Abstract
Aims
Semaglutide is a glucagon‐like peptide‐1 (GLP‐1) analogue approved for the treatment of type 2 diabetes. The impact of switching treatment from another GLP‐1 receptor agonist (GLP‐1RA) to semaglutide was investigated by analyses of exposure‐response models.
Methods
HbA1c and body weight time‐course models were developed, using up to 30 weeks of observations from four trials in the semaglutide phase 3 programme. Given the recommended dosing for each GLP‐1RA, pharmacokinetic profiles were simulated based on published population pharmacokinetic models and exposure was adjusted by the relative potencies to ensure that model predictions matched the effects observed in clinical trials. After 26 weeks of simulated treatment with liraglutide, dulaglutide or exenatide extended‐release, simulated semaglutide treatment was initiated 1 day after the last once‐daily dose of liraglutide and 1 week after the last once‐weekly doses of dulaglutide or exenatide extended‐release.
Results
The potency‐adjusted total effective GLP‐1RA concentration increased after switching from another GLP‐1RA to semaglutide and was associated with reductions ranging from ~0.3% to ~0.8%‐points for HbA1c and from ~2% to ~4% for body weight with semaglutide 1.0 mg. Temporary slight deteriorations in HbA1c were observed after switching to semaglutide 0.25 mg from liraglutide 1.2/1.8 mg or dulaglutide 1.5 mg.
Conclusions
Exposure‐response modelling suggests that switching to semaglutide from liraglutide, dulaglutide or exenatide extended‐release results in further reductions in HbA1c and body weight. Initial slight deterioration in outcome values when switching to semaglutide 0.25 mg could be avoided by initiating semaglutide treatment at a higher dose.
Keywords: antidiabetic drug, GLP‐1, GLP‐1 analogue, population study, type 2 diabetes
1. INTRODUCTION
Glucagon‐like peptide‐1 (GLP‐1) is an incretin hormone that stimulates insulin secretion and inhibits glucagon secretion in a glucose‐dependent manner,1, 2 and is therefore indicated for the treatment of type 2 diabetes mellitus (T2DM).3, 4 Human GLP‐1 is secreted by intestinal L cells in response to food intake.1, 5 In addition to its antihyperglycaemic effects, GLP‐1 has beneficial effects on body weight, unlike many other treatments for T2DM.6 These effects are modulated by slowing the rate of gastric emptying and enhancing satiety, which in turn leads to reduced energy intake.7
Semaglutide (Novo Nordisk, Denmark) is a once‐weekly human GLP‐1 analogue approved for the treatment of T2DM, based on results from the SUSTAIN (Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes) phase 3 clinical development programme. Semaglutide has 94% amino acid sequence homology to native GLP‐1,8 with three structural modifications that prolong the half‐life to approximately 1 week.8, 9
In two phase 3 trials, semaglutide was compared head‐to‐head with dulaglutide and exenatide extended release (ER) and was shown to be superior in reducing HbA1c and body weight10, 11 (Figure 1). However, no studies to date have investigated switching from another GLP‐1RA to semaglutide. When contemplating such a switch between GLP‐1RAs, it is important to consider the GLP‐1RA pharmacokinetics (PK) and the current treatment dosing, once‐daily or once‐weekly. For instance, the once‐daily GLP‐1RA liraglutide is cleared from circulation within a few days,12 whereas exenatide ER provides effective exposure levels for several weeks.13 We considered that sufficient data were available to develop an exposure‐response model which would provide simulations of the expected treatment outcomes, in terms of changes in HbA1c and body weight, after switching from other GLP‐1‐RAs to semaglutide.
Figure 1.
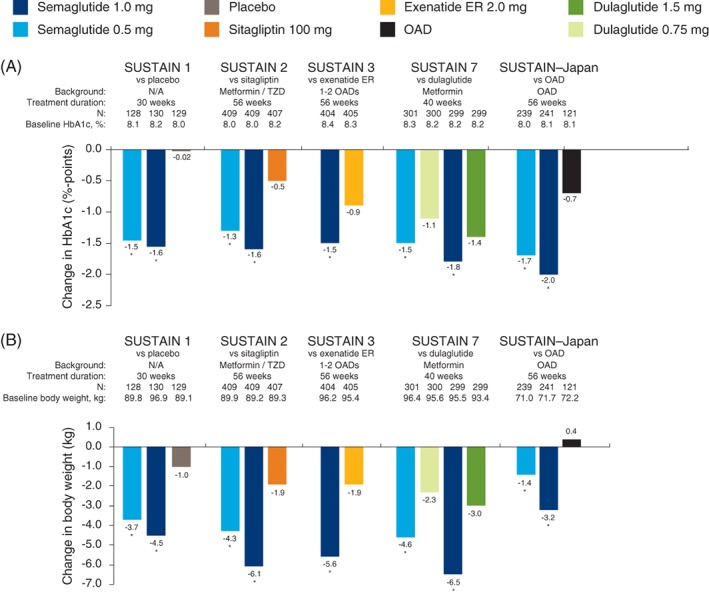
Semaglutide phase 3 SUSTAIN trials included in the analyses with semaglutide 0.5 and 1.0 mg doses. Estimated mean changes in HbA1c (A) and body weight (B) are shown. *P < 0.0001 vs comparator. The phase 2 trial is not included as multiple doses of semaglutide and liraglutide were included. Abbreviations: ER, extended release; HbA1c, glycated haemoglobin; N, number of subjects; N/A, not applicable; OAD, oral anti‐diabetic drug; SUSTAIN, Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes; TZD, thiazolidinediones
The aim with the current analyses was to investigate the impact on HbA1c and body weight of switching to semaglutide from other GLP‐1RAs (liraglutide, dulaglutide and exenatide ER) and to analyse different dose escalation algorithms depending on the PK of each GLP‐1RA.
2. SUBJECTS AND METHODS
2.1. Trial data
A summary of the six randomized, controlled phase 2 and 3 trials included in the analyses is presented in Table 1 and estimated mean changes in HbA1c and body weight are shown in Figure 1. All were conducted in subjects with T2DM.
