu et al., 2011). During early-stage embryonic neurogenesis when microglia have not yet arisen from the developing parenchyma, the principal phagocyte appears to be the neuroepithelial cells themselves (Gu et al., 2015). It is reasonable to suspect these mechanisms of cell removal are retained into adulthood. Observations in both the SVZ and SGZ demonstrate that NPCs are capable of phagocytosing other apoptotic neural progenitors, and this mechanism, seen in doublecortin positive cells, was reliant on intracellular engulfment protein ELMO1, which promotes Rac1 activation and cytoskeletal rearrangements required for engulfment of apoptotic bodies (Lu et al., 2011). It was also recently demonstrated that human embryonic NPCs express P2X7 receptors and that these progenitors are able to phagocytose via a P2X7 mediated pathway (Lovelace et al., 2015). Resting astrocytes were likewise demonstrated to phagocytose via P2X7 receptors (Yamamoto et al., 2013). These data suggest microglial phagocytosis is not the only method used in the clearance of apoptotic NPCs, and that other mechanisms play important roles in the maintenance of the neurogenic niches.
P2X7 Receptors in Phagocytosis
In the absence of ATP, P2X7 receptors have been not only demonstrated to facilitate phagocytosis but can confer the ability to phagocytose. The phagocytic function of P2X7 receptors is mechanistically distinct from its canonical pore function; in addition to agonist presence inhibiting P2X7 phagocytic potential, polymorphic variants conveying changes in pore function do not necessarily alter phagocytic capability, nor is altered by P2X7 antagonists (Ou et al., 2018). P2X7 receptors expressed on the surface of macrophages are involved in the engulfment of latex beads, as well as both live and heat-killed bacteria in the absence of ATP and serum, while transfection of P2X7 receptors into HEK293 cells conferred the ability of HEK293 cells to phagocytose (Wiley and Gu, 2012). Within the CNS, the P2X7 membrane complex is thought to play an important role in innate immunity, as it can mediate the phagocytosis of non-opsonised particles including beads, bacteria and apoptotic neuronal cells (Wiley and Gu, 2012; Lovelace et al., 2015; Leeson et al., 2018).
P2X7 receptors have been shown to tightly associate with heavy chain IIA of the non-muscle myosin complex, a major cytoskeletal component and essential for internalising particles during phagocytosis (Gu et al., 2009). It was later discovered that an intact P2X7-nonmuscle myosin complex was required for phagocytosis, and that extracellular ATP causes the dissociation of the P2X7 complex from myosin IIA, resulting in inhibition of particle uptake (Gu et al., 2010). This suggests that in the absence of ATP and serum proteins, P2X7 receptors may have a function distinct to the inflammatory response, and can act as a scavenger receptor for bacteria, debris and apoptotic cells in the CNS.
It was recently demonstrated that during human embryonic development, NPCs express P2X7 receptors and that these neural progenitors and neuroblasts are able to phagocytose apoptotic ReNcells and apoptotic neuroblasts, as well as latex beads via a P2X7 mediated pathway (Lovelace et al., 2015). Presence of ATP, P2X7 antagonists or siRNA knockdown inhibited this phenomenon, suggesting that P2X7 can act as a scavenger receptor on neural progenitors within the developing human CNS (Lovelace et al., 2015). We have recently expanded on this discovery to show the P2X7 receptor can also facilitate phagocytosis in NPCs derived from the adult hippocampus (Leeson et al., 2018). This alternate function may allow P2X7 to act as a scavenger receptor in the nervous system, where the balance between proliferation and PCD plays a fundamental role in the maintenance of the adult brain.
Dysregulation of Hippocampal Neurogenesis
Adult hippocampal neurogenesis appears to play a role in the brain’s ability to recover from some types of physiological trauma (Jin et al., 2010). Following stroke, there is an increase in progenitor cell proliferation, and these new cells become functionally integrated into the existing hippocampus (Geibig et al., 2012). There is also substantial evidence that psychosocial stress, anxiety, and depression reduces neurogenesis in rodents via the release of stress-related hormones (Dranovsky and Hen, 2006). As an area of the adult brain that constantly remodels its synaptic connectivity in response to sense-data, it is not surprising that these events can also modulate neurogenesis. Chronic corticosterone treatment has been used as a model for anxiety and depression in mice, and inhibits hippocampal neurogenesis, while treatment with antidepressants, such as fluoxetine, reverses this effect (David et al., 2009). Epileptic seizures have been shown to cause abnormal hippocampal neurogenesis, with increased progenitor proliferation but aberrant integration, neuronal hypertrophy, and altered excitability. This may prevent the hippocampus from properly regulating excitatory activity and may prompt further seizures (Danzer, 2012). Inhibition of hippocampal neurogenesis prior to inducing acute seizures with pilocarpine reduced the cognitive impairment associated with epilepsy, and this led to reduced seizure frequency and long term suppression of spontaneous recurrent seizures. The authors concluded that abnormal neurogenesis not only results from but contributes to epileptic episodes (Cho et al., 2015). Whether or not epilepsy can be caused by altered or aberrant neurogenesis as the brain attempts repair following an initial injury remains a debated topic (Jessberger and Parent, 2015).
