Abstract
Background
Non‐adherence to medication is a challenging problem in daily clinical practice.
Objective
To assess reasons for non‐adherence in patients with chronic immune‐mediated inflammatory diseases (IMIDs) in a direct comparison including evaluation of treatment necessity and concerns.
Methods
ALIGN was a non‐interventional, multicountry, multicentre, self‐administered, cross‐sectional, epidemiologic survey study. Here, we investigate the German, Austrian and Swiss (DACH) cohort. Six hundred thirty‐one patients with different IMIDs (rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, plaque psoriasis, Crohn's disease and ulcerative colitis) under systemic therapies were evaluated concerning adherence, beliefs of necessity and concerns towards treatment in patients with IMIDs.
Results
The DACH cohort had significantly different levels of adherence depending on the IMID (P < 0.05) and the type of therapy (P < 0.05). Based on the significant influence of concerns on treatment adherence (P < 0.05) and the high belief of treatment necessity, patients could be classified in four attitudinal segments, which were unequally distributed throughout various IMIDs. High concerns had a significant influence on non‐adherence, whereas necessity did not. Older age, female sex, TNFi mono‐, conventional combination and TNFi combination therapy are positively associated with adherence.
Conclusions
In the DACH region, patients are less concerned about medication and believe in the necessity of treatment. Therefore, we suggest adapting the communication in the various patient groups.
Introduction
Immune‐mediated inflammatory diseases (IMIDs) describe a group of conditions characterized by chronic inflammation, including rheumatoid arthritis (RA), ankylosing spondylitis (AS), psoriatic arthritis (PSA), ulcerative colitis (UC), Crohn's disease (CD) and psoriasis (PSO).1, 2, 3 IMIDs can be treated effectively but often need lifelong medication, which necessitates long‐term adherence. Adherence is defined by the WHO as the extent to which a person's behaviour corresponds with the recommendations from his or her healthcare professional (HCP). Non‐adherence to medication is a challenging problem in daily clinical practice, resulting in increased healthcare costs, reduced quality of life, poor treatment outcomes, higher risk and prolongation of hospitalization, inappropriate therapeutic decisions due to underestimation of treatment efficacy and decreased patient satisfaction.4 In chronic diseases, up to 50% of the medication is not taken as prescribed1, 5, 6, 7 and adherence ranges from 7% to 80% in RA, PSO, UC or CD.2, 4, 8, 9 Non‐adherence can be driven by intentional and unintentional motivations including relationship of the HCP with the patient, lower treatment necessity, treatment concerns and depression.2
ALIGN was a multicountry, cross‐sectional study to determine patient specific and general beliefs towards medication and treatment compliance to selected systemic therapies, as well as illness perceptions in IMIDs.
The primary objective of the ALIGN study was to assess treatment necessity and concerns in patients with chronic IMIDs. Secondary objectives were to define patients’ beliefs and adherence about systemic medications and distribution of adherence among different IMIDs. Herein, the data for the DACH region (Deutschland = Germany, Austria, Confoederatio Helvetica = Switzerland) from the global ALIGN data were analysed.10
Hence, identifying the reasons for non‐adherence in patients with IMIDs is of paramount importance to generate tools and educational programs. Additionally, a practical tool with communication strategies for different patient types was developed.
Materials and methods
The ALIGN study was conducted between June 2012 and October 2013 and the DACH cohort included 631 patients from 7328 patients worldwide.10 Participants were ≥18 years old, attended routine outpatient visits for different IMIDs diagnosed by a rheumatologist (RA, PSA, AS), dermatologist (PSO, PSA), or gastroenterologist (CD, UC), and were being treated with systemic disease‐modifying antirheumatic drugs (DMARDs), glucocorticoids, non‐steroidal anti‐inflammatory drugs (NSAIDs; only AS) and/or tumour necrosis factor inhibitors (TNFi). Written informed consent was obtained before inclusion in the study. The study protocol was conducted in accordance with the Declaration of Helsinki 2013, local regulatory laws and local ethics committee of each participating country.
Data were collected at a single visit during a routine check‐up where each patient had to complete four validated questionnaires in a validated language version: Beliefs about Medicines Questionnaire,11 scoring beliefs about treatment overuse and harm (BMQ‐General), and necessity and concerns (BMQ‐Specific) using a 5‐point Likert scale (1 = strongly disagree; 2 = disagree; 3 = uncertain; 4 = agree; 5 = strongly agree); the 4‐item Morisky Medication Adherence Scale (MMAS‐4),12, 13, 14 consisting of four (yes = 0; no = 1) questions, with high adherence defined as a score of 4; the Brief Illness Perception Questionnaire (BIPQ),15 measuring perception of illness with eight questions using an 11‐point scale; and the Patient Health Questionnaire‐2 (PHQ‐2),16 which consists of two questions measuring depressive symptoms; and a visual analogue scale (VAS),17 measured from 0% to 100% to assess the medication taken during the 3 months before the study visit. After completion, all patient questionnaires were placed into confidential sealed envelopes. The investigators provided the data on patient demographics, social, economic, and educational background, IMID‐related data, previous and current treatment and response to therapy.
