Keywords: neural regeneration, Nogo-66 receptor, Nogo66 receptor-Fc protein, neural progenitor cells, proliferation, differentiation, stroke, photothrombotic cortical injury, transplantation, neurological function, nerve regeneration
Abstract
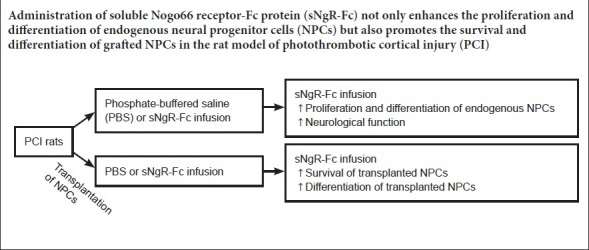
Soluble Nogo66 receptor-Fc protein (sNgR-Fc) enhances axonal regeneration following central nervous system injury. However, the underlying mechanisms remain unclear. In this study, we investigated the effects of sNgR-Fc on the proliferation and differentiation of neural progenitor cells. The photothrombotic cortical injury model of ischemic stroke was produced in the parietal cortex of Sprague-Dawley rats. The rats with photothrombotic cortical injury were randomized to receive infusion of 400 μg/kg sNgR-Fc (sNgR-Fc group) or an equal volume of phosphate-buffered saline (photothrombotic cortical injury group) into the lateral ventricle for 3 days. The effects of sNgR-Fc on the proliferation and differentiation of endogenous neural progenitor cells were examined using BrdU staining. Neurological function was evaluated with the Morris water maze test. To further examine the effects of sNgR-Fc treatment on neural progenitor cells, photothrombotic cortical injury was produced in another group of rats that received transplantation of neural progenitor cells from the hippocampus of embryonic Sprague-Dawley rats. The animals were then given an infusion of phosphate-buffered saline (neural progenitor cells group) or sNgR-Fc (sNgR-Fc + neural progenitor cells group) into the lateral ventricle for 3 days. sNgR-Fc enhanced the proliferation of cultured neural progenitor cells in vitro as well as that of endogenous neural progenitor cells in vivo, compared with phosphate-buffered saline, and it also induced the differentiation of neural progenitor cells into neurons. Compared with the photothrombotic cortical injury group, escape latency in the Morris water maze and neurological severity score were greatly reduced, and distance traveled in the target quadrant was considerably increased in the sNgR-Fc group, indicating a substantial improvement in neurological function. Furthermore, compared with phosphate-buffered saline infusion, sNgR-Fc infusion strikingly improved the survival and differentiation of grafted neural progenitor cells. Our findings show that sNgR-Fc regulates neural progenitor cell proliferation, migration and differentiation. Therefore, sNgR-Fc is a potential novel therapy for stroke and neurodegenerative diseases, The protocols were approved by the Committee on the Use of Live Animals in Teaching and Research of the University of Hong Kong (approval No. 4560-17) in November, 2015.
Chinese Library Classification No. R453; R364
Introduction
Ischemic stroke is a leading cause of morbidity and mortality worldwide, especially in the aging population, and has attracted substantial economic and public health concern (Chang et al., 2018; Faulkner and Wright, 2018; Feng et al., 2018). Despite the development of neuroprotective drugs over the past few decades, most therapeutic interventions have shown limited effectiveness in clinical application, and no definitive strategy is currently available (Minnerup et al., 2014; Zhang et al., 2018). More effective treatments for ischemic stroke are therefore urgently needed.
Ischemic stroke is usually caused by reduced blood supply to the brain, with consequent brain dysfunction. Recovery of specific functions and improvement in activity are dependent on intrinsic changes in existing neurons or the generation of new neurons from neural progenitor cells (NPCs) (Lindvall and Kokaia, 2010). In mammals, NPCs are located in both the subventricular zone and dentate subgranular zone in the adult brain (Zhang et al., 2017, 2018). Indeed, new neurons differentiated from endogenous NPCs greatly alleviate ischemic brain injury (Nakatomi et al., 2002; Ludwig et al., 2018). However, the number of endogenous newborn neurons is insufficient to restore neurological function following stroke (Arvidsson et al., 2002; Kim et al., 2014). Therefore, the transplantation of stem cells may be an effective treatment strategy for stroke (Li et al., 2016; Ryu et al., 2016; Chau et al., 2017; Frid et al., 2018; Mangin and Kubis, 2018; Webb et al., 2018). Accumulating evidence shows that the transplantation of NPCs, including induced pluripotent stem cell-derived NPCs, can enhance structural plasticity and improve functional recovery following ischemia-induced brain injury in both mice and rats (Mine et al., 2013; Chau et al., 2017; Tseng et al., 2018). However, the hostile microenvironment created by the ischemic event is a major obstacle for the survival of transplanted NPCs (Hicks et al., 2009; Wang et al., 2014). Therefore, enhancing the survival of transplanted NPCs in the ischemic region is a major research goal.
The neuronal Nogo66 receptor (NgR1) inhibits axonal regeneration in the presence of its myelin protein ligands (Nogo66, MAG, OMgp) in the central nervous system (Schwab and Strittmatter, 2014). In addition to neurons, NgR1 is also expressed in NPCs derived from the spinal cord of rats (Li et al., 2011), suggesting that it may play a functional role in NPCs. Accumulating evidence shows that soluble NgR-Fc fusion protein (sNgR-Fc), which acts as an NgR1 antagonist, effectively disrupts the interaction of myelin molecules with NgR1 and enhances axon sprouting and functional recovery following central nervous system injury. This indicates that sNgR-Fc promotes neuronal repair and regeneration (Li et al., 2011; Fuentealba et al., 2012; Mi et al., 2013). However, it remains unknown whether NgR1 is involved in the regulation of NPC activity or whether sNgR-Fc treatment modulates the therapeutic effects of NPCs in stroke. Therefore, in this study, we investigated the effects of sNgR-Fc on the proliferation and differentiation of NPCs in vitro and in vivo.
Materials and Methods
Animals
Pregnant (embryonic day 16 (E16)) adult green fluorescent protein (GFP)-transgenic Sprague-Dawley rats [“green rat CZ-004” SD TgN (act-EGFP) OsbCZ-004] were purchased from the Experimental Animal Center of the University of Hong Kong, China (Cap. 340). Thirty-six healthy adult male Sprague-Dawley rats, weighing 250–350 g and 10–12 weeks of age, were also purchased from the Experimental Animal Center of the University of Hong Kong, China. One batch of rats was divided into three groups: sham group (n = 6), phosphate-buffered saline (PBS) group (n = 6; photothrombotic cortical injury (PCI) + PBS infusion) and sNgR-Fc group (n = 6; PCI + sNgR-Fc infusion). Another batch of rats were also divided into three groups: sham group (n = 6), NPCs group (n = 6; PCI + NPCs + PBS infusion) and sNgR-Fc + NPCs group (n = 6; PCI + NPCs + sNgR-Fc infusion). All experimental procedures described here were in accordance with the National Institutes of Health (NIH) guidelines for the Care and Use of Laboratory Animals (Publication No. 85-23, revised 1996). The protocols were approved by the Committee on the Use of Live Animals in Teaching and Research of the University of Hong Kong (approval No. 4560-17) in November, 2015.
