Abstract
Aim
This post hoc analysis of a double‐blind (DB), randomized, placebo‐controlled, relapse‐prevention study evaluated the effects of paliperidone palmitate once‐every‐3‐months (PP3M) in a subpopulation of adults with early illness schizophrenia (duration ≤5 years) from a clinical trial.
Methods
Patients received either PP3M or placebo every 3 months in the DB phase. The primary efficacy variable was time from randomization to first relapse. Symptom severity, patient functioning, and safety were also assessed.
Results
A total of 119 patients who entered the DB phase met the criteria for early illness schizophrenia (PP3M, n = 62; placebo, n = 57). PP3M significantly delayed time to relapse vs placebo (P = .035; hazard ratio, 3.08; 95% CI, 1.08‐8.80). Symptomatic control and patient functioning were maintained in the PP3M group but significantly worsened in the placebo group. There were no unexpected tolerability findings.
Conclusions
PP3M reduced relapse risk and maintained symptomatic and functional improvements compared with placebo in patients with early illness schizophrenia.
Keywords: early illness, paliperidone palmitate, recently diagnosed, relapse, schizophrenia
1. INTRODUCTION
In persons with schizophrenia, the first 5 years following the initial psychotic episode is a critical period for effective intervention (Emsley, Chiliza, & Asmal, 2013; McGorry, 2005). A growing body of evidence indicates that relapse events may cause adverse brain changes and that treatment response tends to diminish with each successive relapse (Emsley et al., 2013; Jeong & Lee, 2013; Lieberman, 2006). Therefore, sustained symptom control through consistent antipsychotic treatment during the early phase of schizophrenia is an important factor in maintaining patient functioning (Lehman et al., 2004). Incomplete adherence to antipsychotic medications is common among patients with schizophrenia, leading to an increased risk of relapse and hospitalization (Correll et al., 2016; Lang et al., 2010; Panish, Karve, Candrilli, & Dirani, 2013). This may be even more common among patients with early illness due to various factors, including lack of social support, negative attitude toward medication, and poor insight (Acosta, Hernandez, Pereira, Herrera, & Rodriguez, 2012; Ceskova, Prikryl, Kasparek, & Ondrusova, 2005; Heres, Lambert, & Vauth, 2014; Quach et al., 2009).
Long‐acting injectable (LAI) formulations of antipsychotic medications provide therapeutic plasma concentrations that are sustained for weeks to months following administration, eliminating the need for daily adherence to oral antipsychotics (Correll et al., 2016; Spanarello & La Ferla, 2014). Paliperidone palmitate once‐every‐3‐months (PP3M) is approved for the treatment of adult patients with schizophrenia who have been adequately treated with paliperidone palmitate once‐monthly (PP1M) for ≥4 months (Janssen Pharmaceuticals Inc, 2017). The results of a multicentre, double‐blind (DB), randomized, placebo‐controlled study showed that PP3M significantly delayed time to relapse compared with placebo in patients adequately treated with PP1M (Berwaerts et al., 2015). Several studies have reported on the feasibility and benefit of LAIs for the treatment of patients with early illness schizophrenia (Alphs, Bossie, Mao, Lee, & Starr, 2015; Schreiner et al., 2015; Subotnik et al., 2015; Weiden et al., 2009), but the effects of PP3M on early illness have not been investigated. This post hoc analysis examines the efficacy and safety of PP3M in a subpopulation of patients diagnosed with schizophrenia ≤5 years before trial enrolment.
