Abstract
A new formulation of levothyroxine sodium has been developed in the form of an oral solution contained in unit‐dose ampules. A study has been conducted to compare the bioavailability of levothyroxine sodium oral solution and levothyroxine sodium soft capsule in healthy volunteers under fasting conditions. The rate and extent of absorption of the new levothyroxine solution were also evaluated when administered on dilution in water or directly into the mouth without water. In each period, according to the randomization scheme, subjects were administered single oral doses of either test, as 4 × 150‐μg unit‐dose ampules, with or without water, or reference, as 4 × 150‐μg capsules in a crossover design. Thirty‐six subjects were randomized and dosed in this study; of these, 31 completed all study periods. When comparing the solution with the capsule (both products administered with water), the 90% confidence intervals for the ratio of log‐transformed values of AUC0‐48 and Cmax were within 90.00% and 111.11%, respectively, for baseline‐corrected levothyroxine. Moreover, the administration of levothyroxine oral solution without water did not affect the rate and extent of its absorption. In conclusion, levothyroxine oral solution unit‐dose ampules were bioequivalent to the levothyroxine capsule when administered with or without water. All formulations were well tolerated, with no major side effects.
Keywords: bioavailability, bioequivalence, levothyroxine, oral solution, pharmacokinetics
Levothyroxine (l‐thyroxine) sodium is the sodium salt of the levo isomer of the thyroid hormone thyroxin (T4). Synthetic T4 is chemically identical to that produced in the human thyroid gland. Levothyroxine is indicated as a replacement or supplemental therapy in congenital or acquired hypothyroidism of any etiology and as an adjunct to surgery and radioiodine therapy in the management of well‐differentiated thyroid cancer. The dose of levothyroxine depends on a variety of factors, including among others body weight and the condition being treated. It must be individualized and adjustments made based on periodic assessments of the patient's clinical response and laboratory parameters.
Absorption of orally administered levothyroxine from the gastrointestinal tract varies from 40% to 80%.1, 2 The majority of the levothyroxine dose is absorbed from the jejunum and upper ileum.3 T4 absorption is increased by fasting4, 5 and decreased in malabsorption syndromes and by certain foods such as soybeans6, 7 and dietary fiber,8 but also grapefruit juice,9 papaya,10 and coffee.11 Many drugs and supplements (such as calcium, iron, and proton pump inhibitors, just to cite a few) are also known to interfere with levothyroxine absorption.12 The major pathway of thyroid hormone metabolism is through sequential deiodination. Approximately 80% of circulating T3 is derived from peripheral T4 by monodeiodination. Levothyroxine is eliminated slowly from the body, with a half‐life of 6‐7 days under normal conditions, whereas T3 half‐life ranges within 1 to 2 days.13, 14
Levothyroxine oral formulations commercially available in the United States include tablets and soft‐gel capsules. The absorption of levothyroxine in soft‐gel capsules has proven to be equivalent to that of tablets in healthy subjects under fasting conditions,15, 16 whereas it appears to be improved with respect to tablets under altered gastric pH conditions.17, 18, 19, 20 Oral solutions are available in some countries in multidose bottles requiring use of a dropper or other devices for dosing. A new formulation of levothyroxine sodium has been developed in the form of an oral solution contained in unit‐dose ampules, with strengths ranging from 13 to 200 μg. Such a formulation (free from ethanol, propylene glycol, and preservatives) aims to facilitate administration in patients who may have difficulty in swallowing tablets or capsules, such as children or the elderly, and to provide the exact dose. This study has been conducted to compare the bioavailability of levothyroxine oral solution and levothyroxine soft‐gel capsule under fasting conditions. A Tirosint (manufactured by IBSA Institut Biochimique SA, Pambio‐Noranco, Switzerland) 150‐μg capsule was selected as the reference product because it is the reference listed drug for levothyroxine capsules as per the Orange Book. Considering the very similar composition of the 2 products, this was considered the most relevant reference for the oral solution. The rate and extent of absorption of the new levothyroxine solution were also evaluated when administered on dilution in water or directly into the mouth without water.
