Abstract
Appropriate documentary standards and reference materials are crucial building blocks for the development of innovative products. In order to support the emerging sector of nanomedicine, relevant standards must be identified and/or developed before the products will enter into the regulatory approval process. The anticipation of standardization needs requires a good understanding on the regulatory information requirements that can be triggered by the particularities of nanomedicines. However, robust datasets allowing firm conclusions on regulatory demands are not yet available due to a lack of regulatory experience with innovative products. Such a catch‐22 situation can only be advanced in an iterative process by monitoring continuously the scientific evidence and by promoting intensive knowledge exchange between all involved stakeholders. In this study, we have compiled information requirements released by regulatory scientists so far and mapped it against available standards that could be of relevance for nanomedicines. Our gap analysis clearly demonstrated that for some endpoints such as drug release/loading and the interaction of nanomedicines with the immune system no standards are available so far. The emerging nanomedicine sector could benefit from cross‐sector collaboration and review the suitability of standards that have been developed for nanomaterials used for other industrial applications. Only a concerted action of all parties can lead to a smooth translation of nanomedicines to clinical application and to the market. This is in particular important because nanotechnology‐based drug delivery systems are key for the development and implementation of personalized medicine.
This article is characterized under:
Toxicology and Regulatory Issues in Nanomedicine > Regulatory and Policy Issues in Nanomedicine
Keywords: information requirements, nanomedicines, personalized medicine, preclinical characterization, regulatory uncertainties, standards
Abbreviations
- AIST
National Institute of Advanced Industrial Science and Technology (Japan)
- BAM
Federal Institute for Materials Research and Testing (Germany)
- BSI
British Standards Institution
- CEN
European Committee for Standardization
- EMA
European Medicines Agency
- FDA
Food and Drug Administration (USA)
- ICH
International Council on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use
- IPRF
International Pharmaceutical Regulators Forum
- ISO
International Organization for Standardization
- JRC
Joint Research Centre of the European Commission
- NIST
National Institute of Standards and Technology (USA)
- NRC
National Research Council (Canada)
- Ph. Eur.
European Pharmacopeia
- USP
US Pharmacopeia
1. INTRODUCTION
The use of nanotechnology in healthcare raises many hopes to address unmet medical needs by contributing to better diagnostic systems (Kim, Mohamed, Zagorovsky, & Chan, 2017; Yoon et al., 2017), supporting regenerative medicines by providing sophisticated scaffolds (Alarçin et al., 2016; Hamdan et al., 2017) and targeting specifically diseased tissue while minimizing adverse effects of pharmaceuticals (Angelakeris, 2017; Ramos, Cruz, Tovani, & Ciancaglini, 2017). However, the full exploitation of nanotechnology for such innovative products comes along with a number of uncertainties until these products can reach the patient and the market. In his systematic review, Jalonen (2011) identified an unclear regulatory environment as one of eight factors that contributed to uncertainties for the innovation process. His evaluation can also be applied to the highly innovative field of nanomedicine as nanomedicines1 are a typical example of the challenging relationship between regulation and innovation (Fleurke & Somsen, 2011). Quandaries in safety assessment can occur, for example, due to an altered biodistribution profile or additional effects triggered by the interaction of the nanomaterial with the immune system (Dobrovolskaia, 2015). The limited availability of robust datasets allowing to draw firm conclusion on the needs for quality and safety assessments as well as the often unproven suitability of test methods assessing innovative nanomedicines are contributing to regulatory challenges (Figure 1). In order to manage eventual risks often the precautionary principle applies which can contribute to the stagnation of innovation in the medical field (Tubiana, 2001).
Figure 1.
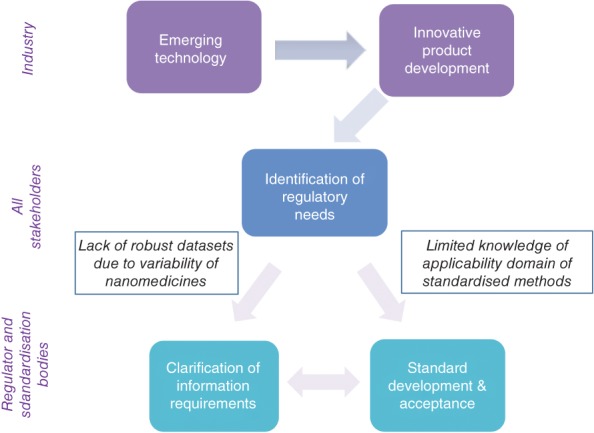
Identified uncertainties related to the regulatory process of innovative products such as nanomedicines
One important parameter contributing to the reduction of uncertainty for the development of innovative nanomedicines is the availability of suitable standardized methods addressing information needs triggered by nano‐specific properties of the formulation. The relevance of standardization for the European single market and the competitiveness of the European economy has been laid down in regulation No. 1025/2012 which mentioned in art 26 the active role of the European Commission's Joint Research Centre in the European standardization process The European Parliament and the Council of the European Union, 2012. For this reason, we have investigated the standardization needs of the emerging sector of nanomedicines. As an initial step we have compiled a number of relevant existing standards with the aim to identify gaps where relevant information cannot be gained due to a lack of reliable and relevant testing methods. Since we observed that only a very limited number of standards were designed for nanomedicines and other existing standards have not been systematically assessed for their suitability to evaluate nanomedicines we included in our study also standards developed in other sectors. The emerging sector of nanomedicines can benefit from a cross‐fertilization with other sectors that are more advanced in exploiting nanotechnology. Developed methods in particular for physicochemical characterization of the nanomaterial and the interaction with biological systems might also be relevant for nanomedicines. Such standards need to be carefully evaluated for their suitability (Gioria et al., 2018).
Since the judgment on the relevance of existing standards required a good understanding on the information requirements that are needed to assess certain particularities of the nanomaterial, we have brought together recommendations proposed by regulatory scientists in dedicated workshops (Global Summit on Regulatory Science: Nanotechnology Standards and Applications; GSRS16, 2016), European Medicines Agency (EMA) reflection papers (European Medicines Agency/Committee for Medicinal Products for Human Use (EMA/CHMP), 2013a, 2013b, 2013c, 2015), or which have been identified in surveys with the regulatory community. These information requirements have been mapped against previously identified standards. Even if a generalization of information requirements is challenging since the field of nanomedicine is progressing fast and a variety of materials, coatings, and functionalizations are used, such exercise can help to identify trends for which standardization needs must be anticipated. The availability of robust and accepted testing methods will not only support a smooth translation of urgently needed nanomedicines from the laboratory environment to clinical applications but it will also contribute to a mutual acceptance of data on various markets.
2. METHODOLOGICAL APPROACH
2.1. Compilation of relevant standards
The search for documentary standards was carried out through web‐based search engines available at Ph. Eur., USP, ISO, CEN, ASTM International, FDA, NIST, ICH and BSI websites by using “nanotechnology,” “nanomedicine,” and “nano” as keywords. The search was further defined considering three categories of methods potentially relevant for nanomedicines: (a) methods that are clearly addressing medical applications; (b)methods not specifying exactly their applicability domain; and (c) methods addressing specific nanomaterials that are potentially relevant for the medical sector. Nanomaterials that are currently used in other industrial sectors were named “manufactured nanomaterials” in the following text. The compilation also included standard methods that were developed and released prior to the definition and adoption of “nano‐specific” terms such as “nanotechnology,” “nanomaterial,” or “nanomedicine.” These standards could not be captured when searching the databases for “nano‐specific” terms. To correct for this, we have added the most important standard methods (labeled with asterisk) that have been used to characterize available nanoscale reference materials (RMs) demonstrating their relevance also for the characterization of nanomedicines.
Due to the complexity and heterogeneity of the documents released by the standardization bodies we propose a distinction between general, “guidance‐like” documents, offering an overview of different methods and more specific documents addressing particular endpoint and defined technique. The general documents should help product developer or other users to understand what kind of measurements are needed and what kind of considerations/special precautions should be taken into account before using a specific test method. Such general documentary standards addressing both physicochemical characterization and safety considerations were gathered in Table 2, whereas more specific documents referring to the physicochemical characterization and safety evaluation were listed in Tables 3 and 4, respectively (Figure 2). The last update was performed in October 2017.
Table 2.