Treatments included TNFi monotherapy (‘TNFi mono’), TNFi combined with conventional therapies (‘TNFi combo’) or conventional therapies only (‘conventional only’; Table 1). The BMQ‐General (Overuse and Harm) were evaluated in three groups: TNFi mono, TNFi combo and conventional only. The BMQ‐Specific (Necessity and Concerns) and MMAS‐4 adherence rates were evaluated in four groups: TNFi mono; conventional only; and TNFi combo, which was split into a TNFi component (‘TNFi combo‐TNFi rating’) as well as the conventional therapy component (‘TNFi combo‐conventional rating’).
Table 1.
Demographics, prior and current disease severity, current therapies, and duration of disease and symptoms of the ALIGN‐DACH population
| Rheumatology n = 209 (33.1%) | Dermatology n = 205 (32.5%) | Gastroenterology n = 217 (34.4%) | ||||
|---|---|---|---|---|---|---|
| RA | AS | PSA | PSO | CD | UC | |
| (n = 100) | (n = 44) | (n = 65) | (n = 205) | (n = 145) | (n = 72) | |
| Female patients, % | 75 | 34.1 | 43.1 | 60.7 | 43.1 | 33.7 |
| Age, | 55.2 | 43.6 | 48.8 | 48.1 | 35.8 | 43.1 |
| Mean (range), years | (23–78) | (24–79) | (23–76) | (20–83) | (18–74) | (19–81) |
| Disease duration, | 9.1 | 7.4 | 9.4 | 18 | 9.3 | 9.6 |
| Mean (range), years | (0.2–37.1) | (0.1–32.7) | (0.3–51.6) | (0.1–38.9) | (0.8–42.4) | (0.12–72.6) |
| Prior initial treatment disease severity, % (number) of patients | ||||||
| Mild | 3 (3) | 4.5 (2) | 3.1 (2) | 6.3 (13) | 0.7 (1) | 1.4 (1) |
| Mild to moderate | 8 (8) | 13.6 (6) | 4.6 (3) | 3.4 (7) | 6.9 (10) | 11.1 (8) |
| Moderate | 35 (35) | 27.3 (12) | 21.5 (14) | 9.3 (19) | 18.6 (27) | 29.2 (21) |
| Moderate to severe | 35 (35) | 34.1 (15) | 47.7 (31) | 41 (81) | 49.7 (72) | 30.6 (22) |
| Severe | 19 (19) | 20.5 (9) | 23.1 (15) | 40 (82) | 24.1 (35) | 27.8 (29) |
| Current disease severity, % (number) of patients | ||||||
| Mild | 44 (44) | 52.3 (23) | 60 (39) | 63.4 (130) | 57.2 (83) | 4.,6 (35) |
| Mild to moderate | 26 (26) | 20.5 (9) | 24.6 (16) | 18.5 (38) | 22.1 (32) | 18.1 (13) |
| Moderate | 21 (21) | 15.9 (7) | 6.2 (4) | 9.3 (19) | 15.2 (22) | 18.1 (13) |
| Moderate to severe | 9 (9) | 6.8 (3) | 6.2 (4) | 7.3 (15) | 4.1 (6) | 11.1 (8) |
| Severe | 0 (0) | 4.5 (2) | 3.1 (2) | 1.5 (3) | 1.4 (2) | 4.2 (3) |
| Current IMID‐related drugs, % (number) of patients | ||||||
| TNFi monotherapy | 15 (15) | 54.5 (24) | 53.8 (35) | 49.8 (102) | 44.8 (65) | 15.3 (11) |
| TNFI combination therapy | 45 (45) | 31.8 (14) | 26.2 (17) | 1 (2) | 28.3 (41) | 33.3 (24) |
| Conventional systemic therapy | 40 (40) | 13.6 (6) | 20 (13) | 49.3 (101) | 26.9 (39) | 51.4 (37) |
| Duration of disease mean years (deviation) | ||||||
| Overall | 10 (9.1) | 8.5 (7.4) | 9,4 (10.5) | 18 (14.1) | 9.3 (8.7) | 9.6 (9.4) |
| Duration of symptoms prior to diagnosis, number (%) of patients | ||||||
| <1 year | 60 (60) | 12 (27.3) | 21 (32.3) | 111 (54.4) | 73 (50.7) | 52 (72.2) |
| 1–3 years | 19 (19) | 11 (25) | 18 (27.7) | 28 (13.7) | 45 (31.3) | 10 (13.9) |
| >3 years | 21 (21) | 21 (47.7) | 26 (40) | 65 (31.9) | 26 (18.1) | 10 (13.9) |
DACH, Deutschland (Germany), Austria, Confoederatio Helvetica (Switzerland); TNFi, tumour necrosis factor inhibitor.