Subjects and Methods
Study Design
This was a single‐center comparative bioavailability, open‐label, randomized, single‐dose, 3‐period, 6‐sequence crossover study under fasting conditions. This study was carried out at inVentiv Health Clinique, Inc., Québec City, Québec, Canada, a Syneos Health company. The study consisted of 3 single‐dose administrations of levothyroxine oral solution (with and without water) or a levothyroxine capsule. Each period was separated by a washout period of 35 days. The study was open label. Because comparative BA studies involve a comparison of pharmacokinetic (PK) profiles, which are not subjective measurements, blinding was not deemed necessary for this study.
All clinical work was conducted in compliance with Good Clinical Practices as referenced in the International Council for Harmonization guidelines (ICH E6), Good Laboratory Practices as referenced in the ICH guidelines, local regulatory requirements, and the recommendations laid down in the most recent version of the Declaration of Helsinki. The clinical study protocol, any related associated documents, and informed consent forms were reviewed and approved by an independent ethics committee (Institutional Review Board Services, Aurora, Ontario, Canada), prior to beginning associated study procedures. All participants provided written informed consent prior to the start of any study procedure.
Study Population
Subjects enrolled in this study were members of the community at large. Subject screening procedures were performed within 28 days prior to first study drug administration and included informed consent, inclusion/exclusion check, medical and medication histories, a concomitant medication check, demographic data (sex, age, race, and ethnicity), body measurements (height, weight, and body mass index [BMI]), physical examinations, measurement of vital signs (blood pressure, heart rate, respiratory rate, and oral temperature), a 12‐lead electrocardiogram (ECG), a urine drug screen, a urine pregnancy test (female subjects), clinical laboratory measurements (biochemistry, hematology, endocrinology in serum [total T3, free T4, and thyroid‐stimulating hormone], serology [human immunodeficiency virus, hepatitis C virus antibodies, and hepatitis B surface antigen], and urinalysis).
All participating subjects were judged eligible for the study when assessed against the inclusion and exclusion criteria.
Study Procedures
For each period, subjects were confined from at least 10 hours before dosing until after the 48‐hour postdose blood draw. Subjects came back for a safety visit approximately 1 month following the last dose of levothyroxine. In each period, according to the randomization scheme, subjects were administered a single oral dose of either the test levothyroxine sodium, as 4 × 150‐μg unit‐dose ampules of oral solution (manufactured by IBSA Institut Biochimique SA) administered with water (treatment A) or without water (treatment B), or the reference levothyroxine sodium (Tirosint) 4 × 150‐μg soft‐gel capsules (treatment C). When administered with water, the oral solution was diluted in 140 mL of water, and the container was rinsed twice with 50 mL of water, which was then consumed by the subjects (total amount of water consumed was therefore 240 mL). When administered without water, the unit‐dose ampules of oral solution were squeezed directly into each subject's mouth. The reference capsules were administered with 240 mL of water. Doses were administered after an overnight fast of at least 10 hours, and subjects subsequently fasted for a period of at least 4 hours.
A total of 19 blood samples were collected in each period: −0.5 hours, −0.25 hours, and within 5 minutes (0 hours) predose and 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 8, 10, 12, 16, 24, and 48 hours postdose. Actual sampling times were used for statistical analyses. A dead volume intravenous catheter was used for blood collection to avoid multiple skin punctures. Otherwise, blood samples were collected by direct venipuncture.
Pregnancy tests were performed for all women at screening, before dosing in each period, and for study exit procedures. Clinical laboratory tests (biochemistry, hematology, endocrinology, and urinalysis) were performed for each subject at the time of screening, before dosing of periods 2 and 3, and for study exit procedures. In addition, endocrinology tests were performed at the safety return visit, which occurred approximately 1 month following the last dose of levothyroxine. Physical examinations were performed at the time of screening procedures and before dosing of periods 2 and 3. ECG measurements were performed at the time of screening, before dosing and approximately 48 hours postdose in each period and at study exit. ECG performed 48 hours postdose of period 3 could have been used as the ECG required at study exit. Measurement of vital signs (blood pressure, heart rate, respiratory rate, and oral temperature) were performed at the time of screening and for study exit procedures. In addition, seated blood pressure and heart rate measurements were performed before dosing and approximately 2, 4, 8, 12, and 24 hours postdose in each period. Throughout the study, subjects were monitored for adverse events (AEs).