Table 1.
Summary of the randomized, controlled trials included in the analysis
| Trial, phase and design | Baseline characteristics (total) |
|---|---|
| SUSTAIN 1 14 | Mean (SD), N = 387 |
| Phase 3 | 54% male |
| Semaglutide vs placebo monotherapy | Age, y: 53.7 (11.3) |
| ClinicalTrials.gov ID: NCT02054897 | HbA1c, %: 8.05 (0.85) |
| BMI, kg/m2: 32.9 (7.7) | |
| Diabetes duration, y: 4.2 (5.5) | |
| SUSTAIN 2 15 | Mean (SD), N = 1225 |
| Phase 3 | 51% male |
| Semaglutide vs sitagliptin as add‐on to | Age, y: 55.1 (10.0) |
| Metformin and/or TZD | HbA1c, %: 8.07 (0.93) |
| ClinicalTrials.gov ID: NCT01930188 | BMI, kg/m2: 32.5 (6.2) |
| Diabetes duration, y: 6.6 (5.1) | |
| SUSTAIN 3 11 | Mean (SD), N = 809 |
| Phase 3 | 55% male |
| Semaglutide vs exenatide ER as add‐on to 1 or 2 OADs | Age, y: 56.6 (10.7) |
| ClinicalTrials.gov ID: NCT01885208 | HbA1c, %: 8.3 (1.0) |
| BMI, kg/m2: 33.8 (6.3) | |
| Diabetes duration, y: 9.2 (6.8) | |
| SUSTAIN–Japan 16 | Mean (SD), N = 601 |
| Phase 3 | 72% male |
| Semaglutide monotherapy or OAD combination | Age, y: 58.5 (10.3) |
| ClinicalTrials.gov ID: NCT02207374 | HbA1c, %: 8.1 (0.9) |
| BMI, kg/m2: 26.4 (4.7) | |
| Diabetes duration, y: 8.8 (6.4) | |
| SUSTAIN 7 10 | Mean (SD), N = 1199 |
| Phase 3 | 55% male |
| Semaglutide vs dulaglutide as add‐on to metformin | Age, y: 56.0 (10.6) |
| ClinicalTrials.gov ID: NCT02648204 | HbA1c, %: 8.20 (0.92) |
| BMI, kg/m2: 33.5 (6.8) | |
| Diabetes duration, y: 7.4 (5.6) | |
| Phase 2 dose‐finding 17 | Mean (SD), N = 705 |
| Semaglutide vs placebo and liraglutide | 54% male |
| ClinicalTrials.gov ID: NCT02461589 | Age, y: 56.7 (9.9) |
| HbA1c, %: 8.06 (0.84) | |
| BMI, kg/m2: 32.8 (4.4) | |
| Diabetes duration, y: 7.2 (5.6) |
Abbreviations: BW, body weight; ER, extended release; HbA1c, glycated haemoglobin; ID, identifier; OAD, oral antidiabetic drug; SD, standard deviation; SUSTAIN, Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes; TZD, thiazolidinediones.
2.2. Predictions of HbA1c and body weight
The following strategy was applied to predict the HbA1c and body weight time courses when switching from another GLP‐1RA to semaglutide. (1) Published PK models were used to predict the exposure time‐course of liraglutide, dulaglutide, exenatide ER and semaglutide for exposure response modelling, including potential switch scenarios.18, 19, 20, 21 (2) Time‐course exposure‐response models for semaglutide were developed based on data from the SUSTAIN trials (Figure 1 and Table S1). (3) Potency differences for each of the GLP‐1RAs and semaglutide were derived and the information was used to convert the exposure of the other GLP‐1RAs into the equivalent exposure of semaglutide. (4) The time‐course exposure‐response models and the adjusted exposure of different switch scenarios were used to predict HbA1c and body weight outcomes. Each of these steps is described in more detail below.
2.3. Simulation of pharmacokinetics for each treatment
For each GLP‐1RA treatment, including semaglutide, population PK models were applied; all used similar techniques and were based on nonlinear mixed‐effects modelling using the software program, NONMEM (ICON Development Solutions, Ellicott City, Maryland) version 7.1.2. Depending on the purpose, PKs were simulated using either individual parameter estimates based on observed concentration values or typical PK parameters based on the demographic information available. Individual demographic data were available and applied for liraglutide, and the exposure‐response across individuals was applied during potency adjustment. For the dulaglutide and exenatide ER potency adjustment and the final simulations, an average demographic profile rather than a distribution was used.
For the analysis of liraglutide, which was compared with semaglutide in the phase 2 trial,17 we applied a PK model based on data from previous trials that investigated liraglutide doses of 1.8 and 3.0 mg in subjects with overweight or obesity, with or without T2DM.18 The volume of distribution was based on a clinical pharmacology trial in adults with obesity and without T2DM,22 to match the peak‐to‐trough ratios observed based on full PK profiles under steady‐state conditions.
For the analysis of dulaglutide, which was compared with semaglutide in the SUSTAIN 7 phase 3 trial,10 we applied the population PK model using data from previous trials with weekly doses of dulaglutide between 0.1 and 3 mg in subjects with T2DM.19
For the analysis of exenatide ER, which was compared with semaglutide in the SUSTAIN 3 phase 3 trial,11 we applied a clinical pharmacology population PK model, using data from previous trials with single‐dose administration of 2.5, 5, 7 and 10 mg of exenatide ER or weekly doses of 0.8 and 2.0 mg in subjects with T2DM.20
For the analysis of semaglutide, we applied the SUSTAIN population PK model, which used data from five phase 3 trials, SUSTAIN 1, 2, 3, 6 and SUSTAIN–Japan, which investigated weekly semaglutide doses of 0.5 and 1.0 mg in subjects with T2DM.21
2.4. HbA1c and body weight time‐course models
Time‐course models of HbA1c and body weight were developed for semaglutide using data up to week 30, which was the duration of the shortest phase 3 trial, based on the SUSTAIN exposure‐response population23 from four clinical trials, SUSTAIN 1, 2, 3 and SUSTAIN–Japan.11, 14, 15, 16 Two of the trials were not included as they either applied daily dosing rather than weekly dosing of semaglutide (phase 2 trial) or did not measure semaglutide concentrations (SUSTAIN 7) (Table S1). Maintenance doses of 0.5 and 1.0 mg semaglutide were used, as well as semaglutide 0.25 mg, which was used in the first 4 weeks during dose escalation. The models were mixed‐effects indirect response models based on simulated exposure levels at each week (obtained via individual PK parameter estimates based on observed concentration values). A previously published cross‐sectional Emax model was used as a starting point,23 and for the present work, we explored each covariate factor to ensure that only clear and statistically significant covariates were included. Graphical analysis of model fit to the time‐course response data, grouped according to covariates, revealed that a number of the pre‐selected covariates could be removed, and additionally, that baseline HbA1c was more likely to influence the Emax for body weight than the placebo effect for body weight. The covariates included in the final model for HbA1c were baseline HbA1c and trial effects; those included in the final model for body weight were sex, HbA1c and trial effects. Model fit across demographic variables influencing treatment response is presented in Figure S1.