2. METHODS
Details of this study are described elsewhere (ClinicalTrials.gov identifier, NCT01529515) (Berwaerts et al., 2015). Men and women (aged 18‐70 years) with schizophrenia (DSM‐IV‐TR criteria) for ≥1 year before screening who had a Positive and Negative Syndrome Scale (PANSS) total score of <120 at screening were eligible for study participation. Patients were excluded from the study if they had an active DSM‐IV axis diagnosis other than schizophrenia or involuntary status in a psychiatric hospital at screening. An independent ethics committee or institutional review board approved the study protocol and amendments at each site. All studies were conducted in compliance with the Declaration of Helsinki and were consistent with Good Clinical Practice and applicable regulatory requirements. All patients provided written informed consent before enrolment. For the present analysis, patients diagnosed with schizophrenia ≤5 years before trial enrolment were considered to have early illness.
This relapse‐prevention, DB, randomized, placebo‐controlled, multicentre study consisted of 4 phases: a 3‐week screening phase; a flexible‐dose, 17‐week, open‐label (OL) transitional phase; a 12‐week OL maintenance phase; and a variable follow‐up DB phase. Patients received PP1M (78, 117, 156, or 234 mg) during the transition phase, followed by a single dose of PP3M (3.5 times the stabilized dose of PP1M) during the maintenance phase. Stabilized patients were randomized to either the same fixed dose of PP3M or placebo every 3 months during the DB phase.
For this post hoc analysis, the primary efficacy variable was time from randomization to first relapse event in the DB phase. Secondary efficacy variables were change from DB baseline to endpoint in PANSS total score; PANSS positive, negative, and general psychopathology subscale scores; Clinical Global Impression of Severity (CGI‐S) scale score; and Personal and Social Performance (PSP) scale total score. Safety assessments included evaluation of treatment‐emergent adverse events (TEAEs).
The DB intention‐to‐treat final analysis set was defined as all those who were randomized and received ≥1 dose of study drug in the DB phase. The safety analysis set was identical to the DB intention‐to‐treat analysis set. Kaplan‐Meier method was used to assess time to relapse, and the log‐rank test was used to compare treatment differences. Risk of relapse between the treatment groups was examined using the Cox proportional hazards model. Treatment comparisons between PP3M and placebo in changes from baseline to DB endpoint of PANSS total, PANSS subscale, CGI‐S, and PSP total scores were performed using an ANCOVA model with fixed effects for treatment, country, and baseline values as covariates. Changes from baseline within treatment groups were examined using paired t ‐ test.
3. RESULTS
Of the 620 screened patients, 506 entered the OL phase. A total of 305 patients who met protocol‐defined stabilization criteria at the end of the OL phase entered the DB phase; 160 of these patients were randomized to PP3M (n = 62 [38.8%] with early illness) and 145 were randomized to placebo (n = 57 [39.3%] with early illness). Demographic and disease characteristics for the early illness population were well balanced between treatment groups (Table 1).
Table 1.
Baseline demographic and disease characteristics for patients in the double‐blind phase with duration of illness ≤5 years
| Double‐blind phase | ||
|---|---|---|
| Parameter | Placebo | PP3M |
| n = 57 | n = 62 | |
| Age, years | ||
| Mean (SD) | 31.8 (9.71) | 31.2 (9.59) |
| Median (min, max) | 29.0 (18, 53) | 29.0 (18, 60) |
| Age category, years, n (%) | ||
| 18‐25 | 19 (33.3) | 22 (35.5) |
| 26‐30 | 10 (17.5) | 11 (17.7) |
| 31‐35 | 10 (17.5) | 17 (27.4) |
| >35 | 18 (31.6) | 12 (19.4) |
| Gender, n (%) | ||
| Male | 46 (80.7) | 40 (64.5) |
| Race, n (%) | ||
| White | 37 (64.9) | 47 (75.8) |
| Black or African American | 7 (12.3) | 5 (8.1) |
| Asian | 6 (10.5) | 3 (4.8) |
| Other | 7 (12.3) | 7 (11.3) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 12 (21.1) | 12 (19.4) |
| Not Hispanic or Latino | 44 (77.2) | 50 (80.6) |
| Not reported/Unknown | 1 (1.8) | 0 (0.0) |
| Country, n (%) | ||
| Colombia | 7 (12.3) | 9 (14.5) |
| Malaysia | 5 (8.8) | 2 (3.3) |
| Mexico | 4 (7.0) | 0 (0.0) |
| Romania | 2 (3.5) | 4 (6.5) |
| South Korea | 1 (1.8) | 1 (1.6) |
| Turkey | 4 (7.0) | 2 (3.2) |
| Ukraine | 27 (47.4) | 36 (58.1) |
| United States | 7 (12.3) | 8 (12.9) |
| Body weight, kg | ||
| Mean (SD) | 77.1 (14.8) | 75.0 (14.1) |
| Median (min, max) | 75.2 (44, 120) | 73.2 (47, 118) |
| BMI (kg/m2) | ||
| Mean (SD) | 25.9 (4.2) | 25.4 (4.6) |
| Median (min, max) | 25.2 (18, 37) | 24.4 (18, 40) |
Abbreviations: BMI, body mass index; max, maximum; min, minimum; PP3M, paliperidone palmitate once‐every‐3‐months; SD, standard deviation.