Analytical Methods
Blood was collected in serum spray‐coated silica tubes and allowed to clot at room temperature for at least 30 minutes. The serum was then separated by centrifugation (approximately 1240g × 10 minutes at room temperature) and harvested within 148 minutes of collection. The serum samples were then stored at −20°C until assayed. The serum concentration of total levothyroxine was determined by a validated high‐performance liquid chromatography/tandem mass spectrometric method, as per the most recent US Food and Drug Administration (FDA) and European Medicines Agency (EMA) validation guidelines.21, 22 During the method validation, the accuracy, precision, within‐run, between‐run, selectivity, and matrix effect as well as the stability (stability in whole blood at 4°C, short‐term stability in the matrix at room temperature, freeze‐thaw stability at −20°C/−80°C, long‐term stability at −20°C/−80°C) were evaluated, and all tests met the acceptance criteria.
The levothyroxine and its internal standard, thyroxine‐13C6, were extracted simultaneously from the human samples using automated solid‐phase extraction. More specifically, 50 μL of serum sample was mixed with 50 μL of 1% ascorbic acid, 1 mL of 0.1 M citric acid, and 50 μL of internal standard solution. After vortex mixing, all the samples were centrifuged at approximately 2000g for 5 minutes at room temperature. A 900‐μL aliquot of each sample was then transferred onto an extraction plate preconditioned with methanol and 0.1N hydrochloric acid. The aliquots in the extraction plate were evaporated under vacuum and washed with 400 μL of methanol and 400 μL of 0.1N hydrochloric acid. The compounds were then eluted with 400 μL of methanol/ammonium hydroxide (95/5). The eluates were subsequently evaporated under an N2 current at 60°C and reconstituted with 150 μL of water/methanol (36/64) and ammonium hydroxide 0.04%. The sample extract was then loaded onto an ACE 3 C18 30 × 4.6 mm, 3 μm (Life Science, Peterborough, Ontario, Canada) for separation. The mobile phase was composed of water/methanol (36/64), ammonium acetate 2 mM, and acetic acid 0.1% (v/v). The high‐pressure liquid chromatographic effluent was introduced into a Sciex API‐5000 Tandem Mass Spectrometer equipped with an electrospray ionization source for levothyroxine. Positive ions were detected in the multiple reaction monitoring mode with precursor → product ion pairs of 777.7 m/z → 731.7 m/z or levothyroxine and 783.7 m/z → 737.7 m/z for thyroxine‐13C6. The analytical range of 25‐250 ng/mL had within‐run coefficient of variation (CV) ranging between 1.14% and 2.48% and a between‐run CV ranging between 3.71% and 10.58%. The randomization scheme was not to be available to the bioanalytical division of inVentiv until the clinical and analytical phases had been completed.
The internal standard, thyroxine‐13C6, was supplied by the Toronto Research Chemical Inc. (North York, Ontario, Canada) and levothyroxine (USP grade) by USP (Rockville, Maryland). The ascorbic acid (CAS50‐81‐7; Sigma grade), citric acid monohydrate (CAS 5949‐29‐1), and ammonium hydroxide (CAS 1336‐21‐6; ACS grade) were purchased from Sigma (Oakville, Ontario, Canada), whereas the methanol (CAS 67‐56‐1; Ominsolv grade) and 0.1N hydrochloric acid (CAS 7647‐01‐0; 0.1000N ± 0.0002 grade), ammonium acetate (CAS 631‐61‐8; AnalaR grade), acetic acid, glacial (CAS 64‐19‐7; AnalaR grade), and 1N sodium hydroxide (CAS 7732‐18‐5; AnalaR grade) were supplied by EMD (Toronto, Ontario, Canada). The T3/T4‐free human serum was obtained from Dia Source (Nivelles, Belgique).
Pharmacokinetic and Statistical Calculations
Pharmacokinetic analyses were performed using Pharsight Knowledgebase Server version 4.0.2 and WinNonlin 5.3, which were validated for bioequivalence studies. The following pharmacokinetic parameters were calculated by standard noncompartmental methods for total levothyroxine: Cmax, Tmax, and AUC0‐48.
