Abstract
Background
Prurigo nodularis (PN) is a chronic skin condition characterized by intensely pruritic, hyperkeratotic papulonodular lesions that dramatically impairs patients’ quality of life. Management of the condition is challenging, and there is no approved therapy in the United States or in Europe.
Objective
The key aim of this survey was to examine current perceptions of PN diagnosis and management among members of the European Academy of Dermatology and Venereology (EADV).
Methods
The survey comprised 29 questions, including multiple choice and open responses, and was sent to clinicians via e‐mail during December 2016. The survey results were collected and analysed.
Results
Responses were received from 30 participants from 14 countries, of whom 22 (73.3%) were members of the EADV Task Force Pruritus. The majority (73.3%) considered PN to be a distinct condition, and the preferred description to explain the most common pathogenesis was ‘chronic pruritus leading to chronic scratching’ (80.0%). Pruritic nodules (n = 23/30, 76.7%) and scratching (n = 12/30, 40.0%) were the most common descriptors used to characterize PN. Most respondents (60.0%) reported seeing ≤5 unique PN patients per month, the majority of whom (68.8%) visited a physician ~2–4 times per year. PN patients reported to the respondents that they experienced persistent, severe pruritus, with a mean ± SD numeric rating scale score of 7.8 ± 1.2, lasting for >6 months in 82.3% of patients and >2 years in 51.0%. The most frequently prescribed therapies by survey respondents for PN symptoms were antihistamines (90.0%), antidepressants (90.0%), gabapentinoids (86.7%) and immunosuppressants (86.7%). Respondents agreed upon the need for new PN therapies (56.7%), revised PN classification and terminology (23.3%) and better understanding of PN pathophysiology (20.0%).
Conclusion
EADV Task Force Pruritus notes several challenges that must be met to improve symptoms and quality of life for patients with PN.
Introduction
Prurigo nodularis (PN) is a chronic skin condition characterized by intensely pruritic papulonodular lesions and is the dominant subtype of chronic prurigo.1 PN is a long‐term outcome of scratching by patients with chronic pruritus.2, 3 Lesions can range from a few millimetres up to 2 cm in diameter and are typically distributed symmetrically along the extensor surface of the extremities.3, 4, 5 The incidence and prevalence of PN are largely unknown, although small epidemiologic studies suggest that it is most common in women and older adults.2, 4, 6 PN has a significant impact on patient quality of life, with studies finding an association between PN and depression and anxiety.7, 8 The pruritus associated with PN is difficult to treat, and there is a significant unmet need for a safe and effective pruritus therapy.4, 8, 9
Opinion varies in the medical literature concerning several issues associated with PN, including whether PN is a separate disease entity or merely a sign of another underlying condition, the exact pathogenesis of PN and the optimal management strategy for patients.2, 4, 5, 6, 8, 10 The pathology associated with PN includes neurologic changes such as dermal nerve hypertrophy and proliferation, and marked increases in populations of calcitonin gene‐related peptide (CGRP)‐ and substance P (SP)‐immunoreactive nerves compared to individuals without PN. It is hypothesized that these neuropeptides may mediate the cutaneous neurogenic inflammation and pruritus in PN.5 Although a variety of systemic and atopy‐related conditions has been associated with PN, the aetiology is unknown in many cases,3, 8 often rendering it impossible to treat the underlying cause. In several cases, treatment of the underlying cause does not resolve PN, and symptomatic relief is needed for many patients.3, 4
The European Academy of Dermatology and Venereology (EADV) recently established a Task Force Pruritus, with members from multiple European countries, to initiate projects of scientific or educational relevance.11, 12 The aims of this task force are to conduct projects and surveys related to symptom prevalence and patient care, to extend knowledge and to fill educational gaps in this area of patient treatment.12 The first‐year focus of the Task Force Pruritus included expanding the validation of pruritus tools (PruNet13) throughout Europe, updating the EADV/EDF (European Dermatology Forum) European Guideline on pruritus, achieving a consensus on the definition, diagnostic criteria and terminology of PN1 and creating a pan‐European registry for PN. Here, we present the results of a survey that was conducted to characterize perceptions of PN held by members of the EADV and Task Force Pruritus, to assess the current state of alignment among physicians regarding PN diagnosis and management and to improve the diagnosis and management of PN and PN‐related pruritus.