Among those with early illness, 12 (21.1%) patients in the placebo group and 5 (8.1%) patients in the PP3M group experienced relapse during the DB phase (Figure 1). Median time to relapse could not be computed because less than 50% of patients relapsed in each treatment group. Continued treatment with PP3M was associated with a significant delay in time to relapse compared with placebo (P = .027) using the log‐rank test. In addition to the Kaplan‐Meier analysis, the estimate of the hazard ratio (risk) and its 95% CI was provided based on the Cox proportional hazards model. The risk of relapse (hazard ratio) was 3.08‐fold higher (95% CI: 1.08, 8.80; P = .035) for patients randomized to placebo after a single dose of PP3M than for those who continued to receive PP3M in the DB phase.
Figure 1.
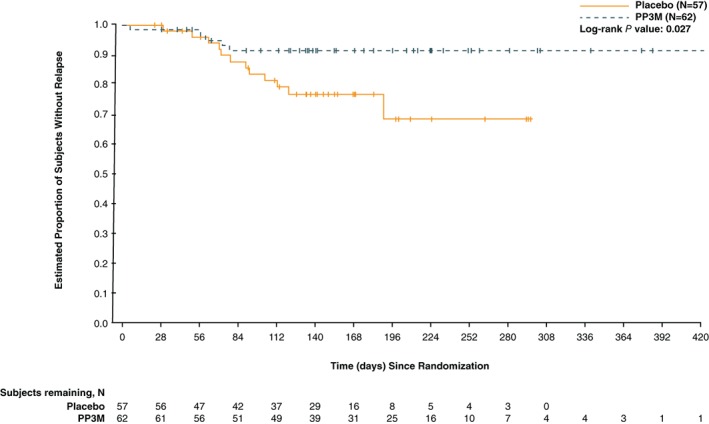
Kaplan‐Meier plot of time to relapse during the double‐blind (DB) phase in patients with early illness: intention‐to‐treat (DB) analysis set. Paliperidone palmitate once‐every‐3‐months (PP3M) significantly delayed time to relapse versus placebo (P = .035; hazard ratio, 3.08; 95% CI, 1.08, 8.80)
Mean changes from DB baseline to DB endpoint in PANSS total and subscale, CGI‐S, and PSP total scores for patients in the placebo and PP3M groups are shown in Table 2. At the end of the DB phase, scores were maintained in the PP3M group. Mean [SD] changes from DB baseline were PANSS total (−0.3 [7.9]; P = .783), PANSS positive subscale (0.4 [2.6]; P = .224), PANSS negative subscale (−0.2 [2.9]; P = .511), PANSS general psychopathology subscale (−0.4 [4.5]; P = .445), CGI‐S (0.1 [0.60]; P = .398), and PSP total (−0.4 [7.2]; P = .660) scores. However, with the exception of the PANSS negative subscale, the scores significantly worsened in the placebo group: PANSS total (5.9 [15.3]; P = .006), PANSS positive subscale (2.5 [5.3]; P = .001), PANSS negative subscale (0.8 [3.8]; P = .115), PANSS general psychopathology subscale (2.6 [8.3]; P = .023), CGI‐S (0.3 [0.86]; P = .021), and PSP total (−3.5 [10.3]; P = .016) scores. A significant between‐group difference in favour of PP3M was demonstrated for PANSS total, positive, negative, and general psychopathology subscale; CGI‐S; and PSP total scores: the LS mean (95% CI) difference in change between the 2 treatment groups were 7.1 (2.5, 11.7; P = .003), 2.1 (0.5, 3.7; P = .011), 1.5 (0.2, 2.7; P = .019), 3.6 (1.0, 6.1; P = .006), 0.3 (0.0, 0.6; P = .025), and −3.8 (−7.2, −0.4; P = .031), respectively.