The models for HbA1c and body weight were validated, based on the four trials used for model development, by visual predictive checks and by model fit to response time‐course in subgroups defined by the covariates. Additionally, an external validation was undertaken by simulating the HbA1c and body weight response for semaglutide, dulaglutide and exenatide ER in SUSTAIN 3 and SUSTAIN 7 (Supplementary Figures S1 and S2).
It was not feasible to investigate effects on safety because the exposure‐dependent effects on safety, mainly gastrointestinal adverse events, with semaglutide were previously observed primarily during the initiation of treatment23 and in the present analysis, subjects were already receiving treatment with another GLP‐1RA.
2.5. Potency adjustment
The concentration of each GLP‐1RA was adjusted by the relative potency to obtain the equivalent semaglutide exposure, that is, the exposure of semaglutide that provides the same response as the original concentration of the GLP‐1RA. As potency differences with respect to both body weight and HbA1c effects were expected, the potency adjustment was performed for both endpoints.
The potency adjustment for liraglutide illustrated in Figure S3 was based on joint exposure‐response analyses of data concerning liraglutide and semaglutide from a phase 2 dose‐finding trial17 for HbA1c and body weight. Liraglutide exposure over 26 weeks (efficacy outcomes were not measured at Week 30) was predicted based on demographic and dosing information for individual subjects, whereas semaglutide exposure was obtained via individual PK parameter estimates based on observed concentration values (see section 2.3). Figure S3 illustrates that liraglutide exposure levels in the clinically relevant range can be potency‐adjusted to match the semaglutide exposure‐response relationship, and hence, the semaglutide model can be used to predict the response to liraglutide treatment.
For dulaglutide and exenatide ER, the simulations for potency adjustment were based on a typical subject approach, performed in the same way as the simulated switch scenarios were performed, to ensure that the differences in response matched specific trials. For dulaglutide, the trial factor and potency adjustment was derived, to ensure that the model predicted the correct effects at Week 28 (efficacy outcomes were not measured at Week 30) for dulaglutide and semaglutide from the SUSTAIN 7 trial.10 For exenatide ER, the potency adjustment was derived to match the effects at Week 30 for exenatide ER and semaglutide from the SUSTAIN 3 trial.11 The potency adjustment for dulaglutide and exenatide ER with respect to HbA1c is shown in Figure 2. The similar potency adjustments for body weight for both treatments are provided in Figure S4.
Figure 2.
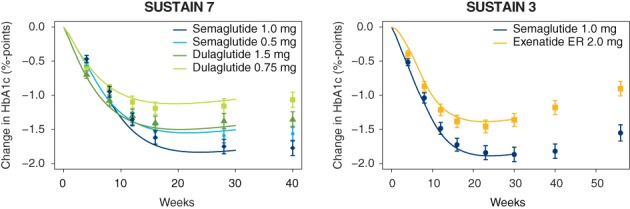
Validation of HbA1c predictions for dulaglutide based on the SUSTAIN 7 trial (left) and for exenatide ER based on the SUSTAIN 3 trial (right).10, 11 Efficacy data from dulaglutide and exenatide ER were not used directly to estimate the model, but data at Week 28 or Week 30 time points were used to adjust the potency of the two compounds. Response data are presented as means with 95% CI, where missing response data or data collected while subjects had discontinued treatment or were receiving rescue medication were imputed using an MMRM analysis, with treatment and country as fixed factors and baseline values as covariates, nested within visits, and adjusted according to the observed baseline distribution. The symbols at the right show changes at the end of the trial (40 weeks for SUSTAIN 7, 56 weeks for SUSTAIN 3). Abbreviations: CI, confidence interval; ER, extended release; MMRM, mixed model for repeated measures; HbA1c, glycated haemoglobin A1c; SUSTAIN, Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes
Once the exposure had been potency adjusted, it was possible to use the semaglutide exposure‐response model to simulate the outcome of liraglutide, dulaglutide and exenatide ER treatment and to obtain the same results as those observed in the clinical trials. In order to simulate the combined effects with semaglutide when subjects were exposed to two long‐acting GLP‐1RAs at the same time, it was considered appropriate, as all treatments targeted the same receptor, to apply the sum of the potency‐adjusted exposure, that is, the total effective GLP‐1RA concentration.
2.6. Simulation of switch scenarios for HbA1c and body weight
A number of scenarios were simulated based on the approach described above. Subjects were assumed to be using stable treatment for 26 weeks with: (1) liraglutide 1.2 mg; (2) liraglutide 1.8 mg; (3) dulaglutide 0.75 mg; (4) dulaglutide 1.5 mg; or (5) exenatide ER 2.0 mg.
Treatment with semaglutide was initiated 1 day after the last once‐daily liraglutide dose and 1 week after the last once‐weekly dose of dulaglutide or exenatide ER. Semaglutide was investigated using either the standard regimen of dose escalation, starting with 0.25 mg for 4 weeks, followed by dose escalations every 4 weeks to 0.5 mg and subsequently to 1.0 mg, or a dose of 0.5 mg was initiated and administered for 4 weeks, with subsequent escalation to 1.0 mg.