P2X7 Receptor Signaling in Inflammation and Disease
The canonical function of P2X7 receptors is to initiate pro-inflammatory responses, and in recent years the evidence implicating extracellular ATP and P2X7 receptors in pathophysiological mechanisms in the brain has rapidly expanded (Ulrich et al., 2012; Di Virgilio et al., 2017; Miras-Portugal et al., 2017). Cytosolic ATP has a relatively high concentration compared to the extracellular milieu and is released in large quantities when membrane integrity is compromised. High ATP concentrations induce the release of plasminogen, tumour necrosis factor-α (TNF-α), and IL-1β via P2X7 receptor-mediated pathways, a process that is regulated by mitogen-activated protein kinases, extracellular signal-regulated kinases and calcium-dependent signaling (Inoue, 2008; Fang et al., 2009). These cytokines and chemokines can act to exacerbate the inflammatory response. In high concentrations, extracellular ATP is neurotoxic and elevated levels strongly correlate with neurological conditions, such as acute spinal cord injury and ischemia, and degenerative diseases, such as Parkinson’s and Alzheimer’s disease (Parvathenani et al., 2003; Wang et al., 2004; Virgilio et al., 2009).
Excessive inflammation caused by high concentrations of ATP may be counterproductive to attempts to repair the acute damage, as the cytotoxic effects of these modulators impact on healthy tissue and exacerbate the initial injury (Fiebich et al., 2014). Higher than normal amounts of ATP released from necrotic cells following an ischemic event activates P2X7 receptors on the surface of both neurons and glial cells, allowing inward current and an overload of cytosolic calcium levels, leading to mitochondrial depolarisation, oxidative stress and cell death. In embryonic NPCs, prolonged ATP exposure resulted in membrane disruption and cell death via activation of the P2X7 receptor (Delarasse et al., 2009). Neuronal vulnerability to high concentrations of extracellular ATP was found to depend on P2X7 expression levels (Ohishi et al., 2016). These studies highlighted the effects of P2X7 receptor activation in cognitive dysfunction and traumatic or ischemic events, and their roles in the neurogenic niches is currently the focus of much interest (Engel et al., 2012; Yu et al., 2013; Liu et al., 2017b).
Subsequently, a number of recent studies demonstrating conferral of neuroprotection by modulation of P2X7 receptor activity have highlighted the therapeutic potential of targeting P2X7 receptors in cerebrovascular diseases (Sperlagh and Illes, 2014). These studies generally focus on ischemic and traumatic brain injury (Nadal-Nicolas et al., 2016; Liu et al., 2017a), epilepsy (Huang et al., 2017) and stroke (reviewed in Zhao et al., 2018), and consistently report that inhibition or blockade of P2X7 receptors decreases the cellular damage, provides neuro-protective qualities and improves functional recovery. Further, in a nerve crush model, pharmacological inhibitors of the P2X7 receptor improved the morphology of regenerating nerves (Ribeiro et al., 2017). Supporting these observations, Choi et al. (2007) showed inhibition of P2X7 receptors to decrease both pro-inflammatory mediators and NFκB activation, subsequently increasing neuronal survival rates in the striatum following lipopolysaccharide injection. These effects are often contributed to a maintained control of membrane potential and integrity, a reduced release of pro-inflammatory mediators such as IL-1β and IL-6 (Savio et al., 2017), and a reduced gliosis (Jimenez-Pacheco et al., 2016).
In mice with pilocarpine and kainic acid induced seizures, an increase in P2X7 receptor immunoreactivity and sensitivity was observed in hippocampal NPCs (Rozmer et al., 2017). Blocking the P2X7 receptor in this study prevented the neuronal degradation of CA3 pyramidal cells, though also caused an increase in the number and severity of subsequent spontaneous seizures. Status epilepticus (prolonged seizures) has also been found to increase levels of P2X7 in the granule neurons of the dentate gyrus, and that antagonising P2X7 receptors reduced both seizure duration and subsequent neuronal death (Engel et al., 2012). Similar observations were made in the CA1 area of the hippocampus, where P2X7 receptor inhibition reduced the amount of delayed neuronal death in ischemic injury (Yu et al., 2013). An increase in receptor expression was also observed.
Contrary to these findings, Kim et al. (2011) reported that activation of P2X7 receptors with BzATP decreased neuron damage following status epilepticus, while inhibition with oxATP or A438079 resulted in increased neuronal death in the CA3 region of the hippocampus. This effect was mediated by release of TNF-α and subsequent NFκB phosphorylation. A possible explanation for this ambiguity is different expression patterns of splice variants, most of which are truncated at the C-terminus and no longer have the ability to form transmembrane pores (Cheewatrakoolpong et al., 2005).
Glial cells are also heavily impacted by P2X7 receptor signaling during inflammatory events (Verkhratsky et al., 2012). Following an acute event, such as ischemia or trauma, as well as chronic neuropathies, such as multiple sclerosis, Parkinson’s and Alzheimer’s disease, expression of P2X7 receptors on the surface of microglia are often upregulated (Franke et al., 2004). This upregulation is also observed in oligodendrocytes, where ischemic damage is partly caused by glutamate toxicity and compounded by increases in extracellular ATP concentrations. The irreversible increase in cytosolic calcium concentrations severely damaged oligodendrocytes and myelin, and the ATP degrading enzyme apyrase and P2X7 receptor antagonists alleviated the damage caused by ischemia, as well as improving action potential recovery (Domercq et al., 2010). Together, these studies heavily implicate P2X7 receptor activity in processes of inflammatory cell death.
