Abstract
The phase 1‐2 study CO‐338‐010 (Study 10; NCT01482715) is evaluating single‐agent rucaparib, a poly(ADP‐ribose) polymerase inhibitor, administered orally to patients with an advanced solid tumor. In the dose escalation phase (Part 1), we characterized the single‐dose and steady‐state pharmacokinetic profiles of rucaparib administered once daily (QD; dose range, 40‐500 mg; n = 16) or twice daily (BID; dose range, 240‐840 mg; n = 30). Across all dosing schedules examined, the plasma exposure of rucaparib was approximately dose proportional; half‐life was approximately 17 hours, and median time to maximum concentration (tmax) ranged from 1.5 to 6.0 hours after a single dose and 1.5 to 4.0 hours following repeated dosing. The steady‐state accumulation ratio ranged from 1.60 to 2.33 following QD dosing and 1.47 to 5.44 following BID dosing. No effect of food on rucaparib pharmacokinetics was observed with a single dose of 40 mg (n = 3) or 300 mg (n = 6). In a phase 2 portion of the study (Part 3), the pharmacokinetic profile of rucaparib was further evaluated at the recommended phase 2 dose of 600 mg BID (n = 26). The mean (coefficient of variation) steady‐state maximum concentration (Cmax) and area under the concentration‐time curve from time zero to 12 hours (AUC0‐12h) were 1940 ng/mL (54%) and 16 900 ng ⋅ h/mL (54%), respectively. A high‐fat meal moderately increased rucaparib exposure. The fed‐to‐fasted geometric mean ratios (90% confidence interval [CI]) for AUC0‐24h and Cmax were 138% (117%‐162%) and 120% (99.1%‐146%); the median (90%CI) tmax delay was 2.5 (0.5‐4.4) hours.
Keywords: food effect, PARP inhibition, pharmacokinetics, rucaparib, tablet
Poly(ADP‐ribose) polymerase (PARP) enzymes make up a 17‐member superfamily of nuclear enzymes, including PARP1, PARP2, and PARP3. Collectively, these enzymes are activated by and promote the repair of DNA damage.1, 2 PARP inhibition results in the accumulation of unrepaired single‐strand breaks, which leads to collapsed replication forks and an accumulation of DNA double‐strand breaks.3, 4 These double‐strand breaks are repaired by the homologous recombination repair pathway, in which BRCA1 and BRCA2 are key proteins that help mediate homologous recombination repair.5, 6, 7 PARP inhibitors may also induce trapped PARP‐DNA complexes, which require homologous recombination for effective bypass.8 These and other mechanisms account for the synthetic lethality between PARP inhibition and homologous recombination repair deficiency.9, 10, 11
Rucaparib (formerly known as CO‐338, AG‐014447, or PF‐01367338) is an oral, small‐molecule inhibitor of PARP1, PARP2, and PARP3.12, 13 Rucaparib has demonstrated antitumor activity in various tumor types, including ovarian, breast, and pancreatic cancers.14, 15, 16 Rucaparib is approved by the US Food and Drug Administration as monotherapy for the maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in a complete or partial response to platinum‐based chemotherapy and for treatment of patients with deleterious BRCA1 or BRCA2 mutation (germline and/or somatic) associated advanced ovarian cancer who have been treated with ≥2 chemotherapies.
The metabolism of rucaparib has been studied in vitro (Clovis Oncology, Inc., data on file). Rucaparib showed slow metabolic turnover rates in incubation with human hepatocytes and liver microsomes. Recombinant human cytochrome P450 (CYP) 2D6 and, to a lesser extent, CYP1A2 and CYP3A4 were able to metabolize rucaparib.
Patients with different phenotypes of CYP2D6 (poor metabolizers, n = 9; intermediate metabolizers, n = 71; normal metabolizers, n = 76; and ultra‐rapid metabolizers, n = 4) showed similar clearance (CL) of rucaparib based on a population pharmacokinetic analysis. Similarly, no apparent effect of CYP1A2 polymorphisms on the pharmacokinetics of rucaparib was observed (normal metabolizers, n = 28; hyperinducers, n = 136).17 Enzymes that contribute to the metabolism and elimination of rucaparib have not been identified in vivo or in humans.
CO‐338‐010 (Study 10; NCT01482715) is a phase 1‐2 clinical trial evaluating oral rucaparib administered as continuous monotherapy to patients with advanced solid tumors, including high‐grade ovarian cancer.14 In Study 10, the recommended phase 2 dose (RP2D) of rucaparib 600 mg twice daily (BID) was established in patients with solid tumors. Subsequently, the RP2D was evaluated for safety and efficacy in women with high‐grade ovarian cancer.14
Study 10 also evaluated the single‐dose and steady‐state pharmacokinetic profiles of oral rucaparib when administered once daily (QD) or BID. The effect of a high‐fat meal on the pharmacokinetic profile of rucaparib was also evaluated after a single dose (40 mg, 300 mg, or 600 mg) was administered. Intensive pharmacokinetic data were collected from patients with solid tumors to evaluate the clinical pharmacokinetics of rucaparib at the RP2D. The comprehensive pharmacokinetic profile of single‐agent rucaparib is reported herein.
Methods
Study Design and Patients
Study 10 is an ongoing, 3‐part, open‐label, phase 1‐2 study that is evaluating single‐agent oral rucaparib (ClinicalTrials.gov identifier, NCT01482715). Study 10 was approved by the institutional review board at each study site (see Supporting Information) and is being conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines of the International Conference on Harmonisation. Patients provided written consent before participating in the study.
Study 10 Part 1 enrolled patients aged ≥18 years who had an advanced solid tumor that progressed on standard treatment and had an Eastern Cooperative Oncology Group Performance Status of 0 to 1. For the dose escalation portion of the study, a standard 3 + 3 design was used. A starting dose of 40 mg QD was used, followed by escalations to 80, 160, 300, and 500 mg QD, followed by further escalation to 240, 360, 480, 600, and 840 mg BID. The primary objectives of this portion of the study were to characterize the safety and pharmacokinetic profile of oral rucaparib (40‐mg, 60‐mg, and/or 120‐mg tablets) administered QD or BID continuously and to establish the maximum tolerated dose and RP2D in patients with an advanced solid tumor.
Study 10 Parts 2A and 2B evaluated the efficacy and safety of oral rucaparib at the RP2D in patients with recurrent, high‐grade, serous or endometrioid ovarian cancer (including primary peritoneal and fallopian tube cancers) with a BRCA1 or BRCA2 mutation.