Levothyroxine is an endogenous compound and therefore was analyzed both with and without baseline correction. For each subject and treatment period, the baseline levothyroxine value was defined as the mean of the ‐0.5‐, ‐0.25‐, and 0‐hour sample concentrations, and this value was attributed to the predose sample concentration. For baseline correction, the baseline value (mean of the 3 predose samples) was subtracted from each measured concentration, including the predose concentration, meaning that the predose concentration was equal to zero. If baseline‐adjusted postdose concentrations were negative, concentrations were set to zero. Uncorrected and baseline‐corrected data were presented for total levothyroxine. Results without baseline correction were provided for information purposes.
Using general linear model procedures in Statistical Analysis System (SAS), analysis of variance (ANOVA) was performed on untransformed Tmax and on ln‐transformed AUC0‐48 and Cmax at the α level of 0.05. Factors incorporated in the model included: sequence, subject (sequence), period, and treatment. Intra‐ and intersubject coefficients of variation were estimated. The ratio of means (A/C, B/A, and B/C) and 90% geometric confidence interval for the ratio of means, based on least‐squares means from the ANOVA of the ln‐transformed data, were calculated for AUC0‐48 and Cmax. Bioequivalence between the test (A) and reference (C) products was to be concluded if, for baseline‐corrected total levothyroxine, the 90% geometric confidence intervals of the ratio (A/C) of least‐squares means from the ANOVA of the ln‐transformed AUC0‐48 and Cmax were within the acceptable range of 80.00% to 125.00%.
Treatment‐emergent adverse events (TEAEs) were summarized descriptively for all subjects who were dosed (safety population).
Results
Participants
In total, 36 subjects (18 women and 18 men) were randomized and dosed. Of these, 31 subjects completed all study periods. Three subjects elected to withdraw for personal reasons, 1 subject did not complete period 2 for personal reasons (but came back for period 3), and 1 subject was withdrawn prior to dosing in period 3 because of a significant AE (alanine aminotransferase increased). Data from all subjects who completed at least 2 study periods, allowing performance of the comparisons between treatments A/C and/or B/C and/or B/A and for whom the PK profile was adequately characterized were used for PK and statistical analyses (n = 34 for comparison A/C, n = 31 for comparison B/C, n = 32 for comparison B/A). Demographic characteristics are presented in Table 1.
Table 1.
Descriptive Statistics of Demographic Data for Subjects Included in the Pharmacokinetic Population
| PK Population | ||||
|---|---|---|---|---|
| Category | Comparison (A/C), n = 34 | Comparison (B/A), n = 32 | Comparison (B/C), n = 31 | |
| Age (years) | Mean ± SD | 35 ± 9 | 36 ± 9 | 36 ± 9 |
| Range | 23–50 | 23–50 | 23–50 | |
| Median | 35 | 35 | 35 | |
| BMI (kg/m2) | Mean ± SD | 24.62 ± 3.01 | 24.51 ± 3.11 | 24.35 ± 3.02 |
| Range | 19.54–29.93 | 19.54–29.93 | 19.54–29.93 | |
| Median | 24.44 | 23.92 | 23.69 | |
| Height (cm) | Mean ± SD | 168.8 ± 7.8 | 168.6 ± 7.6 | 168.5 ± 7.6 |
| Range | 156.5–186.0 | 156.5–186.0 | 156.5–186.0 | |
| Median | 166.3 | 166.3 | 165.5 | |
| Weight (kg) | Mean ± SD | 70.49 ± 12.27 | 70.02 ± 12.26 | 69.43 ± 11.98 |
| Range | 52.70–103.20 | 52.70–103.20 | 52.70–103.20 | |
| Median | 71.10 | 70.50 | 70.50 | |
PK, pharmacokinetic; n, number of observations; SD, standard deviation; BMI, body mass index.
A: levothyroxine sodium oral solution administered with water.
B: levothyroxine sodium oral solution administered without water.
C: levothyroxine sodium capsule.
Pharmacokinetic Assessment
Mean baseline‐corrected serum levothyroxine concentration‐over‐time profiles for all treatments are shown in Figure 1. Key pharmacokinetic parameters for each treatment are summarized in Table 2. When comparing the solution with the capsule (both products administered with water), the 90% geometric confidence intervals (CIs) of the least‐squares means (LSM) ratios were within the prespecified bounds of 80.00%‐125.00% and even between 90.00% and 111.11%, for both Cmax and AUC0‐48, indicating that the solution and capsule formulations were bioequivalent (Figure 2). Moreover, the administration of levothyroxine oral solution without water did not affect the rate and extent of its absorption with respect to administration with water because 90% geometric CIs of the LSM ratios were also within 90.00% to 111.11% for AUC0‐48 and Cmax (Figure 2). An additional analysis (treatment B vs treatment C) also showed that, when administered without water, the solution was bioequivalent to the capsule. The intrasubject CVs for AUC0 48 and Cmax were low, with values of 8.99% and 8.91%, respectively.