Simulations were based on a typical subject approach, simulating the median outcome for a population similar to the trial populations of the two phase 3 GLP‐1RA comparator trials, SUSTAIN 3 and 7.10, 11 The population was 50% male, with a mean baseline body weight of 95 kg and a mean baseline HbA1c of 8.3%.
2.7. Validation of response predictions
In addition to standard model validation for the prediction of semaglutide response following a switch from other GLP‐1RAs that is provided in Appendix S1, the model was validated to provide an accurate time‐course prediction for dulaglutide and exenatide ER with respect to HbA1c (Figure 2) and body weight (Figure S4). Figure 2 illustrates agreement between the model predictions and the observed HbA1c outcome, confirming key differences between semaglutide, dulaglutide and exenatide ER. First, dulaglutide, in particular the 1.5 mg dose, provided a better outcome immediately after initiation of treatment, with a steeper decrease in the first 10 weeks with dulaglutide 1.5 mg compared to semaglutide; however, at steady state, after completion of semaglutide dose escalation, semaglutide clearly had a greater effect on HbA1c. Second, semaglutide had a greater effect than exenatide ER on HbA1c, both during initiation of treatment and at steady state concentrations.
3. RESULTS
3.1. Population characteristics
Baseline HbA1c, body weight and background medication of the population included in the analyses are presented in Figure 1 by trial, together with the estimated treatment effects for HbA1c and body weight. Additional baseline characteristics are included in Table 1.
3.2. Effect of switching from another GLP‐1RA to semaglutide
In the exposure‐response analyses, the potency‐adjusted concentration of GLP‐1RA (liraglutide 1.2 or 1.8 mg, dulaglutide 0.75 or 1.5 mg and exenatide ER 2.0 mg), before and after switching at Week 26 to semaglutide 0.25 mg, and subsequently to semaglutide 0.5 and 1.0 mg, is shown in Figure 3. The increased concentrations of GLP‐1RA associated with semaglutide led to further decreases in HbA1c and body weight, with ultimate reductions at Week 52 ranging from ~0.3% to ~0.8%‐points for HbA1c and from ~2% to ~4% for body weight with semaglutide 1.0 mg. The effect on potency‐adjusted GLP‐1RA concentration of switching directly to semaglutide 0.5 mg, that is, without including the 0.25 mg dose escalation step, is shown in Figure 4, together with the same associated reductions in HbA1c and body weight.
Figure 3.
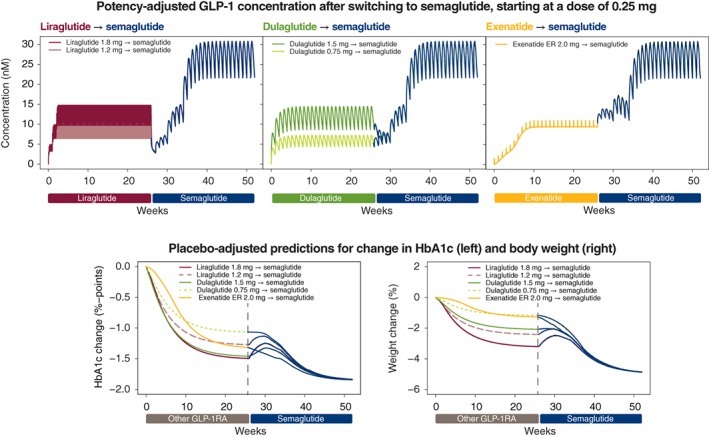
Effect of switching from treatment with another GLP‐1RA to semaglutide treatment, starting at a dose of 0.25 mg. The top three panels show the potency‐adjusted GLP‐1RA concentration before and after switching to semaglutide 0.25 mg at Week 26 and escalating every 4 weeks to 0.5 mg, and subsequently to 1.0 mg. The pink and maroon boxes within the top left panel represent the rapid daily fluctuations in liraglutide 1.2 and 1.8 mg, respectively, at steady state, achieved via a weekly dose escalation of 0.6 mg. The effects on HbA1c (bottom left) and body weight (bottom right) relative to baseline of switching to semaglutide at Week 26 are also shown. Abbreviations: ER, extended release; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated haemoglobin A1c
Figure 4.
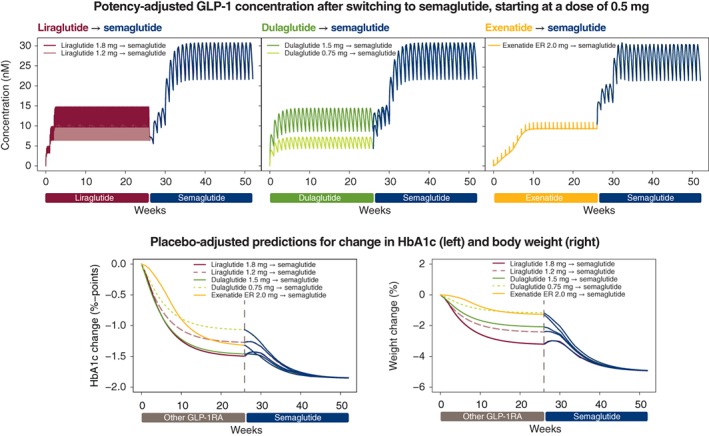
Effect of switching from treatment with another GLP‐1RA to semaglutide treatment, starting at a dose of 0.5 mg. The top three panels show the GLP‐1RA concentration before and after switching to semaglutide 0.5 mg at week 26 and escalating after 4 weeks to 1.0 mg. The pink and maroon boxes within the top left panel represent the rapid daily fluctuations in liraglutide 1.2 and 1.8 mg, respectively, at steady state, achieved via a weekly dose escalation of 0.6 mg. The effects on HbA1c (bottom left) and body weight (bottom right) relative to baseline of switching to semaglutide at Week 26 are also shown. Note: Initiating semaglutide at a dose of 0.5 mg does not conform with prescribing information for semaglutide.24 Abbreviations: ER, extended release; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated haemoglobin A1c
The modelled effects on HbA1c during the transition period of switching from another GLP‐1RA to semaglutide are summarized in Table 2.