Study 10 Part 3 enrolled patients with a relapsed solid tumor that was associated with a germline or somatic BRCA1 or BRCA2 mutation (detected by local or central testing). The primary objectives of this portion of the study were to assess the pharmacokinetic profile of rucaparib, the effect of a high‐fat meal on pharmacokinetics, and the safety profile of rucaparib at the RP2D (600 mg BID) using a higher strength tablet (300 mg).
For this analysis, pharmacokinetic data from patients in Parts 1 and 3 were used to evaluate the clinical pharmacokinetics of rucaparib at the RP2D.
In Study 10 Parts 1 and 3, patients were treated in continuous 21‐day cycles. Two immediate‐release rucaparib camsylate tablet formulations were used. In Part 1, a lower‐strength tablet formulation (40‐ or 60‐mg rucaparib equivalence) was administered at dose levels ranging between 40 and 840 mg. In Part 3, a 300‐mg rucaparib tablet formulation was used for the pharmacokinetic evaluation at the RP2D (600 mg BID).
Pharmacokinetic Sample Analysis
Plasma concentrations of rucaparib were determined by Q Squared Solutions BioSciences (Ithaca, New York; formerly Advion Bioanalytical Laboratories) using validated liquid chromatography and tandem mass spectrometric methods. Blood samples were collected in tubes containing dipotassium ethylenediaminetetraacetic acid. A 96‐well protein precipitation extraction procedure was developed to isolate the analyte from plasma samples of 20‐ or 50‐μL aliquots. The resulting samples were subject to liquid chromatography–tandem mass spectrometry analysis using a selected reaction monitoring (SRM) method with deuterium‐labeled rucaparib (d7‐rucaparib) as the internal standard. The liquid chromatography consisted of a Polaris C18‐A column (2.1 mm × 50 mm; 3 μm, Agilent Technologies, Santa Clara, California) and an isocratic elution with 20% acetonitrile and 0.1% formic acid in water at a flow rate of 500 μL/min. The tandem mass spectrometry consisted of an AB SCIEX API4000 system (SCIEX, Framingham, Massachusetts) and was operated with Analyst version 1.4.2 in Turbo‐ion Spray (400°C) and positive ionization mode (1800 V). Nitrogen was used as the curtain gas, ion source gas, and collision gas. Rucaparib and d7‐rucaparib were quantified by SRMs of m/z 324.1 → 293.1 and m/z 331.2 → 300.2, respectively. The concentration range for quantitation was initially 0.5 to 1000 ng/mL and was later switched to a higher range (5–10 000 ng/mL). Plasma concentrations below the lower limit of quantitation of either 0.5 or 5 ng/mL were treated as “0” when calculating summary statistics.
Pharmacokinetics Evaluation
Pharmacokinetics Design
In Part 1, single‐dose and steady‐state pharmacokinetics were evaluated on cycle 1 day 1 and day 15, respectively, at rucaparib 40 mg to 500 mg QD and 240 mg to 840 mg BID. The effect of a high‐fat meal on the pharmacokinetics of rucaparib was assessed at 40 mg and 300 mg in 2 cohorts in Part 1, in which patients received a single dose of rucaparib under fasting conditions on cycle 1 day –7 and a single dose of rucaparib following a high‐fat meal on cycle 1 day 1. Under fasted conditions, patients fasted overnight for ≥10 hours before predose assessments followed by administration of rucaparib. Under fed conditions, patients fasted overnight for ≥10 hours before predose assessments and then consumed a high‐fat, high‐calorie meal in the clinic 30 minutes prior to administration of oral rucaparib. The meal contained approximately 800 to 1000 calories total, with approximately 500 to 600 calories from fat, approximately 250 calories from carbohydrates, and approximately 150 calories from protein. Under fasted and fed conditions, no water and no food was allowed for ≥2 and ≥4 hours, respectively, following administration of rucaparib. Continuous QD doses of rucaparib were administered to all patients enrolled in the food effect evaluation cohorts beginning on cycle 1 day 2, and steady‐state pharmacokinetics was obtained on cycle 1 day 15.
In Part 3, the effect of a high‐fat meal on the pharmacokinetics of rucaparib after a single 600‐mg dose was also evaluated. In this food effect assessment, patients were randomized to one of the following sequences: sequence 1, fed on cycle 1 day –7 and then fasted on cycle 1 day 1; or sequence 2, fasted on cycle 1 day –7 and then fed on cycle 1 day 1. Thus, the single‐dose pharmacokinetics was evaluated on cycle 1 day –7 and cycle 1 day 1. Patients then started to receive rucaparib 600 mg BID on cycle 1 day 2 with or without food, and steady‐state pharmacokinetics was evaluated on cycle 1 day 15.
Serial blood samples for full pharmacokinetic profile generation were obtained from patients during cycle 1 day –7 (Part 1: food effect cohorts only; Part 3: all patients), cycle 1 day 1 (Parts 1 and 3: all patients), and cycle 1 day 15 (Parts 1 and 3: all patients) prior to dosing and at 15 (±2) minutes, 30 (±3) minutes, 1 hour (±5 minutes), 1.5 hours (±5 minutes), 2.5 hours (±5 minutes), 4 hours (±15 minutes), 6 hours (±15 minutes), 8 hours (±15 minutes), 10 hours (±30 minutes), and 24 hours (±30 minutes) after dosing. For patients in the BID dose cohorts, the 24‐hour sample was collected approximately 24 hours after the morning dose on day 1 or day 15 and prior to the next morning dose. Blood samples for trough concentrations were collected prior to dosing on cycle 1 day 8 and cycle 1 day 22 (or cycle 2 day 1).
Analysis Populations
A patient was included if he or she had sufficient pharmacokinetic data to derive ≥1 primary pharmacokinetic parameter and had no major protocol deviations that would affect pharmacokinetic evaluation. Analysis of pharmacokinetics was stratified by study part, dosing regimen (QD or BID dose escalation cohorts), and fed/fasted status (food effect analysis cohorts). For the food effect analysis, only patients who completed both fed and fasted assessments were included.
Pharmacokinetic Parameters
Pharmacokinetic parameters were calculated using noncompartmental analysis methods in Phoenix WinNonLin (V6.3 or higher; Certara, Princeton, New Jersey). Actual pharmacokinetic sampling times were used in the pharmacokinetic analysis; data points with missing dosing or sampling time information were excluded from the analysis. Plasma concentration values below the lower limit of quantitation following the same dose were set as “0” for the first occurrence and as “missing” thereafter.