Table 2.
Change from baseline to endpoint in Positive and Negative Syndrome Scale, Clinical Global Impression of Severity scale, and Personal and Social Performance scale scores for patients in the double‐blind phase with duration of illness ≤5 years
| Double‐blind phase | |||
|---|---|---|---|
| Parameter | Placebo | PP3M | Estimatea |
| n = 54 | n = 61 | ||
| PANSS scores | |||
| Total score | |||
| Baseline, mean (SD) | 54.0 (9.7) | 54.9 (10.9) | |
| Endpoint, mean (SD) | 60.0 (18.3) | 54.6 (13.2) | |
| Change from baseline, mean (SD) | 5.9 (15.3) | −0.3 (7.9) | |
| P valueb | .006 | .783 | |
| Change from baseline, LS mean (SE) | 5.2 (2.1) | −1.9 (2.2) | |
| LS mean difference (95% CI) | 7.1 (2.5, 11.7) | ||
| P value | .003 | ||
| Positive subscale score | |||
| Baseline, mean (SD) | 11.4 (3.1) | 11.4 (3.3) | |
| Endpoint, mean (SD) | 13.9 (6.2) | 11.8 (4.1) | |
| Change from baseline, mean (SD) | 2.5 (5.3) | 0.4 (2.6) | |
| P valueb | .001 | .224 | |
| Change from baseline, LS mean (SE) | 2.7 (0.7) | 0.6 (0.8) | |
| LS mean difference (95% CI) | 2.1 (0.5, 3.7) | ||
| P value | .011 | ||
| Negative subscale score | |||
| Baseline, mean (SD) | 16.1 (4.0) | 16.4 (4.8) | |
| Endpoint, mean (SD) | 16.9 (5.2) | 16.2 (5.0) | |
| Change from baseline, mean (SD) | 0.8 (3.8) | −0.2 (2.9) | |
| P valueb | .115 | .511 | |
| Change from baseline, LS mean (SE) | 0.1 (0.6) | −1.4 (0.6) | |
| LS mean difference (95% CI) | 1.5 (0.2, 2.7) | ||
| P value | .019 | ||
| General psychopathology subscale score | |||
| Baseline, mean (SD) | 26.6 (4.9) | 27.0 (5.1) | |
| Endpoint, mean (SD) | 29.2 (9.6) | 26.6 (6.4) | |
| Change from baseline, mean (SD) | 2.6 (8.3) | −0.4 (4.5) | |
| P valueb | .023 | .445 | |
| Change from baseline, LS mean (SE) | 2.4 (1.2) | −1.2 (1.2) | |
| LS mean difference (95% CI) | 3.6 (1.0, 6.1) | ||
| P value | .006 | ||
| CGI‐S scale score | |||
| Baseline, mean (SD) | 2.8 (0.70) | 2.8 (0.62) | |
| Endpoint, mean (SD) | 3.1 (1.00) | 2.8 (0.88) | |
| Change from baseline, mean (SD) | 0.3 (0.86) | 0.1 (0.60) | |
| P valueb | .021 | .398 | |
| Change from baseline, LS mean (SE) | 0.2 (0.12) | −0.1 (0.13) | |
| LS mean difference (95% CI) | 0.3 (0.0, 0.6) | ||
| P value | .025 | ||
| PSP total score | |||
| Baseline, mean (SD) | 68.1 (9.1) | 69.7 (8.7) | |
| Endpoint, mean (SD) | 64.6 (12.7) | 69.2 (11.8) | |
| Change from baseline, mean (SD) | −3.5 (10.3) | −0.4 (7.2) | |
| P valueb | .016 | .660 | |
| Change from baseline, LS mean (SE) | −3.8 (1.6) | −0.1 (1.6) | |
| LS mean difference (95% CI) | −3.8 (−7.2, −0.4) | ||
| P value | .031 | ||
Abbreviations: CGI‐S, Clinical Global Impression of Severity; PANSS, Positive and Negative Symptom Scale; PP3M, paliperidone palmitate once‐every‐3‐months; PSP, Personal and Social Performance; SD, standard deviation.