List of general and “guidance‐like” documentary standards on physicochemical and biological characterization of nanomaterials
| Name of the standard, test method or guidance document | Scope | References | Comments |
|---|---|---|---|
| Medical applications | |||
| Biological evaluation of medical devices—Part 22 Guidance on nanomaterials | Physicochemical and biological evaluation | ISO/TR 10993‐22:2017 | For medical devices |
| Guidance on the determination of potential health effects of nanomaterials used in medical devices | Physicochemical and biological evaluation | Scientific Committee on Emerging and Newly Identified Health Risks (2015) | For medical devices |
| Other applications | |||
| Guidance on physicochemical characterization of engineered nanoscale materials for toxicological assessment | Physicochemical characterization | ISO/TR 13014:2012 | |
| Compilation and description of sample preparation and dosing methods for engineered and manufactured nanomaterials | Sample preparation, dosing methods | ISO/TR 16196:2016 | |
| Surface chemical analysis—Characterization of nanostructured materials | Overview of surface characterization methods | ISO/TR 14187:2011 | |
| Nanotechnologies—Guidance on measurands for characterizing nano‐objects and materials that contain them | Characterization of nano‐objects and materials containing nano‐objects | CEN/TS 17010:2016 | |
| Characteristics of working suspensions of nano‐objects for in vitro assays to evaluate inherent nano‐object toxicity | Characterization in the biological medium (stability, dissolution, protein corona, etc.) | ISO/TS 19337:2016 | Refers to ISO 29701 (endotoxin test) |
| Measurement technique matrix for the characterization of nano‐objects | Matrix of available measurement methods/techniques/instruments | ISO/TR 18196:2016 | |
| Compilation and description of toxicological screening methods for manufactured nanomaterials | In vitro, in vivo and ecotoxicological screening methods | ISO/TR 16197:2014 | |
| Use and application of acellular in vitro tests and methodologies to assess nanomaterial biodurability | Biodurability in biological and environmental media | ISO/TR 19057:2017 | |
| Nanoparticles in powder form—Characteristics and measurements | Material specifications and the methods to measure these characteristics | ISO/TS 17200:2013 | |
| Guidelines for the characterization of dispersion stabilitya | Characterization in the biological medium | ISO/TR 13097:2013 | |
| Liquid suspension of magnetic nanoparticles—Characteristics and measurements | Material specifications and the methods to measure these characteristics | ISO/DTS 19807 | Under development |
| Separation and size fractionation for the characterization of metal‐based nanoparticles in water samples | Separation and size fractionation | ISO/AWI TR 20489 | Under development |
| Nanostructured layers for enhanced electrochemical bio‐sensing applications—Characteristics and measurements | Material specifications and the methods to measure these characteristics | ISO/AWI TS 21412 | Under development |
| Nanotechnologies—Guidance on detection and identification of nano‐objects in complex matrices | Identification of nanoparticles in complex matrices | prCEN/TS—PWI 00352012 | Under development |
| Considerations in performing toxicokinetic studies of nanomaterials | Toxicokinetics | ISO/AWI TR 22019 | Under development |
| New guide for collection and generation of environment, health, and safety information for nanomaterials and nano‐enabled products | Environment, health, and safety information | ASTM WK48313 | Under development |
Note. AWI, approved work item (ISO); CEN, adopted by European Committee for Standardization; DTS, draft technical specification; PWI, preliminary work item; TR, technical report; TS, technical specification; WK, work item.
Not designed specifically for nanoscale but relevant for nanomaterials.
Table 3.
Selection of standardized test methods and guides for the assessment of physicochemical properties of nanomaterials relevant for the nanomedicine field
| Name of standard test method or guide | Endpoint | Nanomaterial type | References | Comments |
|---|---|---|---|---|
| Medical applications | ||||
| Standard guide for measurement of electrophoretic mobility and zeta potential of nanosized biological materials | Zeta potential | Biological materials | ASTM E2865‐12 | Refers to biological materials such as proteins, DNA, liposomes |
| New test method for measuring the size of nanoparticles in aqueous media using batch‐mode dynamic light scattering | Particle size | NPs for biomedical applications | ASTM WK54872 | Under development |
| New guide for standard practice for performing electron cryo‐microscopy of liposomes | Particle shape and size distribution | Liposomes | ASTM WK54615 | Under development |
| Other applications | ||||
| Characterization of single‐wall carbon nanotubes using transmission electron microscopy | Particle size, morphology | SWCNTs | ISO/TS 10797:2012 | Last review in 2015; could be adapted to cover other types of NPs |
| Characterization of single‐wall carbon nanotubes using scanning electron microscopy and energy dispersive X‐ray spectrometry analysis | Morphology, elemental composition of impurities including catalysts | SWCNTs, MWCNTs | ISO/TS 10798:2011 | Being adapted to include MWCNTs |
| Characterization of single‐wall carbon nanotubes using near infrared photoluminescence spectroscopy | Chiral indices of semi‐conducting SWCNTs | SWCNTs | ISO/TS 10867:2010 | To be adapted based on review in 2017 |
| Characterization of single‐wall carbon nanotubes using ultraviolet–visible–near infrared (UV–Vis–NIR) absorption spectroscopy | Particle size, purity, and ratio of metallic content to the total SWCNT content in the sample | SWCNTs | ISO/TS 10868:2011 | Could be adapted to other inorganic NPs |
| Characterization of volatile components in single‐wall carbon nanotube samples using evolved gas analysis/gas chromatograph‐mass spectrometry | Characterization of volatile components in SWCNTs samples | SWCNTs | ISO/TS 11251:2010 | To be adapted based on review in 2017 |
| Characterization of single‐wall carbon nanotubes using thermogravimetric analysis | Purity assessment | SWCNTs | ISO/TS 11308:2011 | Being adapted to also include MWCNTs, could be adapted to other inorganic and organic NPs |
| Determination of elemental impurities in samples of carbon nanotubes using inductively coupled plasma mass spectrometry | Purity assessment, chemical composition | SWCNTs and MWCNTs | ISO/TS 13278:2011 | New version approved for publication; could be adapted to other inorganic and organic NPs |
| Characterization of multiwall carbon nanotubes—mesoscopic shape factors | Description of shape using SEM, TEM, viscometry, and light scattering analysis | MWCNTs | ISO/TS 11888:2017 | Relevant also for other types of NPs |
| Surface characterization of gold nanoparticles for nanomaterial specific toxicity screening: FT‐IR method | Surface characteristics | Gold NPs | ISO/TS 14101:2012 | Last review in 2016; relevant also for other types of NPs |
| Use of UV–Vis absorption spectroscopy in the characterization of cadmium chalcogenide colloidal quantum dots | Particle size and concentration | Quantum dots | ISO/TS 17466:2015 | Could be adapted to other metallic NPs |
| Size distribution and concentration of inorganic nanoparticles in aqueous media via single particle inductively coupled plasma mass spectrometry | Particle size and concentration | Inorganic NPs | ISO/TS 19590:2017 | |
| Determination of the specific surface area of solids by gas adsorption—BET methoda | Specific surface area | Powders including nanopowders | ISO 9277:2010 | |
| Colloidal systems—methods for zeta‐potential determination—Parts 1, 2, 3a | Zeta potential | Colloidal systems | ISO 13099‐1:2012, ‐2:2012, ‐3:2014 | |
| Determination of particle size distribution by centrifugal liquid sedimentation methodsa | Particle size | ISO 13318‐1:2001, ‐2:2007, ‐3:2004 | ||
| Small‐angle X‐ray scattering | Particle size | ISO 17867:2015 | ||
| Particle tracking analysis methoda | Particle size | ISO 19430:2016 | ||
| Dynamic light scatteringa | Particle size | ISO 22412:2017 | ||
| Standard guide for measurement of particle size distribution of nanomaterials in suspension by nanoparticle tracking analysis (NTA) | Particle size distribution (guide) | ASTM E2834‐12 | ||
| Standard guide for measurement of particle size distribution of nanomaterials in suspension by photon correlation spectroscopy | Particle size distribution (guide) | ASTM E2490‐09(2015) | ||
| Standard guide for size measurement of nanoparticles using atomic force microscopy | Particle size | ASTM E2859‐11(2017) | ||
| Measurements of particle size and shape distributions by scanning electron microscopy | Particle size and shape distribution | ISO/WD 19749 | Under development | |
| Application of field flow fractionation for characterization of nanomaterial contents | Separation and size distribution | ISO/DTS 21362 | Under development | |
| Protocol for particle size distribution by transmission electron microscopy | Particle size, morphology | ISO/WD 21363 | Under development | |
| New guide for standard guide for the analysis of nanoparticles by single particle inductively coupled plasma mass spectrometry (SP‐ICP‐MS) | Chemical composition | ASTM WK54613 | Under development | |
Note. DTS, draft technical specification; MWCNTs, multiwall carbon nanotubes; SWCNTs, single‐wall carbon nanotubes; TS, technical specification; WD, working draft; WK, work item.
Not designed specifically for nanoscale but relevant for nanomaterials.
Table 4.
Selection of standard test methods for the evaluation of safety of nanoparticles relevant for the nanomedicine field
| Name of standard, test method | Endpoint | Reference | Comments |
|---|---|---|---|
| Medical applications | |||
| Standard test method for analysis of hemolytic properties of nanoparticles | Biocompatibility, hemolytic properties | ASTM E2524‐08(2013) | Similar to practice F756 but modified to accommodate nanoparticulate materials |
| Standard test method for evaluation of cytotoxicity of nanoparticulate materials in porcine kidney cells and human hepatocarcinoma cells | Cytotoxicity assessment using MTT and LDH | ASTM E2526‐08(2013) | |
| Standard test method for evaluation of the effect of nanoparticulate materials on the formation of mouse granulocyte‐macrophage colonies | Immunological response | ASTM E2525‐08(2013) | |
| New test method for quantitative measurement of the chemoattractant capacity of a nanoparticulate material in vitro | Chemoattractant capacity | ASTM WK60373 | Under development |
| Other applications | |||
| Endotoxin test on nanomaterial samples for in vitro systems—Limulus amoebocyte lysate test | Contamination by endotoxin | EN ISO 29701:2010 | Last review in 2016; applies to nanomaterials intended for in vitro tests |
| 5‐(and 6)‐Chloromethyl‐2′,7′‐dichloro‐dihydrofluorescein diacetate (CM‐H2DCF‐DA) assay for evaluating nanoparticle‐induced intracellular reactive oxygen species (ROS) production in RAW 264.7 macrophage cell line | Oxidative stress | ISO/TS 19006:2016 | |
| Electron spin resonance as a method for measuring ROS generated by metal oxide nanomaterials | Oxidative stress | ISO/TS 18827:2017 | |
| In vitro MTS assay for measuring the cytotoxic effect of nanoparticles | Cytotoxicity | ISO/FDIS 19007 | Under development |
| High throughput screening method for nanoparticles toxicity using 3D cells | Cytotoxicity | ISO/AWI TS 22455 | Under development |
Note. AWI, approved work item; DIS: draft international standard; TS, technical specification.