Methods
The survey was designed in collaboration with experts of the EADV Task Force Pruritus steering committee (M. Augustin, C. Forner, F. Legat, M. Pereira, C. Riepe, S. Ständer, S. Steinke, J. Szepietowski, J. Wallengren, E. Weisshaar, C. Zeidler) and staff at Navigant Life Sciences (San Francisco, CA, USA). The survey was programmed and hosted online by Navigant Life Sciences and comprised 29 questions, including multiple choice and open responses (Table S1). It was sent via e‐mail to select clinicians who were members of the EADV Task Force Pruritus and/or were associated colleagues of the Task Force members as of December 2016. The initial list of potential respondents was assembled by the Task Force steering committee. All survey results were collected and analysed by staff at Navigant Life Sciences.
Results
Respondents
Responses were received from 30 participants in 14 countries, of whom almost three quarters (n = 22 #bib73.3%) were members of EADV Task Force Pruritus. Most participants (n = 25, 83.3%) were from European countries (Austria, Finland, France, Germany, Italy, Norway, Poland, Portugal, Spain, Sweden, UK); the remaining five (16.7%) were from India, Russia and Turkey.
Definition and terminology
Most respondents (73.3%) considered PN to be a distinct condition. When asked to select a concept to explain the most common pathogenesis, there was overwhelming support from all respondents (80.0%) for ‘chronic pruritus leading to chronic scratching’. A total of 10.0% preferred ‘subtype of atopic dermatitis’, and 10.0% supported ‘idiopathic chronic scratching’. There was no support for PN as a psychiatric disease or a subtype of perforating collagenosis.
When asked to describe PN in their own words, respondents commonly mentioned itchy/pruritic nodules (n = 23/30, 76.7%), scratching (n = 12/30, 40.0%), excoriation (n = 8/30, 26.7%), chronic pruritus (n = 6/30, 20.0%), atopic background (n = 5/30, 16.7%) and hyperpigmentation (n = 3/30, 10.0%). Four (13.3%) respondents indicated that PN nodules should have a standard definition, variously suggesting a minimum diameter of ≥1 cm, >1 cm or >0.5 cm for a PN nodule.
Descriptions of PN focused mainly on itch (n = 12/30, 40.0%) and scratch‐related papules/nodules/lesions (n = 13/30, 43.3%). When respondents were asked to describe lichen simplex chronicus (LSC), another cutaneous condition associated with scratching,14 the terminology that was used was somewhat different, with respondents mentioning lichenification (n = 13/30 #bib43.3%), plaques (n = 12/30, 40.0%), thickened skin (n = 6/30, 20.0%) and localization (n = 10/30, 33.3%), as well as itch (n = 11/30, 36.7%), hyperpigmentation (n = 4/30, 13.3%) and scratching (n = 15/30, 50.0%).
The respondents considered that, in current clinical practice and teaching, ‘prurigo nodularis’ (100.0%) and ‘prurigo’ (73.3%) are the most regularly used diagnostic terms for PN. Overall, respondents indicated a marked preference for using either the general term ‘prurigo’ (30.0%), or terminology related to the clinical appearance of the disease (e.g. ‘prurigo nodularis’, ‘papular prurigo’ or ‘plaque prurigo’; 40.0%). There was little support for terminology related to the comorbidity (e.g. ‘atopic prurigo’, ‘diabetic prurigo’; 13.3%).
Prevalence of PN
Most respondents (60.0%) reported seeing ≤5 unique PN patients per month; however, 30.0% of respondents saw >10 unique PN patients per month.
Patient demographics and disease burden
In line with the published finding that PN affects mostly older adults,6 most of the respondents’ PN patients were aged ≥40 years (78.6%); most were in the 40–60 years (38.4%) and 60–80 years (29.9%) age groups. Conversely, only 4.4% of patients were in the <20 years age group.