Plasma pharmacokinetic parameters were calculated after a single dose on cycle 1 day –7 and included maximum plasma concentration (Cmax), time of occurrence of Cmax (tmax), and area under the concentration‐time curve (AUC) from time 0 to the time (t) of the last quantifiable concentration (AUC0‐t). Parameters calculated after repeated doses (cycle 1 days 1 and 15) included Cmax, tmax, minimum plasma concentration (Cmin), AUC0‐t (t = 24 hours for QD dose; t = 10 hours for BID dose), AUC from time 0 to 12 hours (AUC0‐12h) based on extrapolation (for BID dose), the accumulation ratio of Cmax (Rac_Cmax), and accumulation ratio of AUC (Rac_AUC) at steady state. The food effect analysis included fed‐to‐fasted comparison of Cmax, tmax, and AUC0‐24h. The linear trapezoid rule was used in the AUC calculation. AUC0‐12h at steady state was derived from pharmacokinetic data up to 10 hours after dosing using extrapolation. Additional pharmacokinetic parameters, such as apparent total plasma CL at steady state (CLss/F) and elimination half‐life (t1/2), were derived when data allowed.
All statistical analyses were conducted in SAS Version 9.3 (SAS Institute, Cary, North Carolina).
Dose Proportionality (Part 1)
Dose proportionality on log‐transformed pharmacokinetic parameters derived at steady state vs the log‐transformed dose was assessed separately for QD and BID doses. The log‐transformed pharmacokinetic parameters and the log‐transformed dose were fitted to the equation:
Y represents the Cmax or AUC0‐t of one dosing interval at steady state, and ε represents the error with assumed normal distribution. Intercept β0 represents the coefficient of the dose before the log transformation. Slope β1, which is the power term of the dose (Doseβ1), and its 90% confidence interval (CI) were calculated to assess the dose proportionality.18 The statistical analysis was considered descriptive instead of inferential.
Steady‐State Pharmacokinetics
Time to reach the steady state was evaluated using plasma trough levels from Part 1 and Part 3, specifically the predose concentrations measured on cycle 1 days 8, 15, 16 (24 hours post day 15 dosing), and 22. Accumulation of rucaparib at steady state was calculated as Rac_Cmax and Rac_AUC following QD and BID dosing, respectively. For the QD dose, AUC0‐24h was used to calculate Rac_AUC. For the BID dose, AUC0‐12h was used to calculate Rac_AUC.
Effect of Food on Pharmacokinetics
In Part 1, pharmacokinetic parameters (AUC0‐t, Cmax, and tmax) derived from a single dose of rucaparib 40 mg or 300 mg with or without a high‐fat meal (fed vs fasted) were used for preliminary assessment of food effect on pharmacokinetics. In Part 3, the effect of a high‐fat meal (fed vs fasted) was examined in patients receiving a single dose of rucaparib 600 mg. Part 3 pharmacokinetic parameters (AUC0‐t and Cmax) were log‐transformed prior to analysis and were analyzed using a linear mixed‐effects model. The model included food effect, period, and sequence as fixed effects and the subject as a random effect. Point estimates (least‐squares means) for food effect and their 90%CIs were calculated on a log scale and then back‐transformed to provide estimates of and CIs for the geometric mean ratios. The residual variance from the linear mixed‐effects model was used to calculate the 90%CIs. For tmax, nonparametric analysis was performed using a Wilcoxon signed‐rank test, and the corresponding 95%CIs for differences in medians were determined using Walsh averages.
Results
Patients
The first patient was enrolled on December 14, 2011; the visit cutoff dates for this analysis were November 30, 2015 (Part 1), and December 10, 2015 (Part 3). In the phase 1 dose‐escalation portion of Study 10 (Part 1), 56 patients with a locally advanced or metastatic solid tumor who had progressed on standard treatment were enrolled. In Part 1, single‐dose and steady‐state pharmacokinetics were evaluated in 25 patients who received rucaparib QD at doses ranging from 40 to 500 mg and in 30 patients who received rucaparib BID at doses ranging from 240 to 840 mg (pharmacokinetic analysis; Figure 1). One patient from Part 1 was excluded from the analysis because the pharmacokinetic profile was incomplete (only 3 data points during cycle 1 day 1 were reported). The effect of a high‐fat meal on single‐dose pharmacokinetics (food effect analysis) was evaluated in 9 patients treated with a single dose of rucaparib 40 mg (n = 3) or rucaparib 300 mg QD (n = 6) (food effect cohorts).
Figure 1.

Patient flow diagram. BID, twice daily; PK, pharmacokinetics; QD, once daily.
In the phase 2 pharmacokinetic portion of Study 10 (Part 3), 26 patients with an advanced solid tumor and evidence of a germline or somatic BRCA1 or BRCA2 mutation were enrolled. All patients received rucaparib 600 mg BID. Full pharmacokinetic profiles were characterized after a single dose of rucaparib was administered and at steady state. All Part 3 patients were evaluated for the effect of a high‐fat meal on single‐dose pharmacokinetics (food effect analysis). Steady‐state pharmacokinetics (cycle 1 day 15) was evaluated in 18 patients from Part 3 (Figure 1).
The baseline characteristics of patients who enrolled in Study 10 Parts 1 and 3 are presented in Table S1.
Part 1 Pharmacokinetics
Overall, a dose‐dependent increase in the plasma concentration of rucaparib was observed following QD or BID dosing schedules (Figure 2). At 80 mg QD, rucaparib exposure on cycle 1 day 1 appeared to be lower than what was observed at 40 mg QD, likely due to intersubject pharmacokinetic variability.
Figure 2.