Figure 2.
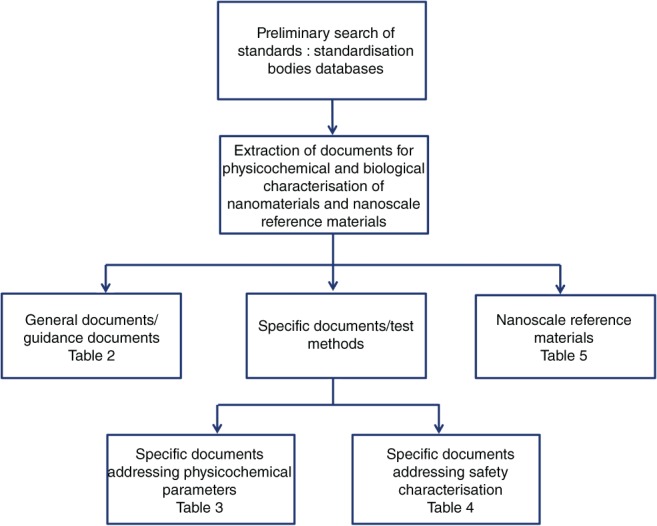
Search strategy and classification applied to extract standards (methods and materials) relevant for nanomedicine
The search for nanoscale RMs was performed at NIST, BAM, JRC, NRC, and AIST websites exploring the category of nanomaterials or via searchable catalogs using “nano,” “nanoparticles,” and “nanomaterials” as keywords. The last update was performed in October 2017.
2.2. Identification of information needs for regulatory purposes
Regulatory needs were identified by extracting: (a) recommendations from EMAs reflection papers (EMA/CHMP, 2013a, 2013b, 2013c, 2015); (b) the workshop report of 16th meeting of the Global Summit of Regulatory Science (GSRS16) which discussed scientific challenges to utilize progresses in nanomaterial measurement science and develop practical standards and the results of three surveys that have been performed with regulatory scientists from European and international institutions (Table 1).
Table 1.
Regulatory bodies invited to participate in the survey
| No. | First survey (IPRF) | Second survey (EU‐innovation network) | Third survey (workshop on “Bridging communities” organized by the JRC) |
|---|---|---|---|
| 1 | Health Canada (Market Health Products), Canada | National Institute of Pharmacy and Nutrition, Hungary | National Institute for Public Health and the Environment, the Netherlands |
| 2 | European Medicines Agency | BfArM—Federal Institute for Drugs and Medical Devices, Germany | BfArM—Federal Institute for Drugs and Medical Devices, Germany |
| 3 | Swiss Agency for Therapeutic Products, Switzerland | Health Products Regulatory Authority, Ireland | Swiss Agency for Therapeutic Products, Switzerland |
| 4 | Health Canada (Health Products and Food Branch), Canada | MEB Medicines Evaluation Board, the Netherlands | Istituto Superiore di Sanità, Roma, Italy |
| 5 | United States Food and Drug Administration, USA | FIMEA Finnish Medicines Agency, Finland | Université de Genève, Switzerland |
| 6 | Pharmaceuticals and Medical Devices Agency, Office of New Drug II, Japan | Austrian Medicines and Medical Devices Agency, Austria | The National Institute of Standards and Technology, USA |
| 7 | Brazilian Health Surveillance Agency, Brazil | State Institute for Drug Control, Czech Republic | Chemistry Manufacturing and Control (CMC), Sanofi, France |
| 8 | Ministry of Food and Drug Administration, Korea | Spanish Agency for Medicines and Medical Devices | Commissariat à l'énergie atomique et aux énergies alternatives, France |
| 9 | Center for Drug Evaluation, Taiwan | MPA Medical Product Agency, Sweden | Swedish Toxicology Sciences Research Center, Sweden |
| 10 | National Institute for Public Health and the Environment, the Netherlands | Infarmed National Authority of Medicines and Health Products, Portugal | University of Liverpool, UK |
| 11 | Swiss Federal Laboratories for Materials Science and Technology, Switzerland | ||
| 12 | Université de Lorraine, France |
The first survey was performed with the working group on nanomedicine of the International Pharmaceutical Regulators Forum (IPRF) (www.i-p-r-f.org/index.php/en) which is discussing emerging questions in order to anticipate regulatory needs specifically related to nanomedicines. This survey was launched in October 2015 and finalized in November 2015 (Bremer, Halamoda‐Kenzaoui, & Borgos, 2018). A second survey (July 2017–September 2017) was done with the European Innovation Offices Network that has been formally established in 2016 in order to facilitate the development of innovative medicines in Europe (http://www.hma.eu/495.html). Finally, a third survey was performed as a follow up of a technical meeting on “Bridging communities in the field of nanomedicine” in which regulatory and academic scientists discussed regulatory needs and scientific/technical solutions (JRC Expert Workshop, 2017). The questions were based on recommendations of the Scientific Committee on Emerging and Newly Identified Health Risks (2015), EMAs reflection papers related to nanomedicines and a similar questionnaire performed within the EU project “NANoREG” on manufactured nanoparticles (NANoREG, 2013). In order to avoid any bias, interviewees had the possibility (and were encouraged) to include additional information not covered by the predefined questions.
3. AVAILABLE STANDARDS FOR NANOMEDICINE
3.1. Guidance documents
Nanomaterials used in the medical field represent a broad range of materials including polymers, liposomes, dendrimers, nanocrystals, metal colloids, etc. Their comprehensive physicochemical and biological characterization is critical to ensure the reproducibility of the manufacturing process and to produce an intended biological effect.
The International Organization for Standardization (ISO) as well as ASTM International have developed and published several standardized test methods, guidance, and reports dedicated to the physicochemical and biological characterization of engineered nanomaterials. ISO, for example, releases three levels of documents: (a) international standards (IS), the highest level normative documents requiring approval by the national standardization bodies; (b) technical specifications (TS), intermediate level normative documents that must be evaluated 3 years after their publication; and (c) technical reports (TR), which do not have a normative status, but rather are informative documents. Most other standardization bodies make a similar distinction between informative, guidance documents, and normative documents.
Table 2 compiles more general informative or normative documents and guidelines offering an overview of existing methods to determine basic physicochemical and toxicological characteristics of nanomaterials. These documents highlight the relevance and the limitations of different techniques and include special considerations for testing of nanomaterials. Only two of them are explicitly intended for medical applications, but also those designed for other applications could be considered relevant for the evaluation of nanomaterials used in the medical field.
The guidance document on the determination of potential health effects of nanomaterials used in medical devices released by the European Commission Scientific Committee on Emerging and Newly Identified Health Risks (2015) provides an update on existing methods for physicochemical and toxicological evaluation of medical devices and provides information about possible difficulties and adaptations required when testing nanomaterials. It refers particularly to the ISO multipart document for the biological evaluation of medical devices (ISO 10993). The latter was recently amended by an additional part 22 containing Guidance on Nanomaterials (Table 2). In fact, a number of medical devices may specifically contain nanostructures or can involuntarily release nanomaterials following degradation, wear or mechanical treatment processes. The guidance covers all aspects of testing including nanomaterial characterization, sample preparation, toxicological evaluation, and risk assessment considerations. An important issue is the biopersistence of inorganic NPs which could cause the risk of long‐term accumulation in tissues. One document, related to the issue of biodurability, is currently under development by ISO (Table 2).
In addition, many guidance documents were developed by the OECD's Working Party on Manufactured Nanomaterials (WPMN), also within the frame of the testing program of manufactured nanomaterials, running from 2007 to 2015. OECD published a series of documents, expert meetings reports and guidance documents covering physicochemical characterization as well as methods for toxicological and ecotoxicological assessments. Moreover, the existing test guidelines and guidance documents for chemicals have been assessed regarding their suitability for nanomaterials (Organisation for Economic Co‐operation and Development, 2009; Rasmussen et al., 2016) and to evaluate the need for developing new test guidelines and guidance documents. It remains an open question whether a similar exercise should be performed for the medical guidance documents and methods to evaluate their suitability for nano‐enabled medical products.
3.2. Test methods for quality assessment
The assessment of the quality of the pharmaceutical product including physicochemical characterization, stability, purity, and sterility of the formulation is an indispensable part of the preclinical evaluation. The relevant guidelines are provided by the International conference on harmonization of technical requirements for registration of pharmaceuticals for human use (ICH). In case of nano‐enabled medicines physicochemical characterization covering a series of properties such as size distribution, shape, surface coating and charge, hydrophobicity, chemical composition, dispersion stability, and others can be particularly complex. Furthermore, these properties should be evaluated not only in the pristine state but also in the relevant biological medium, where the presence of organic molecules can modify them and lead to processes such as dissolution, agglomeration, sedimentation or formation of a protein corona (Halamoda‐Kenzaoui et al., 2015). Because of the very small size and unique properties of NPs many techniques for particle characterization can lead to inaccurate results, particularly in complex media or in the presence of proteins.