Physicians estimated that they see the majority of their PN patients (68.8%) every 3–6 months, resulting in ~2–4 visits per year: 13.5% of PN patients visited their physician every month. When queried about their PN patients’ three most common complaints, all respondents (100%) stated that itch is the number one symptom reported; followed by sleep disturbance (43.3%), pain/burning/bleeding (36.7%) and psychological distress/shame (33.3%) (Fig. 1).
Figure 1.
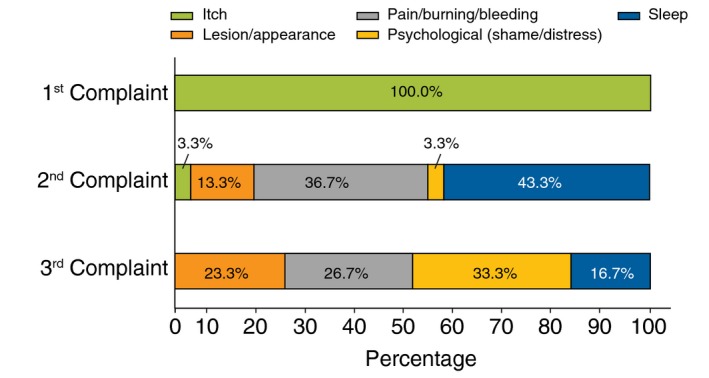
Top three complaints reported by patients with prurigo nodularis to physicians (N = 30).
Based on responding physicians’ estimates, patients with PN reported experiencing pruritus of high severity, with a mean ± SD score on a numeric rating scale of 0–10 (where 0 is no itch and 10 is the worst itch imaginable) of 7.8 ± 1.2. PN‐associated pruritus was persistent, lasting for >6 months in most patients (82.3%) and >2 years in more than half (51.0%) (Fig. 2).
Figure 2.
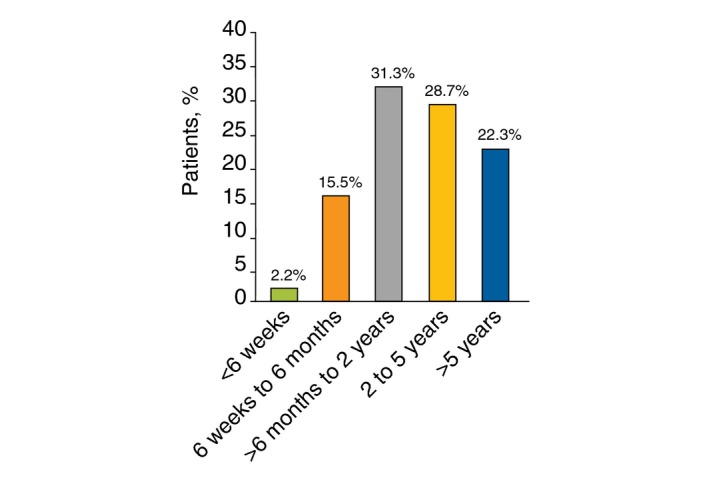
Duration of time patients with prurigo nodularis experienced pruritus (N = 30).
Diagnosis
Overall, the survey responses indicated that most patients with PN are diagnosed by clinical evaluation only (57.8%), while 40.4% are diagnosed by a combination of clinical and histological evaluation. Very few patients (1.8%) are diagnosed solely based on histology. According to the survey respondents, most PN patients present with ≥20 lesions (74.3%); almost one‐third (29.2%) are affected in ≥5 areas (trunk and each extremity) at presentation, and nearly half (49.0%) have three or four affected areas.
Atopic predisposition (44.9%) and dry skin (31.6%) were cited by respondents as the most common dermatologic findings in patients with PN. Atopic dermatitis (AD) is the most common underlying atopic condition and is diagnosed as the only atopic condition in approximately half of PN patients with atopic predisposition (51.5%), whereas 25.5% of PN patients with atopic predisposition have a combination of AD plus asthma and/or allergic rhinitis.