P values for between‐group comparisons are from an analysis of covariance model with fixed effects for treatment and country and the baseline value as a covariate.
P values for within‐group comparisons are based on a paired t ‐ test.
Among patients with early illness, the most common TEAEs (≥2%) occurring more frequently in the PP3M group than in the placebo group during the DB phase were weight increased (12.9% vs 3.5%), anxiety (9.7% vs 8.8%), nasopharyngitis (8.1% vs 3.5%), headache (8.1% vs 1.8%), urinary tract infection (6.5% vs 0.0%), and akathisia (3.2% vs 0.0%), respectively (Table 3). In the DB phase, 3 serious adverse events were experienced by 8 patients: paranoid‐type schizophrenia (n = 1) in the PP3M group and schizophrenia (n = 6) and cellulitis (n = 1) in the placebo group. There were no deaths during the DB phase and no patient withdrew due to a TEAE.
Table 3.
Most common treatment‐emergent adverse events (≥2% in either treatment group during the double‐blind phase)
| Double‐blind phase | ||
|---|---|---|
| Parameter | Placebo | PP3M |
| n = 57 | n = 62 | |
| Patients with AEs, n (%) | 28 (50.9) | 39 (62.9) |
| Weight increased | 2 (3.5) | 8 (12.9) |
| Anxiety | 5 (8.8) | 6 (9.7) |
| Headache | 1 (1.8) | 5 (8.1) |
| Nasopharyngitis | 2 (3.5) | 5 (8.1) |
| Insomnia | 9 (15.8) | 4 (6.5) |
| Urinary tract infection | 0 (0.0) | 4 (6.5) |
| Akathisia | 0 (0.0) | 2 (3.2) |
| Influenza | 3 (5.3) | 1 (1.6) |
| Schizophrenia | 8 (14.0) | 1 (1.6) |
| Weight decreased | 2 (3.5) | 1 (1.6) |
| Dyskinesia | 2 (3.5) | 0 (0.0) |
| Cough | 2 (3.5) | 0 (0.0) |
| Hyperglycaemia | 2 (3.5) | 0 (0.0) |
Abbreviations: AEs, adverse events; PP3M, paliperidone palmitate once‐every‐3‐months.
4. DISCUSSION
This post hoc analysis demonstrates the efficacy and safety of PP3M in a subpopulation of patients with early illness schizophrenia and is consistent with the primary results of the trial (Berwaerts et al., 2015). Specifically, the use of PP3M significantly delayed time to relapse compared with placebo, and PP3M maintained both symptomatic and functional status in early illness patients. Conversely, symptom severity and patient functioning worsened in patients randomized to placebo. There were no unexpected tolerability findings in patients with early illness.