The European and American Pharmacopeias (Ph. Eur. and USP) provide standardized methods for the assessment of quality parameters of medicinal products and the requirements relative to the use of adequate materials and methods in the manufacturing process. Up to date they do not contain the methods specifically addressing nanotechnology‐based products. This may well be due to the fact that most of the existing approved nanomedicines are still under patent protection. However, several described techniques for particle size measurement such as dynamic light scattering, laser light diffraction or image analysis are relevant and currently used for the characterization of nanomedicines. They are based on standardized ISO methods: ISO 22412 (Dynamic light scattering), ISO 13320‐1 (Laser diffraction methods) and ISO 13322 (Image analysis) (ISO/TC 24, 1999, ISO/TC 24, 2014). However, their suitability for testing NP‐based medicinal products should be proven.
Table 3 lists ISO and ASTM International standardized test methods and guides specifically addressing nanomaterials. They describe specific methods addressing particular nanomaterial properties, such as size, shape, or purity. Particle size is the most frequent endpoint (followed by shape/morphology) addressed by the methods listed in Table 3 and one of a few endpoints referring to all classes of nanomaterials (Figure 3). Size measurement by dynamic light scattering and by electron cryo‐microscopy are the only methods so far, being developed explicitly for biomedical applications (ASTM WK54872 and WK54615). There is also one method related to the measurement of zeta potential, developed for nanosized biological materials, with a potential to be used for organic NPs such as liposomes. It must be noted that it is not the intention of standard organizations to develop standard methods for a specific class of materials, if the same method can be used for other materials as well. This is reflected, for example, in the check‐list for new work item proposals developed in ISO/TC 229 (ISO/TC 229 N 673). That is why Table 3 contains a few documents (footnote a) that are useful also for nanoparticles even if they were not designed specifically for the nanoscale.
Figure 3.
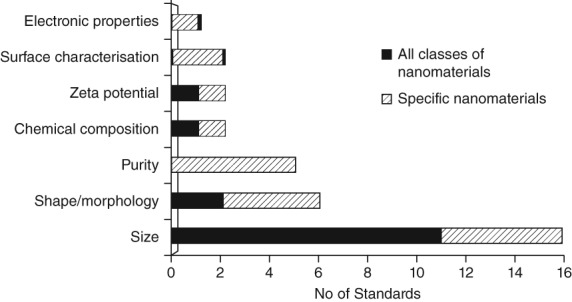
List of current endpoints addressed by the standardized test methods for physicochemical characterization of nanomaterials based on the information given in Table 3
Many methods address one specific type of NPs, particularly carbon nanotubes which are used in industry but their real impact in the nanomedicine field remains limited so far (Hassan et al., 2016; John et al., 2015; Serpell, Kostarelos, & Davis, 2016). However, by slightly modifying the procedures and adapting sample preparation, these methods could be employed for other applications more relevant for nanomedicines. For example, methods assessing the purity of carbon nanotubes with thermogravimetric analysis (TGA) or with TGA coupled with mass spectrometry or with inductively coupled plasma mass spectrometry (ICP‐MS) could be adapted to analyze chemical composition, surface coating or impurities present in nanomedicine formulations based on inorganic or organic nanomaterials. The latter is particularly relevant for nanomedicines, since a major part of them consist of polymers, liposomes or protein‐based formulations. On the other hand, several methods measuring particle size and chemical composition in all types of nanomaterials are now being standardized by ISO and ASTM International (Table 3). Additionally, other standardized methods for particle characterization, but not specifically designed for nanoscale, are also available (ISO/TC 24/SC 4). They address several particle properties such as size, specific surface area or zeta potential and thus are helpful for the characterization of medical NPs when nano‐specific methods are missing.
3.3. Drug loading and drug release evaluation
A crucial property of drug delivery systems is the efficiency of drug loading and the ratio of carrier‐bound or encapsulated drug to free drug after purification determining the quality and the biological activity of the product. These properties need to be evaluated in a physiologically relevant medium or in vivo in order to assess the safety and efficacy of the nanomedicine and to compare the pharmacokinetics (PKs) of the free drug with that of the nanoformulation. The possibility that the free drug is in equilibrium with the protein‐bound drug and as well with the drug in its nanoformulation adds an additional challenge to this task (Stern, Martinez, & Stevens, 2016). When targeting the diseased tissues, the carrier should ideally deliver the drug directly to the diseased cells. Thus, free drug in blood is a sign of imperfect targeting. It appears that the conceptual differences between traditional pharmacology and nanopharmacology even require a consensus on a new terminology to be used (Stern et al., 2016).
Internationally accepted consensus guidelines on how to determine encapsulation efficiency and drug release from the nanoscale delivery systems in parenteral applications do not exist. Due to the peculiarities of systemic release and targeting, an analysis is needed to understand whether new guidelines might be developed on the basis of existing ones dealing with modified‐release of pharmaceutical products (European Medicines Agency, 2013, 2014).
So far, most nanomedicines are based on liposomal carriers and most methods to determine liposomal encapsulation efficiency start with the separation of the encapsulated drug from the free drug by chromatographic techniques, centrifugation, (ultra)filtration or dialysis and analyze both fractions separately (Gómez‐Hens & Fernández‐Romero, 2006). In many cases, the encapsulated drug is quantified as released drug after destruction of the liposomes. Proton nuclear magnetic resonance, electron spin resonance and fluorescence methods were suggested as methods avoiding the physical separation of encapsulated and free drug, but is was argued that these methods are not generally applicable, lack the required sensitivity and are subject to interferences (Itoh, Santa, & Kato, 2015). Recent efforts focus on the development of fast, robust and fully automated procedures capable to ensure that regulatory requirements can be met for a specific product (Deshpande, Gangrade, Kekare, & Vaidya, 2010; Itoh et al., 2015; Yamamoto et al., 2011) rather than on finding methods that could fit a broader range of products with the potential for standardization. Additionally, there is a concern whether chromatographic and extraction techniques that alter the matrix in which free, encapsulated and protein‐bound drug are in equilibrium, are affected by process‐induced artifacts, suggesting ultracentrifugation, equilibrium dialysis, and ultrafiltration as preferable separation methods (Skoczen, McNeil, & Stern, 2015; Stern et al., 2016).
Recently, one broadly applicable alternative method has been proposed that spikes the plasma to be analyzed with a known quantity of stable isotope‐labeled (e.g., 2H or 13C) free drug which then equilibrates with the drug bound to proteins and formulation components (Skoczen et al., 2015). The free drug is separated from the plasma by ultrafiltration and can then be quantified unambiguously in the ultrafiltrate using the ratio of labeled to unlabeled free drug determined by mass spectrometry. Also radiolabelling of carrier and drug with different radioactive labels was used in the assessment of stability and release properties of nanomedicines and their biodistribution patterns in vivo (Crist et al., 2013).
3.4. Test methods for safety assessment
Pharmaceutical products need to undergo a preclinical characterization of their safety and efficacy profile before they can be accepted for clinical trials. Such studies, involving in vitro and in vivo testing, allow the determination of the starting dose for tests in humans and are designed to minimize any risks before the clinical trials (European Medicines Agency, 2017). The guidelines for the preclinical safety assessment, released by the International conference on harmonization of technical requirements for registration of pharmaceuticals for human use (2009), are now applied also to candidate nanomedicines. However, since developed for small molecule drugs, they may not be suitable to address nano‐specific safety issues (Giannakou et al., 2016). Moreover, nanosize‐related properties, such as high reactivity and adsorption capacity, modified optical properties, interaction with other materials, in particular proteins, are not considered in existing methodologies. Indeed, special precautions are required, including appropriate controls and complementary assays, for the reliable testing of nanomaterials (Krug, 2014; Stone, Johnston, & Schins, 2009).
Currently, only three standardized test methods, addressing NPs for biomedical applications are available and one method is under development by ASTM (Table 4). They concern the evaluation of hemolytic and chemo‐attractive properties of NPs, their cytotoxicity and immunological response in vitro.
Other methods, dedicated to toxicity testing of manufactured nanomaterials, are available or are being developed by ISO (Table 4). The presence of endotoxin originating from bacterial contamination has to be excluded before proceeding with other tests as it can invalidate subsequent toxicity studies. Moreover, when introduced to the human body it can lead to serious health disorders (Morris & Li, 2012). Standard methods for the detection and quantification of the endotoxin content in pharmaceutical products are provided in Ph. Eur. One of the commonly used assays is the Limulus amoebocyte lysate‐based assay. However, several types of NPs can interfere with the conventional LAL assay giving an enhancement or an inhibition effect (Dobrovolskaia, 2015; Dobrovolskaia, Neun, Clogston, Grossman, & McNeil, 2014; Li et al., 2015). An adapted version of the LAL assay, suitable for testing nanomaterial samples, was published by ISO and adopted by the European Committee for Standardization (EN ISO 29701:2010) (Table 4). Similar adjustments and modifications may be required for other in vitro test methods.