Multiple regression analyses were performed to estimate factors affecting BMQ‐Specific (linear regression) and high medication adherence (logistic regression). Covariates in these analyses consisted of various sociodemographic, clinical or attitudinal/psychologic variables. A backward selection approach, based on removal when P > 0.05, was used to determine predictors of high BMQ‐Specific scores and high medication adherence. To account for the within‐subject correlations, the final model was refitted by a random effects linear logistic model, with a patient indicator as a random intercept.
With backward selection, the model complexity of the prediction model was automatically determined from the data. A best subset selection approach was applied to determine a simpler prognostic model for high adherence with only four to six predictors. All variables (except type of treatment) were dichotomized using the cut‐off maximizing the sum of sensitivity and specificity. The model with the highest cross‐validated area under the receiver operating characteristic curve among all candidate models (treatment and three dichotomous predictors for CD and RA, treatment and five dichotomous predictors for PSO in monotherapy) was chosen as the final prediction model.
Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
The DACH population consists of 631 patients from Germany (n = 298), Austria (n = 138) and Switzerland (n = 195). Demographics, disease severity and treatments are shown in Table 1 and adherence between IMIDs and medications in Fig. 1. Other characteristics such as rural or urban locations, living arrangements, children or years of education were not significantly different (not shown). Forgetting to take the medication (MMAS‐4 question 1) was significantly more prevalent in patients receiving conventional treatment (alone 15.9% or in combination 12.9%) compared with patients receiving TNFi monotherapy (11.3%) or TNFi combination therapy (6.6%).
Figure 1.
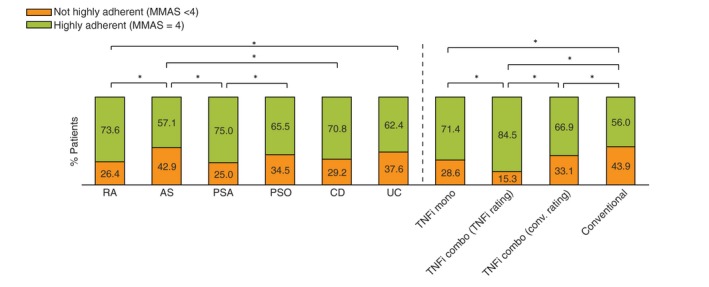
Adherence in percentage by disease and treatment of highly and not highly adherent participants. AS, ankylosing spondylitis; CD, Crohn's disease; MMAS‐4, Morisky Medication Adherence Scale; PSA, psoriatic arthritis; PSO, psoriasis; RA, rheumatoid arthritis, TNFi, tumour necrosis factor inhibitor; UC, ulcerative colitis. *p < 0.05.
The results for the BMQ‐General (Overuse and Harm) and BMQ‐Specific (Necessity and Concerns) questionnaire are shown in Fig. S1 (Supporting Information) by IMID and Fig. S2 (Supporting Information) by treatment. BMQ‐Specific results can be translated into a Necessity–Concerns framework, which divides patients into four attitudinal dimensions: sceptical (low necessity, high concerns; 2.4%), indifferent (low necessity, low concerns; 9.7%), ambivalent (high necessity, high concerns, 26.1%) and accepting (high necessities, low concerns; 61.8%) (Fig. 2).18 The highest percentage of acceptance was found in patients with AS, the highest percentage of those who were sceptical and indifferent occurred in patients with PSO, and the highest ambivalence was found in patients with RA (Fig. S3, Supporting Information). Younger participants (≤45 years) had a tendency to believing in a lower treatment necessity (3.1% were sceptical and 9.7% were indifferent vs. 1.7% and 8.4% in participants >45 years, not significant).
Figure 2.
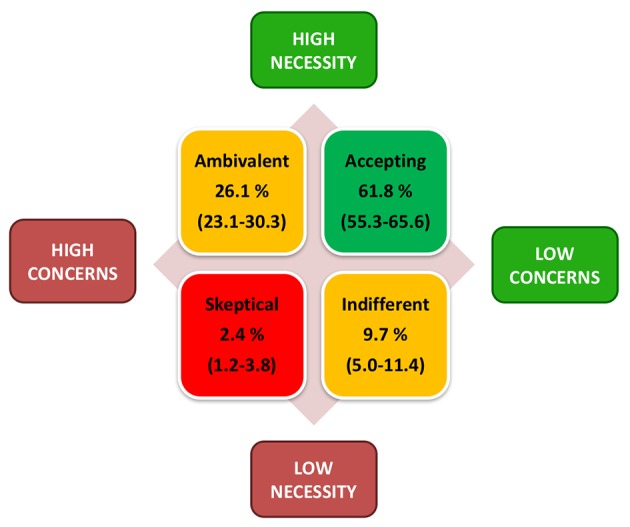
Necessity–Concerns framework grouping patients into four attitudinal segments by classifying results for BMQ‐Specific Necessity and Concerns into the two categories defined by ‘low’ (<3.0) and ‘high’ (≥3.0) and combining the results for each patient. Values in brackets presented depict the range for the proportion of patients classified in each of the four attitudinal segments across all treatment groups. BMQ, Beliefs about Medicines Questionnaire.