Patients with early illness schizophrenia are often nonadherent to antipsychotic medications due to poor awareness and knowledge of their illness and the importance of continuous, effective medication therapy (Ceskova et al., 2005; Quach et al., 2009). This population is therefore particularly vulnerable to relapse, with the potential for irreversible cognitive deterioration (Nuechterlein, Ventura, Subotnik, & Bartzokis, 2014). Psychiatrists may be reluctant to treat early episode schizophrenia with LAIs (Altamura et al., 2012; Correll et al., 2016; Jeong & Lee, 2013). However, patients with first‐episode schizophrenia may be surprisingly open to LAI treatment after stabilization on oral antipsychotic medication (Weiden et al., 2009). Given the high prevalence of nonadherence to oral medications and the potential negative consequences of relapses in this population, clinician reluctance to offer LAIs to patients with early episode schizophrenia is not supported by available evidence (Weiden et al., 2009). The findings presented here are also consistent with findings from studies of patients with early illness receiving PP1M (Alphs et al., 2015; Bossie, Fu, Sliwa, Ma, & Alphs, 2011; Fu, Bossie, Sliwa, Ma, & Alphs, 2014; Schreiner et al., 2015). Additionally, the Canadian Schizophrenia Guidelines acknowledge that use of LAIs earlier in the course of treatment has been advocated and that discussions regarding LAI use should not be limited to patients with poor adherence (Remington et al., 2017).
This post hoc analysis has several limitations. First, the study was not designed to evaluate the efficacy and safety of PP3M in patients with early illness. Second, the present analysis used a 5‐year cutoff to define early illness; however, there is no consensus on the definition of “early illness” and it can be defined in several ways (Canuso et al., 2010; Dubois, Peuskens, Geerts, & Detraux, 2014; Kreyenbuhl et al., 2016; Macfadden, Bossie, Turkoz, & Haskins, 2010). Finally, this was an international study, and the duration of illness as reported by the investigators may be influenced by local cultural norms that could accelerate or delay the receipt of an appropriate diagnosis following symptom onset (Berwaerts et al., 2015).
In conclusion, results of this post hoc analysis demonstrate that in adults with ≤5 years' duration of schizophrenia, PP3M is efficacious and tolerated; these findings are consistent with the primary analysis. Use in this population could help patients maintain sustained symptom control during a critical period when relapses can be disruptive yet potentially avoidable.
ACKNOWLEDGEMENTS
This research was supported by Janssen Scientific Affairs, LLC. Editorial support was provided by Matthew Grzywacz, PhD, and Lynn Brown, PhD, of ApotheCom (Yardley, PA, USA).
Author disclosures
K.S.B.L. and E.K. are employees of Janssen Scientific Affairs, LLC, and are stockholders of Johnson & Johnson. I.T. is an employee of Janssen Research & Development, LLC, and is a stockholder of Johnson & Johnson.