3.5. Reference materials
The need for RMs for the development and validation of measurement methods and for calibration of instruments is particularly important in the nanotechnology field. A RM is, according to the ISO definition, a “material sufficiently homogeneous and stable with respect to one or more specified properties, which has been established to be fit for its intended use in a measurement process” (International Organization for Standardization/Committee on Reference Materials (ISO/REMCO), 2015). Certified reference materials (CRMs) are not only RMs (sufficiently homogeneous and stable for a specified intended use) but they also have been characterized in a thorough manner that has allowed the RM producer to certify one or more values of the material properties, including their metrological traceability and an estimate of their uncertainty (ISO/REMCO, 2015). Metrologically valid procedures for the production and certification of RMs are given in, among others, ISO 17034 and ISO Guide 35 (International Organization for Standardization/Committee on Conformity Assessment, 2016; ISO/REMCO, 2017). The certification process requires considerable effort and investment and is particularly difficult at the nanoscale, given the complexity of the specific properties of nanomaterials influencing final outcome of the measurements. In many cases, the result of the measurement depends on the applied method, limiting the comparability with other methods and increasing the need of additional reference property values.
An overview of the available nanoscale RMs is given in Table 5. It includes titanium dioxide, single‐wall carbon nanotubes, gold, silica, silver and polystyrene NPs, as well as cellulose nanocrystals and nanoalumina. Most of the assigned property values refer to the size of the particles. For some nanoscale RMs (such as certain titanium dioxide, cellulose nanocrystals, and alumina nanopowders), the specific surface area is the referenced property. Nanomaterials relevant for the nanomedicine field such as liposomes or biodegradable polymeric NPs are still lacking today. In the unfortunate but not uncommon situation where only a limited number of nanoscale RMs and CRMs are available, other materials, so called representative test materials (RTMs) (JRC Nanomaterials Repository—European Commission, 2016), can be used in test method development. RTMs are materials checked for homogeneity and stability in terms of one or more specified properties, but used in a range of properties for which their homogeneity and stability are not tested (Roebben et al., 2013).
Table 5.
Nanoscale reference materials (RMs) and certified reference materials (CRMs)
| Nanomaterial type | Form | Main investigated properties (status) | Nominal value | Organization | Reference number | Additional information |
|---|---|---|---|---|---|---|
| Gold nanoparticles | Aqueous suspension | Particle size (RM) | 3 sizes: 10, 30, and 60 nm | NIST | RM 8011, RM 8012, RM 8013 | Chemical and electrochemical properties |
| Polyvinylpyrrolidone coated silver nanoparticles | Lyophilized | Particle size (RM) | 75 nm | NIST | RM 8017 | Morphology, elemental impurities, zeta potential |
| Nanosilver | Aqueous solution | Particle size distribution (d 10, d 50, d 90) (CRM) | 13 nma | BAM | BAM‐N001 | |
| Nano‐crystalline zinc oxide | Powder | Crystallite size (column lengths and lattice parameters) (CRM) | 2 sizes: 15 and 60 nm | NIST | SRM 1979 | Particle size distribution |
| Colloidal silica | Aqueous suspension | Particle size (CRM) | 20 nm | JRC | ERM‐FD100 | Zeta potential, pH |
| Colloidal silica | Aqueous suspension | Particle size (CRM) | 85 nm | JRC | ERM‐FD101b | Zeta potential, pH, electrolytic conductivity |
| Colloidal silica | Aqueous suspension | Particle size (CRM) | Bimodal: 20 and 85 nm | JRC | ERM‐FD102 | Zeta potential, pH, electrolytic conductivity |
| Colloidal silica | Aqueous suspension | Particle size (CRM) | 40 nm | JRC | ERM‐FD304 | Zeta potential, pH |
| Silicon dioxide | Particle size (RM) | 4 sizes: 951, 458, 296, 143 nm | AQSIQ | GBW 12013, 12014, 12015, 12016 | ||
| Silicon nanoparticles | Toluene suspension | Particle size, Si mass fraction (RM) | 2 nm | NIST | RM 8027 | Size distribution |
| Polystyrene spheres | Aqueous suspension | Particle size (CRM) | 100 nm | NIST | SRM 1963a | Size distribution |
| Polystyrene spheres | Aqueous suspension | Particle size (CRM) | 60 nm | NIST | SRM 1964 | Size distribution |
| Polystyrene particle | Aqueous suspension | Particle size (RM) | 3 sizes: 948, 352, 61 nm | AQSIQ | GBW 12009, 12010, 12011 | |
| Titanium dioxide nanomaterial | Dry powder | Specific surface area (CRM) | 55 m2/g (multipoint BET) or 53 m2/g (single point BET) | NIST | SRM 1898 | Dispersion protocol, pH, particle diameter |
| Titanium dioxide | Dry powder | Specific surface area (CRM) | 8 m2/g (nitrogen BET) | JRC | BCR‐173 | |
| Cellulose nanocrystals | Powder | Impurity mass fractions (CRM), specific surface area, particle size, zeta potential (RM) | 1.29 m2/g (nitrogen BET), 95 nm (DLS) | NRC | CNC‐1 | Oxidation temperature |
| Cellulose nanocrystals | Powder | Mass fraction of elemental sulfur (CRM), particle sizes, crystalline fraction (RM) | 8.7 g/kg S, 70 nm (DLS), 3.4 nm (height by AFM), 88% crystalline (XRD) | NRC | CNCD‐1 | Fiber length, zeta potential |
| Cellulose nanocrystals | Aqueous suspension | Impurity mass fractions (CRM), mass fraction of sulfur, particle size, zeta potential (RM) | 0.5 g/kg S, 80 nm (DLS), 100 nm (length by AFM, TEM), 5 nm (height by AFM, TEM) | NRC | CNCS‐1 | |
| Single‐wall carbon nanotubes | Dry soot | Impurity mass fractions (CRM and RM), specific surface area (RM) | 329 m2/g (nitrogen BET) | NRC | SWCNT‐1 | Morphology, optical absorption, photoluminescence |
| Single‐wall carbon nanotubes | Raw soot | Mass fractions (CRM and RM), oxidation temperature (RM) | NIST | SRM 2483 | ||
| Multiwall carbon nanotubes | Raw soot | Mass fractions of cobalt (CRM) and iron (RM), oxidation temperature (RM) | Co: 0.1821% ± 0.0071 | NIST | SRM 2484 | Morphology (tube length, tube diameter) |
| Single‐wall carbon nanotubes | Surfactant solution | 3‐Length populations (RM) | Long, medium, and short | NIST | RM 8281 | Fluorescence spectra, micrographs |
| Nanoalumina | Powder | Specific surface area (CRM) | 445.4, 359.4, 515.3 m2/g | NCNST | GBW 13901, 13906, 13907 |
Note. AQSIQ, General Administration of Quality Supervision Inspection and Quarantine (China); BAM, Federal Institute for Materials Research and Testing (Germany); JRC, Joint Research Centre of the European Commission; NCNST, National Center for Nanoscience and Technology (China); NIST, National Institute of Standards and Technology (USA); NRC, National Research Council (Canada).
Number of weighted distribution.
4. INFORMATION NEEDS FOR REGULATORY APPROVAL PROCESSES
The regulatory community is well aware on the potential of nanomedicines and actively support activities leading to advancements of the regulatory science in the field of nanomedicine (Ehmann et al., 2013; Pita, Ehmann, & Papaluca, 2016). In order to better anticipate what kind of information should be considered as relevant for regulatory decision‐making, EMA's CHMP released four reflection papers related to block copolymer micelle products (EMA/CHMP, 2013a) and to the coating of nanomedicinal products (EMA/CHMP, 2013b), as well as to intravenous liposomal products (EMA/CHMP, 2013c), and iron‐based products (EMA/CHMP, 2015) developed with reference to innovator products. They provide a list of quality and safety parameters specifically addressing the properties of the nanomedicinal formulation that should be considered during preclinical characterization. A similar approach is reflected in a draft guidance related to liposomal drug products and to drug products containing nanomaterials issued by the USA FDA Center for Drug Evaluation and Research in 2015 and in 2017, respectively (US FDA Center for Drug Evaluation and Research, 2015; US FDA Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), 2017).
Furthermore, the Global Summit of Regulatory Science held a workshop on nanotechnology in Bethesda in 2016 (GSRS16), in which information and standardization needs were discussed. The IPRF has established a working group on nanomedicine that shares the experiences on regulating nano‐enabled products and contributes to harmonization efforts allowing a mutual acceptance of data on various markets. This group responded to a survey aiming to collect the regulators opinion on the relevance of information needs for decision‐making (Bremer, Halamoda‐Kenzaoui, & Borgos, 2018). This survey was one out of three surveys organized by the European Nanomedicine Characterization Laboratory (EU‐NCL) (http://www.euncl.eu). The other two involved the European innovation network and the “Bridging communities” workshop participants, respectively. The results of all the three surveys related to the relevance of the physicochemical parameters of nano‐enabled products are demonstrated in Figure 4.
Figure 4.