Primary NPC isolation and culture
NPCs were isolated as previously described (Guo et al., 2007). Briefly, the hippocampi of E16 Sprague-Dawley rats were harvested immediately after decapitation and dissected mechanically in cold Hank’s Balanced Salt Solution supplemented with 16 mM Na-HEPES (pH 7.2). Cells were collected after centrifugation and cultured in Dulbecco’s modified Eagle’s medium/F12 supplemented with epidermal growth factor, basic fibroblast growth factor, 2% B27 supplement and 1% N2 supplement. Culture medium was replaced every 3 days. Cultured neurospheres were immunostained for nestin (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The dissociated NPCs were immunostained for rat NgR1 (1:200; Biogen Idec, Inc., Cambridge, MA, USA) and nestin.
In vitro NPC differentiation assay
The neurospheres described above were harvested, dissociated and cultured in medium supplemented with 500 ng/mL sNgR-Fc, but without epidermal growth factor or basic fibroblast growth factor to initiate and promote differentiation (sNgR-Fc group). For the control group, cells were differentiated without sNgR-Fc. After 7 days, NPC differentiation was assessed by immunocytochemistry. The following primary antibodies (neural stem cell-specific markers) were used: mouse monoclonal anti-nestin (1:100), rabbit monoclonal anti-glial fibrillary acidic protein (GFAP; 1:100; Chemicon, Hofheim, Germany), mouse monoclonal anti-RIP (1:50; kind gift from Dr Xu XM, University of Louisville School of Medicine, Louisville, Kentucky) and mouse monoclonal anti-β-tubulin III (1:100; Sigma-Aldrich, St. Louis, MO, USA). Incubations were performed at 4°C overnight. After washing with PBS, the sections were incubated with goat anti-rabbit IgG Alexa Fluor 594 and goat anti-Mouse IgG Alexa Fluor 594 (1:1000; Abcam, Cambridge, UK) at room temperature for 1 hour. The number of immunoreactive cells in each culture was calculated. Five visual fields (200×) were selected for each slide, and a total of 30 visual fields were counted for each parameter. The number of DAPI-stained cells was taken to represent the total number of cells. The percentage of immunoreactive cells was then calculated for each antibody.
Preparation of sNgR-Fc
The sNgR-Fc fusion protein was prepared as previously described (Li et al., 2011). Briefly, the form of sNgR-Fc used in this study, AA-rNgR (310)-rFc, consists of a 310 amino acid fragment of rat NgR1 fused to a rat IgG1-Fc fragment (Biogen Idec, Inc.). Rat IgG1 (control protein) was purchased from Protos Immunoresearch (San Francisco, CA, USA). sNgR-Fc does not directly interact with NgR1. Instead, it binds to the ligands of NgR1 (Nogo66, MAG, OMgp) and inhibits PirB–Nogo and MAG–sialic acid interactions. This modified protein has been shown to inhibit the Nogo66–NgR interaction and promote neurite outgrowth from rat dorsal root ganglia and cerebellar granule neurons in vitro, with a potency similar to that of unmodified sNgR-Fc (Li et al., 2011).
PCI model
The PCI model of cerebral ischemic injury was produced in the rat parietal cortex according to the method of Watson et al., with a minor modification (Watson et al., 1985). Briefly, 36 Sprague-Dawley rats were anesthetized by intraperitoneal injection of ketamine (80 mg/kg) and xylazine (8 mg/kg). Diluted rose bengal (40 mg/kg) was infused into the femoral vein. The exposed skull, 5 mm posterior to the bregma and 5 mm lateral to the midline, was illuminated with a cold, white light beam (Volpi Intralux 6000, 150 W; Volpi AG, Schlieren, Switzerland) for 8 minutes at maximum output via a fiber-optic bundle with a 10 mm aperture. In the sham group, animals were operated as above, but without injection of rose bengal. The PCI rats were randomly allocated to receive PBS (n = 6; PCI group) or an sNgR-Fc infusion (n = 6; sNgR-Fc group) for 3 days. One day after PCI, rats were implanted with a mini-osmotic pump (model 1003D, flow rate 0.1 μL/h; Alzet, Palo Alto, CA, USA) that infused either sNgR-Fc (400 μg/kg) or the same volume of PBS (10 µL) continuously into the ipsilateral (lesioned) ventricle. The pumps were removed 4 days after PCI to avoid local inflammation. Throughout the experiment, room temperature was maintained at 25°C, and core body temperature was monitored and maintained at 37°C by a thermostatically-controlled heating pad.
Transplantation of NPCs into the brain after PCI
To further clarify the effects of sNgR-Fc on NPCs, another batch of PCI rats were prepared as above. NPCs from the hippocampus of E16 GFP-transgenic Sprague-Dawley rats [“green rat CZ-004” SD TgN (act-EGFP) OsbCZ-004] were prepared as described above for transplantation. On the day of transplantation, NPCs were dissociated and re-suspended in Hank’s Balanced Salt Solution at a density of 50,000 viable cells/μL (Arvidsson et al., 2002). One day after PCI, 2 μL (5 × 104 cells/μL) NPCs were grafted into the brain tissue around the infarct area, 0.5 mm to the right of the infarct area at a depth of 1 mm (Buhnemann et al., 2006). Rats were then randomly assigned to receive PBS (n = 6; NPCs group) or sNgR-Fc infusion (n = 6; sNgR-Fc+ NPCs group) into the ipsilateral (lesioned) ventricle as described above for 3 days.
5-Bromodeoxyuridine (BrdU) labeling
Intraperitoneal injection of BrdU (50 mg/kg body weight; Sigma-Aldrich) was administered twice daily for 4–6 days at 24 hours following PCI, as previously described (Kang et al., 2013). Animals were sacrificed to determine cell proliferation at day 7 and for differentiation assays at day 21 after PCI. After BrdU staining, the images were captured using AxioVision (Zeiss, Thornwood, NY, USA). All BrdU-positive cells (rat monoclonal anti-BrdU, 1:500; Accurate Chemical & Scientific, Westbury, NY, USA) were counted in the brain tissue. Counts were expressed as the average cell number per field at 100× magnification.
Behavioral assessments
The behavioral tests were administered before PCI and 1, 7, 14 and 21 days after PCI by two investigators who were blinded to group assignment. The Morris water maze comprised a circular pool (150 cm in diameter, 60 cm in depth) filled with water at 24 ± 1°C to a depth of 35 cm (WinFast PVR, Jiliang, Nanjing, China). The pool was divided into four quadrants with four starting locations (north, east, south and west). An invisible platform (12 cm in diameter) was placed 1 cm below the water surface in the center of the northern quadrant. Water in the pool was made opaque with milk powder (Full Cream Milk Powder; Nestle Shuangcheng Ltd., China) prior to each test to eliminate visual cues. The rats were trained at 13:00–15:00 for 5 days before PCI. At the beginning of each trial, the rats were placed in the water facing the pool wall in one of the four quadrants. Each rat was allowed to swim until it found the hidden platform or until 120 seconds had elapsed. All groups were trained from each of the starting positions (north, east, south and west). There was a 30-second period between the two trials. Rats were allowed to rest for 5 minutes between two consecutive trials. The time taken to reach the hidden platform (escape latency) and the length of the swim path (travelled distance) were recorded. On day 21, a probe trial was performed in which the platform was removed from the pool and each rat was allowed to swim for 120 seconds. For these probe trials, the distance traveled in the target quadrant and the former platform location passing times within 120 seconds were recorded. The neurological severity score was calculated as described previously (Tang et al., 2014). Neurological function was graded on a scale of 0 to 14 (normal score, 0; maximal deficit score, 14). The neurological severity score is a composite of motor and sensory assessments, and reflex and beam balance scores. One point is awarded for the inability to perform the test or the lack of a tested reflex. Thus, a higher score indicates a more severe deficit.