Figure 1.
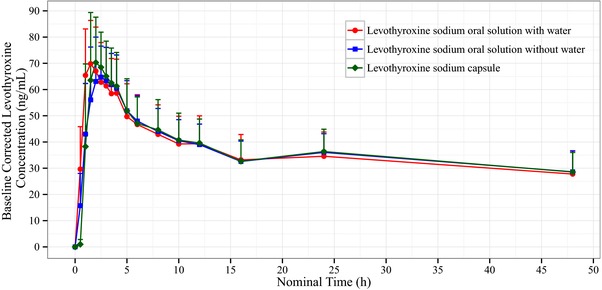
Mean concentration‐time profile for baseline‐corrected levothyroxine for each treatment (n = 35 for the levothyroxine sodium oral solution with water, n = 32 for the levothyroxine sodium oral solution without water, and n = 34 for the levothyroxine sodium capsule).
Table 2.
Summary of Pharmacokinetic Parameters for Baseline‐Corrected Serum Levothyroxine for Each Treatment
| Levothyroxine Sodium Oral Solution With Water (A) | Levothyroxine Sodium Oral Solution Without Water (B) | Levothyroxine Sodium Capsule (C) | |
|---|---|---|---|
| n | 35 | 32 | 34 |
| AUC0–48 a | 1739.26 ± 438.25 | 1755.86 ± 330.86 | 1764.14 ± 380.88 |
| (ng·h/mL) | (25.20) | (18.84) | (21.59) |
| Cmax a | 72.66 ± 16.67 | 71.30 ± 14.19 | 76.64 ± 16.48 |
| (ng/mL) | (22.95) | (19.91) | (21.51) |
| Tmax b | 1.50 | 2.50 | 2.00 |
| (h) | (1.00–4.00) | (1.00–5.00) | (1.00–4.00) |
Mean ± SD (CV%).
Median (Min‐Max).
Figure 2.
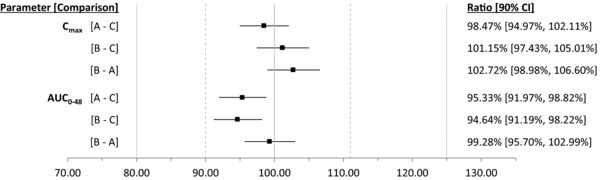
Ratios and 90% geometric confidence intervals for AUC0‐48 and Cmax for baseline‐corrected serum levothyroxine. (A) Levothyroxine sodium oral solution administered with water. (B) Levothyroxine sodium oral solution administered without water. (C) Levothyroxine sodium capsule.
Statistical results obtained on data uncorrected for baseline were comparable to those described above, as all 90%CIs were also within 90.00% to 111.11% for both Cmax and AUC0‐48. These results are not shown, as this constituted a secondary analysis.
Safety Assessment
No serious adverse events were reported during this study. A total of 47 TEAEs were reported by 24 of the 36 subjects who received at least 1 dose of the study medication (safety population). The breakdown by treatment group was as follows: 17 TEAEs reported by 31.4% (n = 11) of the 35 subjects who received levothyroxine oral solution with water (A), 9 TEAEs reported by 26.5% (n = 9) of the 34 subjects who received levothyroxine oral solution without water (B), and 21 TEAEs reported by 47.1% (n = 16) of the 34 subjects who received the levothyroxine capsule (C). There were no relevant differences between each treatment group when comparing the number of subjects for each TEAE. The most commonly reported TEAEs by subjects who constituted the safety population were headache, reported by 27.8% (n = 10); nasopharyngitis, reported by 13.9% (n = 5); and back pain, reported by 8.3% (n = 3) . All other TEAEs were reported by 2 subjects or fewer. The majority of the TEAEs were resolved spontaneously without any countermeasure.