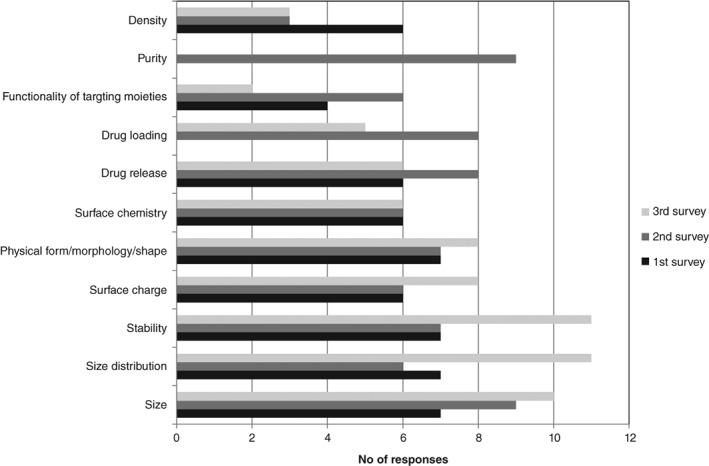
Relevance of the physicochemical parameters for the regulatory decision‐making evaluated in surveys performed within regulatory communities: International Pharmaceutical Regulators Forum (IPRF, first survey), EU‐innovation network (second survey), and “Bridging communities in the field of nanomedicine” workshop of the Joint Research Centre of the European Commission (JRC, third survey)
Most of the recommendations formulated by GSRS16 workshop were confirmed as highly relevant and some additional recommendations were added (Figure 4). Even if a generalization of information needs is very challenging and the information requirements are depending on individual product, the collection of information requirements already elucidated some trends on information needs which are particular for nanomedicines.
5. MAPPING OF INFORMATION NEEDS AGAINST AVAILABLE STANDARDS
As an initial step for the identification of standardization needs, information requirements gathered within the regulatory scientists were mapped against publically available documentary standards (Table 6). The heatmap shows substantial agreement of the regulatory community on information needs specifically addressing the nano‐specific properties of a formulation. The mapping of those information requirements against identified standards demonstrates in which areas new standard development is necessary and for which endpoints standardization activities are ongoing. Moreover, it shows which standardization bodies are active in development of standards relevant for nanomedicine.
Table 6.
Mapping of information requirements against available standards
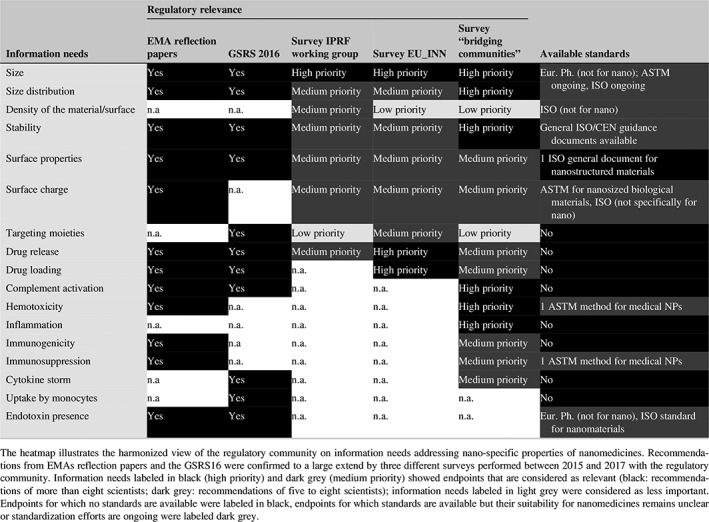
A more detailed view on the type and number of identified standards is provided in Figure 5. For most of the information requirements very few standards designed specifically for nanomedicines are available (Figure 5, black bars). They address size and shape measurement (still under development), surface charge as well as safety parameters including hemolytic and immunological effect.
Figure 5.
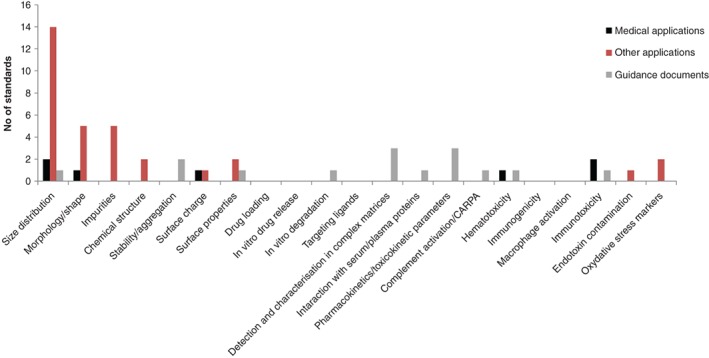
Mapping of the available documentary standards against regulatory needs included in European Medicines Agency (EMA) reflection papers, GSRS16 workshop and surveys within the regulatory community; standards are classified as standardized methods developed for medical applications (black bars) or for not specified applications (red bars) and general documentary standards (gray bars)
However, the mapping exercise allows not only the identification of gaps but it also indicates the endpoints for which standards have been developed in other industrial sectors (Figure 5, red bars) and that could be of relevance for nanomedicines. For example, many standardized methods for size, shape, and purity measurements were developed for nanomaterials, but their suitability to characterize nanomedicines remains unclear. Specific standardized methods addressing surface chemistry, surface charge, or stability are very few. Furthermore, methods assessing drug loading and drug release profiles as well as presence of targeting ligands, which are particularly important for drug delivery systems at nanoscale, are not existing so far (Figure 5).
Detection and characterization of nanomaterials in complex matrices was considered an important issue by the regulatory community (GSRS16). Detection and quantification of nanomaterials in blood and other biological tissues is relevant for the determination of the biodistribution and PK parameters of nanomedicinal product (EMA/CHMP, 2013a; US FDA Center for Drug Evaluation and Research, 2015). Such studies are particularly important for nanocarriers whose PK profile will substantially differ from the active molecule administered in free form. Furthermore, PK parameters of follow‐on nanomedicines will be compared to corresponding innovator products (Tinkle et al., 2014). The elucidation of the final fate of an active substance and of a nanocarrier is also dependent on the possibility to detect the nanomaterials in biological tissues. Guidance documents related to the detection of nanomaterials in complex matrices are in preparation by CEN and ISO (Table 2) but their relevance for the evaluation of toxicokinetic and PKs of nanomedicines has to be first understood and tested in practice. Toxicokinetic parameters are also considered in two guidance documents addressing the medical devices containing nanomaterials (Table 2) but their relevance for nanomedicines needs to be proven.
In addition, nanomedicines might trigger particular effects in biological systems which have to be evaluated in addition to standard toxicity studies. Effects on hematotoxicity, antigenicity, and immunotoxicity including complement activation should be considered in the preclinical development for liposomes and polymeric micelles according to EMA reflection papers (EMA/CHMP, 2013a, EMA/CHMP, 2013c). Moreover, complement activation, cytokine release and other immunological and hematological reactions were identified by regulatory scientists as very relevant for nanomedicines (Table 6). Similarly, the formation of protein corona as the effect of the interaction of NPs with the blood proteins was shown to highly influence the effect on the immune system (Chen et al., 2017; Corbo et al., 2016), even if the exact molecular mechanism is not known up to now. In the recent guidance released by the Food and Drug Administration (FDA) the analysis of the protein corona is recommended for the drug products that contains nanomaterials (US FDA Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), 2017). Among the developed standards dedicated to the nanotechnology field only two methods address immunological response and one method refers to the hemocompatibility of medical NPs. Moreover, no standardized test methods exist for the interaction of NPs with plasma proteins, nor for the complement activation or immunogenicity (Table 6, Figure 5). The assessment of the interaction of a nanomaterial with the immune system can be highly species‐specific (Dobrovolskaia & McNeil, 2013; Najafi‐Hajivar et al., 2016) and standardized methods based on human in vitro systems are needed to complement preclinical testing for intravenously administered nanomedicine products (Halamoda‐Kenzaoui & Bremer‐Hoffmann, in press).
Test method standardization as well as the calibration of instruments would benefit from a pool of nanoscale RMs relevant to medical applications, including liposomes and materials with well characterized surface properties (GSRS16). As stated above, currently the certified properties of the available RMs refer mainly to particle size and there are no nanomaterials with reference values for coating and other surface characteristics (Table 5).
The US NCL (https://nanolab.cancer.gov) and the EU‐NCL have developed and optimized protocols for the physicochemical and biological characterization of candidate nanomedicines. Although, they would need to undergo a thorough validation/standardization process these protocols are promising tools in order to support the regulatory decision‐making. Also, a recently launched H2020 project REFINE (http://refine-nanomed.com/) will support development and validation of experimental methods and approaches relevant for the regulatory review of nanomedicines.
Additional challenges are related to the assessment of generic versus innovative nanomedicines, which requires strategies to assess the bioequivalence of the formulations. The development of strategies, guidance and appropriate methodologies are highly relevant. Clearly, supplementary efforts are necessary to develop and implement a harmonized and standardized methodology for the characterization of quality and safety of nanomedicines.
6. CONCLUSION
With our mapping study we confirmed that also in the field of nanomedicine there is a lack of accepted standards, which could contribute to the uncertainty for product developers. A cross‐fertilization between sectors could be beneficial to enlarge the portfolio of standards relevant for nanomedicines in particular for physicochemical characterization. This would require a review process for the suitability of standards developed for industrial applications. For several endpoints recommended by the regulatory community, no corresponding standards do exist so far (Box 1). This concerns particularly methods assessing drug loading/drug release of drug delivery systems at nanoscale and the interaction with the blood and immune system. Adequate reference nanomaterials representing the major platforms used in nanomedicine need to be developed. Moreover, other important aspects such as critical quality attributes for nanomedicines, prioritization of method development, and method comparability would require consideration in order to advance the field of nanomedicine.