Recently P2X7 receptors have also emerged as a new target for depression and cognitive dysfunction studies (Liu et al., 2017b). Inflammation is a key pathophysiological mechanism contributing to neuropsychiatric disorders, and pro-inflammatory cytokines IL-6, IL-1β and TNF-α can mediate many of the psychological changes associated with depression. Chrysophanol, a traditional Chinese medicine with anti-inflammatory properties, was demonstrated to impart anti-depressant effects in lipopolysaccharide depression models, and reduced the expression of P2X7 receptors, as well as serum levels of IL-6, IL-1β and TNF-α (Zhang et al., 2016). The authors speculated that the antidepressant effect of Chrysophanol was mediated by a P2X7/NFκB signaling pathway. This hypothesis is supported by P2X7 receptor knock out mice that display antidepressant-like profiles in forced swim and tail suspension tests (Basso et al., 2009).
Conclusion
There are at least three distinct functions P2X7 receptors may play in the adult hippocampus, depending on the conditions present in the extracellular environment. The first is the initiation of cell death in the presence of inflammation and extracellular ATP. The second is calcium-mediated signal transduction in response to ATP signaling, which may, in turn, regulate biological functions, such as proliferation and differentiation. The third and non-canonical function of P2X7 receptors is to promote phagocytosis in the absence of extracellular ATP. These alternate facets of P2X7 signaling have somewhat juxtaposed outcomes in terms of function. Understanding these mechanisms is essential to addressing important questions that remain regarding neurogenesis and regeneration in the adult brain.
Footnotes
Conflicts of interest: The authors declare that they have no conflict of interest.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Yu J; T-Editor: Jia Y
References
- 1.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signaling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson L, Batten TF, Moores TS, Varoqui H, Erickson JD, Deuchars J. Differential co-localisation of the P2X7 receptor subunit with vesicular glutamate transporters VGLUT1 and VGLUT2 in rat CNS. Neuroscience. 2004;123:761–768. doi: 10.1016/j.neuroscience.2003.08.065. [DOI] [PubMed] [Google Scholar]
- 3.Barros-Barbosa AR, Lobo MG, Ferreirinha F, Correia-de-Sa P, Cordeiro JM. P2X7 receptor activation downmodulates Na(+)-dependent high-affinity GABA and glutamate transport into rat brain cortex synaptosomes. Neuroscience. 2015;306:74–90. doi: 10.1016/j.neuroscience.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 4.Basso AM, Bratcher NA, Harris RR, Jarvis MF, Decker MW, Rueter LE. Behavioral profile of P2X7 receptor knockout mice in animal models of depression and anxiety: relevance for neuropsychiatric disorders. Behav Brain Res. 2009;198:83–90. doi: 10.1016/j.bbr.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Berridge MJ. The AM and FM of calcium signaling. Nature. 1997;386:759–760. doi: 10.1038/386759a0. [DOI] [PubMed] [Google Scholar]
- 6.Berridge MJ, Bootman MD, Roderick HL. Calcium signaling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi BR, Lynch KJ, Touma E, Niforatos W, Burgard EC, Alexander KM, Park HS, Yu H, Metzger R, Kowaluk E, Jarvis MF, van Biesen T. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur J Pharmacol. 1999;376:127–138. doi: 10.1016/s0014-2999(99)00350-7. [DOI] [PubMed] [Google Scholar]
- 8.Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur J Neurosci. 2005;21:513–521. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- 9.Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- 10.Burnstock G. Introduction to purinergic signaling in the brain. Adv Exp Med Biol. 2013;986:1–12. doi: 10.1007/978-94-007-4719-7_1. [DOI] [PubMed] [Google Scholar]
- 11.Burnstock G. An introduction to the roles of purinergic signaling in neurodegeneration, neuroprotection and neuroregeneration. Neuropharmacology. 2016;104:4–17. doi: 10.1016/j.neuropharm.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 12.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 13.Cheewatrakoolpong B, Gilchrest H, Anthes JC, Greenfeder S. Identification and characterization of splice variants of the human P2X7 ATP channel. Biochem Biophys Res Commun. 2005;332:17–27. doi: 10.1016/j.bbrc.2005.04.087. [DOI] [PubMed] [Google Scholar]
- 14.Cho KO, Lybrand ZR, Ito N, Brulet R, Tafacory F, Zhang L, Good L, Ure K, Kernie SG, Birnbaum SG, Scharfman HE, Eisch AJ, Hsieh J. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat Commun. 2015;6:6606. doi: 10.1038/ncomms7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi HB, Ryu JK, Kim SU, McLarnon JG. Modulation of the purinergic P2X7 receptor attenuates lipopolysaccharide-mediated microglial activation and neuronal damage in inflamed brain. J Neurosci. 2007;27:4957–4968. doi: 10.1523/JNEUROSCI.5417-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung KM, Yu SW. Interplay between autophagy and programmed cell death in mammalian neural stem cells. BMB Rep. 2013;46:383–390. doi: 10.5483/BMBRep.2013.46.8.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collo G, Neidhart S, Kawashima E, Kosco-Vilbois M, North RA, Buell G. Tissue distribution of the P2X7 receptor. Neuropharmacology. 1997;36:1277–1283. doi: 10.1016/s0028-3908(97)00140-8. [DOI] [PubMed] [Google Scholar]