Treatment
When respondents were asked to indicate all systemic therapies they are using to treat PN, most respondents reported prescribing antihistamines (90.0%), antidepressants (90.0%), gabapentinoids (86.7%) and immunosuppressants (86.7%) (Fig. 3a). Despite unanimous agreement among respondents that antihistamines are generally ineffective for PN‐associated pruritus, they were prescribed for the majority of their PN patients irrespective of symptom severity (73.0% for mild and moderate pruritus; 51.5% for severe pruritus) (Fig. 3b). The majority of respondents indicated that immunosuppressants and gabapentinoids are the most effective systemic treatments for PN (26.7% and 16.7%, respectively; Fig. 3a).
Figure 3.
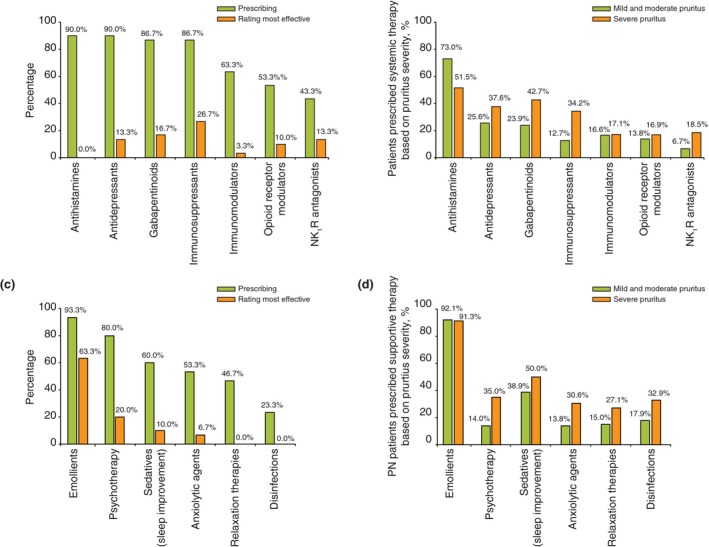
The most commonly used therapies for prurigo nodularis. (a) Systemic therapies: prescription frequency and efficacy rating. (b) Systemic therapies: use according to condition severity. (c) Supportive therapies: prescription and efficacy rating. (d) Supportive therapies: use according to condition severity.
The most frequently used supportive therapy for PN‐associated pruritus are emollients (93.3%), followed by psychotherapy (80.0%) and sedatives (60.0%) (Fig. 3c). Emollients were almost universally used as supportive therapy for pruritus in patients with PN (92.1% of patients with mild or moderate pruritus and 91.3% of those with severe pruritus); sedatives to help improve sleep were the second choice (38.9% and 50.0%) (Fig. 3d). Respondents reported prescribing all other supportive therapies (e.g. psychotherapy, anxiolytic agents, relaxation therapies and disinfectants) at similar rates based on pruritus severity (i.e. 13.8–17.9% for mild/moderate pruritus and 27.1–35.0% for severe pruritus).
Most respondents indicated that the treatments that they prescribe for both PN overall (84.6%) and PN‐associated pruritus (70.0%) in patients with atopic predisposition and dry skin as underlying conditions are similar to the treatments that they prescribe for patients who do not have one of the underlying conditions listed in the survey (Fig. 4a and b). For certain rarer underlying conditions in PN (e.g. bullous pemphigoid, mycosis fungoides), respondents may use specific treatments (Fig. 4a and b), if available.
Figure 4.