Single attitudinal segment had no significant influence on adherence. By grouping the attitudinal segments into high (sceptical and ambivalent) vs. low (indifferent and accepting) concerns, high concerns were associated with significantly less adherence (P < 0.05), whereas grouping on necessity had no significant influence on adherence.
The highest BIPQ score (a more threatening view towards the illness) was reported in patients with UC and PSO, whereas less in AS and PSA (Fig. S4, Supporting Information). Irrespective of the treatment, the results showed that participants were aware that their disease is a chronic disease, that the medication is helpful, and especially, the TNFi combo group felt like they understand their disease (data not shown).
In a multivariable regression analysis model based on variable selection, different factors influenced the treatment adherence of patients directly and indirectly in a positive or negative way (Fig. 3). Older age (>45 years, 61.3% of younger vs. 75.5% of older patients were highly adherent; P < 0.0001), TNFi combo (within the combination: TNFi, P < 0.0001; conventional, P = 0.21) or TNFi mono (P = 0.0006) had a higher likelihood to influence treatment adherence directly and positively (odds ratio [OR] are 1.70, 12.6, 2.53 and 3.30, respectively). More than three pretreatments (P = 0.03) or a higher rating for the overuse (P = 0.0003) of medication (OR of 0.42 and 0.82, respectively) had a higher likelihood of influencing treatment adherence in a direct and negative way. Positive influence factors of treatment concerns, which are indirect factors on adherence, were female sex (P = 0.044), ‘Consequences’ (BIPQ‐1, P = 0.0007), and ‘Illness concerns’ (BIPQ‐6, P < 0.0001); negative influence factors were ‘Control’ (BIPQ‐4, P < 0.0001), ‘Symptoms’ (BIPQ‐5, P = 0.0016), and ‘Understanding’ (BIPQ‐7, P < 0.0001). Positive influence factors of necessity of treatment that were indirect factors on adherence were higher age (P = 0.0002), use of TNFi therapy (mono, P = 0.015, or combination, P < 0.0001), duration (BIPQ‐2, P = 0.0004), treatment control (BIPQ‐4, P < 0.0001) and illness concerns (BIPQ‐6, P = 0.0008). Necessity was negatively influenced by a disease duration of >1 year (P = 0.0066; Fig. 3, Tables S1, S2 and S3, Supporting Information).
Figure 3.
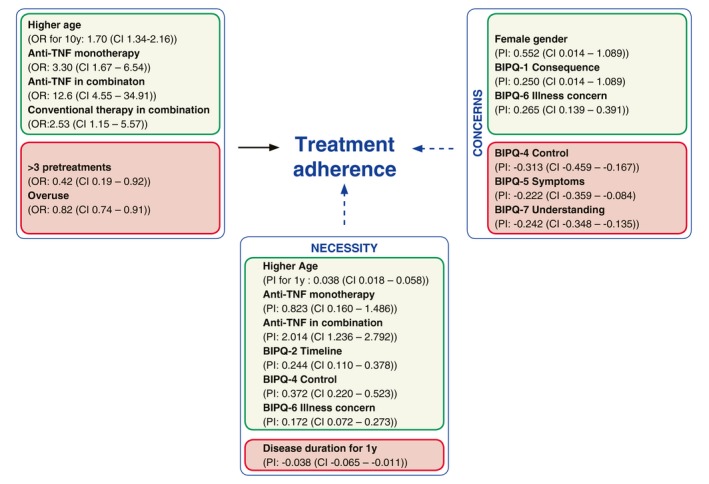
Multiple regression analysis showing the factors having a positive (green) or negative (red) effect on adherence either directly (black arrow, left box) or indirectly (dotted arrow) on Necessity (lower box) or Concerns (right box). As a matter of interpretation, for each 10 years’ increase in age, the OR for being highly adherent increases 1.63. Conversely, for each year of age, an increase of 0.038 points in the BMQ‐Specific is expected based on the probability of increase (PI). BIPQ, Brief Illness Perceptions Questionnaire; BMQ, Beliefs about Medicines Questionnaire; CI, confidence interval; OR, odds ratio.
Additionally, a risk matrix winner model was performed to show probability of non‐adherence. As the patient numbers were limited, reasonable matrices could only be developed for RA and CD (Figs S5 and S6, Supporting Information).