Discussion
This study was properly designed to compare the bioavailability of different levothyroxine formulations. A supratherapeutic dose of 600 μg was administered to minimize the impact of endogenous levothyroxine levels.23, 24 The mean of 3 baseline concentrations (‐0.5, ‐0.25, and 0 hours) was used to calculate baseline‐adjusted pharmacokinetic parameters, which was considered an adequate method to characterize levothyroxine baseline. Although levothyroxine is considered a drug with a long half‐life, the comparison of extent of bioavailability was based on AUC truncated at 48 hours. This ensured that levothyroxine concentrations were maintained above baseline levels, making the baseline correction method more reliable. A washout of 35 days was also deemed sufficient to avoid any carryover effect. The above study design considerations were in line with the FDA guidance on levothyroxine pharmacokinetic and bioavailability studies that was applicable at the time the study was conducted.25
The aim of that study was to assess the bioequivalence between levothyroxine oral solution contained in unit‐dose ampules diluted in water and a reference levothyroxine capsule under fasting conditions. It was demonstrated in that study that levothyroxine oral solution was bioequivalent when diluted in water to the capsule formulation. This was based on the finding that 90%CIs for both AUC0‐48 and Cmax were within the prespecified bounds of 80.00%‐125.00% and even within 90.00% to 111.11% (as required for narrow therapeutic index drugs by the current EMA guideline on bioequivalence studies26) for both Cmax and AUC0‐48, indicating that the 2 products were bioequivalent. The rate and extent of absorption of the new solution were also evaluated when administered on dilution in water or directly into the mouth without water. This study has also shown that the levothyroxine solution can be administered directly into the mouth from the unit‐dose ampule because this mode of administration does not change the rate and extent of absorption of levothyroxine.
It has been suggested that levothyroxine in the form of an oral solution may be less prone to the impact of some factors on the absorption of this drug compared with conventional tablets.27, 28 For instance, previous studies suggested that an oral solution was less affected than the tablet formulation with regard to levothyroxine malabsorption that could be caused by proton pump inhibitors29 or other interfering drugs.30, 31, 32 It was also reported that oral liquid levothyroxine formulations could diminish the problem of levothyroxine malabsorption caused by lactose intolerance33 or Helicobacter pylori 34 infection or bariatric surgery when using traditional tablet formulations. Other reports showed better stabilization in the thyroid hormones profile with liquid thyroxine as opposed to tablet formulation.35, 36, 37, 38
In conclusion, the levothyroxine oral solution unit‐dose ampules were bioequivalent to levothyroxine capsules within tightened confidence intervals when administered with or without water under fasting conditions. This new formulation may offer an additional treatment option for patients who have difficulty in swallowing solid dosage forms, potentially increasing patients’ adherence to therapy. The unit‐dose containers allow delivery of the exact dose of the oral solution, thus minimizing the risks related to drops count or use of a pipette.
Acknowledgments
We are grateful to Olivier Barrière (Syneos Health) for providing graphical design support. We thank Betty Stamatiou (Syneos Health) for her bioanalytical expertise and her contribution to the analytical methods section.
Declaration of Conflicting Interests
Mario Tanguay, Johanne Girard, and Richard Larouche report that their employer, Syneos Health, was the contract research organization contracted to conduct the present study. Claudia Scarsi and Giuseppe Mautone report that their employer, IBSA Institut Biochimique SA, funded the present study.
Funding
IBSA Institut Biochimique SA funded the present study.