Immunohistochemistry
Immunohistochemical staining was performed as previously reported (Zhang et al., 2015). On day 7 or 21 following PCI, the brains were removed, and 5-µm frozen coronal sections were prepared. After antigen retrieval, sections were immunolabeled with rat monoclonal anti-BrdU (1:500; Accurate Chemical & Scientific), rabbit polyclonal anti-GFP (1:200; Santa Cruz Biotechnology), mouse monoclonal anti-NeuN (1:500; Chemicon), rabbit monoclonal anti-GFAP (1:200; Chemicon), mouse monoclonal anti-RIP (1:50; kind gift from Dr Xu XM, University of Louisville School of Medicine) and mouse monoclonal anti-NgR1 (1:200; Biogen Idec, Inc.) overnight at 4°C. After washing with PBS, the sections were incubated with goat anti-rabbit IgG Alexa Fluor 488, goat anti-rat IgG Alexa Fluor 488, goat anti-mouse IgG Alexa Fluor 594 and donkey anti-rabbit IgG Alexa Fluor 594 (1:1000; Abcam) at room temperature for 1 hour. Cells double-positive for BrdU/NeuN, GFP/GFAP and GFP/RIP were counted. Positive cells were also visualized using a motorized inverted microscope (IX81-ZDC2; Olympus, Hamburg, Germany).
Western blot assay
The hippocampus on the lesioned side was collected and homogenized 7 days after PCI to extract protein. After calculating the concentration, the protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane (Millipore Corp, Billerica, MA, USA). The membrane was washed with Tris-buffered saline containing 0.1% Tween-20 three times, blocked with 5% fat-free milk in Tris-buffered saline containing 0.1% Tween-20, and then incubated with mouse monoclonal anti-NgR1 (1:500; Biogen Idec, Inc.) overnight at 4°C. Subsequently, after washing with Tris-buffered saline containing 0.1% Tween-20, the membrane was incubated with horseradish peroxidase-conjugated secondary antibodies (1:10,000; Santa Cruz Biotechnology) at room temperature for 1 hour, and then developed. Optical densitometry on the blots was performed with Quantity One software (Bio-Rad Laboratories, Shanghai, China).
Statistical analysis
Values are presented as the mean ± SD. Data were analyzed using SPSS 12.0 software (SPSS, Chicago, IL, USA). Statistical analysis was performed using Student’s t-test for comparisons between two groups, or one-way analysis of variance followed by the Bonferroni post hoc test for comparisons of more than two groups. A P-value < 0.05 was considered statistically significant.
Results
sNgR-Fc enhances the proliferation and differentiation of NPCs in vitro
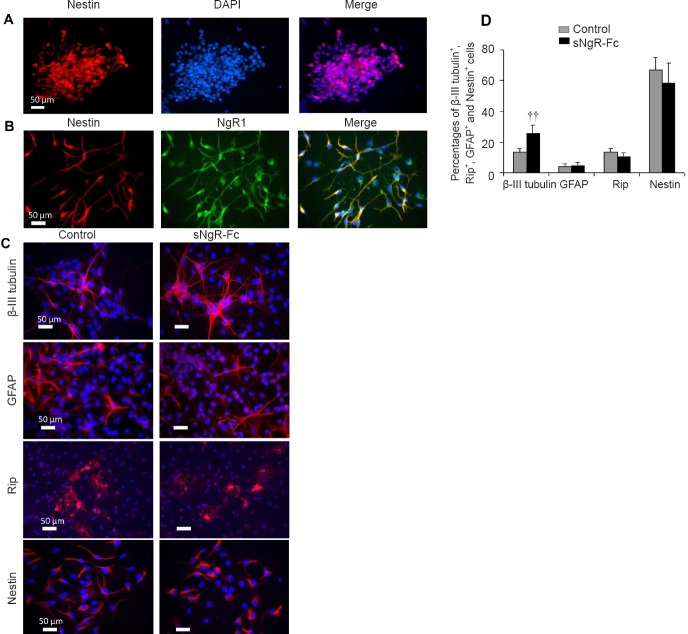
We characterized the NPCs isolated from the hippocampus of embryonic (E16) Sprague-Dawley rats. In the presence of epidermal growth factor and basic fibroblast growth factor, the NPCs proliferated into larger neurospheres floating in the culture medium after 3–7 days. The cells in the neurospheres were positive for nestin (Figure 1A). Next, we examined NgR1 expression in the NPCs. Most of the NPCs were positive for NgR1 (Figure 1B). Our previous study showed that sNgR-Fc promotes the proliferation of NPCs in vitro (Li et al., 2011). We found that sNgR-Fc enhanced the proliferation of NPCs in vitro (data not shown). Next, we investigated whether sNgR-Fc can induce NPCs to differentiate into neurons. Compared with control NPCs, there was significant differentiation of NPCs into β-III tubulin-positive neurons following sNgR-Fc treatment (P < 0.01; Figure 1C and D). There was no significant difference in the numbers of GFAP, RIP or nestin-positive cells between control and sNgR-Fc-treated NPCs (Figure 1C and D). Collectively, these results show that sNgR-Fc stimulates NPCs to differentiate into neurons.
Figure 1.
sNgR-Fc enhances the differentiation of NPCs in vitro.
(A) Representative immunofluorescence images showing Nestin (red) staining of cultured neurospheres. (B) Representative immunofluorescence images showing the co-localization of Nestin (red) and NgR1 (green) in NPCs. (C) After treatment with phosphate-buffered saline or sNgR-Fc, cultured NPCs were stained with antibodies against β-III tubulin (red), GFAP (red), Rip (red) and Nestin (red). Scale bars: 50 μm in A–C. (D) Percentages of β-III tubulin+, Rip+, GFAP+ and Nestin+ cells among the total number of cells. All data are expressed as the mean ± SD (n = 3; Student’s t-test). ††P < 0.01, vs. control group. sNgR-Fc: Soluble Nogo66 receptor-Fc protein; NPCs: neural progenitor cells; DAPI: 4′,6-diamidino-2-phenylindole; GFAP: glial fibrillary acidic protein; NgR1: Nogo66 receptor.
NgR1 expression increases after PCI in rats
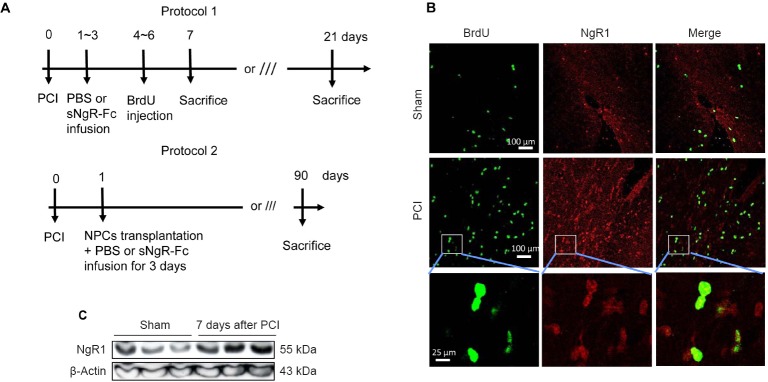
The animal experimental protocol is shown in Figure 2A. We examined NgR1 expression in rats with PCI. Compared with sham rats, NgR1 expression was greatly increased in the brain 7 days post PCI (Figure 2B). Notably, most of the NgR1-positive cells were also positive for BrdU (Figure 2B). Western blot assay showed that NgR1 was significantly upregulated in the hippocampus 7 days post-injury (Figure 2C). Taken together, these findings demonstrate that NgR1 expression is upregulated in rats following PCI.