BOX 1. MAJOR GAPS IN STANDARDS FOR NANOMEDICINES.
-
Methods for:
Drug loading and drug release from nanocarriers
Evaluation of the interaction with the immune system, in particular immunogenicity
Investigation of the protein corona
Detection of nanomaterial in biological tissues
Guidance for assessment of the comparability of methods
Reference materials relevant for nanomedicine
Evaluation of the suitability and recognition of standards developed in other sectors
Currently, only limited robust datasets describing the physicochemical properties and toxicological effects are available which makes it difficult to identify information requirements. A close monitoring of the scientific evidence on the next generation nanomedicines is necessary to anticipate these information requirements and to design the corresponding testing methods which will reduce the uncertainty for product developer.
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
RELATED WIREs ARTICLES
Product quality for nanomaterials: Current U.S. experience and perspective
ACKNOWLEDGMENTS
The authors would like to thank Dr Anil Patri from the US Food and Drug Administration for his encouragement to enter in the field of standardization for nanomedicines and his supportive comments on the document. Furthermore, we would like to acknowledge Dr Kirsten Rasmussen and Dr Douglas Gilliland for the helpful discussion and the constructive comments of the manuscript. The work was partially funded by the European Union's Horizon 2020 Framework Programme, under grant agreement no. 654190 referring to EU‐NCL project: “Nanomedicine Characterization Laboratory (EU‐NCL).” Any opinions expressed in this publication are those of the authors only, and this paper does not represent an official position of the European Commission.
Halamoda‐Kenzaoui B, Holzwarth U, Roebben G, Bogni A, Bremer‐Hoffmann S. Mapping of the available standards against the regulatory needs for nanomedicines. WIREs Nanomed Nanobiotechnol. 2019;11:e1531. 10.1002/wnan.1531
Funding information EC Joint Research Centre
NOTE
EMA working definition of nanomedicines: purposely designed systems for clinical applications with at least one component at nano‐scale size; resulting in definable specific properties and characteristics (a) related to the specific nanotechnology application and characteristics for the intended use (route of admin, dose) and (b) associated with the expected clinical advantages of the nanoengineering (e.g., preferential organ/tissue distribution); needs to meet definition as a medicinal product according to European legislation (http://www.ema.europa.eu/docs/en_GB/document_library/Presentation/2014/04/WC500165444.pdf)
REFERENCES
- Alarçin, E. , Guan, X. , Kashaf, S. S. , Elbaradie, K. , Yang, H. , Jang, H. L. , & Khademhosseini, A. (2016). Recreating composition, structure, functionalities of tissues at nanoscale for regenerative medicine. Regenerative Medicine, 11(8), 849–858. 10.2217/rme-2016-0120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelakeris, M. (2017). Magnetic nanoparticles: A multifunctional vehicle for modern theranostics. Biochimica et Biophysica Acta, 1861(6), 1642–1651. 10.1016/j.bbagen.2017.02.022 [DOI] [PubMed] [Google Scholar]
- Bremer, S. , Halamoda‐Kenzaoui, B. , & Borgos, S. E. (2018). Identification of regulatory needs for nanomedicines. Journal of Interdisciplinary Nanomedicine, 3(1), 4–15. [Google Scholar]
- Chen, F. , Wang, G. , Griffin, J. I. , Brenneman, B. , Banda, N. K. , Holers, V. M. , … Simberg, D. (2017). Complement proteins bind to nanoparticle protein corona and undergo dynamic exchange in vivo. Nature Nanotechnology, 12(4), 387–393. 10.1038/nnano.2016.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbo, C. , Molinaro, R. , Parodi, A. , Toledano Furman, N. E. , Salvatore, F. , & Tasciotti, E. (2016). The impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug delivery. Nanomedicine, 11(1), 81–100. 10.2217/nnm.15.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist, R. M. , Grossman, J. H. , Patri, A. K. , Stern, S. T. , Dobrovolskaia, M. A. , Adiseshaiah, P. P. , … McNeil, S. E. (2013). Common pitfalls in nanotechnology: Lessons learned from NCI's nanotechnology characterization laboratory. Integrative Biology, 5(1), 66–73. 10.1039/c2ib20117h [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande, N. M. , Gangrade, M. G. , Kekare, M. B. , & Vaidya, V. V. (2010). Determination of free and liposomal amphotericin B in human plasma by liquid chromatography‐mass spectroscopy with solid phase extraction and protein precipitation techniques. Journal of Chromatography B, 878(3–4), 315–326. 10.1016/j.jchromb.2009.11.036 [DOI] [PubMed] [Google Scholar]
- Dobrovolskaia, M. A. (2015). Pre‐clinical immunotoxicity studies of nanotechnology‐formulated drugs: Challenges, considerations and strategy. Journal of Controlled Release, 220, 571–583. 10.1016/j.jconrel.2015.08.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrovolskaia, M. A. , & McNeil, S. E. (2013). Understanding the correlation between in vitro and in vivo immunotoxicity tests for nanomedicines. Journal of Controlled Release, 172(2), 456–466. 10.1016/j.jconrel.2013.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrovolskaia, M. A. , Neun, B. W. , Clogston, J. D. , Grossman, J. H. , & McNeil, S. E. (2014). Choice of method for endotoxin detection depends on nanoformulation. Nanomedicine (London, England), 9(12), 1847–1856. 10.2217/nnm.13.157 [DOI] [PubMed] [Google Scholar]
- Ehmann, F. , Sakai‐Kato, K. , Duncan, R. , Hernán Pérez De La Ossa, D. , Pita, R. , Vidal, J.‐M. , … Amati, M. P. (2013). Next‐generation nanomedicines and nanosimilars: EU regulators' initiatives relating to the development and evaluation of nanomedicines. Nanomedicine (London, England), 8(5), 849–856. 10.2217/nnm.13.68 [DOI] [PubMed] [Google Scholar]
- European Medicines Agency . (2013, February). Guideline on the pharmacokinetic and clinical evaluation of modified release dosage forms, EMA/CHMP/EWP/280/96.
- European Medicines Agency . (2014, March). Guideline on quality of oral modified release products, 44, EMA/CHMP/QWP/428693/2013. 10.1016/S0022-3913(12)00047-9 [DOI]
- European Medicines Agency . (2017). Guideline on strategies to identify and mitigate risks for first‐in‐human and early clinical trials with investigational medicinal products, EMEA/CHMP/SWP/28367/07 Rev. 1.
- European Medicines Agency/Committee for Medicinal Products for Human Use . (2013a). Joint MHLW/EMA reflection paper on the development of block copolymer micelle medicinal products, EMA/CHMP/13099/2013.
- European Medicines Agency/Committee for Medicinal Products for Human Use . (2013b). Reflection paper on surface coatings: General issues for consideration regarding parenteral administration of coated nanomedicine products, EMA/325027/2013.
- European Medicines Agency/Committee for Medicinal Products for Human Use . (2013c). Reflection paper on the data requirements for intravenous liposomal products developed with reference to an innovator liposomal product, EMA/CHMP/806058/2009/Rev.02.
- European Medicines Agency/Committee for Medicinal Products for Human Use . (2015). Reflection paper on the data requirements for intravenous iron‐based nano‐colloidal products developed with reference to an innovator medicinal product, EMA/CHMP/SWP/620008/2012.
- Fleurke, F. , & Somsen, H. (2011). Precautionary regulation of chemical risk: How REACH confronts the regulatory challenges of scale, uncertainty, complexity and innovation. Common Market Law Review, 48, 357–393. [Google Scholar]
- Giannakou, C. , Park, M. V. D. Z. , De Jong, W. H. , Van Loveren, H. , Vandebriel, R. J. , & Geertsma, R. E. (2016). A comparison of immunotoxic effects of nanomedicinal products with regulatory immunotoxicity testing requirements. International Journal of Nanomedicine, 11, 2935–2952. 10.2147/IJN.S102385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioria, S. , Caputo, F. , Urbán, P. , Maguire, C. M. , Bremer‐Hoffmann, S. , Prina‐Mello, A. , … Mehn, D. (2018). Are existing standard methods suitable for the evaluation of nanomedicines: Some case studies. Nanomedicine (London, England), 13(5), 539–554. 10.2217/nnm-2017-0338 [DOI] [PubMed] [Google Scholar]
- Gómez‐Hens, A. , & Fernández‐Romero, J. M. (2006). Analytical methods for the control of liposomal delivery systems. TrAC, Trends in Analytical Chemistry, 25, 167–178. 10.1016/j.trac.2005.07.006 [DOI] [Google Scholar]
- GSRS16 . (2016). Global summit on regulatory science: Nanotechnology standards and applications. Final Report (pp. 1–35).