Discussion
Adherence to therapy is a central factor for controlling a disease. Within the global ALIGN study, the DACH region was analysed to determine general and specific beliefs towards medication and treatment adherence in patients simultaneously in different IMIDs.10
Treatment adherence is within previously reported ranges (Fig. 1).2, 4, 7, 8 By therapy, the highest adherence was seen in the TNFi combo‐TNFi rating (84.5%), vs. lowest in conventional (56.0%, significant). Also, our data confirm that older age has a positive direct effect on treatment adherence and necessity; female sex has a positive effect on concerns, whereas disease duration of 1 year has a negative impact on treatment necessity and more than three previous treatments have a negative impact on treatment adherence (Table 1, Fig. 3).2, 19, 20 Less than one‐third of the patients across all of the IMIDs were worried about overuse and harm of their medication. Most of the patients did not believe that they use too much medication or consider the medication harmful (Fig. S1, Supporting Information). Overuse has a direct negative impact on treatment adherence (Fig. 3). In our cohort, the probability of being treatment adherent decreases 0.8% with each 1‐point increase on the BMQ‐General Overuse score (Fig. S1, Supporting Information).
Concerns were quite low (C1, C3–5); however, the fear about long‐term effects (C2) was present in up to 64%. This indicates that in DACH, an additional emphasis should be given to education on long‐term outcome and side‐effects. Necessity was quite high with health depending on medicine (>70%, N1) and medicine protects from becoming worse (>85%, N5). Interestingly, 81% of patients in the TNFi combo group agree to strongly agree with being very ill without medicine (N3) vs. 56% of patients in the conventional group. Combination treatments are normally prescribed to patients where monotherapy was insufficient, underlining their higher belief in treatment necessity.
In DACH‐ALIGN, the BMQ‐Specific Necessity–Concerns framework21 shows a significant influence on adherence by comparing high concern (sceptical, ambivalent) vs. low concern groups (indifferent, accepting). This indicates that a higher emphasis on treatment concerns in different IMIDs in the DACH region should be given (Fig. 2). These findings are in accordance with previous studies, where lower necessity2, 21, 22, 23 and higher concerns2, 24, 25, 26 have a negative influence on adherence. As the attitudinal segments sceptical, ambivalent, and indifferent have a higher risk of non‐adherence,21 participants in those segments need special attention to increase treatment necessity and to lower concerns.
In a multiple regression analysis (Fig. 3), older age (>45 years), TNFi mono, TNFi in combination and conventional therapy in combination have a positive direct effect, whereas the belief of overuse and >3 pretreatments have a negative direct effect on adherence. Necessity and concerns both influence treatment adherence. Positive effects on necessity are higher age, TNFi mono, TNFi combination, BIPQ‐2 (Duration), BIPQ‐4 (Control) and BIPQ‐6 (Illness concerns), whereas disease duration of 1 year has a negative effect. Female sex, BIPQ‐1 (Consequences) and BIPQ‐6 (Illness concerns) have positive effects on concerns, whereas BIPQ‐4 (Control), BIPQ‐5 (Symptoms) and BIPQ‐7 (Understanding) have negative effects. It is important for HCPs in the DACH region to be aware of these characteristics and keep them in mind when communicating with patients. Based on our findings and previous studies, we suggest a quick tool to identify patient types (Fig. S7, Supporting Information) and a strategy to empower certain patient types (Fig. S8, Supporting Information).
Depending on the attitudinal patient type, the time to invest in a good HCP–patient relationship may improve patient satisfaction and treatment adherence.27, 28 A patient‐centred approach increases patient knowledge, education, motivation, self‐efficacy, decisioning, adherence, self‐care, quality of life, treatment survival, outcome and reduces care costs.29, 30, 31, 32, 33, 34 About 75% of HCPs employ a physician‐directed communication35 and about 70% of patients complain about the communication itself and not about competency.36 Patients often place higher value on prognosis, diagnosis and causation, and the HCP overestimates the patients’ wishes for information about treatment.37 As shown here, long‐term effects and side‐effects also require special attention (Fig. S1, Supporting Information, C2). Desired information within a patient should be identified and kept SIMPLE (Simplify regimen characteristics, Impart knowledge, Modifying patient beliefs, Patient communication, Leave bias, Evaluating adherence).38
Limitations of the study are the limited patient numbers where possible confounding factors such as severity, treatment response, comorbidities, possible unequal distribution, no randomization or stratification could not be evaluated. Recall and self‐representational biases cannot be ruled out with the use of self‐reported questionnaires. A single, randomly chosen visit without follow‐up might lead to variable patient responses influenced by different disease stages. Other reasons for treatment non‐adherence (e.g. side‐effects or loss of response) were not evaluated. Although confidential, patients may have overrated their adherence to conform to the doctor's expectations and to avoid negative appraisal.
The ALIGN study was the first study to simultaneously and extensively analyse psychosocial factors besides demographical and treatment‐related factors across six different IMIDs. In the DACH population, high treatment concerns were significantly associated with treatment non‐adherence. Therefore, we suggest tools to identify (Fig. S7, Supporting Information) and to empower patient types (Fig. S8, Supporting Information). An improvement in communication with a patient‐centred approach, focusing on patients concerns and needs, could enhance patients’ adherence more than improvement in specific treatments alone.