References
- 1. Wonsick C, Hays MT. Absorption of oral thyroxine. Endocrinologist, 1995;5:222–228. [Google Scholar]
- 2. Hays MT. Thyroid hormone and the gut. Endocr Res. 1988;14:203–224. [DOI] [PubMed] [Google Scholar]
- 3. Hays MT. Localization of human thyroid absorption. Thyroid. 1991;1:241–248. [DOI] [PubMed] [Google Scholar]
- 4. Wenzel KW, Kirschsieper HE. Aspects of the absorption of oral L‐thyroxine in normal man. Metabolism. 1977;26:1–8. [DOI] [PubMed] [Google Scholar]
- 5. Bach‐Huynh TG, Nayak B, Loh J, et al. Timing of levothyroxine administration affects serum thyrotropin concentrations. J Clin Endocrinol Metab. 2009;94:3905–3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bell DS, Ovalle F. Use of soy protein supplement and resultant need for increased dose of levothyroxine. Endocr Pract. 2001;7:193–194. [DOI] [PubMed] [Google Scholar]
- 7. Pinchera A, Macgillivray MH, Crawford JD, Freeman AG. Thyroid refractoriness in an athyreotic cretin fed soybean formula. N Engl J Med. 1965;273:83–87. [DOI] [PubMed] [Google Scholar]
- 8. Liel Y, Harman‐Boehm I, Shany S. Evidence for a clinically important adverse effect of fiber‐enriched diet on the bioavailability of levothyroxine in adult hypothyroid patients. J Clin Endocrinol Metab. 1996;81:857–859. [DOI] [PubMed] [Google Scholar]
- 9. Lilja JJ, Laitinen K, Neuvonen PJ. Effect of the grapefruit juice on the absorption of levothyroxine. Br J Clin Pharmacol. 2005;60:337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deiana L, Marini S, Mariotti S. Ingestion of large amounts of papaya fruit and impaired effectiveness of levothyroxine therapy. Endocr Pract. 2012;18:98–100. [DOI] [PubMed] [Google Scholar]
- 11. Benvenga S, Bartolone L, Pappalardo MA, et al. Altered intestinal absorption of levothyroxine caused by coffee. Thyroid. 2008;18:293–301. [DOI] [PubMed] [Google Scholar]
- 12. Benvenga S, Ruggeri RM, Trimarchi F. Thyroid and drugs In: Monaco F, ed. Thyroid Diseases. Boca Raton, FL; CRC Press; 2012:482–483. [Google Scholar]
- 13. Fish LH, Schwartz HL, Cavanaugh J, et al. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316(13):764–770. [DOI] [PubMed] [Google Scholar]
- 14. Nicoloff JT, Low JC, Dussault JH, Fisher DA. Simultaneous measurement of thyroxine and triiodothyronine peripheral turnover kinetics in man. J Clin Invest. 1972;51(3):473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Colucci P, D'Angelo P, Mautone G, Scarsi C, Ducharme MP. Pharmacokinetic equivalence of a levothyroxine sodium soft capsule manufactured using the new food and drug administration potency guidelines in healthy volunteers under fasting conditions. Ther Drug Monit. 2011;33(3):355–361. [DOI] [PubMed] [Google Scholar]
- 16. Al‐Numani D, Scarsi C, Ducharme MP. Levothyroxine soft capsules demonstrate bioequivalent pharmacokinetic exposure with the European reference tablets in healthy volunteers under fasting conditions. Int J Clin Pharmacol Ther. 2016;54(2):135–143. [DOI] [PubMed] [Google Scholar]
- 17. Pabla D, Akhlaghi F, Zia H. A comparative pH‐dissolution profile study of selected commercial levothyroxine products using inductively coupled plasma mass spectrometry. Eur J Pharm Biopharm. 2009;72(1):105–110. [DOI] [PubMed] [Google Scholar]
- 18. Seng Yue C, Benvenga S, Scarsi C, Loprete L, Ducharme MP. When bioequivalence in healthy volunteers may not translate to bioequivalence in patients: differential effects of increased gastric pH on the pharmacokinetics of levothyroxine capsules and tablets. J Pharm Pharm Sci. 2015;18(5):844–855. [DOI] [PubMed] [Google Scholar]
- 19. Santaguida MG, Virili C, Del Duca SC, et al. Thyroxine softgel capsule in patients with gastric‐related T4 malabsorption. Endocrine. 2015;49(1):51–57. [DOI] [PubMed] [Google Scholar]
- 20. Vita R, Benvenga S. Tablet levothyroxine (L‐T4) malabsorption induced by proton pump inhibitor; a problem that was solved by switching to L‐T4 in soft gel capsule. Endocr Pract. 2014;20(3):e38–e41. [DOI] [PubMed] [Google Scholar]
- 21. European Medicines Agency (EMA) , Committee for medicinal products for human use (CHMP) ‐ Guideline on Bioanalytical Method Validation, February 2012. Ref.: CHMP/EWP/192217/2009 Rev. 1 Corr. 2**. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf.
- 22. Food and Drug Administration (FDA) , Center for Drug Evaluation and Research (CDER) ‐ Guidance for Industry Bioanalytical method validation, May 2018. https://www.fda.gov/downloads/drugs/guidances/ucm070107.pdf.