Figure 2.
NgR1 expression increases in rats after PCI.
(A) Procedure to create a PCI model in rats with either PBS or sNgR-Fc infusion (protocol 1) or transplantation of NPCs together with PBS or sNgR-Fc infusion for 3 days (protocol 2). (B) Representative immunofluorescence images showing BrdU labeling (green) and NgR1 expression (red) in the brain. Scale bars: 100 and 25 μm. (C) NgR1 protein levels assessed by western blot assay in the brain (sham rats or 7 days post injury). BrdU: 5-Bromodeoxyuridine; sNgR-Fc: soluble Nogo66 receptor-Fc protein; NgR1: Nogo66 receptor; PBS: phosphate-buffered saline; PCI: photothrombotic cortical injury.
Administration of sNgR-Fc improves neurological function following PCI in rats
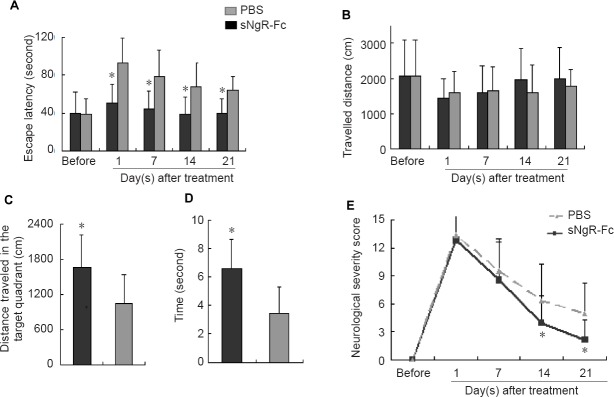
The escape latency was greatly increased 1 day after PCI, especially in the PBS group (Figure 3A). Compared with the PBS group, escape latency was significantly reduced in rats treated with sNgR-Fc at 1, 7, 14 and 21 days after PCI (P < 0.05; Figure 3A). However, there was no significant difference in travelled distance between the sNgR-Fc and PBS groups either before or 1, 7, 14 and 21 days after PCI (Figure 3B). The spatial memory test showed that in comparison with the PBS group, sNgR-Fc-treated rats spent more time passing through the former platform location and swam a greater distance in the target quadrant (P < 0.05; Figure 3C, D). The neurological severity score revealed that in both the sNgR-Fc and PBS groups, neurological severity score was significantly increased 1 day after PCI, and then gradually decreased at 7, 14 and 21 days after PCI (Figure 3E). More importantly, the neurological severity score was greatly reduced in the sNgR-Fc group compared with the PBS group at 14 and 21 days (P < 0.05; Figure 3E). These results indicate that administration of sNgR-Fc improves neurological function in rats following PCI.
Figure 3.
Administration of sNgR-Fc improves neurological function in rats after PCI.
(A) Escape latency after treatment with PBS or sNgR-Fc in rats with PCI at various time points. (B) Travelled distance following treatment with PBS or sNgR-Fc in rats with PCI at the indicated time points. (C) Distance traveled in the target quadrant in the PBS and sNgR-Fc groups. (D) Total time spent in passing through the former platform location in the PBS and sNgR-Fc groups. (E) Neurological severity score in the PBS and sNgR-Fc groups. All data are expressed as the mean ± SD (n = 6; Student’s t-test). *P < 0.05, vs. PBS group. sNgR-Fc: Soluble Nogo66 receptor-Fc protein; PBS: phosphate-buffered saline.
Administration of sNgR-Fc promotes the proliferation and differentiation of endogenous NPCs in rats with PCI
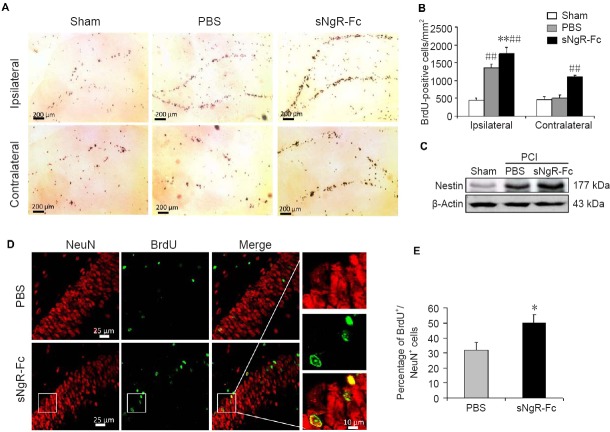
We sought to determine whether sNgR-Fc impacts the proliferation or differentiation of endogenous NPCs in rats with PCI. Compared with the sham group, the number of BrdU-positive cells was significantly increased in the ipsilateral hemisphere in the PBS and sNgR-Fc groups (both P < 0.01; Figure 4A and B). The number of BrdU-positive cells was significantly higher in the sNgR-Fc group than in the PBS group (P < 0.01; Figure 4A and B). A similar result was found for the contralateral hemisphere. Compared with the PBS group, the number of BrdU-positive cells was significantly increased in the sNgR-Fc group (P < 0.01; Figure 4A and B). Next, we evaluated nestin expression in the hippocampus by western blot assay. Nestin expression was upregulated after PCI injury, and was further increased by sNgR-Fc treatment (Figure 4C). Subsequently, we examined the effect of sNgR-Fc on the differentiation of NPCs in our stroke model. In the lesioned dentate gyrus, more cells were positive for BrdU in the sNgR-Fc group than in the PBS group (Figure 4D and E). Furthermore, the ratio of NeuN/BrdU double-positive cells in the dentate gyrus in the lesioned area was higher in the sNgR-Fc group (P < 0.05; Figure 4D, E). These results show that sNgR-Fc enhances the proliferation and differentiation of endogenous NPCs in rats with PCI.
Figure 4.
Administration of sNgR-Fc promotes the proliferation and differentiation of endogenous NPCs in rats with PCI.
(A) Representative images showing BrdU staining (brown) in the dentate gyrus on the ipsilateral (ischemic lesion) and contralateral sides after ischemic injury. Scale bars: 200 μm. (B) Quantification of BrdU-positive cells in the dentate gyrus on each side (n = 6; one-way analysis of variance followed by the Bonferroni test). (C) Nestin expression in the hippocampus measured by western blot assay. (D) Representative immunofluorescence images showing BrdU+ (green)/NeuN+ (red) cells on the side of the ischemic lesion in the PBS and sNgR-Fc groups. Scale bars: 25 and 10 μm. (E) Percentage of BrdU+/NeuN+ cells on the side of the ischemic lesion in the PBS and sNgR-Fc groups (n = 6; Student’s t-test). All data are expressed as the mean ± SD. *P < 0.05, **P < 0.001, vs. PBS group; ##P < 0.01, vs. sham group. sNgR-Fc: Soluble Nogo66 receptor-Fc protein; BrdU: 5-bromodeoxyuridine; NPCs: neural progenitor cells; PCI: photothrombotic cortical injury; PBS: phosphate-buffered saline.