- 19.Danzer SC. Depression, stress, epilepsy and adult neurogenesis. Exp Neurol. 2012;233:22–32. doi: 10.1016/j.expneurol.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- 22.Delarasse C, Gonnord P, Galante M, Auger R, Daniel H, Motta I, Kanellopoulos JM. Neural progenitor cell death is induced by extracellular ATP via ligation of P2X7 receptor. J Neurochem. 2009;109:846–857. doi: 10.1111/j.1471-4159.2009.06008.x. [DOI] [PubMed] [Google Scholar]
- 23.Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 receptor in infection and inflammation. Immunity. 2017;47:15–31. doi: 10.1016/j.immuni.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 24.Diaz-Hernandez M, del Puerto A, Diaz-Hernandez JI, Diez-Zaera M, Lucas JJ, Garrido JJ, Miras-Portugal MT. Inhibition of the ATP-gated P2X7 receptor promotes axonal growth and branching in cultured hippocampal neurons. J Cell Sci. 2008;121:3717–3728. doi: 10.1242/jcs.034082. [DOI] [PubMed] [Google Scholar]
- 25.Diez-Zaera M, Diaz-Hernandez JI, Hernandez-Alvarez E, Zimmermann H, Diaz-Hernandez M, Miras-Portugal MT. Tissue-nonspecific alkaline phosphatase promotes axonal growth of hippocampal neurons. Mol Biol Cell. 2011;22:1014–1024. doi: 10.1091/mbc.E10-09-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Domercq M, Perez-Samartin A, Aparicio D, Alberdi E, Pampliega O, Matute C. P2X7 receptors mediate ischemic damage to oligodendrocytes. Glia. 2010;58:730–740. doi: 10.1002/glia.20958. [DOI] [PubMed] [Google Scholar]
- 27.Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehninger D, Kempermann G. Neurogenesis in the adult hippocampus. Cell Tissue Res. 2008;331:243–250. doi: 10.1007/s00441-007-0478-3. [DOI] [PubMed] [Google Scholar]
- 29.Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci U S A. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engel T, Gomez-Villafuertes R, Tanaka K, Mesuret G, Sanz-Rodriguez A, Garcia-Huerta P, Miras-Portugal MT, Henshall DC, Diaz-Hernandez M. Seizure suppression and neuroprotection by targeting the purinergic P2X7 receptor during status epilepticus in mice. FASEB J. 2012;26:1616–1628. doi: 10.1096/fj.11-196089. [DOI] [PubMed] [Google Scholar]
- 31.Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisen J. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156:1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 32.Fang KM, Yang CS, Sun SH, Tzeng SF. Microglial phagocytosis attenuated by short-term exposure to exogenous ATP through P2X receptor action. J Neurochem. 2009;111:1225–1237. doi: 10.1111/j.1471-4159.2009.06409.x. [DOI] [PubMed] [Google Scholar]
- 33.Ferrari D, Stroh C, Schulze-Osthoff K. P2X7/P2Z purinoreceptor-mediated activation of transcription factor NFAT in microglial cells. J Biol Chem. 1999;274:13205–13210. doi: 10.1074/jbc.274.19.13205. [DOI] [PubMed] [Google Scholar]
- 34.Ferrari D, Wesselborg S, Bauer MK, Schulze-Osthoff K. Extracellular ATP activates transcription factor NF-kappaB through the P2Z purinoreceptor by selectively targeting NF-kappaB p65. J Cell Biol. 1997;139:1635–1643. doi: 10.1083/jcb.139.7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiebich BL, Akter S, Akundi RS. The two-hit hypothesis for neuroinflammation: role of exogenous ATP in modulating inflammation in the brain. Front Cell Neurosci. 2014;8:260. doi: 10.3389/fncel.2014.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Firth J, Stubbs B, Vancampfort D, Schuch F, Lagopoulos J, Rosenbaum S, Ward PB. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. Neuroimage. 2018;166:230–238. doi: 10.1016/j.neuroimage.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Franke H, Gunther A, Grosche J, Schmidt R, Rossner S, Reinhardt R, Faber-Zuschratter H, Schneider D, Illes P. P2X7 receptor expression after ischemia in the cerebral cortex of rats. J Neuropathol Exp Neurol. 2004;63:686–699. doi: 10.1093/jnen/63.7.686. [DOI] [PubMed] [Google Scholar]
- 38.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geibig CS, Keiner S, Redecker C. Functional recruitment of newborn hippocampal neurons after experimental stroke. Neurobiol Dis. 2012;46:431–439. doi: 10.1016/j.nbd.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Glaser T, Cappellari AR, Pillat MM, Iser IC, Wink MR, Battastini AM, Ulrich H. Perspectives of purinergic signaling in stem cell differentiation and tissue regeneration. Purinergic Signal. 2012;8:523–537. doi: 10.1007/s11302-011-9282-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glaser T, de Oliveira SL, Cheffer A, Beco R, Martins P, Fornazari M, Lameu C, Junior HM, Coutinho-Silva R, Ulrich H. Modulation of mouse embryonic stem cell proliferation and neural differentiation by the P2X7 receptor. PLoS One. 2014;9:e96281. doi: 10.1371/journal.pone.0096281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goritz C, Frisén J. Neural stem cells and neurogenesis in the adult. Cell Stem Cell. 2012;10:657–659. doi: 10.1016/j.stem.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Götz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- 45.Grimm I, Ullsperger SN, Zimmermann H. Nucleotides and epidermal growth factor induce parallel cytoskeletal rearrangements and migration in cultured adult murine neural stem cells. Acta Physiol (Oxf) 2010;199:181–189. doi: 10.1111/j.1748-1716.2010.02092.x. [DOI] [PubMed] [Google Scholar]