Table 2.
Summary of the effects on HbA1c during transition after switching from another GLP‐1RA to semaglutide at a starting dose of 0.25 or 0.5 mg
| Initial treatment |
Switch to semaglutide
0.25 mg |
Switch to semaglutide
0.5 mg a |
|---|---|---|
| Liraglutide 1.2 mg | Small transient HbA1c increase | Stable HbA1c |
| Liraglutide 1.8 mg | Small transient HbA1c increase | Stable HbA1c |
| Dulaglutide 0.75 mgb | Stable HbA1c | Immediate improvement in HbA1c |
| Dulaglutide 1.5 mg | Small transient HbA1c increase | Stable HbA1c |
| Exenatide ER 2.0 mgc | Gradual improvement in HbA1c | Immediate improvement in HbA1c |
Abbreviations: ER, extended release; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated haemoglobin.
It should be noted that semaglutide is currently marketed at a starting dose of 0.25 mg.24
Cautious initiation of semaglutide is recommended because of low effect compared to semaglutide 0.5 mg.
Cautious initiation of semaglutide is recommended as exenatide ER pharmacokinetics are extended beyond 1 week.13
3.2.1. Switching from liraglutide to semaglutide
Liraglutide at once‐daily doses of 1.2 and 1.8 mg was eliminated from the bloodstream within days in the model following cessation of treatment (Figure 3). After switching from the lower dose of liraglutide 1.2 mg to once‐weekly semaglutide 0.25 mg, there was a temporary small deterioration (increase) in HbA1c of up to ~0.25%‐points. This deterioration was greater when switching from liraglutide 1.8 mg to semaglutide 0.25 mg. If treatment with semaglutide was initiated at a dose of 0.5 mg, the HbA1c deterioration was negligible (Figure 4). A similar pattern was observed for body weight (Figure 4), also with the lower dose of liraglutide 0.9 mg in Japanese subjects (Figure S5).
3.2.2. Switching from dulaglutide to semaglutide
When switching from the lowest dose of once‐weekly dulaglutide, 0.75 mg, to the semaglutide 0.25 mg regimen in the model, the effective GLP‐1RA concentration gradually increased and HbA1c remained stable during the transition before decreasing (Figure 3). Body weight immediately decreased after switching to semaglutide 0.25 mg. In contrast, initiation of semaglutide 0.25 mg 1 week after cessation of the higher dose of dulaglutide 1.5 mg led to an initial reduction in the potency‐adjusted GLP‐1RA concentration and a concomitant small temporary deterioration in HbA1c level, but not in body weight loss. This deterioration in HbA1c was avoided by initiating semaglutide treatment at a dose of 0.5 mg (Figure 4).
3.2.3. Switching from exenatide ER to semaglutide
Exenatide ER 2.0 mg remained in circulation at effective concentration levels for ~6 weeks after the last dose. Switching to semaglutide at a dose of 0.25 mg 1 week after cessation of exenatide ER resulted in gradual increases in the total effective GLP‐1RA concentration and in gradual improvements (reductions) in HbA1c and body weight in the model (Figure 3). Switching directly to semaglutide 0.5 mg led to a more rapid increase in GLP‐1RA concentration and concomitant immediate reductions in HbA1c and body weight (Figure 4).
4. DISCUSSION
The present exposure‐response analyses investigated the simulated effects of switching from treatment with liraglutide, dulaglutide or exenatide ER to treatment with semaglutide. After switching, short‐term excursions were observed with some treatments when initiating semaglutide treatment at a dose of 0.25 mg, but ultimately, improvements in glycaemic control and body weight were achieved, with an ultimate reduction at 52 weeks ranging from ~0.3% to ~0.8%‐points for HbA1c and from ~2% to ~4% for body weight. A more rapid reduction in HbA1c and body weight was observed when switching directly to semaglutide 0.5 mg.
The knowledge obtained from this study is of potential benefit to clinicians and patients in providing guidance concerning management of T2DM. A variety of treatment options are available for the management of T2DM and a patient‐centred individual approach is required.25, 26 GLP‐1RAs in general are of interest, not only because of their effect on glycaemic control, but also because of their effect on weight loss and the low risk of hypoglycaemia as well as cardiovascular effects.3, 27, 28 The effects of long‐acting GLP‐1RAs generally appear to be greater than those achieved with their short‐acting counterparts, making the once‐weekly GLP‐1RAs particularly attractive for patients with inadequate glycaemic control.3, 29 Other benefits such as greater convenience and, thus, better adherence to treatment have also been reported with once‐weekly GLP‐1RAs.30 Furthermore, semaglutide has demonstrated superior efficacy to other GLP‐1RAs in the SUSTAIN programme, with statistically significant reductions in HbA1c and body weight with semaglutide vs dulaglutide and exenatide ER.10, 11
The present study used simulated exposure‐response analyses to provide additional information about the clinical effects during the transition period when switching from another GLP‐1RA to semaglutide. When simulating the switch to semaglutide at a starting dose of 0.25 mg from liraglutide 1.2 or 1.8 mg, a temporary slight increase in HbA1c was observed. This effect was abrogated when simulating initiation of semaglutide at a dose of 0.5 mg. A similar pattern was observed with dulaglutide 1.5 mg. Conversely, the temporary increase in HbA1c was not observed when switching from dulaglutide 0.75 mg to semaglutide 0.25 mg. It should be noted that, according to the label,24 the dose escalation regimen for semaglutide starts at a dose of 0.25 mg to ameliorate gastrointestinal side effects. It is not known how switching directly to semaglutide 0.5 mg might impact gastrointestinal tolerability; however, long‐term treatment with GLP‐1RAs is associated with development of tolerance.10, 11, 14, 15, 23, 31
When switching to semaglutide from once‐weekly exenatide ER 2.0 mg, the protracted half‐life of several weeks of exenatide ER is worth noting.13 However, based on our analysis, switching to the semaglutide 0.25 mg dose resulted in gradual increases in the total effective GLP‐1 concentration and led to a gradual improvement in HbA1c and body weight. We did not simulate tolerability when switching to semaglutide as tolerability depends on many factors, including previous treatment duration and tolerability of the GLP‐1RA treatment.32 In the present simulation, subjects were already receiving treatment with another GLP‐1RA. In general, we would expect limited gastrointestinal tolerability issues if switching between two GLP1‐RAs can be achieved without sudden increases in GLP‐1RA concentration.