Administration of sNgR-Fc promotes the survival of grafted NPCs in the rat model of PCI
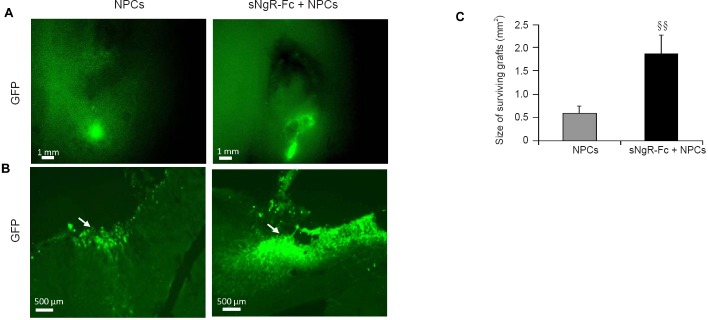
To further elucidate the effects of sNgR-Fc on grafted NPCs, the PCI rats given NPC transplantation were administered PBS or sNgR-Fc infusion. Then, 3 months after transplantation, the brains were harvested to assess cell survival. The size of the NPC engraftment was significantly greater in the sNgR-Fc + NPCs group compared with the NPC group, suggesting that sNgR-Fc promotes the survival of NPCs in the PCI rats (P < 0.01; Figure 5A, C). Furthermore, in a high-magnification field, compared with the NPC group, more transplanted NPCs were observed in the sNgR-Fc + NPCs group (P < 0.01; Figure 5B, C). Taken together, these findings show that administration of sNgR-Fc enhances the survival of grafted NPCs in PCI rats.
Figure 5.
Administration of sNgR-Fc enhances the survival of grafted NPCs in the rat model of PCI.
(A, B) Representative immunofluorescence images showing the surviving grafts in a low-magnification field (A, scale bar: 1 mm) and grafted GFP-positive NPCs in a high-magnification field (B, scale bar: 500 μm) in response to sNgR-Fc or PBS treatment 3 months after NPC transplantation in the stroke injury model. Arrows point to GFP-positive NPCs. Green staining indicates GFP-positive cells. (C) Size of surviving grafts measured in the sNgR-Fc and PBS-treated rats at 3 months after NPC transplantation in the stroke injury model. All data are expressed as the mean ± SD (n = 6; Student’s t-test). §§P < 0.01, vs. NPCs group. sNgR-Fc: Soluble Nogo66 receptor-Fc protein; PCI: photothrombotic cortical injury; NPCs: neural progenitor cells; GFP: green fluorescent protein; PBS: phosphate-buffered saline.
Treatment with sNgR-Fc promotes the differentiation of engrafted NPCs into neurons
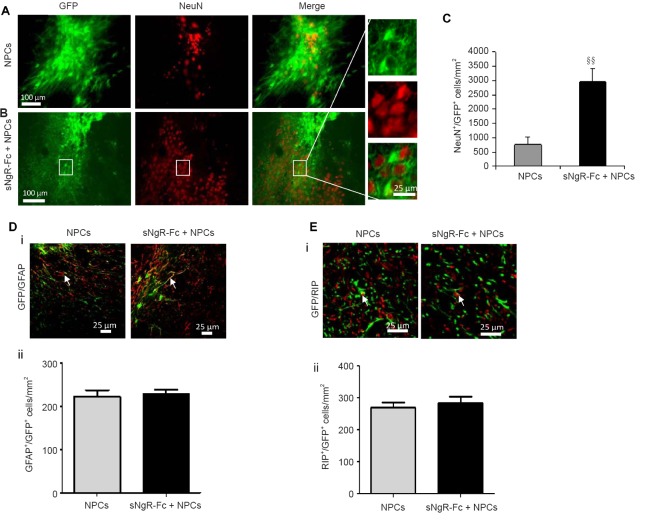
Next, we examined the effect of sNgR-Fc on the differentiation of grafted NPCs 3 months after transplantation. As shown in Figure 6A, more GFP-positive NPCs were observed in rats infused with sNgR-Fc after NPC transplantation (Figure 6A, B). A higher number of GFP-positive NPCs also expressed NeuN in the sNgR-Fc + NPCs group, indicating that sNgR-Fc can promote not only survival but also differentiation of engrafted NPCs in vivo (P < 0.01; Figure 6A, B). More importantly, the density of NeuN/GFP double-positive cells was much higher in the sNgR-Fc + NPCs group compared with the NPC group, suggesting that sNgR-Fc treatment promotes the differentiation of NPCs into neurons (Figure 6C). In contrast, compared with the NPC group, the number of GFAP/GFP double-positive cells was not increased in the sNgR-Fc + NPCs group (Figure 6D–i, ii). There was also no difference in the number of RIP/GFP double-positive cells between the two groups (Figure 6E–i, ii). These results indicate that sNgR-Fc treatment also promotes the differentiation of engrafted NPCs into neurons in rats with PCI.
Figure 6.
Treatment with sNgR-Fc promotes the differentiation of engrafted NPCs into neurons.
(A, B) Representative immunofluorescence images showing the expression of NeuN (red) in GFP + NPCs (green) in the NPCs and sNgR-Fc + NPCs groups at 3 months post transplantation in the rat model of PCI. Scale bars: 100 and 25 μm. (C) Density of NeuN+/GFP+ cells in the injured area in the NPCs and sNgR-Fc + NPCs groups. (D) GFAP (red) and GFP (green) detected by immunofluorescence staining at 3 months post-PCI in the NPCs and sNgR-Fc + NPCs groups (i). Scale bar: 25 μm. Arrows point to GFAP+/RIP+ cells. The density of GFAP+/GFP+ cells in the injured area in the NPCs and sNgR-Fc + NPCs groups (ii). (E) Expression of RIP (red) and GFP (green) at 3 months post-PCI in the NPCs and sNgR-Fc + NPCs groups (i). Scale bar: 25 μm. Arrows point to RIP+/GFP+ cells. The density of RIP+/GFP+ cells in the injured area in the NPCs and sNgR-Fc + NPCs groups (ii). (C, Dii, Eii) All data are expressed as the mean ± SD (n = 6; Student’s t-test). §§P < 0.01, vs. NPCs group. sNgR-Fc: Soluble Nogo66 receptor-Fc protein; PCI: photothrombotic cortical injury; NPCs: neural progenitor cells; GFP: green fluorescent protein; GFAP: glial fibrillary acidic protein.
Discussion
There are several major findings in this study. First, we found that NgR1 is expressed by endogenous NPCs and is upregulated in the ischemic brain. Second, the sNgR-Fc fusion protein has a strong neurogenic effect when administered into the ischemic brain. Third, sNgR-Fc stimulates the proliferation of NPCs and promotes their differentiation into neurons. Last, but not least, administration of sNgR-Fc promotes the survival of grafted NPCs in rats with PCI.