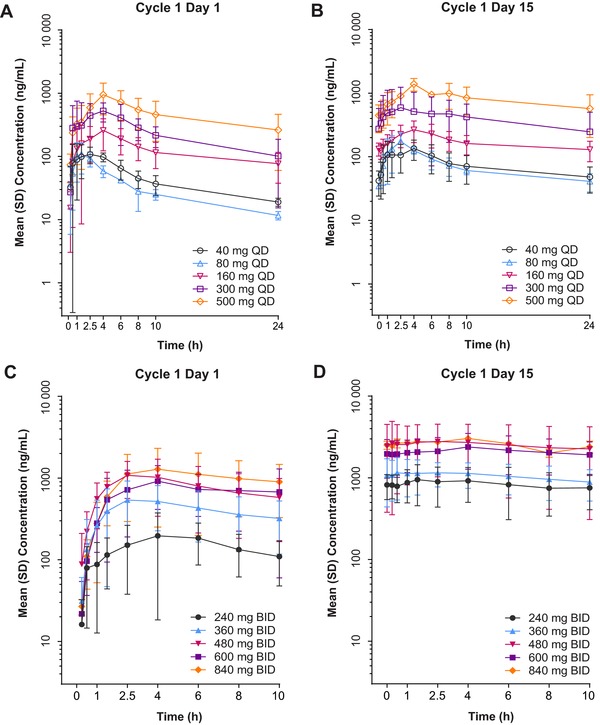
Rucaparib plasma concentration‐time profiles following once daily (QD [A, B]) and twice daily (BID [C, D]) oral administration. Error bars represent standard deviation (SD). Adapted from Kristeleit R, Shapiro GI, Burris HA, et al. A phase I‐II study of the oral poly(ADP‐ribose) polymerase inhibitor rucaparib in patients with germline BRCA1/2‐mutated ovarian carcinoma or other solid tumors. Clin Cancer Res. 2017;23(15):4095‐4106 [Supplementary Appendix, Figures S1 and S2].14
In the QD cohorts (40‐500 mg), mean Cmax on cycle 1 day 1 (single dose) ranged from 114 to 949 ng/mL, and AUC0‐24h ranged from 800 to 11 000 ng ⋅ h/mL (Table 1). Steady state for the QD dosing schedule was reached by cycle 1 day 8 (Figure 3A). At steady state (cycle 1 day 15), mean Cmax (range, 138‐1390 ng/mL) and AUC0‐24h (range, 1740‐19 900 ng ⋅ h/mL) exhibited similar dose‐dependent increases as a single dose of rucaparib (Table 1). Median tmax for the Part 1 QD cohorts ranged from 1.5 to 4 hours, suggesting relatively fast absorption of rucaparib following oral administration. Mean t1/2 ranged from 11.0 to 19.9 hours during cycle 1 day 1 and 19.5 to 33.6 hours during cycle 1 day 15 across all dose levels. Noncompartmental analysis showed that the percentage of extrapolated AUC (%AUCex) was <20% for only 3 subjects in the Part 1 QD cohorts following administration of a single dose of rucaparib. Therefore, AUC from time 0 extrapolated to infinity (AUC∞) and pharmacokinetic parameters related to AUC∞ such as CL/F are not reported. There was no apparent dose‐associated trend in mean CLss/F reported based on the pharmacokinetic profile of cycle 1 day 15 (Table 1). The Rac_Cmax at steady state ranged from 1.06 to 1.8 and the Rac_AUC0‐24h ranged from 1.6 to 2.33, which was consistent with the corresponding ranges of t1/2 following QD dosing.
Table 1.
Single‐Dose and Steady‐State Plasma Pharmacokinetic Parameters of Rucaparib Following Once‐ or Twice‐Daily Continuous Oral Administration (Part 1 QD and BID Cohorts)
| Dosage | N | Day | Arithmetic Mean Cmax (CV%), ng/mL | Median tmax (range), h | Arithmetic Mean AUC0‐ t (CV%), ng ⋅ h/mL | Arithmetic Mean CLss/F (CV%), L/h | Arithmetic Mean t1/2 (CV%), h | Rac_Cmax (CV%) | Rac_AUC0‐ t (CV%) |
|---|---|---|---|---|---|---|---|---|---|
| 40 mg QD | 3 | 1 | 129 (28) | 2.5 (1‐4) | 915a | NR | 13.9 (57) | NA | NA |
| 15 | 138 (36) | 4 (1‐4.05) | 1810 (44) | 26.7 (59) | 25.7 (23) | 1.06 (24) | 1.68a | ||
| 80 mg QD | 3 | 1 | 114 (41) | 1.5 (1‐2.5) | 800 (27) | NR | 11.0a | NA | NA |
| 15 | 175 (37) | 2.5 (2.5‐2.57) | 1740 (20) | 47.5 (23) | 19.5a | 1.8 (58) | 2.33 (42) | ||
| 160 mg QD | 4 | 1 | 261 (51) | 4.0 (4‐6.05) | 3050 (51) | NR | 19.9 (21) | NA | NA |
| 15 | 288 (29)b | 3.75 (2.5‐4)b | 4110 (33)b | 41.6 (29)b | 33.6 (12)b | 1.54 (35) | 1.84 (31)b | ||
| 300 mg QD | 3 | 1 | 629 (37) | 2.5 (1‐4.08) | 5740 (38) | NR | 15.2 (72) | NA | NA |
| 15 | 693 (76) | 2.53 (2.5‐8) | 9610 (83) | 46.7 (63) | 29.8a | 1.09 (51) | 1.60 (53) | ||
| 500 mg QD | 3 | 1 | 949 (52) | 4 (4‐4) | 11 000 (61) | NR | 15.0 (32) | NA | NA |
| 15 | 1390 (23) | 4 (4‐4.17) | 19 900 (41) | 27.8 (35) | 20.8 (38) | 1.6 (24) | 1.94 (17) | ||
| 240 mg BID | 3 | 1 | 219 (72) | 6 (4.05‐6) | 2800c | NR | NRh | NA | NA |
| 15 | 971 (49) | 1.5 (1‐4) | 10 700a | 27.3a | NRh | 4.91 (22) | 5.44c | ||
| 360 mg BID | 8 | 1 | 666 (58) | 3.23 (1.5‐6) | 4860 (58)d | NR | NRh | NA | NA |
| 15 | 1300 (43)d | 3.3 (0‐6.33)d | 9430a | 40.4a | NRh | 2.6 (63)d | 4.08a | ||
| 480 mg BID | 9 | 1 | 1150 (57) | 2.5 (1.5‐4) | 8810 (63)e | NR | NRh | NA | NA |
| 15 | 3170 (69)e | 1.51 (0‐6)e | 26 300 (73)d | 26.2 (63)d | NRh | 2.72 (23)e | 3.97 (38)f | ||
| 600 mg BID | 7 | 1 | 1030 (61) | 4 (2.42‐10) | 7200 (66)g | NR | NRh | NA | NA |
| 15 | 2420 (45) | 4 (2.53‐10) | 21 400 (61)g | 58.6 (123)g | NRh | 2.81 (54) | 3.23 (66)g | ||
| 840 mg BID | 3 | 1 | 1380 (69) | 4 (2.5‐8) | 13 200a | NR | NRh | NA | NA |
| 15 | 3030a | 4.04 (4‐4.07)a | 29 000c | 29c | NRh | 2.75a | 1.47c |
AUC0‐t, area under the plasma concentration‐time curve from 0 to time t (t = 24 hours for QD; t = 12 hours for BID; for BID dosing schedule, concentration at 12 hours was calculated by extrapolation from last observed concentration in the same dosing interval); BID, twice daily; CLss/F, apparent total plasma clearance at steady state; Cmax, maximum plasma concentration; CV, coefficient of variation; NA, not available; NR, not reportable; QD, once daily; Rac, accumulation rate; t1/2, half‐life; tmax, time of occurrence of maximum plasma concentration.