- 46.Gu BJ, Saunders BM, Jursik C, Wiley JS. The P2X7-nonmuscle myosin membrane complex regulates phagocytosis of nonopsonized particles and bacteria by a pathway attenuated by extracellular ATP. Blood. 2010;115:1621–1631. doi: 10.1182/blood-2009-11-251744. [DOI] [PubMed] [Google Scholar]
- 47.Gu BJ, Rathsam C, Stokes L, McGeachie AB, Wiley JS. Extracellular ATP dissociates nonmuscle myosin from P2X(7) complex: this dissociation regulates P2X(7) pore formation. Am J Physiol Cell Physiol. 2009;297:C430–439. doi: 10.1152/ajpcell.00079.2009. [DOI] [PubMed] [Google Scholar]
- 48.Gu BJ, Lovelace MD, Weible MW, 2nd, Allen DG, Eamegdool SS, Chan-Ling T, Wiley JS. P2X7 is an archaic scavenger receptor recognizing apoptotic neuroblasts in early human neurogenesis. Receptor Clin Invest. 2015;2:e699. [Google Scholar]
- 49.Hogg RC, Chipperfield H, Whyte KA, Stafford MR, Hansen MA, Cool SM, Nurcombe V, Adams DJ. Functional maturation of isolated neural progenitor cells from the adult rat hippocampus. Eur J Neurosci. 2004;19:2410–2420. doi: 10.1111/j.0953-816X.2004.03346.x. [DOI] [PubMed] [Google Scholar]
- 50.Huang C, Chi XS, Li R, Hu X, Xu HX, Li JM, Zhou D. Inhibition of P2X7 receptor ameliorates nuclear factor-kappa B mediated neuroinflammation induced by status epilepticus in rat hippocampus. J Mol Neurosci. 2017;63:173–184. doi: 10.1007/s12031-017-0968-z. [DOI] [PubMed] [Google Scholar]
- 51.Hueston CM, Cryan JF, Nolan YM. Stress and adolescent hippocampal neurogenesis: diet and exercise as cognitive modulators. Transl Psychiatry. 2017;7:e1081. doi: 10.1038/tp.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Illes P, Khan TM, Rubini P. Neuronal P2X7 receptors revisited: do they really exist? J Neurosci. 2017;37:7049–7062. doi: 10.1523/JNEUROSCI.3103-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inoue K. Purinergic systems in microglia. Cell Mol Life Sci. 2008;65:3074–3080. doi: 10.1007/s00018-008-8210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iso H, Simoda S, Matsuyama T. Environmental change during postnatal development alters behaviour, cognitions and neurogenesis of mice. Behav Brain Res. 2007;179:90–98. doi: 10.1016/j.bbr.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 55.Jessberger S, Parent JM. Epilepsy and Adult Neurogenesis. Cold Spring Harb Perspect Biol. 2015;7:a020677. doi: 10.1101/cshperspect.a020677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jhaveri DJ, Tedoldi A, Hunt S, Sullivan R, Watts NR, Power JM, Bartlett PF, Sah P. Evidence for newly generated interneurons in the basolateral amygdala of adult mice. Mol Psychiatry. 2018;23:521–532. doi: 10.1038/mp.2017.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang LH, Baldwin JM, Roger S, Baldwin SA. Insights into the molecular mechanisms underlying mammalian P2X7 receptor functions and contributions in diseases, revealed by structural modeling and single nucleotide polymorphisms. Front Pharmacol. 2013;4:55. doi: 10.3389/fphar.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jimenez-Pacheco A, Diaz-Hernandez M, Arribas-Blazquez M, Sanz-Rodriguez A, Olivos-Ore LA, Artalejo AR, Alves M, Letavic M, Miras-Portugal MT, Conroy RM, Delanty N, Farrell MA, O’Brien DF, Bhattacharya A, Engel T, Henshall DC. Transient P2X7 receptor antagonism produces lasting reductions in spontaneous seizures and gliosis in experimental temporal lobe epilepsy. J Neurosci. 2016;36:5920–5932. doi: 10.1523/JNEUROSCI.4009-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jin K, Wang X, Xie L, Mao XO, Greenberg DA. Transgenic ablation of doublecortin-expressing cells suppresses adult neurogenesis and worsens stroke outcome in mice. Proc Natl Acad Sci U S A. 2010;107:7993–7998. doi: 10.1073/pnas.1000154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaczmarek-Hajek K, Lorinczi E, Hausmann R, Nicke A. Molecular and functional properties of P2X receptors--recent progress and persisting challenges. Purinergic Signal. 2012;8:375–417. doi: 10.1007/s11302-012-9314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang E, Wen Z, Song H, Christian KM, Ming GL. Adult neurogenesis and psychiatric disorders. Cold Spring Harb Perspect Biol. 2016;8:a019026. doi: 10.1101/cshperspect.a019026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kempermann G, Gage FH. Genetic determinants of adult hippocampal neurogenesis correlate with acquisition, but not probe trial performance, in the water maze task. Eur J Neurosci. 2002;16:129–136. doi: 10.1046/j.1460-9568.2002.02042.x. [DOI] [PubMed] [Google Scholar]