- Halamoda‐Kenzaoui, B., & Bremer‐Hoffmann, S. (in press). Main trends of immune effects triggered by nanomedicines in preclinical studies. International Journal of Nanomedicine. [DOI] [PMC free article] [PubMed]
- Halamoda‐Kenzaoui, B. , Ceridono, M. , Colpo, P. , Valsesia, A. , Urbán, P. , Ojea‐Jiménez, I. , … Kinsner‐Ovaskainen, A. (2015). Dispersion behaviour of silica nanoparticles in biological media and its influence on cellular uptake. PLoS One, 10(10), e0141593 10.1371/journal.pone.0141593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan, S. , Pastar, I. , Drakulich, S. , Dikici, E. , Tomic‐Canic, M. , Deo, S. , & Daunert, S. (2017). Nanotechnology‐driven therapeutic interventions in wound healing: Potential uses and applications. ACS Central Science, 3(3), 163–175. 10.1021/acscentsci.6b00371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan, H. A. F. M. , Smyth, L. , Wang, J. T.‐W. , Costa, P. M. , Ratnasothy, K. , Diebold, S. S. , … Al‐Jamal, K. T. (2016). Dual stimulation of antigen presenting cells using carbon nanotube‐based vaccine delivery system for cancer immunotherapy. Biomaterials, 104, 310–322. 10.1016/j.biomaterials.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International conference on harmonization of technical requirements for registration of pharmaceuticals for human use . (2009). ICH guideline M3(R2) on non‐clinical safety studies for the conduct of human clinical trials and marketing authorisation for pharmaceuticals ICH Guidance, https://doi.org/EMA/CPMP/ICH/286/1995
- International Organization for Standardization/Committee on Conformity Assessment . (ISO/CASCO) (2016). General requirements for the competence of reference material producers, ISO 17034:2016.
- International Organization for Standardization/Committee on Reference Materials . (ISO/REMCO) (2015). Reference materials—Selected terms and definitions, ISO Guide 30:2015.
- International Organization for Standardization/Committee on Reference Materials . (ISO/REMCO) (2017). Reference materials—Guidance for characterization and assessment of homogeneity and stability, ISO Guide 35:2017.
- ISO/TC 24 . (1999). Particle size analysis—Laser diffraction methods, ISO 13320‐1.
- ISO/TC 24 . (2014). Particle size analysis—Image analysis method, ISO 13322‐1.
- Itoh, N. , Santa, T. , & Kato, M. (2015). Rapid evaluation of the quantity of drugs encapsulated within nanoparticles by high‐performance liquid chromatography in a monolithic silica column. Analytical and Bioanalytical Chemistry, 407(21), 6429–6434. 10.1007/s00216-015-8805-0 [DOI] [PubMed] [Google Scholar]
- Jalonen, H. (2011). The uncertainty of innovation: A systematic review of the literature. Journal of Management Research, 4(1), e12 10.5296/jmr.v4i1.1039 [DOI] [Google Scholar]
- John, A. A. , Subramanian, A. P. , Vellayappan, M. V. , Balaji, A. , Mohandas, H. , & Jaganathan, S. K. (2015). Carbon nanotubes and graphene as emerging candidates in neuroregeneration and neurodrug delivery. International Journal of Nanomedicine, 10, 4267–4277. 10.2147/IJN.S83777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- JRC Expert Workshop . (2017). Bridging communities in the field of nanomedicine. Retrieved from https://ec.europa.eu/jrc/en/science-update/expert-workshop-nanomedicines-hosted-jrc-27-28-september-2017
- JRC Nanomaterials Repository—European Commission . (2016). Retrieved from https://ec.europa.eu/jrc/en/scientific-tool/jrc-nanomaterials-repository
- Kim, J. , Mohamed, M. A. A. , Zagorovsky, K. , & Chan, W. C. W. (2017). State of diagnosing infectious pathogens using colloidal nanomaterials. Biomaterials, 146, 97–114. 10.1016/j.biomaterials.2017.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug, H. F. (2014). Nanosafety research—Are we on the right track? Angewandte Chemie, 53, 12304–12319. 10.1002/anie.201403367 [DOI] [PubMed] [Google Scholar]
- Li, Y. , Italiani, P. , Casals, E. , Tran, N. , Puntes, V. F. , & Boraschi, D. (2015). Optimising the use of commercial LAL assays for the analysis of endotoxin contamination in metal colloids and metal oxide nanoparticles. Nanotoxicology, 9(4), 462–473. 10.3109/17435390.2014.948090 [DOI] [PubMed] [Google Scholar]
- Morris, M. , & Li, L. (2012). Molecular mechanisms and pathological consequences of endotoxin tolerance and priming. Archivum Immunologiae et Therapiae Experimentalis, 60(1), 13–18. 10.1007/s00005-011-0155-9 [DOI] [PubMed] [Google Scholar]
- Najafi‐Hajivar, S. , Zakeri‐Milani, P. , Mohammadi, H. , Niazi, M. , Soleymani‐Goloujeh, M. , Baradaran, B. , & Valizadeh, H. (2016). Overview on experimental models of interactions between nanoparticles and the immune system. Biomedicine & Pharmacotherapy, 83, 1365–1378. 10.1016/j.biopha.2016.08.060 [DOI] [PubMed] [Google Scholar]
- NANoREG . (2013). Report on a Virtual Workshop to identify, formulate and prioritize issues/questions Retrieved from https://rivm.nl/en/About_RIVM/Mission_and_strategy/International_Affairs/International_Projects/Completed/NANoREG/deliverables/NANoREG_D1_01_DR_Report_on_a_Virtual_Workshop_to_identify_formulate_and_prioritize_issues_questions.org
- Organisation for Economic Co‐operation and Development . (2009). Preliminary review of OECD test guidelines for their applicability to manufactured nanomaterials. Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology. ENV/JM/MONO(2009)21
- Pita, R. , Ehmann, F. , & Papaluca, M. (2016). Nanomedicines in the EU‐regulatory overview. The AAPS Journal, 18, 1576–1582. 10.1208/s12248-016-9967-1 [DOI] [PubMed] [Google Scholar]
- Ramos, A. P. , Cruz, M. A. E. , Tovani, C. B. , & Ciancaglini, P. (2017). Biomedical applications of nanotechnology. Biophysical Reviews, 9(2), 79–89. 10.1007/s12551-016-0246-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen, K. , González, M. , Kearns, P. , Sintes, J. R. , Rossi, F. , & Sayre, P. (2016). Review of achievements of the OECD working party on manufactured nanomaterials’ testing and assessment programme. From exploratory testing to test guidelines. Regulatory Toxicology and Pharmacology, 74, 147–160. 10.1016/j.yrtph.2015.11.004 [DOI] [PubMed] [Google Scholar]
- Roebben, G. , Rasmussen, K. , Kestens, V. , Linsinger, T. P. J. , Rauscher, H. , Emons, H. , & Stamm, H. (2013). Reference materials and representative test materials: The nanotechnology case. Journal of Nanoparticle Research, 15(3), 1455 10.1007/s11051-013-1455-2 [DOI] [Google Scholar]
- Scientific Committee on Emerging and Newly Identified Health Risks . (2015). Opinion on the guidance on the determination of potential health effects of nanomaterials used in medical devices.
- Serpell, C. J. , Kostarelos, K. , & Davis, B. G. (2016). Can carbon nanotubes deliver on their promise in biology? Harnessing unique properties for unparalleled applications. ACS Central Science, 2(4), 190–200. 10.1021/acscentsci.6b00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoczen, S. , McNeil, S. E. , & Stern, S. T. (2015). Stable isotope method to measure drug release from nanomedicines. Journal of Controlled Release, 220(Pt A), 169–174. 10.1016/j.jconrel.2015.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, S. T. , Martinez, M. N. , & Stevens, D. M. (2016). When is it important to measure unbound drug in evaluating nanomedicine pharmacokinetics? Drug Metabolism and Disposition, 44(12), 1934–1939. 10.1124/dmd.116.073148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone, V. , Johnston, H. , & Schins, R. P. F. (2009). Development of in vitro systems for nanotoxicology: Methodological considerations. Critical Reviews in Toxicology, 39(7), 613–626. 10.1080/10408440903120975 [DOI] [PubMed] [Google Scholar]
- The European Parliament and the Council of the European Union . (2012). Regulation (EU) No 1025/2012 of the European Parliament and of the Council on European Standardisation. Official Journal of the European Union.
- Tinkle, S. , McNeil, S. E. , Uhlebach, S. , Bawa, R. , Borchard, G. , Barenholz, Y. , … Desai, N. (2014). Nanomedicines: Addressing the scientific and regulatory gap. Annals of the New York Academy of Sciences, 1313, 35–56. 10.1111/nyas.12403 [DOI] [PubMed] [Google Scholar]
- Tubiana, M. (2001). The precautionary principle: Advantages and risks. Journal de Chirurgie, 138(2), 68–80. [PubMed] [Google Scholar]
- US FDA Center for Drug Evaluation and Research . (2015). Liposome drug products. Guidance for industry.
- US FDA Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) . (2017). Guidance for industry on drug products, including biological products, that contain nanomaterials. Guidance for industry.
- Yamamoto, E. , Hyodo, K. , Ohnishi, N. , Suzuki, T. , Ishihara, H. , Kikuchi, H. , & Asakawa, N. (2011). Direct, simultaneous measurement of liposome‐encapsulated and released drugs in plasma by on‐line SPE‐SPE‐HPLC. Journal of Chromatography B, 879(30), 3620–3625. 10.1016/j.jchromb.2011.10.004 [DOI] [PubMed] [Google Scholar]
- Yoon, H. Y. , Jeon, S. , You, D. G. , Park, J. H. , Kwon, I. C. , Koo, H. , & Kim, K. (2017). Inorganic nanoparticles for image‐guided therapy. Bioconjugate Chemistry, 28(1), 124–134. 10.1021/acs.bioconjchem.6b00512 [DOI] [PubMed] [Google Scholar]