an = 2; bn = 3; cn = 1; dn = 6; en = 8; fn = 5; gn = 4; ht1/2 is too long to allow for accurate estimate for the BID dosing schedule.
Adapted from Kristeleit R, Shapiro GI, Burris HA, et al. A phase I‐II study of the oral poly(ADP‐ribose) polymerase inhibitor rucaparib in patients with germline BRCA1/2‐mutated ovarian carcinoma or other solid tumors. Clin Cancer Res. 2017;23(15):4095‐4106 [Table 4].14
Figure 3.

Mean (standard error [SE]) rucaparib plasma trough concentrations vs time by cohort (QD [A] and BID [B] dosing schedules). Note: Preliminary food effect test cohorts were excluded. BID, twice daily; QD, once daily.
Across the BID dose cohorts in Part 1 (240‐840 mg BID), the mean Cmax on cycle 1 day 1 ranged from 219 to 1380 ng/mL and the AUC0‐12h ranged from 2800 to 13 200 ng ⋅ h/mL (Table 1). Steady state was reached by cycle 1 day 8 (Figure 3B) for the BID dosing schedule. At steady state (cycle 1 day 15), mean Cmax ranged from 971 to 3170 ng/mL and AUC0‐12h ranged from 9430 to 29 000 ng ⋅ h/mL (Table 1). The median tmax ranged from 1.5 to 6 hours across all dose levels tested. Due to the limited pharmacokinetics collection duration and relatively slow elimination of rucaparib, t1/2 was poorly estimated and is not reported here. Noncompartmental analysis showed that no subjects in the Part 1 BID cohorts had a %AUCex <20%; therefore, AUC∞ was not analyzed, and pharmacokinetic parameters related to AUC∞ such as CL/F are not reported. Following BID dosing, the Rac_Cmax at steady state ranged from 2.6 to 4.9 and the Rac_AUC0‐12h ranged from 1.47 to 5.44. Accumulation following BID dosing was approximately twice that following QD dosing.
Dose Proportionality
For the QD and BID dosing schedules, dose proportionality was assessed at steady state for both Cmax and AUC0‐t (Figure 4). For the QD dose, the slopes (β1) of Cmax and AUC0‐24h were 0.92 (90%CI, 0.69–1.15; R2 = 0.795) and 0.98 (90%CI, 0.72–1.25; R2 = 0.775); for the BID dose, these values were 1.03 (90%CI, 0.42–1.63; R2 = 0.261) and 1.04 (90%CI, 0.42–1.67; R2 = 0.253), respectively. In both dosing schedules, the slopes were approximately 1, suggesting that for both Cmax and AUC0‐t, the relationships between dose and exposure were proportional.
Figure 4.

Observed and predicted relationship between rucaparib dose and exposure at steady state on once daily (QD [A, B]) and twice daily (BID [C, D]) dosing schedules. Open circles represent observed individual steady‐state Cmax or AUC0‐t, solid lines represent model prediction, and shaded areas represent 90% confidence intervals (CIs). AUC0‐t, area under the concentration‐time curve from time 0 to last measurable concentration (t = 24 hours for QD dosing schedule; t = 12 hours for BID dosing schedule); AUC0‐12h, area under the concentration‐time curve from 0 to 12 hours; AUC0‐24h, area under the concentration‐time curve from 0 to 24 hours; Cmax, maximum plasma concentration. Adapted from Kristeleit R, Shapiro GI, Burris HA, et al. A phase I‐II study of the oral poly(ADP‐ribose) polymerase inhibitor rucaparib in patients with germline BRCA1/2‐mutated ovarian carcinoma or other solid tumors. Clin Cancer Res. 2017;23(15):4095‐4106 [Supplementary Appendix, Figure S3].14
Food Effect (Parts 1 and 3)
Nine patients in Part 1 (3 from the 40 mg QD group and 6 from the 300 mg QD group) were evaluated for the effect of a high‐fat meal on single‐dose pharmacokinetics (Figure 1). Mean rucaparib plasma concentration profiles and pharmacokinetic parameters following administration of a single dose of rucaparib (Figure 5A; Table 2) were similar between fed and fasted conditions. Minimal food effect was observed at each dose level (Figure S1). No formal food effect analysis was conducted due to the small sample sizes. (Steady‐state pharmacokinetic data without food restriction for these patients are provided in Figure 5B and Table 3.)
Figure 5.

Mean (standard deviation [SD]) rucaparib plasma concentration‐time profiles under fasted conditions and with a high‐fat meal following a single dose of rucaparib at 40 and 300 mg (Part 1) and 600 mg (Part 3) (A), and steady‐state pharmacokinetic profiles following rucaparib 40 and 300 mg QD and 600 mg BID with or without food (B). Food effect was evaluated on cycle 1 days –7 and 1. Steady‐state pharmacokinetics was evaluated on cycle 1 day 15. BID, twice a day; QD, once daily.
Table 2.