- 23. Bolton S. Bioequivalence studies for levothyroxine. AAPS J. 2005;7(1):E47–E53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gibaldi M. Bioequivalence of thyroid preparations: the final word? AAPS J. 2005;7(1):E59–E60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Food and Drug Administration (FDA) , Center for Drug Evaluation and Research (CDER) ‐ Guidance to industry Levothyroxine Sodium Tablets – In vivo Pharmacokinetic and Bioavailability Studies and In Vitro Dissolution Testing, December 2000. https://www.fda.gov/downloads/drugs/guidances/ucm071946.pdf.
- 26. European Medicines Agency (EMA) , Committee for medicinal products for human use (CHMP) ‐ Guideline on the investigation of bioequivalence, August 2010. Ref.: CPMP/EWP/QWP/1401/98 Rev. 1/ Corr **. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070039.pdf.
- 27. Vita R, Fallahi P, Antonelli A, Benvenga S. The administration of l‐thyroxine as soft gel capsule or liquid solution. Expert Opin Drug Deliv. 2014;11(7):1103–1111. [DOI] [PubMed] [Google Scholar]
- 28. Skelin M, Lucijanić T, Amidžić Klarić D, et al. Factors affecting gastrointestinal absorption of levothyroxine: a review. Clin Ther. 2017;39(2):378–340. [DOI] [PubMed] [Google Scholar]
- 29. Vita R, Saraceno G, Trimarchi F, Benvenga S. Switching Levothyroxine from the tablet to the oral solution formulation corrects the impaired absorption of levothyroxine induced by proton‐pump inhibitors. J Clin Endocrinol Metab. 2014;99(12):4481–4486. [DOI] [PubMed] [Google Scholar]
- 30. Guglielmi V, Bellia A, Bianchini E, et al. Drug interactions in users of tablet vs. oral liquid levothyroxine formulations: a real‐world evidence study in primary care. Endocrine. 2018;59(3):585–592. [DOI] [PubMed] [Google Scholar]
- 31. Vita R, Di Bari F, Benvenga S. Oral liquid levothyroxine solves the problem of tablet levothyroxine malabsorption due to concomitant intake of multiple drugs. Expert Opin Drug Deliv. 2017;14(4):467–472. [DOI] [PubMed] [Google Scholar]
- 32. Fallahi P, Ferrari SM, Camastra S, et al. TSH normalization in bariatric surgery patients after the switch from L‐thyroxine in tablet to an oral liquid formulation. Obes Surg. 2017;27(1):78–82. [DOI] [PubMed] [Google Scholar]
- 33. Fallahi P, Ferrari SM, Marchi S, De Bortoli N, Ruffilli I, Antonelli A. Patients with lactose intolerance absorb liquid levothyroxine better than tablet levothyroxine. Endocrine 2017;57(1):175–178. [DOI] [PubMed] [Google Scholar]
- 34. Ribichini D, Fiorini G, Repaci A, Castelli V, et al. Tablet and oral liquid L‐thyroxine formulation in the treatment of naïve hypothyroid patients with Helicobacter pylori infection. Endocrine, 2017;57(3):394–401. [DOI] [PubMed] [Google Scholar]
- 35. Cappelli C, Pirola I, Daffini L, Gandossi E, Agosti B, Castellano M. Thyroid hormonal profile in elderly patients treated with two different levothyroxine formulations: A single institute survey. Eur Geriatr Med. 2014;5(6):382–385. [Google Scholar]
- 36. Cappelli C, Pirola I, Gandossi E, et al. TSH variability of patients affected by differentiated thyroid cancer treated with levothyroxine liquid solution or tablet form. [published online ahead of print 2017] Int J Endocrinol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ferrara R, Lentile V, Arcoraci V, et al. Treatment pattern and frequency of serum TSH measurement in users of different levothyroxine formulations: a population‐based study during the years 2009–2015. Endocrine. 2017;58(s1):143–152. [DOI] [PubMed] [Google Scholar]
- 38. Virili C, Giovanella L, Fallahi P, Antonelli A, et al. Levothyroxine therapy: changes of TSH levels by switching patients from tablet to liquid formulation. A systematic review and meta‐analysis. Front Endocrinol (Lausanne). 2018;9: Article ID 10. 10.3389/fendo.2018.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]