- 63.Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- 65.Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 66.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 67.Kim JE, Ryu HJ, Kang TC. P2X7 receptor activation ameliorates CA3 neuronal damage via a tumor necrosis factor-alpha-mediated pathway in the rat hippocampus following status epilepticus. J Neuroinflammation. 2011;8:62. doi: 10.1186/1742-2094-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim JE, Kim DS, Jin Ryu H, Il Kim W, Kim MJ, Won Kim D, Young Choi S, Kang TC. The effect of P2X7 receptor activation on nuclear factor-kappaB phosphorylation induced by status epilepticus in the rat hippocampus. Hippocampus. 2013;23:500–514. doi: 10.1002/hipo.22109. [DOI] [PubMed] [Google Scholar]
- 69.Leeson HC, Kasherman MA, Chan-Ling T, Lovelace MD, Brownlie JC, Toppinen KM, Gu BJ, Weible MW., 2nd P2X7 receptors regulate phagocytosis and proliferation in adult hippocampal and SVZ neural progenitor cells: implications for inflammation in neurogenesis. Stem Cells. 2018;36:1764–1777. doi: 10.1002/stem.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Y, Mu Y, Gage FH. Development of neural circuits in the adult hippocampus. Curr Top Dev Biol. 2009;87:149–174. doi: 10.1016/S0070-2153(09)01205-8. [DOI] [PubMed] [Google Scholar]
- 71.Liu X, Zhao Z, Ji R, Zhu J, Sui QQ, Knight GE, Burnstock G, He C, Yuan H, Xiang Z. Inhibition of P2X7 receptors improves outcomes after traumatic brain injury in rats. Purinergic Signal. 2017a;13:529–544. doi: 10.1007/s11302-017-9579-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Y, Chen GQ, Liu BY, Chen Q, Qian YM, Qin SS, Liu CL, Xu CS. P2X7 receptor in the hippocampus is involved in gp120-induced cognitive dysfunction. Genet Mol Res. 2017b;16 doi: 10.4238/gmr16019356. [DOI] [PubMed] [Google Scholar]
- 73.Lovelace MD, Gu BJ, Eamegdool SS, Weible MW, 2nd, Wiley JS, Allen DG, Chan-Ling T. P2X7 receptors mediate innate phagocytosis by human neural precursor cells and neuroblasts. Stem Cells. 2015;33:526–541. doi: 10.1002/stem.1864. [DOI] [PubMed] [Google Scholar]
- 74.Lu Z, Elliott MR, Chen Y, Walsh JT, Klibanov AL, Ravichandran KS, Kipnis J. Phagocytic activity of neuronal progenitors regulates adult neurogenesis. Nat Cell Biol. 2011;13:1076–1083. doi: 10.1038/ncb2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ma CL, Ma XT, Wang JJ, Liu H, Chen YF, Yang Y. Physical exercise induces hippocampal neurogenesis and prevents cognitive decline. Behav Brain Res. 2017;317:332–339. doi: 10.1016/j.bbr.2016.09.067. [DOI] [PubMed] [Google Scholar]
- 76.McEown K, Treit D. Alpha2 GABAA receptor sub-units in the ventral hippocampus and alpha5 GABAA receptor sub-units in the dorsal hippocampus mediate anxiety and fear memory. Neuroscience. 2013;252:169–177. doi: 10.1016/j.neuroscience.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 77.Merkle FT, Alvarez-Buylla A. Neural stem cells in mammalian development. Curr Opin Cell Biol. 2006;18:704–709. doi: 10.1016/j.ceb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 78.Messemer N, Kunert C, Grohmann M, Sobottka H, Nieber K, Zimmermann H, Franke H, Norenberg W, Straub I, Schaefer M, Riedel T, Illes P, Rubini P. P2X7 receptors at adult neural progenitor cells of the mouse subventricular zone. Neuropharmacology. 2013;73:122–137. doi: 10.1016/j.neuropharm.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 79.Ming GL, Song HJ. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 80.Ming GL, Song HJ. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miras-Portugal MT, Sebastian-Serrano A, de Diego Garcia L, Diaz-Hernandez M. Neuronal P2X7 receptor: involvement in neuronal physiology and pathology. J Neurosci. 2017;37:7063–7072. doi: 10.1523/JNEUROSCI.3104-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nadal-Nicolas FM, Galindo-Romero C, Valiente-Soriano FJ, Barbera-Cremades M, deTorre-Minguela C, Salinas-Navarro M, Pelegrin P, Agudo-Barriuso M. Involvement of P2X7 receptor in neuronal degeneration triggered by traumatic injury. Sci Rep. 2016;6:38499. doi: 10.1038/srep38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132:288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nokia MS, Lensu S, Ahtiainen JP, Johansson PP, Koch LG, Britton SL, Kainulainen H. Physical exercise increases adult hippocampal neurogenesis in male rats provided it is aerobic and sustained. J Physiol. 2016;594:1855–1873. doi: 10.1113/JP271552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 86.Ohishi A, Keno Y, Marumiya A, Sudo Y, Uda Y, Matsuda K, Morita Y, Furuta T, Nishida K, Nagasawa K. Expression level of P2X7 receptor is a determinant of ATP-induced death of mouse cultured neurons. Neuroscience. 2016;319:35–45. doi: 10.1016/j.neuroscience.2016.01.048. [DOI] [PubMed] [Google Scholar]
- 87.Oliveira SL, Pillat MM, Cheffer A, Lameu C, Schwindt TT, Ulrich H. Functions of neurotrophins and growth factors in neurogenesis and brain repair. Cytometry A. 2013;83:76–89. doi: 10.1002/cyto.a.22161. [DOI] [PubMed] [Google Scholar]