Summary of Single‐Dose Pharmacokinetic Parameters of Rucaparib Administered Under Fed and Fasted Conditions in Food Effect Cohorts
| Dose | Visit | Statistics | Cmax, ng/mL | tmax, h | AUC0‐24h, ng ⋅ h/mL | t1/2, h |
|---|---|---|---|---|---|---|
| 40 mg QD (Part 1) | Cycle 1, day –7 fasted | n | 3 | 3 | 3 | 3 |
| Arithmetic mean | 77.9 | 741 | 10.6 | |||
| Arithmetic mean SD | 46.4 | 580 | 5.3 | |||
| Arithmetic mean CV% | 60 | 78 | 50 | |||
| Median | 57.6 | 4 | 468 | 8.16 | ||
| Min, max | 45.2, 131 | 2.5, 4.05 | 347, 1410 | 6.9, 16.6 | ||
| Cycle 1, day 1 fed | n | 3 | 3 | 2 | 3 | |
| Arithmetic mean | 64.8 | 794 | 12.6 | |||
| Arithmetic mean SD | 40.7 | 536 | 5.3 | |||
| Arithmetic mean CV% | 63 | 68 | 42 | |||
| Median | 71.1 | 2.55 | 794 | 12.7 | ||
| Min, max | 21.3, 102 | 1, 4.08 | 415, 1170 | 7.21, 17.8 | ||
| 300 mg QD (Part 1) | Cycle 1, day –7 fasted | n | 6 | 6 | 6 | 4 |
| Arithmetic mean | 415 | 5320 | 23.5 | |||
| Arithmetic mean SD | 190 | 2490 | 10.9 | |||
| Arithmetic mean CV% | 46 | 47 | 46 | |||
| Median | 424 | 4.09 | 5410 | 24.1 | ||
| Min, max | 182, 638 | 2.5, 24.22 | 2390, 8680 | 11.9, 33.8 | ||
| Cycle 1, day 1 fed | n | 6 | 6 | 5 | 3 | |
| Arithmetic mean | 502 | 6890 | 17.4 | |||
| Arithmetic mean SD | 377 | 3740 | 5.9 | |||
| Arithmetic mean CV% | 75 | 54 | 34 | |||
| Median | 393 | 5.95 | 6000 | 20.5 | ||
| Min, max | 177, 1210 | 2.53, 10 | 2670, 12 100 | 10.6, 21.1 | ||
| 600 mg BID (Part 3) | Fasteda | n | 26 | 26 | 26 | 19 |
| Arithmetic mean | 819 | 10 000 | 18.7 | |||
| Arithmetic mean SD | 689 | 7590 | 9.9 | |||
| Arithmetic mean CV% | 84 | 76 | 53 | |||
| Median | 585 | 4.02 | 7050 | 18.8 | ||
| Min, max | 127, 3100 | 0.53, 24.83 | 1110, 33 000 | 6.65, 52.2 | ||
| Feda | n | 26 | 26 | 26 | 11 | |
| Arithmetic mean | 959 | 13 900 | 16.8 | |||
| Arithmetic mean SD | 698 | 10 300 | 9.5 | |||
| Arithmetic mean CV% | 73 | 74 | 57 | |||
| Median | 746 | 7.83 | 10 900 | 14.4 | ||
| Min, max | 198, 2640 | 1.5, 24.45 | 1990, 40 400 | 7.96, 42.3 |
Adapted from Kristeleit R, Shapiro GI, Burris HA, et al. A phase I‐II study of the oral poly(ADP‐ribose) polymerase inhibitor rucaparib in patients with germline BRCA1/2‐mutated ovarian carcinoma or other solid tumors. Clin Cancer Res. 2017;23(15):4095‐4106 [Supplementary Appendix, Table S1].14
AUC0‐24h, area under the plasma concentration‐time curve from 0 to 24 hours; BID, twice daily; Cmax, maximum plasma concentration; CV, coefficient of variation; QD, once daily; SD, standard deviation; t1/2, half‐life; tmax, time of occurrence of maximum plasma concentration.
In Part 3 patients were randomized to 1 of the following: sequence 1, fed on day –7 and then fasted on cycle 1 day 1; or sequence 2, fasted on day –7 and then fed on cycle 1 day 1.
Table 3.
Summary of Steady‐State Pharmacokinetic Parameters of Rucaparib in Food Effect Cohorts
| Dose | Visit | Statistics | Cmax, ng/mL | tmax, h | AUC0‐t, ng ⋅ h/mL | AUC0‐12h, ng ⋅ h/mLa | t1/2, h |
|---|---|---|---|---|---|---|---|
| 40 mg QD (Part 1) | Cycle 1, day 15 | n | 3 | 3 | 1 | 2 | |
| Arithmetic mean | 129 | 1050 | 9.23 | ||||
| Arithmetic mean CV% | 62 | 86 | |||||
| Median | 103 | 4 | 1050 | 9.23 | |||
| Min, max | 65.3, 220 | 1, 4.03 | 1050, 1050 | 3.63, 14.8 | |||
| 300 mg QD (Part 1) | Cycle 1, day 15 | n | 5 | 5 | 3 | 3 | |
| Arithmetic mean | 727 | 13 300 | 20 | ||||
| Arithmetic mean CV% | 79 | 106 | 68 | ||||
| Median | 444 | 2.47 | 5220 | 27.5 | |||
| Min, max | 341, 1720 | 1.08, 6 | 5070, 29 600 | 4.24, 28.2 | |||
| 600 mg BID (Part 3) | Cycle 1, day 15 | n | 18 | 18 | 18 | 12 | 12 |
| Arithmetic mean | 1940 | 15 800 | 16 900 | 12.6 | |||
| Arithmetic mean CV% | 54 | 58 | 54 | 54 | |||
| Median | 1480 | 1.92 | 12 900 | 14 700 | 10.9 | ||
| Min, max | 626, 4050 | 0, 5.98 | 5400, 34 200 | 7580, 39 300 | 5.31, 26.6 |
AUC0‐t, area under the plasma concentration‐time curve from 0 to time t (t = 24 hours for QD; t = 10 hours for BID); AUC0‐12h, area under the plasma concentration‐time curve from 0 to 12 hours; BID, twice daily; Cmax, maximum plasma concentration; CV, coefficient of variation; QD, once daily; t1/2, half‐life; tmax, time of occurrence of maximum plasma concentration.
For the BID dosing schedule, concentration at 12 hours was calculated by extrapolation from last observed concentration in the same dosing interval.
In Part 3, the mean plasma exposure of a single dose of rucaparib 600 mg was higher when administered with a high‐fat meal than under fasted conditions (Figure 5A; Table 2). The fed‐to‐fasted geometric mean ratios (90%CI) were 138% (117%‐162%) and 120% (99.1%‐146%) for AUC0‐24h and Cmax, respectively. The median tmax was 7.83 and 4.02 hours after dosing under fed and fasted conditions, respectively. The median delay of tmax by a high‐fat meal was 2.5 hours (95%CI, 0.500–4.405; P ≤ .05 [Wilcoxon signed‐rank test]). Under fed conditions, the intersubject variability (CV%) of the geometric mean AUC0‐24h was 86.8% and that of the Cmax was 82.8%; these values were similar under fasted conditions (84.6% and 87.5%, respectively), suggesting that a high‐fat meal did not reduce the intersubject pharmacokinetic variability in the absorption of rucaparib.