Several methods of model validation were applied for HbA1c and body weight, which generally indicated consistency in data, with the exception of the body weight response over time in the SUSTAIN 3 trial, indicating some bias in prediction during the first weeks of the study. Due to the variability among subjects and among trials, this finding may be artificial, and as the discrepancies among models were small and were present only at low concentrations, they would not be expected to significantly influence the simulated switch scenarios or the conclusions drawn.
Data on HbA1c and body weight from the SUSTAIN 3 and 7 clinical trials10, 11 were adequately predicted up to Week 30 in the present analyses. Part of the treatment effects on these endpoints decreased in the clinical trials when approaching 1 year.10, 11 In the SUSTAIN 3 trial, the reduced effects, which were not incorporated into the present models, appeared more pronounced for exenatide ER compared to semaglutide.11 Therefore, potentially even greater benefits of switching to semaglutide may be seen in the longer term.
Previous exposure‐response analyses for the SUSTAIN trials revealed a clear reduction in HbA1c and weight across exposures after 30 weeks, the duration of the shortest trial, irrespective of baseline values.23 The exposure‐response for HbA1c was influenced by baseline HbA1c, and body weight exposure‐response was influenced by sex (Figure S1); other factors had limited impact. The present analyses extended previous work by including analysis of the complete exposure‐response time course for semaglutide, and by including outcome simulations for liraglutide, dulaglutide and exenatide ER.
It was assumed that the pharmacodynamic models and parameter values describing the response of the other GLP‐1RAs were the same as those estimated for semaglutide, with the exception of a difference in potency. Although rate parameters are more likely to be consistent across treatments,33 it is possible that differences exist in maximal efficacy, reflecting the differences in response seen across therapeutic dose levels for the different GLP‐1RAs. Because of the potency calibration method, a different maximal effect for one of the other GLP1‐RAs would not change the steady‐state response predictions but would lead to some bias in predictions at lower concentrations.
One limitation of our analyses is that data were obtained from different trials with differences in trial design and populations; hence, variability could be an issue. This has been accounted for by the inclusion of trial as a covariate factor, and results are validated by predictions of the direct comparisons of the GLP‐1 treatments available from phase 3 trials. The model was able to reflect the data obtained from clinical trials comparing the respective GLP‐1RAs, dulaglutide and exenatide ER, to semaglutide.10, 11 Simulations also provided an indirect comparison between liraglutide and dulaglutide, with similar results as observed in the AWARD‐6 trial, in which a mean treatment difference of −0.06%‐points was observed for HbA1c.34 Moreover, in the DURATION 6 trial, a better response by 0.21%‐points with liraglutide 1.8 mg was demonstrated, compared with exenatide ER, similar to the predictions based on our analysis.35
An additional limitation is that our results provide only predictions for the mean outcomes and not for the individual patient. No data are available for the correlation between semaglutide and the other GLP‐1RAs in parameters for exposure and response, which could predict the variability among patients with regard to the potential benefits of switching to semaglutide. Undoubtedly, differences among patients do exist. However, we believe that the mean outcome will be the most informative when evaluating the potential future outcome for patients who may switch to semaglutide.
In summary, pharmacokinetic and pharmacodynamic differences exist between liraglutide, dulaglutide and exenatide ER, at the approved dose levels, and it is relevant to consider these when switching to semaglutide. Exposure‐response modelling can facilitate simulation of HbA1c and weight loss outcomes following a switch from other GLP‐1RAs to semaglutide treatment. Significant and clinically relevant improvements in HbA1c and body weight are expected to occur following a switch from any of the other GLP‐1RAs to semaglutide.
Supporting information
Table S1 Randomised, controlled trials included in the exposure‐response analyses.
Figure S1 Time course models for HbA1c (A) and body weight (B) relative to baseline based on the SUSTAIN exposure‐response pool of data with semaglutide 0.5 mg (left) and semaglutide 1.0 mg (right).
Figure S2 Visual predictive check for the time course models for HbA1c (top) and body weight (bottom) relative to baseline based on the SUSTAIN exposure‐response pool of data grouped according to study ID and semaglutide dose level (0.5 and 1.0 mg) or placebo. Solid lines are the mean and the two dotted lines are the 5th and 95th percentiles of the observed response. Shaded areas are the 95% confidence interval of the mean, the 5th and 95th percentiles as computed based on 1000 simulations of the exposure‐response data set.
Figure S3 Potency adjustment for liraglutide for mean changes from baseline in HbA1c (A) and body weight (B). Lines through the data show the estimated exposure‐response in a joint analysis of liraglutide and semaglutide, where a potency adjustment factor was estimated for liraglutide. Response data are mean and 95% CI in exposure quantiles, with a value of 0 exposure assigned to subjects treated with placebo. Horizontal lines with diamonds along the x‐axis represent median semaglutide exposure and median potency‐adjusted liraglutide exposure. Missing response data or data collected while subjects had stopped treatment or were on rescue medication were imputed using an MMRM analysis, with treatment and country as fixed factors and baseline values as covariates, nested within visits, and adjusted according to the observed baseline distribution.
Figure S4 Validation of body weight predictions for dulaglutide based on the SUSTAIN 7 trial (left) and for exenatide ER based on the SUSTAIN 3 trial (right). Efficacy data from dulaglutide and exenatide ER were not used directly to estimate the model, but data at the week 28 time point was used to adjust the potency of the two compounds.