Supporting information
Figure S1. Percentage of patients in each indication who agreed or strongly agreed to the respective statements out of the BMQ‐General questionnaire containing Overuse (O1 to O4) and Harm (H1 to H4) statements or BMQ‐Specific questionnaire containing Concerns (C1 to C5) and Necessity (N1 to N5) statements.
Figure S2. Percentage of patients in each treatment group who agreed or strongly agreed to the respective statements out of the BMQ‐General questionnaire containing Overuse (O1 to O4) and Harm (H1 to H4) statements or BMQ‐Specific questionnaire containing Concerns (C1 to C5) and Necessity (N1 to N5) statements.
Figure S3. Percentage of patients for each indication distributed in the four attitudes by stacked columns.
Figure S4. BIPQ by diagnosis. The total BIPQ score was calculated as the sum of the score values of the individual items.
Figure S5. Risk matrix showing the probability of being highly adherent (MMAS = 4) for patients with RA depending on their age, treatment, the number of comorbidities and their answer to BIPQ‐6 (Illness concerns).
Figure S6. Risk matrix showing the probability of being highly adherent (MMAS = 4) for patients with CD depending on their sex, treatment, the BMQ Overuse score and the BMQ Necessity score.
Figure S7. Factors identifying patient types by attitudinal segments.
Figure S8. Strategies for empowering a certain patient type based on attitudinal segments.
Table S1. Parameter (variable selection) for multivariable logistic regression analysis to Adherence.
Table S2. Parameter (variable selection) for multivariable logistic regression analysis to Concerns.
Table S3. Parameter (variable selection) for Multivariable Logistic Regression Analysis to Necessity.
Acknowledgements
The ALIGN study was sponsored by AbbVie Inc. We are grateful to Dr. Julia Sommer (GSK, Munich, Germany) for statistical support.
Conflict of interest
Antonios Kolios has served as an investigator, speaker and/or advisor for AbbVie, Abbott, Janssen, Eli Lilly, MSD, Pfizer, Celgene, Novartis, Actelion, Leo, Amgen and Alk‐Abello; Dr. Kolios does not hold any shares or other financial interest in any related pharmaceutical company. Axel Hueber has been an advisor and/or received speaker honoraria and/or travel expense reimbursements and/or received grants and/or participated in clinical trials for AbbVie, Celgene, Janssen, Lilly, Novartis, Pfizer, Roche, Sanofi and UCB. Pierre Michetti has served as an investigator, speaker and/or advisor for AbbVie, AstraZeneca, Calypso, Delenex, Falk, Ferring, Hospira, MSD, Merck‐Serono, Nestlé Health Sciences, Pfizer, Takeda, UCB Pharma and Vifor Pharma and has received grants from UCB, MSD and Takeda. Dr. Michetti does not hold any shares or other financial interest in any related pharmaceutical company. Ulrich Mrowietz has served as an advisor and/or received speakers honoraria and/or received grants and/or participated in clinical trials for AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Dr. Reddy's, Eli Lilly, Foamix, Formycon, Forward Pharma, Janssen, Leo Pharma, Medac, MSD, Novartis, VBL and Xenoport. Monika Mustak‐Blagusz has served as an investigator, speaker and/or advisor for AbbVie, MSD, Pfizer, Actelion and Roche. Dr. Mustak‐Blagusz does not hold any shares or financial interest in any pharmaceutical company. Paul‐Gunther Sator has been an advisor and/or received speaker honoraria or travel expense reimbursements and/or received grants and/or participated in clinical trials for AbbVie, Actelion, Amgen, Almirall, Janssen, Novartis, Leo Pharma, Pfizer, MSD, Celgene, Maruho, ALK, Galderma, Abbott, UCB,Gilead and Lilly. Max Reinshagen has served as an investigator, speaker and/or advisor for AbbVie, MSD, Takeda, Janssen, Dr. Falk Pharma, Boehringer Ingelheim and Bristol‐Myers Squibb. Dr. Reinshagen does not hold any shares or other financial interest in any related pharmaceutical company. Dagmar Wilsmann‐Theis has been an advisor and/or received speaker honoraria or travel expense reimbursements and/or received grants and/or participated in clinical trials for AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Forward Pharma, GlaxoSmithKline, Janssen‐Cilag, Leo, Lilly, Medac, MSD, Novartis, Pfizer, UCB Pharma and VBL. Susana Gomis‐Kleindienst, Ulrike Luckey and Alexander Rössler are employees of AbbVie Deutschland GmbH & Co. KG., shareholders of AbbVie Inc., and may own stock options. Ingolf Schiefke has served as an investigator, speaker and or advisor for AbbVie, Vifor Pharma, Shire, Baxter, Fresenius, MSD, Takeda, Nutricia, Gilead, Pharmawerk Weinböhla and Dr. Falk Pharma. Dr. Schiefke does not hold any shares or other financial interest in any related pharmaceutical company.
Funding source
This study was sponsored by AbbVie Inc. AbbVie Inc. contributed to the study design, research, and interpretation of data, and writing, reviewing, and approving the publication. The authors determined the final content. No payments were made to the authors for writing this publication.