Bell Lynum KS, Turkoz I, Kim E. Paliperidone palmitate once‐every‐3‐months in adults with early illness schizophrenia. Early Intervention in Psychiatry. 2019;13:667–672. 10.1111/eip.12685
Funding information Janssen Scientific Affairs, LLC, Grant/Award Number: No grant/award number
REFERENCES
- Acosta, F. J. , Hernandez, J. L. , Pereira, J. , Herrera, J. , & Rodriguez, C. J. (2012). Medication adherence in schizophrenia. World Journal of Psychiatry, 2(5), 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alphs, L. , Bossie, C. , Mao, L. , Lee, E. , & Starr, H. L. (2015). Treatment effect with paliperidone palmitate compared with oral antipsychotics in patients with recent‐onset versus more chronic schizophrenia and a history of criminal justice system involvement. Early Intervention in Psychiatry, 12(1), 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamura, A. C. , Aguglia, E. , Bassi, M. , Bogetto, F. , Cappellari, L. , De Giorgi, S. , … Girardi, P. (2012). Rethinking the role of long‐acting atypical antipsychotics in the community setting. International Clinical Psychopharmacology, 27(6), 336–349. [DOI] [PubMed] [Google Scholar]
- Berwaerts, J. , Liu, Y. , Gopal, S. , Nuamah, I. , Xu, H. , Savitz, A. , … Hough, D. W. (2015). Efficacy and safety of the 3‐month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry, 72(8), 830–839. [DOI] [PubMed] [Google Scholar]
- Bossie, C. A. , Fu, D. J. , Sliwa, J. K. , Ma, Y. W. , & Alphs, L. (2011). Tolerability of initiation doses of once‐monthly paliperidone palmitate in patients with recently diagnosed schizophrenia in an acute treatment trial. Therapeutic Advances in Psychopharmacology, 1(4), 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canuso, C. M. , Bossie, C. A. , Amatniek, J. , Turkoz, I. , Pandina, G. , & Cornblatt, B. (2010). Paliperidone extended‐release tablets in patients with recently diagnosed schizophrenia. Early Intervention in Psychiatry, 4(1), 64–78. [DOI] [PubMed] [Google Scholar]
- Ceskova, E. , Prikryl, R. , Kasparek, T. , & Ondrusova, M. (2005). Psychopathology and treatment responsiveness of patients with first‐episode schizophrenia. Neuropsychiatric Diseases and Treatment, 1(2), 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll, C. U. , Citrome, L. , Haddad, P. M. , Lauriello, J. , Olfson, M. , Calloway, S. M. , & Kane, J. M. (2016). The use of long‐acting injectable antipsychotics in schizophrenia: evaluating the evidence. Journal of Clinical Psychiatry, 77(suppl 3), 1–24. [DOI] [PubMed] [Google Scholar]
- Dubois, V. , Peuskens, J. , Geerts, P. , & Detraux, J. (2014). Clinical outcomes of long‐acting risperidone in recent versus long‐term diagnosed Belgian schizophrenic patients: results from electronic Schizophrenia Treatment Adherence Registry (e‐STAR) and Trial for the Initiation and Maintenance Of REmission in Schizophrenia with risperidone (TIMORES). Early Intervention in Psychiatry, 8(1), 39–49. [DOI] [PubMed] [Google Scholar]
- Emsley, R. , Chiliza, B. , & Asmal, L. (2013). The evidence for illness progression after relapse in schizophrenia. Schizophrenia Research, 148(1–3), 117–121. [DOI] [PubMed] [Google Scholar]
- Fu, D. J. , Bossie, C. A. , Sliwa, J. K. , Ma, Y. W. , & Alphs, L. (2014). Paliperidone palmitate versus oral risperidone and risperidone long‐acting injection in patients with recently diagnosed schizophrenia: a tolerability and efficacy comparison. International Clinical Psychopharmacology, 29(1), 45–55. [DOI] [PubMed] [Google Scholar]
- Heres, S. , Lambert, M. , & Vauth, R. (2014). Treatment of early episode in patients with schizophrenia: the role of long acting antipsychotics. European Psychiatry, 29(Suppl 2), 1409–1413. [DOI] [PubMed] [Google Scholar]
- Janssen Pharmaceuticals, Inc . (2017). Invega Trinza (paliperidone palmitate) extended‐release injectable suspension, for intramuscular use [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc. [Google Scholar]