Although numerous studies have investigated the mechanisms of ischemic stroke, current therapies are far from ideal (Cassella and Jagoda, 2017; Russo et al., 2018). Novel therapeutic strategies for ischemic stroke are therefore urgently needed. Neuronal loss as a result of ischemic stroke has devastating effects on the development and outcome of the disease (Radak et al., 2017; Flippo et al., 2018; Sekerdag et al., 2018; Tuo et al., 2018). Neurogenesis post-ischemic stroke contributes to spontaneous neuroplasticity, attenuating neurological dysfunction (Tobin et al., 2014; Wu et al., 2017; Zhu et al., 2018). In response to neuronal damage or death, adult neural stem cells/NPCs in the central nervous system can proliferate and differentiate into neural cells, and thereby replenish the lost or injured neural cells and help restore the brain’s functional and structural integrity (Daadi et al., 2010; Jahanbazi Jahan-Abad et al., 2018). However, the limited proliferative and differentiation capacities of endogenous NPCs curtail their protective effects (Tseng et al., 2018; Zhou et al., 2018). Stimulating the proliferation and differentiation of NPCs is a new therapeutic approach for stroke (Dadwal et al., 2015).
NgR1 has been reported to regulate neurological function in the central nervous system. Nogo66 has been shown to inhibit axonal regeneration in the zebrafish optic nerve (Abdesselem et al., 2009). Moreover, the LINGO-1 receptor, a functional component of the NgR1, promotes neuronal apoptosis by inhibiting the activity of WNK3 kinase (Zhang et al., 2013). These findings suggest that NgR1 plays a critical role in the regulation of neurological function. Consistent with a previous study (Zhang et al., 2013), we found that NgR1 is highly expressed in NPCs after stroke, indicating that NgR1 regulates their function. Furthermore, NgR1 expression is highly upregulated in PCI rats. Therefore, targeting NgR1 to modulate the function of NPCs is a potential novel strategy for treating stroke. Indeed, pre-treatment with LINGO-1-Fc dramatically reduces potassium-induced cerebellar granular neuron apoptosis by inhibiting glycogen synthase kinase-3 beta activation (Zhao et al., 2008). In the current study, treatment with sNgR-Fc markedly restored neurological function in rats with PCI by increasing the proliferation of endogenous NPCs and their differentiation into neurons, suggesting that sNgR-Fc promotes neurogenesis from NPCs by inhibiting NgR1 signaling. Nonetheless, this strategy is unable to adequately functionally compensate for the lost and injured neural cells following stroke.
Over the past few decades, stem cell-based therapies have shown beneficial effects in brain repair (Ludwig et al., 2018; Marei et al., 2018). Neural stem cell/NPC transplantation greatly reduces the size of the infarcted region in the brain and improves neurological outcomes as determined by neurological severity score in a rat model of acute ischemic stroke (Zhang et al., 2017). Notably, the transplanted NPCs can be detected using magnetic resonance imaging and fluorescence imaging, 6 weeks after transplantation. Importantly, some transplanted neural stem cells have already differentiated into neurons by this time. Transplantation of induced pluripotent stem cell-derived NPCs increases functional recovery in mice with cerebral ischemia-induced brain injury, and these protective effects are further augmented by transplantation of induced pluripotent stem cell-NPCs overexpressing stromal cell-derived factor 1 alpha (Chau et al., 2017). Nevertheless, the hostile microenvironment of the infarcted area is a major obstacle to the survival of the transplanted NPCs, thereby heavily limiting their therapeutic efficacy. Pre-treatment of NPCs with adjudin enhances the therapeutic efficacy of injected NPCs in the infarct area by increasing their survival (Zhang et al., 2017). Therefore, exploring novel methods to promote NPC survival after transplantation should greatly advance treatment strategies for stroke.
It has been reported that NgR1 overexpression results in a hostile environment that inhibits the survival, proliferation and differentiation of NPCs (Lu et al., 2012). In the current study, the increased expression of NgR1 after PCI may produce a hostile environment that reduces the survival of transplanted NPCs. Modulating NgR1 expression in the brain following stroke may enhance the survival of implanted NPCs. Indeed, in the current study, we showed that compared with PBS infusion, sNgR-Fc infusion improved the survival of transplanted NPCs 3 months post-PCI, suggesting that suppressing NgR1 expression or activation may enhance the engraftment of NPCs in the ischemic brain. Combined treatment with sNgR-Fc and exogenous NPCs further ameliorated the therapeutic efficacy of these cells by promoting their survival and differentiation.
There are some limitations to the current study. First, the dose of sNgR-Fc was adapted from our previous study. Whether the dose of sNgR-Fc affects the differentiation of NPCs into neurons is unknown. Second, because NgR1 is upregulated by stroke, it is necessary to determine whether conditional knockdown of NgR1 can influence the differentiation capacity of NPCs. Third, although we demonstrated that sNgR-Fc improves the survival of transplanted NPCs, the underlying mechanisms are unknown. Further studies are required to address these issues.
In summary, sNgR-Fc enhances the proliferation and differentiation of endogenous NPCs into neurons, and promotes neurological functional recovery after stroke. More importantly, sNgR-Fc increases the survival of transplanted NPCs in the ischemic brain and augments their therapeutic efficacy. sNgR-Fc may therefore have therapeutic potential for ischemic stroke and neurodegenerative diseases.
Additional file: Open peer review reports 1 (106.3KB, pdf) and 2 (102.2KB, pdf) .
Acknowledgments
We thank Biogen Idec, Inc. for providing the sNgR-Fc in this study. We also appreciate the useful discussions from Dr Daniel H.S. Lee from Biogen Idec Inc., USA for this project.
Footnotes
Conflicts of interest: The authors declare that they have no conflicts of interest.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81671882, 81471832 (to XL); the Natural Science Foundation of Guangdong Province of China, No. 2016A030311039 (to XL); the Science and Technology Foundation of Guangdong Province of China, No. 2015A020212012, 2017A020224012 (to XL); the Science and Technology Foundation of Guangzhou City of China, No.201707010373 (to XL). The conception, design, execution, and analysis of experiments, as well as the preparation of and decision to publish this manuscript, were made independent of any funding organization.
Institutional review board statement: All experimental procedures and protocols were approved by the Committee on the Use of Live Animals in Teaching and Research of the University of Hong Kong (approval No. 4560-17) in November, 2015. All experimental procedures described here were in accordance with the National Institutes of Health (NIH) guidelines for the Care and Use of Laboratory Animals (Publication No. 85-23, revised 1996).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Olga Chechneva, University of California Davis, USA; Sergei Fedorovich, Institute of Biophysics and Cell Engineering NASB, Laboratory of Biophysics and Engineering of Cell, Belarus.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81671882, 81471832 (to XL); the Natural Science Foundation of Guangdong Province of China, No. 2016A030311039 (to XL); the Science and Technology Foundation of Guangdong Province of China, No. 2015A020212012, 2017A020224012 (to XL); the Science and Technology Foundation of Guangzhou City of China, No. 201707010373 (to XL).