Trial registration: ACTRN12612000977875. Funded by AbbVie Inc.
References
- 1. Depont F, Berenbaum F, Filippi J et al Interventions to improve adherence in patients with immune‐mediated inflammatory disorders: a systematic review. PLoS ONE 2015; 10: e0145076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vangeli E, Bakhshi S, Baker A et al A systematic review of factors associated with non‐adherence to treatment for immune‐mediated inflammatory diseases. Adv Ther 2015; 32: 983–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kolios AG, Yawalkar N, Anliker M et al Swiss S1 guidelines on the systemic treatment of psoriasis vulgaris. Dermatology 2016; 232: 385–406. [DOI] [PubMed] [Google Scholar]
- 4. Thorneloe RJ, Bundy C, Griffiths CE, Ashcroft DM, Cordingley L. Adherence to medication in patients with psoriasis: a systematic literature review. Br J Dermatol 2013; 168: 20–31. [DOI] [PubMed] [Google Scholar]
- 5. Cramer JA, Benedict A, Muszbek N, Keskinaslan A, Khan ZM. The significance of compliance and persistence in the treatment of diabetes, hypertension and dyslipidaemia: a review. Int J Clin Pract 2008; 62: 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Makela MJ, Backer V, Hedegaard M, Larsson K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med 2013; 107: 1481–1490. [DOI] [PubMed] [Google Scholar]
- 7. Storm A, Andersen SE, Benfeldt E, Serup J. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol 2008; 59: 27–33. [DOI] [PubMed] [Google Scholar]
- 8. van den Bemt BJ, Zwikker HE, van den Ende CH. Medication adherence in patients with rheumatoid arthritis: a critical appraisal of the existing literature. Expert Rev Clin Immunol 2012; 8: 337–351. [DOI] [PubMed] [Google Scholar]
- 9. Jackson CA, Clatworthy J, Robinson A, Horne R. Factors associated with non‐adherence to oral medication for inflammatory bowel disease: a systematic review. Am J Gastroenterol 2010; 105: 525–539. [DOI] [PubMed] [Google Scholar]
- 10. Michetti P, Weinman J, Mrowietz U et al Impact of treatment‐related beliefs on medication adherence in immune‐mediated inflammatory diseases: results of the global ALIGN study. Adv Ther 2017; 34: 91–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health 1999; 14: 1–24. [Google Scholar]
- 12. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self‐reported measure of medication adherence. Med Care 1986; 24: 67–74. [DOI] [PubMed] [Google Scholar]
- 13. Morisky DE, DiMatteo MR. Improving the measurement of self‐reported medication nonadherence: response to authors. J Clin Epidemiol 2011; 64(3): 255–257; discussion 58‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morisky DE. Donald Morisky's website: MMAS scale. Available at: http://dmorisky.bol.ucla.edu/MMAS_scale.html (last accessed: 9 November 2015).
- 15. Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res 2006; 60: 631–637. [DOI] [PubMed] [Google Scholar]
- 16. Kroenke K, Spitzer RL, Williams JB. The patient health questionnaire‐2: validity of a two‐item depression screener. Med Care 2003; 41: 1284–1292. [DOI] [PubMed] [Google Scholar]
- 17. Walsh JC, Mandalia S, Gazzard BG. Responses to a 1 month self‐report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS 2002; 16: 269–277. [DOI] [PubMed] [Google Scholar]
- 18. Aikens JE, Nease DE Jr, Nau DP, Klinkman MS, Schwenk TL. Adherence to maintenance‐phase antidepressant medication as a function of patient beliefs about medication. Ann Fam Med 2005; 3: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martinez‐Santana V, Gonzalez‐Sarmiento E, Calleja‐Hernandez M, Sanchez‐Sanchez T. Comparison of drug survival rates for tumor necrosis factor antagonists in rheumatoid arthritis. Patient Prefer Adherence 2013; 7: 719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zaghloul SS, Goodfield MJ. Objective assessment of compliance with psoriasis treatment. Arch Dermatol 2004; 140: 408–414. [DOI] [PubMed] [Google Scholar]
- 21. Horne R, Parham R, Driscoll R, Robinson A. Patients’ attitudes to medicines and adherence to maintenance treatment in inflammatory bowel disease. Inflamm Bowel Dis 2009; 15: 837–844. [DOI] [PubMed] [Google Scholar]
- 22. de Thurah A, Norgaard M, Harder I, Stengaard‐Pedersen K. Compliance with methotrexate treatment in patients with rheumatoid arthritis: influence of patients’ beliefs about the medicine. A prospective cohort study. Rheumatol Int 2010; 30: 1441–1448. [DOI] [PubMed] [Google Scholar]
- 23. Selinger CP, Eaden J, Jones DB et al Modifiable factors associated with nonadherence to maintenance medication for inflammatory bowel disease. Inflamm Bowel Dis 2013; 19: 2199–2206. [DOI] [PubMed] [Google Scholar]