- 88.Ou A, Gu BJ, Wiley JS. The scavenger activity of the human P2X7 receptor differs from P2X7 pore function by insensitivity to antagonists, genetic variation and sodium concentration: Relevance to inflammatory brain diseases. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1051–1059. doi: 10.1016/j.bbadis.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 89.Papp L, Vizi ES, Sperlagh B. Lack of ATP-evoked GABA and glutamate release in the hippocampus of P2X7 receptor-/- mice. Neuroreport. 2004;15:2387–2391. doi: 10.1097/00001756-200410250-00017. [DOI] [PubMed] [Google Scholar]
- 90.Parvathenani LK, Tertyshnikova S, Greco CR, Roberts SB, Robertson B, Posmantur R. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer’s disease. J Biol Chem. 2003;278:13309–13317. doi: 10.1074/jbc.M209478200. [DOI] [PubMed] [Google Scholar]
- 91.Rezaie P, Male D. Colonisation of the developing human brain and spinal cord by microglia: a review. Microsc Res Tech. 1999;45:359–382. doi: 10.1002/(SICI)1097-0029(19990615)45:6<359::AID-JEMT4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 92.Ribeiro T, Oliveira JT, Almeida FM, Tomaz MA, Melo PA, Marques SA, de Andrade GM, Martinez AMB. Blockade of ATP P2X7 receptor enhances ischiatic nerve regeneration in mice following a crush injury. Brain Res. 2017;1669:69–78. doi: 10.1016/j.brainres.2017.05.025. [DOI] [PubMed] [Google Scholar]
- 93.Rozmer K, Gao P, Araujo MG, Khan MT, Liu J, Rong W, Tang Y, Franke H, Krugel U, Fernandes MJ, Illes P. Pilocarpine-induced status epilepticus increases the sensitivity of P2X7 and P2Y1 receptors to nucleotides at neural progenitor cells of the juvenile rodent hippocampus. Cereb Cortex. 2017;27:3568–3585. doi: 10.1093/cercor/bhw178. [DOI] [PubMed] [Google Scholar]
- 94.Ruitenberg MJ, Wells J, Bartlett PF, Harvey AR, Vukovic J. Enrichment increases hippocampal neurogenesis independent of blood monocyte-derived microglia presence following high-dose total body irradiation. Brain Res Bull. 2017;132:150–159. doi: 10.1016/j.brainresbull.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 95.Ryu JR, Hong CJ, Kim JY, Kim EK, Sun W, Yu SW. Control of adult neurogenesis by programmed cell death in the mammalian brain. Mol Brain. 2016;9:43. doi: 10.1186/s13041-016-0224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 98.Savio LEB, Andrade MGJ, de Andrade Mello P, Santana PT, Moreira-Souza ACA, Kolling J, Longoni A, Feldbrugge L, Wu Y, Wyse ATS, Robson SC, Coutinho-Silva R. P2X7 receptor signaling contributes to sepsis-associated brain dysfunction. Mol Neurobiol. 2017;54:6459–6470. doi: 10.1007/s12035-016-0168-9. [DOI] [PubMed] [Google Scholar]
- 99.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 100.Schoenfeld TJ, McCausland HC, Morris HD, Padmanaban V, Cameron HA. Stress and loss of adult neurogenesis differentially reduce hippocampal volume. Biol Psychiatry. 2017;82:914–923. doi: 10.1016/j.biopsych.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Seo DO, Carillo MA, Chih-Hsiung Lim S, Tanaka KF, Drew MR. Adult hippocampal neurogenesis modulates fear learning through associative and nonassociative mechanisms. J Neurosci. 2015;35:11330–11345. doi: 10.1523/JNEUROSCI.0483-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Smedler E, Uhlen P. Frequency decoding of calcium oscillations. Biochim Biophys Acta. 2014;1840:964–969. doi: 10.1016/j.bbagen.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 104.Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci. 2002;5:438–445. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- 105.Southwell DG, Paredes MF, Galvao RP, Jones DL, Froemke RC, Sebe JY, Alfaro-Cervello C, Tang Y, Garcia-Verdugo JM, Rubenstein JL, Baraban SC, Alvarez-Buylla A. Intrinsically determined cell death of developing cortical interneurons. Nature. 2012;491:109–113. doi: 10.1038/nature11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H, Frisen J. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sperlagh B, Illes P. P2X7 receptor: an emerging target in central nervous system diseases. Trends Pharmacol Sci. 2014;35:537–547. doi: 10.1016/j.tips.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 108.Sperlagh B, Vizi ES, Wirkner K, Illes P. P2X7 receptors in the nervous system. Prog Neurobiol. 2006;78:327–346. doi: 10.1016/j.pneurobio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 109.Sperlagh B, Kofalvi A, Deuchars J, Atkinson L, Milligan CJ, Buckley NJ, Vizi ES. Involvement of P2X7 receptors in the regulation of neurotransmitter release in the rat hippocampus. J Neurochem. 2002;81:1196–1211. doi: 10.1046/j.1471-4159.2002.00920.x. [DOI] [PubMed] [Google Scholar]
- 110.Steiner B, Zurborg S, Horster H, Fabel K, Kempermann G. Differential 24 h responsiveness of Prox1-expressing precursor cells in adult hippocampal neurogenesis to physical activity, environmental enrichment, and kainic acid-induced seizures. Neuroscience. 2008;154:521–529. doi: 10.1016/j.neuroscience.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 111.Sun L, Sun Q, Qi J. Adult hippocampal neurogenesis: an important target associated with antidepressant effects of exercise. Rev Neurosci. 2017;28:693–703. doi: 10.1515/revneuro-2016-0076. [DOI] [PubMed] [Google Scholar]