P-Reviewers: Fedorovich S; Chechneva O; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Patel B, Wysong S, Qiu Y, Song LP; T-Editor: Liu XL
References
- 1.Abdesselem H, Shypitsyna A, Solis GP, Bodrikov V, Stuermer CA. No Nogo66- and NgR-mediated inhibition of regenerating axons in the zebrafish optic nerve. J Neurosci. 2009;29:15489–15498. doi: 10.1523/JNEUROSCI.3561-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 3.Buhnemann C, Scholz A, Bernreuther C, Malik CY, Braun H, Schachner M, Reymann KG, Dihne M. Neuronal differentiation of transplanted embryonic stem cell-derived precursors in stroke lesions of adult rats. Brain. 2006;129:3238–3248. doi: 10.1093/brain/awl261. [DOI] [PubMed] [Google Scholar]
- 4.Cassella CR, Jagoda A. Ischemic stroke: advances in diagnosis and management. Emerg Med Clin North Am. 2017;35:911–930. doi: 10.1016/j.emc.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Chang QY, Lin YW, Hsieh CL. Acupuncture and neuroregeneration in ischemic stroke. Neural Regen Res. 2018;13:573–583. doi: 10.4103/1673-5374.230272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chau M, Deveau TC, Song M, Wei ZZ, Gu X, Yu SP, Wei L. Transplantation of iPS cell-derived neural progenitors overexpressing SDF-1alpha increases regeneration and functional recovery after ischemic stroke. Oncotarget. 2017;8:97537–97553. doi: 10.18632/oncotarget.22180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daadi MM, Davis AS, Arac A, Li Z, Maag AL, Bhatnagar R, Jiang K, Sun G, Wu JC, Steinberg GK. Human neural stem cell grafts modify microglial response and enhance axonal sprouting in neonatal hypoxic-ischemic brain injury. Stroke. 2010;41:516–523. doi: 10.1161/STROKEAHA.109.573691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dadwal P, Mahmud N, Sinai L, Azimi A, Fatt M, Wondisford FE, Miller FD, Morshead CM. Activating endogenous neural precursor cells using metformin leads to neural repair and functional recovery in a model of childhood brain injury. Stem Cell Rep. 2015;5:166–173. doi: 10.1016/j.stemcr.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faulkner J, Wright A. Using robotic-assisted technology to improve lower-limb function in people with stroke. Clin Trials Degener Dis. 2018;3:111–114. [Google Scholar]
- 10.Feng R, Badgeley M, Mocco J, Oermann EK. Deep learning guided stroke management: a review of clinical applications. J Neurointerv Surg. 2018;10:358–362. doi: 10.1136/neurintsurg-2017-013355. [DOI] [PubMed] [Google Scholar]
- 11.Flippo KH, Gnanasekaran A, Perkins GA, Ajmal A, Merrill RA, Dickey AS, Taylor SS, McKnight GS, Chauhan AK, Usachev YM, Strack S. AKAP1 protects from cerebral ischemic stroke by inhibiting Drp1-dependent mitochondrial fission. J Neurosci. 2018;38:8233–8242. doi: 10.1523/JNEUROSCI.0649-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frid K, Binyamin O, Fainstein N, Keller G, Ben-Hur T, Gabizon R. Autologous neural progenitor cell transplantation into newborn mice modeling for E200K genetic prion disease delays disease progression. Neurobiol Aging. 2018;65:192–200. doi: 10.1016/j.neurobiolaging.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Guo J, Zeng Y, Liang Y, Wang L, Su H, Wu W. Cyclosporine affects the proliferation and differentiation of neural stem cells in culture. Neuroreport. 2007;18:863–868. doi: 10.1097/WNR.0b013e32811d6d36. [DOI] [PubMed] [Google Scholar]
- 14.Hicks AU, Lappalainen RS, Narkilahti S, Suuronen R, Corbett D, Sivenius J, Hovatta O, Jolkkonen J. Transplantation of human embryonic stem cell-derived neural precursor cells and enriched environment after cortical stroke in rats: cell survival and functional recovery. Eur J Neurosci. 2009;29:562–574. doi: 10.1111/j.1460-9568.2008.06599.x. [DOI] [PubMed] [Google Scholar]
- 15.Jahanbazi Jahan-Abad A, Sahab Negah S, Hosseini Ravandi H, Ghasemi S, Borhani-Haghighi M, Stummer W, Gorji A, Khaleghi Ghadiri M. Human neural stem/progenitor cells derived from epileptic human brain in a self-assembling peptide nanoscaffold improve traumatic brain injury in rats. Mol Neurobiol. 2018;55:9122–9138. doi: 10.1007/s12035-018-1050-8. [DOI] [PubMed] [Google Scholar]
- 16.Kang C, Yang CY, Kim JH, Moon SK, Lee S, Park SA, Han EH, Zhang LQ. The effect of continuous epidural electrical stimulation on neuronal proliferation in cerebral ischemic rats. Ann Rehabil Med. 2013;37:301–310. doi: 10.5535/arm.2013.37.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim YR, Kim HN, Ahn SM, Choi YH, Shin HK, Choi BT. Electroacupuncture promotes post-stroke functional recovery via enhancing endogenous neurogenesis in mouse focal cerebral ischemia. PLoS One. 2014;9:e90000. doi: 10.1371/journal.pone.0090000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li G, Yu F, Lei T, Gao H, Li P, Sun Y, Huang H, Mu Q. Bone marrow mesenchymal stem cell therapy in ischemic stroke: mechanisms of action and treatment optimization strategies. Neural Regen Res. 2016;11:1015–1024. doi: 10.4103/1673-5374.184506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Su H, Fu QL, Guo J, Lee DH, So KF, Wu W. Soluble NgR fusion protein modulates the proliferation of neural progenitor cells via the Notch pathway. Neurochem Res. 2011;36:2363–2372. doi: 10.1007/s11064-011-0562-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders--time for clinical translation? J Clin Invest. 2010;120:29–40. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, Conner JM, Marsala M, Tuszynski MH. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ludwig PE, Thankam FG, Patil AA, Chamczuk AJ, Agrawal DK. Brain injury and neural stem cells. Neural Regen Res. 2018;13:7–18. doi: 10.4103/1673-5374.224361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mangin G, Kubis N. Cell therapy for ischemic stroke: how to turn a promising preclinical research into a successful clinical story. Stem Cell Rev. 2018 doi: 10.1007/s12015-018-9864-3. doi: 10.1007/s.12015-018-9864-3. [DOI] [PubMed] [Google Scholar]
- 24.Marei HE, Hasan A, Rizzi R, Althani A, Afifi N, Cenciarelli C, Caceci T, Shuaib A. Potential of stem cell-based therapy for ischemic stroke. Front Neurol. 2018;9:34. doi: 10.3389/fneur.2018.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mi S, Pepinsky RB, Cadavid D. Blocking LINGO-1 as a therapy to promote CNS repair: from concept to the clinic. CNS Drugs. 2013;27:493–503. doi: 10.1007/s40263-013-0068-8. [DOI] [PubMed] [Google Scholar]