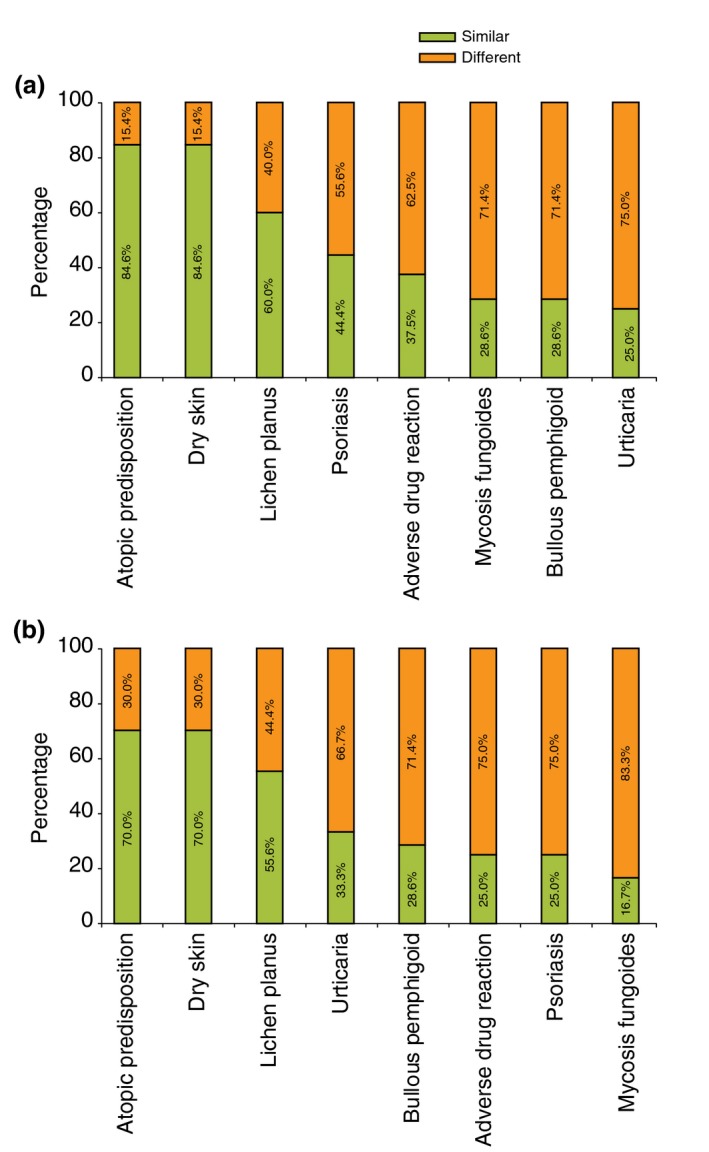
Physicians who reported treating patients similarly or differently, according to the underlying disease. (a) Treatment of prurigo nodularis. (b) Treatment of prurigo nodularis‐associated pruritus.
Unmet need
Respondents agreed that several key topics in PN need to be addressed, including a need for new therapies (56.7%), revised classification and terminology (23.3%) and better understanding of the pathophysiology of this condition (20.0%). Respondents commented that the first step towards addressing these unmet needs should be to clarify and unify the nomenclature, and once achieved, this should render the development of consensus algorithms for diagnosis and management easier and enable the design of robust clinical trials to test therapeutic agents.
Discussion
One of the aims of EADV Task Force Pruritus is to improve knowledge about PN,12 and the aim of the present survey was to examine current perceptions of and experiences managing PN among EADV members. Although opinion varies in the medical literature regarding whether PN is a distinct condition – because it evolves from a subset of patients with chronic pruritus – or a sign of an underlying condition,5, 6, 8, 10 nearly three quarters (73.3%) of our survey respondents considered PN to be a distinct condition.