- 112.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 113.Tang Y, Illes P. Regulation of adult neural progenitor cell functions by purinergic signaling. Glia. 2017;65:213–230. doi: 10.1002/glia.23056. [DOI] [PubMed] [Google Scholar]
- 114.Tonelli FM, Santos AK, Gomes DA, da Silva SL, Gomes KN, Ladeira LO, Resende RR. Stem cells and calcium signaling. Adv Exp Med Biol. 2012;740:891–916. doi: 10.1007/978-94-007-2888-2_40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17:11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 116.Tsao HK, Chiu PH, Sun SH. PKC-dependent ERK phosphorylation is essential for P2X7 receptor-mediated neuronal differentiation of neural progenitor cells. Cell Death Dis. 2013;4:e751. doi: 10.1038/cddis.2013.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tsukimoto M, Maehata M, Harada H, Ikari A, Takagi K, Degawa M. P2X7 receptor-dependent cell death is modulated during murine T cell maturation and mediated by dual signaling pathways. J Immunol. 2006;177:2842–2850. doi: 10.4049/jimmunol.177.5.2842. [DOI] [PubMed] [Google Scholar]
- 118.Ulrich H, Abbracchio MP, Burnstock G. Extrinsic purinergic regulation of neural stem/progenitor cells: implications for CNS development and repair. Stem Cell Rev. 2012;8:755–767. doi: 10.1007/s12015-012-9372-9. [DOI] [PubMed] [Google Scholar]
- 119.van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular Med. 2008;10:128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- 120.Verkhratsky A, Pankratov Y, Lalo U, Nedergaard M. P2X receptors in neuroglia. Wiley Interdiscip Rev Membr Transp Signal. 2012;1 doi: 10.1002/wmts.12. 10.1002/wmts.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 122.Virgilio F, Ceruti S, Bramanti P, Abbracchio MP. Purinergic signaling in inflammation of the central nervous system. Trends Neurosci. 2009;32:79–87. doi: 10.1016/j.tins.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 123.Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A. Pore dilation of neuronal P2X receptor channels. Nat Neurosci. 1999;2:315–321. doi: 10.1038/7225. [DOI] [PubMed] [Google Scholar]
- 124.von Allmen DY, Wurmitzer K, Martin E, Klaver P. Neural activity in the hippocampus predicts individual visual short-term memory capacity. Hippocampus. 2013;23:606–615. doi: 10.1002/hipo.22121. [DOI] [PubMed] [Google Scholar]
- 125.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, Li P, Xu Q, Liu QS, Goldman SA, Nedergaard M. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10:821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- 127.West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 128.Wiley JS, Gu BJ. A new role for the P2X7 receptor: a scavenger receptor for bacteria and apoptotic cells in the absence of serum and extracellular ATP. Purinergic Signal. 2012;8:579–586. doi: 10.1007/s11302-012-9308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wu PY, Lin YC, Chang CL, Lu HT, Chin CH, Hsu TT, Chu D, Sun SH. Functional decreases in P2X7 receptors are associated with retinoic acid-induced neuronal differentiation of Neuro-2a neuroblastoma cells. Cell Signal. 2009;21:881–891. doi: 10.1016/j.cellsig.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 130.Yamamoto M, Kamatsuka Y, Ohishi A, Nishida K, Nagasawa K. P2X7 receptors regulate engulfing activity of non-stimulated resting astrocytes. Biochem Biophys Res Commun. 2013;439:90–95. doi: 10.1016/j.bbrc.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 131.Yau SY, Li A, So KF. Involvement of adult hippocampal neurogenesis in learning and forgetting. Neural Plast. 2015;2015:717958. doi: 10.1155/2015/717958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yu Q, Guo Z, Liu X, Ouyang Q, He C, Burnstock G, Yuan H, Xiang Z. Block of P2X7 receptors could partly reverse the delayed neuronal death in area CA1 of the hippocampus after transient global cerebral ischemia. Purinergic Signal. 2013;9:663–675. doi: 10.1007/s11302-013-9379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zang J, Liu Y, Li W, Xiao D, Zhang Y, Luo Y, Liang W, Liu F, Wei W. Voluntary exercise increases adult hippocampal neurogenesis by increasing GSK-3beta activity in mice. Neuroscience. 2017;354:122–135. doi: 10.1016/j.neuroscience.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 134.Zhang K, Liu J, You X, Kong P, Song Y, Cao L, Yang S, Wang W, Fu Q, Ma Z. P2X7 as a new target for chrysophanol to treat lipopolysaccharide-induced depression in mice. Neurosci Lett. 2016;613:60–65. doi: 10.1016/j.neulet.2015.12.043. [DOI] [PubMed] [Google Scholar]
- 135.Zhao H, Chen Y, Feng H. P2X7 receptor-associated programmed cell death in the pathophysiology of hemorrhagic stroke. Curr Neuropharmacol. 2018;16:1282–1295. doi: 10.2174/1570159X16666180516094500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zheng J, Jiang YY, Xu LC, Ma LY, Liu FY, Cui S, Cai J, Liao FF, Wan Y, Yi M. Adult hippocampal neurogenesis along the dorsoventral axis contributes differentially to environmental enrichment combined with voluntary exercise in alleviating chronic inflammatory pain in mice. J Neurosci. 2017;37:4145–4157. doi: 10.1523/JNEUROSCI.3333-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]