Steady‐State Exposure at the RP2D (Part 3)
The mean rucaparib plasma concentration at steady state (cycle 1 day 15) for patients receiving rucaparib 600 mg BID in Part 3 is shown in Figure 5B. Mean (CV%) steady‐state Cmax and AUC0‐12h were 1940 ng/mL (54%) and 16 900 ng ⋅ h/mL (54%), respectively, and median tmax was 1.92 (range, 0‐5.98) hours (Table 3).
Safety (Parts 1 and 3)
In Part 1, the median duration of treatment in patients with advanced solid tumors was 3.2 months (range, 1 day to 37.9 months) across all dose levels. The most common treatment‐emergent adverse events (any grade) were nausea, asthenia/fatigue, vomiting, and anemia (Table S2). The majority (57.1%) of treatment‐emergent events were grade 1 or 2. No grade 4 events were reported. Three deaths due to disease progression were reported.14 No treatment‐related deaths were reported in Part 1.14
In Part 3, the median duration of treatment in patients with relapsed solid tumors was 2.9 months (range, 8 days to 7.6 months). The most common treatment‐emergent adverse events (any grade) were asthenia/fatigue, nausea, decreased appetite, and vomiting (Table S2). Four (15.4%) patients died due to a treatment‐emergent adverse event, including 2 (7.7%) due to disease progression, 1 (3.8%) due to dyspnea, and 1 (3.8%) due to pulmonary embolism. No treatment‐related deaths were reported in Part 3.
Discussion
The intensive pharmacokinetic data collected in Study 10 allowed for sufficient evaluation of the pharmacokinetic profile of the PARP inhibitor rucaparib. The analysis demonstrates that plasma exposure of rucaparib is approximately dose proportional across the entire dose range tested (40–500 mg QD and 240–840 mg BID).
The estimated t1/2 was approximately 17 (range, 11‐33.6) hours. As the t1/2 values were based on a relatively short pharmacokinetics sampling duration of 24 hours, the t1/2 should be interpreted with caution. Nevertheless, the observed time to steady state (day 8) and the magnitude of steady‐state accumulation (range, 1.6‐2.33 for QD, 1.47‐5.44 for BID) are consistent with the estimated t1/2, suggesting time‐independent pharmacokinetics.
Early data showed that a high‐fat meal had minimal effect on rucaparib pharmacokinetics following single‐dose administration of rucaparib 40 mg or 300 mg. Given this early finding, patients ingested rucaparib with or without food at the higher doses evaluated in Part 1 (dose‐escalation portion) and in Part 2 (phase 2 portion) of Study 10, as well as in other Clovis‐sponsored phase 2 or phase 3 trials. Later, a formal evaluation of food effect was conducted with rucaparib 600 mg in Part 3. A high‐fat meal moderately increased rucaparib exposure, with the fed‐to‐fasted geometric mean ratios (90%CI) of 138% (117%‐162%) and 120% (99.1%‐146%) for AUC0‐24h and Cmax, respectively, and delayed tmax by a median of 2.5 hours. The increase in exposure may be due to increased intestinal solubility following consumption of a high‐fat meal. Despite the observed moderate food effect on Cmax and AUC0‐24h, collective clinical data indicate that rucaparib is efficacious with an acceptable safety profile when administered to patients at 600 mg BID without food restriction.14, 16, 19 As a result, the moderate food effect on pharmacokinetics is not considered clinically significant; thus, rucaparib can be administered with or without food.
The intersubject pharmacokinetic variability at the RP2D was moderate, with 54% CV for both steady‐state Cmax and AUC0‐12h. The oral bioavailability of rucaparib was previously determined to be moderately low (36%).20 The magnitude of intersubject pharmacokinetic variability observed in this study is not uncommon for small molecule oncology drugs with low to moderate oral bioavailability. Despite the observed effect of food on rucaparib exposure, there was no apparent difference in intersubject pharmacokinetic variability when rucaparib was taken with a high‐fat meal vs under fasted conditions.
In this analysis, pharmacokinetics was examined in patients receiving lower strength tablets (40 mg and 60 mg, Part 1) and higher strength tablets (300 mg, Part 3). Across all doses and tablet strengths tested, the tmax was relatively short, with a cohort median tmax of 1.5 to 6 hours, suggesting relatively fast absorption with comparable kinetics regardless of dose or tablet strength. At the RP2D of 600 mg BID, steady‐state rucaparib exposures were comparable between patients in Part 1 (60 mg tablets) and Part 3 (300 mg tablets). These results suggest similar absorption kinetics between the two immediate‐release formulations that were used in Study 10.
Estimated mean CLss/F ranged from 26.7 to 47.5 L/h for the QD dosing schedule and 26.2 to 58.6 L/h for the BID dosing schedule. Given rucaparib's oral bioavailability of 36%,20 the CLss at tested oral dose levels was estimated to be ≤350 mL/min, which is approximately ≤24% of the normal liver blood flow rate (1450 mL/min),21 suggesting relatively low systemic elimination. Following continuous rucaparib 600 mg BID dosing, median tmax was 1.92 (range, 0‐5.98) hours. Mean (CV%) steady‐state Cmax, AUC0‐12h, and CLss/F were 1940 ng/mL (54%), 16 900 ng ⋅ h/mL (54%), and 44.2 L/h (45%), respectively. The steady‐state pharmacokinetic variability appeared to be moderate. Effects of intrinsic and extrinsic factors on rucaparib pharmacokinetic variability were evaluated by population pharmacokinetic modeling, which will be reported separately.
To date, there have been no formal drug‐drug interaction studies in patients to determine the effect of any metabolic enzymes on rucaparib pharmacokinetics. In vitro and preliminary in vivo metabolite profiling data are suggestive of oxidative deamination and formation of a carboxylic acid metabolite. No conjugate metabolite has been identified in vitro or in vivo. A [14C] rucaparib mass balance and metabolite profiling study in patients is under way.
Conclusion
Rucaparib pharmacokinetics was characterized in patients with an advanced solid tumor, including high‐grade ovarian cancer. Rucaparib demonstrated time‐independent and dose‐linear pharmacokinetics, with moderate pharmacokinetic variability over the dose levels examined. A high‐fat meal showed moderate but clinically insignificant increases in rucaparib Cmax and AUC at the recommended clinical dose of 600 mg.