- 24. Chan SA, Hussain F, Lawson LG, Ormerod AD. Factors affecting adherence to treatment of psoriasis: comparing biologic therapy to other modalities. J Dermatolog Treat 2013; 24: 64–69. [DOI] [PubMed] [Google Scholar]
- 25. Shale MJ, Riley SA. Studies of compliance with delayed‐release mesalazine therapy in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2003; 18: 191–198. [DOI] [PubMed] [Google Scholar]
- 26. Spruill TM, Ogedegbe G, Harrold LR et al Association of medication beliefs and self‐efficacy with adherence in urban Hispanic and African‐American rheumatoid arthritis patients. Ann Rheum Dis 2014; 73: 317–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Augustin M, Holland B, Dartsch D, Langenbruch A, Radtke MA. Adherence in the treatment of psoriasis: a systematic review. Dermatology 2011; 222: 363–374. [DOI] [PubMed] [Google Scholar]
- 28. Umar N, Schaarschmidt M, Schmieder A, Peitsch WK, Schollgen I, Terris DD. Matching physicians’ treatment recommendations to patients’ treatment preferences is associated with improvement in treatment satisfaction. J Eur Acad Dermatol Venereol 2013; 27: 763–770. [DOI] [PubMed] [Google Scholar]
- 29. Levinson W, Lesser CS, Epstein RM. Developing physician communication skills for patient‐centered care. Health Aff (Millwood) 2010; 29: 1310–1318. [DOI] [PubMed] [Google Scholar]
- 30. de Bes J, Legierse CM, Prinsen CA, de Korte J. Patient education in chronic skin diseases: a systematic review. Acta Derm Venereol 2011; 91: 12–17. [DOI] [PubMed] [Google Scholar]
- 31. Zirwas MJ, Holder JL. Patient education strategies in dermatology: part 1: benefits and challenges. J Clin Aesthet Dermatol 2009; 2: 24–27. [PMC free article] [PubMed] [Google Scholar]
- 32. Bostoen J, Bracke S, De Keyser S, Lambert J. An educational programme for patients with psoriasis and atopic dermatitis: a prospective randomized controlled trial. Br J Dermatol 2012; 167: 1025–1031. [DOI] [PubMed] [Google Scholar]
- 33. Stewart M, Brown JB, Donner A et al The impact of patient‐centered care on outcomes. J Fam Pract 2000; 49: 796–804. [PubMed] [Google Scholar]
- 34. Lebrun‐Harris LA, Shi L, Zhu J, Burke MT, Sripipatana A, Ngo‐Metzger Q. Effects of patient‐centered medical home attributes on patients’ perceptions of quality in federally supported health centers. Ann Fam Med 2013; 11: 508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roter DL, Stewart M, Putnam SM, Lipkin M Jr, Stiles W, Inui TS. Communication patterns of primary care physicians. JAMA 1997; 277: 350–356. [PubMed] [Google Scholar]
- 36. Meryn S. Improving doctor‐patient communication. Not an option, but a necessity. BMJ 1998; 316: 1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kindelan K, Kent G. Concordance between patient's information preferences and general practitioners’ perceptions. Psychol Health 1987; 1: 399–409. [Google Scholar]
- 38. Atreja A, Bellam N, Levy SR. Strategies to enhance patient adherence: making it simple. MedGenMed 2005; 7: 4. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Percentage of patients in each indication who agreed or strongly agreed to the respective statements out of the BMQ‐General questionnaire containing Overuse (O1 to O4) and Harm (H1 to H4) statements or BMQ‐Specific questionnaire containing Concerns (C1 to C5) and Necessity (N1 to N5) statements.
Figure S2. Percentage of patients in each treatment group who agreed or strongly agreed to the respective statements out of the BMQ‐General questionnaire containing Overuse (O1 to O4) and Harm (H1 to H4) statements or BMQ‐Specific questionnaire containing Concerns (C1 to C5) and Necessity (N1 to N5) statements.
Figure S3. Percentage of patients for each indication distributed in the four attitudes by stacked columns.
Figure S4. BIPQ by diagnosis. The total BIPQ score was calculated as the sum of the score values of the individual items.
Figure S5. Risk matrix showing the probability of being highly adherent (MMAS = 4) for patients with RA depending on their age, treatment, the number of comorbidities and their answer to BIPQ‐6 (Illness concerns).
Figure S6. Risk matrix showing the probability of being highly adherent (MMAS = 4) for patients with CD depending on their sex, treatment, the BMQ Overuse score and the BMQ Necessity score.
Figure S7. Factors identifying patient types by attitudinal segments.
Figure S8. Strategies for empowering a certain patient type based on attitudinal segments.
Table S1. Parameter (variable selection) for multivariable logistic regression analysis to Adherence.
Table S2. Parameter (variable selection) for multivariable logistic regression analysis to Concerns.
Table S3. Parameter (variable selection) for Multivariable Logistic Regression Analysis to Necessity.