- 26.Mine Y, Tatarishvili J, Oki K, Monni E, Kokaia Z, Lindvall O. Grafted human neural stem cells enhance several steps of endogenous neurogenesis and improve behavioral recovery after middle cerebral artery occlusion in rats. Neurobiol Dis. 2013;52:191–203. doi: 10.1016/j.nbd.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Minnerup J, Wersching H, Schilling M, Schabitz WR. Analysis of early phase and subsequent phase III stroke studies of neuroprotectants: outcomes and predictors for success. Exp Transl Stroke Med. 2014;6:2. doi: 10.1186/2040-7378-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 29.Radak D, Katsiki N, Resanovic I, Jovanovic A, Sudar-Milovanovic E, Zafirovic S, Mousad SA, Isenovic ER. Apoptosis and acute brain ischemia in ischemic stroke. Curr Vasc Pharmacol. 2017;15:115–122. doi: 10.2174/1570161115666161104095522. [DOI] [PubMed] [Google Scholar]
- 30.Russo E, Nguyen H, Lippert T, Tuazon J, Borlongan CV, Napoli E. Mitochondrial targeting as a novel therapy for stroke. Brain Circ. 2018;4:84–94. doi: 10.4103/bc.bc_14_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryu S, Lee SH, Kim SU, Yoon BW. Human neural stem cells promote proliferation of endogenous neural stem cells and enhance angiogenesis in ischemic rat brain. Neural Regen Res. 2016;11:298–304. doi: 10.4103/1673-5374.177739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwab ME, Strittmatter SM. Nogo limits neural plasticity and recovery from injury. Curr Opin Neurobiol. 2014;27:53–60. doi: 10.1016/j.conb.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sekerdag E, Solaroglu I, Gursoy-Ozdemir Y. Cell death mechanisms in stroke and novel molecular and cellular treatment options. Curr Neuropharmacol. 2018;16:1396–1415. doi: 10.2174/1570159X16666180302115544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang G, Liu Y, Zhang Z, Lu Y, Wang Y, Huang J, Li Y, Chen X, Gu X, Wang Y, Yang GY. Mesenchymal stem cells maintain blood-brain barrier integrity by inhibiting aquaporin-4 upregulation after cerebral ischemia. Stem Cells. 2014;32:3150–3162. doi: 10.1002/stem.1808. [DOI] [PubMed] [Google Scholar]
- 35.Tobin MK, Bonds JA, Minshall RD, Pelligrino DA, Testai FD, Lazarov O. Neurogenesis and inflammation after ischemic stroke: what is known and where we go from here. J Cereb Blood Flow Metab. 2014;34:1573–1584. doi: 10.1038/jcbfm.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tseng KY, Anttila JE, Khodosevich K, Tuominen RK, Lindahl M, Domanskyi A, Airavaara M. MANF Promotes differentiation and migration of neural progenitor cells with potential neural regenerative effects in stroke. Mol Ther. 2018;26:238–255. doi: 10.1016/j.ymthe.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tuo QZ, Liuyang ZY, Lei P, Yan X, Shentu YP, Liang JW, Zhou H, Pei L, Xiong Y, Hou TY, Zhou XW, Wang Q, Wang JZ, Wang XC, Liu R. Zinc induces CDK5 activation and neuronal death through CDK5-Tyr15 phosphorylation in ischemic stroke. Cell Death Dis. 2018;9:870. doi: 10.1038/s41419-018-0929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Jiang F, Li Q, He X, Ma J. Mild hypothermia combined with neural stem cell transplantation for hypoxic-ischemic encephalopathy: neuroprotective effects of combined therapy. Neural Regen Res. 2014;9:1745–1752. doi: 10.4103/1673-5374.143417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watson BD, Dietrich WD, Busto R, Wachtel MS, Ginsberg MD. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann Neurol. 1985;17:497–504. doi: 10.1002/ana.410170513. [DOI] [PubMed] [Google Scholar]
- 40.Webb RL, Kaiser EE, Jurgielewicz BJ, Spellicy S, Scoville SL, Thompson TA, Swetenburg RL, Hess DC, West FD, Stice SL. Human neural stem cell extracellular vesicles improve recovery in a porcine model of ischemic stroke. Stroke. 2018;49:1248–1256. doi: 10.1161/STROKEAHA.117.020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu KJ, Yu S, Lee JY, Hoffer B, Wang Y. Improving neurorepair in stroke brain through endogenous neurogenesis-enhancing drugs. Cell Transplant. 2017;26:1596–1600. doi: 10.1177/0963689717721230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang F, Duan X, Lu L, Zhang X, Chen M, Mao J, Cao M, Shen J. In vivo long-term tracking of neural stem cells transplanted into an acute ischemic stroke model with reporter gene-based bimodal mr and optical imaging. Cell Transplant. 2017a;26:1648–1662. doi: 10.1177/0963689717722560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang G, Guo X, Chen L, Li B, Gu B, Wang H, Wu G, Kong J, Chen W, Yu Y. Interferon-gamma promotes neuronal repair by transplanted neural stem cells in ischemic rats. Stem Cells Dev. 2018;27:355–366. doi: 10.1089/scd.2017.0225. [DOI] [PubMed] [Google Scholar]
- 44.Zhang JJ, Zhu JJ, Hu YB, Xiang GH, Deng LC, Wu FZ, Wei XJ, Wang YH, Sun LY, Lou XQ, Shao MM, Mao M, Zhang HY, Xu YP, Zhu SP, Xiao J. Transplantation of bFGF-expressing neural stem cells promotes cell migration and functional recovery in rat brain after transient ischemic stroke. Oncotarget. 2017b;8:102067–102077. doi: 10.18632/oncotarget.22155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang L, Cheng H, Leng MH, Pan JH, He XC, Zhang YM, Lu S, Chen H. Effect of formyl peptide receptor on the differentiation of neural stem/progenitor cells into neurons. Zhongguo Zuzhi Gongcheng Yanjiu. 2018;22:107–112. [Google Scholar]
- 46.Zhang T, Yang X, Liu T, Shao J, Fu N, Yan A, Geng K, Xia W. Adjudin-preconditioned neural stem cells enhance neuroprotection after ischemia reperfusion in mice. Stem Cell Res Ther. 2017c;8:248. doi: 10.1186/s13287-017-0677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Chiu S, Liang X, Gao F, Zhang Z, Liao S, Liang Y, Chai YH, Low DJ, Tse HF, Tergaonkar V, Lian Q. Rap1-mediated nuclear factor-kappaB (NF-kappaB) activity regulates the paracrine capacity of mesenchymal stem cells in heart repair following infarction. Cell Death Discov. 2015;1:15007. doi: 10.1038/cddiscovery.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z, Xu X, Xiang Z, Yu Z, Feng J, He C. LINGO-1 receptor promotes neuronal apoptosis by inhibiting WNK3 kinase activity. J Biol Chem. 2013;288:12152–12160. doi: 10.1074/jbc.M112.447771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao XH, Jin WL, Wu J, Mi S, Ju G. Inactivation of glycogen synthase kinase-3beta and up-regulation of LINGO-1 are involved in LINGO-1 antagonist regulated survival of cerebellar granular neurons. Cell Mol Neurobiol. 2008;28:727–735. doi: 10.1007/s10571-007-9258-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Y, Zhu J, Lv Y, Song C, Ding J, Xiao M, Lu M, Hu G. Kir6.2 deficiency promotes mesencephalic neural precursor cell differentiation via regulating miR-133b/GDNF in a parkinson’s disease mouse model. Mol Neurobiol. 2018;55:8550–8562. doi: 10.1007/s12035-018-1005-0. [DOI] [PubMed] [Google Scholar]
- 51.Zhu SZ, Szeto V, Bao MH, Sun HS, Feng ZP. Pharmacological approaches promoting stem cell-based therapy following ischemic stroke insults. Acta Pharmacol Sin. 2018;39:695–712. doi: 10.1038/aps.2018.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.