Figure S5 Effect of switching from treatment with liraglutide 0.9 mg to semaglutide treatment in Japanese subjects. The top panel shows the GLP‐1RA concentration before and after switching to semaglutide at week 26 starting at a dose of 0.25 or 0.5 mg. The light blue box in the top panel represents the rapid daily fluctuations in liraglutide 0.9 mg at steady state, which is achieved via weekly dose escalations of 0.3 and 0.6 mg. The effects on HbA1c (middle) and body weight (bottom) relative to baseline of switching to semaglutide at week 26 are also shown. Note: starting at a semaglutide dose of 0.5 mg is not according to the prescribing information for semaglutide (FDA. Ozempic [semaglutide], US Prescribing Information. https://wwwaccessdatafdagov/drugsatfda_docs/label/2017/209637lblpdf2017). ER, extended release; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated haemoglobin A1c.
ACKNOWLEDGMENTS
We thank Anders Strathe, PhD, Modelling Specialist, Quantitative Clinical Pharmacology, Novo Nordisk, for his input to the modelling analysis and liraglutide potency adjustment as well as Angela Stocks, PhD, Larix A/S, Copenhagen, Denmark, for editorial and medical writing services, which were funded by Novo Nordisk.
Conflict of interest
R. V. O., S. L. and D. T. are employed by and hold stock in Novo Nordisk.
Author contributions
R. V. O., S. L. and D. T. contributed to the conceptual design. R. V. O. was responsible for the data set collection. R. V. O. planned the statistical and modelling analyses. All authors contributed to the interpretation of the data. All authors were involved in the writing, reviewing and editing of the manuscript, gave final approval and agree to be accountable for all aspects of the work.
Overgaard RV, Lindberg Søren Ø., Thielke D. Impact on HbA1c and body weight of switching from other GLP‐1 receptor agonists to semaglutide: A model‐based approach. Diabetes Obes Metab. 2019;21:43–51. 10.1111/dom.13479
Funding information Funding for the trials used in the analyses and the trial products were provided by Novo Nordisk A/S, Bagsværd, Denmark.
REFERENCES
- 1. Holst JJ. The physiology of glucagon‐like peptide 1. Physiol Rev. 2007;87:1409‐1439. [DOI] [PubMed] [Google Scholar]
- 2. Drucker DJ, Nauck MA. The incretin system: glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696‐1705. [DOI] [PubMed] [Google Scholar]
- 3. Tella SH, Rendell MS. Glucagon‐like polypeptide agonists in type 2 diabetes mellitus: efficacy and tolerability, a balance. Ther Adv Endocrinol Metab. 2015;6:109‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Madsbad S. Review of head‐to‐head comparisons of glucagon‐like peptide‐1 receptor agonists. Diabetes Obes Metab. 2016;18:317‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Orskov C, Wettergren A, Holst JJ. Secretion of the incretin hormones glucagon‐like peptide‐1 and gastric inhibitory polypeptide correlates with insulin secretion in normal man throughout the day. Scand J Gastroenterol. 1996;31:665‐670. [DOI] [PubMed] [Google Scholar]
- 6. Potts JE, Gray LJ, Brady EM, Khunti K, Davies MJ, Bodicoat DH. The effect of glucagon‐like peptide 1 receptor agonists on weight loss in type 2 diabetes: a systematic review and mixed treatment comparison meta‐analysis. PLoS One. 2015;10:e0126769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flint A, Raben A, Astrup A, Holst JJ. Glucagon‐like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lau J, Bloch P, Schaffer L, et al. Discovery of the once‐weekly glucagon‐like peptide‐1 (GLP‐1) analogue semaglutide. J Med Chem. 2015;58:7370‐7380. [DOI] [PubMed] [Google Scholar]
- 9. Kapitza C, Nosek L, Jensen L, Hartvig H, Jensen CB, Flint A. Semaglutide, a once‐weekly human GLP‐1 analog, does not reduce the bioavailability of the combined oral contraceptive, ethinylestradiol/levonorgestrel. J Clin Pharmacol. 2015;55:497‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open‐label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6:275‐286. [DOI] [PubMed] [Google Scholar]
- 11. Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once‐weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56‐week, open‐label, randomized clinical trial. Diabetes Care. 2017;41:258‐266. [DOI] [PubMed] [Google Scholar]
- 12. Agerso H, Jensen LB, Elbrond B, Rolan P, Zdravkovic M. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long‐acting GLP‐1 derivative, in healthy men. Diabetologia. 2002;45:195‐202. [DOI] [PubMed] [Google Scholar]
- 13. Fineman M, Flanagan S, Taylor K, et al. Pharmacokinetics and pharmacodynamics of exenatide extended‐release after single and multiple dosing. Clin Pharmacokinet. 2011;50:65‐74. [DOI] [PubMed] [Google Scholar]
- 14. Sorli C, Harashima S, Tsoukas GM, et al. Efficacy and safety of once‐weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double‐blind, randomised, placebo‐controlled, parallel‐group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5:251‐260. [DOI] [PubMed] [Google Scholar]
- 15. Ahren B, Masmiquel L, Kumar H, et al. Efficacy and safety of once weekly semaglutide versus sitagliptin as add‐on to metformin and/or thiazolidinediones in subjects with type 2 diabetes (SUSTAIN 2): a 56‐week randomised, controlled clinical trial. Lancet Diabetes Endocrinol. 2017;5:341‐354. [DOI] [PubMed] [Google Scholar]
- 16. Kaku K, Yamada Y, Watada H, et al. Safety and efficacy of once‐weekly semaglutide versus additional oral antidiabetic drugs, in Japanese subjects with inadequately controlled T2D: a randomised trial. Diabetes Obes Metab. 2018;20:1202‐1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lingvay I, Desouza CV, Lalic KS, et al. A 26‐week, randomized, controlled trial of semaglutide once daily versus liraglutide and placebo in patients with type 2 diabetes suboptimally controlled on diet and exercise with or without metformin. Diabetes Care. 2018; Published online, 10.2337/dc17-2381. [DOI] [PubMed] [Google Scholar]
- 18. Overgaard RV, Petri KC, Jacobsen LV, Jensen CB. Liraglutide 3.0 mg for weight management: a population pharmacokinetic analysis. Clin Pharmacokinet. 2016;55:1413‐1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Geiser JS, Heathman MA, Cui X, et al. Clinical pharmacokinetics of dulaglutide in patients with type 2 diabetes: analyses of data from clinical trials. Clin Pharmacokinet. 2016;55:625‐634. [DOI] [PubMed] [Google Scholar]
- 20. Cirincione B, Edwards J, Mager DE. Population pharmacokinetics of an extended‐release formulation of exenatide following single‐ and multiple‐dose administration. AAPS J. 2017;19:487‐496. [DOI] [PubMed] [Google Scholar]
- 21. Petri KCC, Ingwersen SH, Flint A, Zacho J, Overgaard RV. Semaglutide s.c. once‐weekly in type 2 diabetes: a population pharmacokinetic analysis. Diabetes Ther. 2018;9:1533‐1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Can J, Sloth B, Jensen C, Flint A, Blaak EE, Saris WHM. Effects of the once‐daily GLP‐1 analog liraglutide on gastric emptying, glycemic parameters, appetite, and energy metabolism in obese, non‐diabetic adults. Int J Obes (Lond). 2014;38:784‐793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Petri KCC, Ingwersen SH, Flint A, Zacho J, Overgaard RV. Exposure‐response analysis for evaluation of semaglutide dose levels in type 2 diabetes. Diabetes Obes Metab. 2018;20:2238‐2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. FDA . Ozempic (semaglutide), US Prescribing Information. https://wwwaccessdatafdagov/drugsatfda_docs/label/2017/209637lblpdf2017. Accessed April 24, 2018.