- Jeong, H. G. , & Lee, M. S. (2013). Long‐acting injectable antipsychotics in first‐episode schizophrenia. Clinical Psychopharmacology and Neuroscience, 11(1), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreyenbuhl, J. A. , Medoff, D. R. , McEvoy, J. P. , Smith, T. E. , Hackman, A. L. , Nossel, I. R. , … Buchanan, R. W. (2016). The RAISE Connection Program: psychopharmacological treatment of people with a first episode of schizophrenia. Psychiatric Services, 67(12), 1300–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, K. , Meyers, J. L. , Korn, J. R. , Lee, S. , Sikirica, M. , Crivera, C. , … Menzin, J. (2010). Medication adherence and hospitalization among patients with schizophrenia treated with antipsychotics. Psychiatric Services, 61(12), 1239–1247. [DOI] [PubMed] [Google Scholar]
- Lehman, A. F. , Lieberman, J. A. , Dixon, L. B. , McGlashan, T. H. , Miller, A. L. , Perkins, D. O. , … American Psychiatric Association, & Steering Committee on Practice Guidelines . (2004). Practice guideline for the treatment of patients with schizophrenia, second edition. American Journal of Psychiatry, 161(2 Suppl), 1–56. [PubMed] [Google Scholar]
- Lieberman, J. A. (2006). Neurobiology and the natural history of schizophrenia. Journal of Clinical Psychiatry, 67(10), e14. [PubMed] [Google Scholar]
- Macfadden, W. , Bossie, C. A. , Turkoz, I. , & Haskins, J. T. (2010). Risperidone long‐acting therapy in stable patients with recently diagnosed schizophrenia. International Clinical Psychopharmacology, 25(2), 75–82. [DOI] [PubMed] [Google Scholar]
- McGorry, P. D. (2005). Early intervention in psychotic disorders: beyond debate to solving problems. British Journal of Psychiatry, 187(Suppl 48), s108–s110. [DOI] [PubMed] [Google Scholar]
- Nuechterlein, K. H. , Ventura, J. , Subotnik, K. L. , & Bartzokis, G. (2014). The early longitudinal course of cognitive deficits in schizophrenia. Journal of Clinical Psychiatry, 75(Suppl 2), 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panish, J. , Karve, S. , Candrilli, S. D. , & Dirani, R. (2013). Association between adherence to and persistence with atypical antipsychotics and psychiatric relapse among US Medicaid‐enrolled patients with schizophrenia. Journal of Pharmaceutical Health Services Research, 4(1), 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quach, P. L. , Mors, O. , Christensen, T. O. , Krarup, G. , Jorgensen, P. , Bertelsen, M. , … Nordentoft, M. (2009). Predictors of poor adherence to medication among patients with first‐episode schizophrenia‐spectrum disorder. Early Intervention in Psychiatry, 3(1), 66–74. [DOI] [PubMed] [Google Scholar]
- Remington, G. , Addington, D. , Honer, W. , Ismail, Z. , Raedler, T. , & Teehan, M. (2017). Guidelines for the Pharmacotherapy of Schizophrenia in Adults. Canadian Journal of Psychiatry, 62(9), 604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner, A. , Aadamsoo, K. , Altamura, A. C. , Franco, M. , Gorwood, P. , Neznanov, N. G. , … Hargarter, L. (2015). Paliperidone palmitate versus oral antipsychotics in recently diagnosed schizophrenia. Schizophrenia Research, 169(1–3), 393–399. [DOI] [PubMed] [Google Scholar]
- Spanarello, S. , & La Ferla, T. (2014). The pharmacokinetics of long‐acting antipsychotic medications. Current Clinical Pharmacology, 9(3), 310–317. [DOI] [PubMed] [Google Scholar]
- Subotnik, K. L. , Casaus, L. R. , Ventura, J. , Luo, J. S. , Hellemann, G. S. , Gretchen‐Doorly, D. , … Nuechterlein, K. H. (2015). Long‐acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry, 72(8), 822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiden, P. J. , Schooler, N. R. , Weedon, J. C. , Elmouchtari, A. , Sunakawa, A. , & Goldfinger, S. M. (2009). A randomized controlled trial of long‐acting injectable risperidone vs continuation on oral atypical antipsychotics for first‐episode schizophrenia patients: initial adherence outcome. Journal of Clinical Psychiatry, 70(10), 1397–1406. [DOI] [PubMed] [Google Scholar]