Supporting information
List of institutional review boards for all sites involved in Study 10
Table S1. Baseline Patient and Disease Characteristics
Table S2. Treatment‐Emergent Adverse Events Occurring in ≥15% of Patients and All Grade 5 Events in Part 1 (Overall) or Part 3 by Rucaparib Dose
Figure S1. Cmax (A) and AUC0‐24 (B) following a single dose of rucaparib administered under fed and fasted conditions in the Part 1 food effect cohorts.
Acknowledgments
Clinical operations support for the study was provided by Samantha McGuinness. Medical writing and editorial support, funded by Clovis Oncology, was provided by Nathan Yardley, PhD, and Shannon Davis of Ashfield Healthcare Communications (Middletown, Connecticut). All authors contributed to the conception and design, or acquisition of data, or analysis and interpretation o data and drafted or revised the article for important intellectual content. All authors approved the final draft for publication.
Funding
The study was funded by Clovis Oncology, Inc. The study was also supported by the NIHR/Wellcome UCH Clinical Research Facility, the UCH/UCL Biomedical Research Centre, and UCL Experimental Cancer Medicine Centre.
Declaration of Conflicting Interests
G.I.S.’s institution received reimbursement of study costs from Clovis Oncology for this clinical trial. R.S.K. received an honorarium from Clovis Oncology for attending an advisory board relating to rucaparib, and her institution received reimbursement of study costs from Clovis Oncology for this clinical trial. Y.D. received an honorarium from Clovis Oncology for attending an advisory board relating to rucaparib, and her institution received reimbursement of study costs from Clovis Oncology for this clinical trial. H.G., L.M., S.W., S.G., and J.X. are employees of Clovis Oncology and may hold stock or have stock options in the company. S.J.‐T. is a former employee of Clovis Oncology and may hold stock in the company. No potential conflicts of interest were disclosed by the other authors.
References
- 1. Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP‐ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7(7):517–528. [DOI] [PubMed] [Google Scholar]
- 2. Ryu KW, Kim DS, Kraus WL. New facets in the regulation of gene expression by ADP‐ribosylation and poly(ADP‐ribose) polymerases. Chem Rev. 2015;115(6):2453–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Helleday T, Lo J, van Gent DC, Engelward BP. DNA double‐strand break repair: from mechanistic understanding to cancer treatment. DNA Repair. 2007;6(7):923–935. [DOI] [PubMed] [Google Scholar]
- 4. Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8(3):193–204. [DOI] [PubMed] [Google Scholar]
- 5. Moynahan ME, Chiu JW, Koller BH, Jasin M. BRCA1 controls homology‐directed DNA repair. Mol Cell. 1999;4(4):511–518. [DOI] [PubMed] [Google Scholar]
- 6. Moynahan ME, Pierce AJ, Jasin M. BRCA2 is required for homology‐directed repair of chromosomal breaks. Mol Cell. 2001;7(2):263–272. [DOI] [PubMed] [Google Scholar]
- 7. Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108(2):171–182. [DOI] [PubMed] [Google Scholar]
- 8. Murai J, Huang SY, Renaud A, et al. Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol Cancer Ther. 2014;13(2):433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ashworth A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double‐strand break repair. J Clin Oncol. 2008;26(22):3785–3790. [DOI] [PubMed] [Google Scholar]
- 10. Helleday T. The underlying mechanism for the PARP and BRCA synthetic lethality: Clearing up the misunderstandings. Mol Oncol. 2011;5(4):387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D'Andrea AD. Homologous recombination deficiency: exploiting the fundamental vulnerability of ovarian cancer. Cancer Discov. 2015;5(11):1137–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wahlberg E, Karlberg T, Kouznetsova E, et al. Family‐wide chemical profiling and structural analysis of PARP and tankyrase inhibitors. Nat Biotechnol. 2012;30(3):283–288. [DOI] [PubMed] [Google Scholar]
- 13. Thomas HD, Calabrese CR, Batey MA, et al. Preclinical selection of a novel poly(ADP‐ribose) polymerase inhibitor for clinical trial. Mol Cancer Ther. 2007;6(3):945–956. [DOI] [PubMed] [Google Scholar]
- 14. Kristeleit R, Shapiro GI, Burris HA, et al. A phase I‐II study of the oral poly(ADP‐ribose) polymerase inhibitor rucaparib in patients with germline BRCA1/2‐mutated ovarian carcinoma or other solid tumors. Clin Cancer Res. 2017;23(15):4095–4106. [DOI] [PubMed] [Google Scholar]
- 15. Domchek SM, Hendifar AE, McWilliams RR, et al. RUCAPANC: An open‐label, phase 2 trial of the PARP inhibitor rucaparib in patients (pts) with pancreatic cancer (PC) and a known deleterious germline or somatic BRCA mutation. J Clin Oncol. 2016;34(15 suppl):abst 4110.27863191 [Google Scholar]
- 16. Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum‐sensitive high‐grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open‐label, phase 2 trial. Lancet Oncol. 2017;18(1):75–87. [DOI] [PubMed] [Google Scholar]
- 17. Xiao JJ, Green M, Ma SC, et al. Population pharmacokinetics (PK) of rucaparib (CO‐338) in patients with advanced ovarian cancer (AOC) or other solid tumors. Clin Pharmacol Ther. 2017;101(S1):S5–S99. [DOI] [PubMed] [Google Scholar]
- 18. Smith BP, Vandenhende FR, DeSante KA, et al. Confidence interval criteria for assessment of dose proportionality. Pharm Res. 2000;17(10):1278–1283. [DOI] [PubMed] [Google Scholar]
- 19. Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2017;390(10106):1949–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilson RH, Evans TJ, Middleton MR, et al. A phase I study of intravenous and oral rucaparib in combination with chemotherapy in patients with advanced solid tumours. Br J Cancer. 2017;116(7):884–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10(7):1093–1095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of institutional review boards for all sites involved in Study 10
Table S1. Baseline Patient and Disease Characteristics
Table S2. Treatment‐Emergent Adverse Events Occurring in ≥15% of Patients and All Grade 5 Events in Part 1 (Overall) or Part 3 by Rucaparib Dose
Figure S1. Cmax (A) and AUC0‐24 (B) following a single dose of rucaparib administered under fed and fasted conditions in the Part 1 food effect cohorts.