- 25. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient‐centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2012;55:1577‐1596. [DOI] [PubMed] [Google Scholar]
- 26. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient‐centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58:429‐442. [DOI] [PubMed] [Google Scholar]
- 27. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 29. Garber AJ. Long‐acting glucagon‐like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care. 2011;34(suppl 2):S279‐S284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Polonsky WH, Fisher L, Hessler D, Bruhn D, Best JH. Patient perspectives on once‐weekly medications for diabetes. Diabetes Obes Metab. 2011;13:144‐149. [DOI] [PubMed] [Google Scholar]
- 31. Blonde L, Russell‐Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1‐5 studies. Diabetes Obes Metab. 2009;11(suppl 3):26‐34. [DOI] [PubMed] [Google Scholar]
- 32. Bailey TS, Takacs R, Tinahones FJ, et al. Efficacy and safety of switching from sitagliptin to liraglutide in subjects with type 2 diabetes (LIRA‐SWITCH): a randomized, double‐blind, double‐dummy, active‐controlled 26‐week trial. Diabetes Obes Metab. 2016;18:1191‐1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moller JB, Overgaard RV, Kjellsson MC, et al. Longitudinal modeling of the relationship between mean plasma glucose and HbA1c following antidiabetic treatments. CPT Pharmacometrics Syst Pharmacol. 2013;2:e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dungan K, Povedano S, Forst T, et al. Once‐weekly dulaglutide versus once‐daily liraglutide in metformin‐treated patients with type 2 diabetes (AWARD‐6): a randomised, open‐label, phase 3, non‐inferiority trial. Lancet. 2014;384:1349‐1357. [DOI] [PubMed] [Google Scholar]
- 35. Buse JB, Nauck M, Forst T, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION‐6): a randomised, open‐label study. Lancet. 2013;381:117‐124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Randomised, controlled trials included in the exposure‐response analyses.
Figure S1 Time course models for HbA1c (A) and body weight (B) relative to baseline based on the SUSTAIN exposure‐response pool of data with semaglutide 0.5 mg (left) and semaglutide 1.0 mg (right).
Figure S2 Visual predictive check for the time course models for HbA1c (top) and body weight (bottom) relative to baseline based on the SUSTAIN exposure‐response pool of data grouped according to study ID and semaglutide dose level (0.5 and 1.0 mg) or placebo. Solid lines are the mean and the two dotted lines are the 5th and 95th percentiles of the observed response. Shaded areas are the 95% confidence interval of the mean, the 5th and 95th percentiles as computed based on 1000 simulations of the exposure‐response data set.
Figure S3 Potency adjustment for liraglutide for mean changes from baseline in HbA1c (A) and body weight (B). Lines through the data show the estimated exposure‐response in a joint analysis of liraglutide and semaglutide, where a potency adjustment factor was estimated for liraglutide. Response data are mean and 95% CI in exposure quantiles, with a value of 0 exposure assigned to subjects treated with placebo. Horizontal lines with diamonds along the x‐axis represent median semaglutide exposure and median potency‐adjusted liraglutide exposure. Missing response data or data collected while subjects had stopped treatment or were on rescue medication were imputed using an MMRM analysis, with treatment and country as fixed factors and baseline values as covariates, nested within visits, and adjusted according to the observed baseline distribution.
Figure S4 Validation of body weight predictions for dulaglutide based on the SUSTAIN 7 trial (left) and for exenatide ER based on the SUSTAIN 3 trial (right). Efficacy data from dulaglutide and exenatide ER were not used directly to estimate the model, but data at the week 28 time point was used to adjust the potency of the two compounds.
Figure S5 Effect of switching from treatment with liraglutide 0.9 mg to semaglutide treatment in Japanese subjects. The top panel shows the GLP‐1RA concentration before and after switching to semaglutide at week 26 starting at a dose of 0.25 or 0.5 mg. The light blue box in the top panel represents the rapid daily fluctuations in liraglutide 0.9 mg at steady state, which is achieved via weekly dose escalations of 0.3 and 0.6 mg. The effects on HbA1c (middle) and body weight (bottom) relative to baseline of switching to semaglutide at week 26 are also shown. Note: starting at a semaglutide dose of 0.5 mg is not according to the prescribing information for semaglutide (FDA. Ozempic [semaglutide], US Prescribing Information. https://wwwaccessdatafdagov/drugsatfda_docs/label/2017/209637lblpdf2017). ER, extended release; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated haemoglobin A1c.