As expected, respondents agreed that there are several critical areas of uncertainty in PN that should be addressed, including terminology, pathophysiology and treatment. In particular, respondents indicated that the most urgent topics in PN are the need for consensus regarding the classification and terminology of the condition, and new therapies. New terminology would be welcome, as it would better enable physicians to construct diagnosis and treatment algorithms as well as aid in the development and testing of new therapeutic options. Recently, the EADV published an expert consensus document on the definition, classification and terminology of chronic prurigo.1
The results of this survey also support the established finding that PN confers a heavy burden on patients and that these patients likely require more frequent use of healthcare resources.2, 15, 16 In this survey, the primary complaint among all respondents’ patients was pruritus, which was also likely responsible for the second most common complaint, sleep disturbance. Sleep disturbance has been demonstrated to have serious consequences, including poor health, missed workdays and doctor visits owing to other skin conditions.17 For many patients with PN, the most important symptom to treat is often pruritus because of its impact on quality of life;16, 18, 19, 20, 21, 22, 23 however, pruritus is difficult to treat.4, 8, 9
Treatment for PN is commonly standardized, and the goal of therapy is often to manage pruritus. Most of the respondents’ patients with PN are prescribed systemic treatments and supportive therapy. In most cases, the therapies used to treat PN are relatively ineffective,8, 24 as evidenced in this study by the far higher rates of therapy prescription compared with the respective rates of effectiveness. An exception would be emollients, which are prescribed in the vast majority of cases and were found to be most effective as supportive agents. Despite the consensus among the respondents that antihistamines are not particularly effective for PN, they remain commonly prescribed. In line with what is reported in the literature,8, 25, 26 respondents rated immunosuppressants and gabapentinoids as the most effective systemic treatments for pruritus in PN. However, the evidence base for many of the prescribed treatments is not from randomized controlled trials.8 The success of agents such as gabapentin and pregabalin may be attributable to the potentially neuropathic basis of PN‐associated itch.2, 25, 26, 27, 28, 29 Of particular note is the use of antidepressants.30
PN is a chronic skin condition with a significant impact on patient quality of life;19 however, there are currently no approved therapies. Treatments that are used for patients with PN often have inadequate efficacy, and some are associated with side‐effects that may limit their use.4, 8 Thus, there is a significant unmet need for effective treatment options for patients with PN.
Conclusion
The results of this clinician survey largely concur with what is known about PN and PN‐associated pruritus and its treatment: prurigo nodularis is a distressing chronic condition of often unknown aetiology and ambiguous pathogenesis that impacts patient mental health and quality of life, and for which there is a dearth of epidemiologic data, no currently approved treatment and little clinical trial evidence. The findings emphasize the need for a clear consensus on the definition and terminology for PN, and for well‐powered, randomized controlled trials to establish the best treatment for PN and PN‐associated pruritus, as well as specific therapies for PN or the associated pruritus of PN. EADV Task Force Pruritus has identified several challenges that must be met to improve the symptoms and quality of life of patients with PN.
Supporting information
Table S1. Survey Questions for the EADV and Task Force Pruritus.
Acknowledgements
The authors would like to thank the European Academy of Dermatology and Venereology for the grant provided to Dr. Pereira. In addition, medical writing and editorial assistance were provided by Nathan Hutcheson, PhD, and Davelene Israel‐Hanniford, PhD, of ApotheCom (New York, NY), and funded by Menlo Therapeutics Inc.
Conflict of Interests
Dr. Pereira declares no conflict of interests. Mr. Basta is Chief Executive Officer at Menlo Therapeutics Inc. Mr. Moore is Director of Life Sciences Strategy at Navigant Life Sciences, which has provided consulting services to Menlo Therapeutics Inc. Dr. Ständer has received grants/research support and honoraria for her role as an investigator and consultant from Menlo Therapeutics Inc, Galderma, Kiniksa Pharmaceuticals, Pierre Fabre Dermo‐Cosmetique, Trevi Therapeutics, and Vanda Pharmaceuticals; has received honoraria for participation in advisory boards for Menlo Therapeutics Inc, Beiersdorf AG, Celgene GmbH, Galderma, Kneipp‐Werke, NeRRe Therapeutics and Sienna Biopharmaceuticals; and has received patient fees from Menlo Therapeutics Inc, Dermasence, Trevi Therapeutics, and Vanda Pharmaceuticals.
Funding Sources
Menlo Therapeutics Inc, Redwood City, California, USA, supported this study. The authors gratefully acknowledge the grant from the European Academy of Dermatology and Venereology (EADV, No. 2016‐012 to MP).
The copyright line for this article was changed on 23 July 2018 after original online publication.
References
- 1. Pereira MP, Steinke S, Zeidler C et al European academy of dermatology and venereology European prurigo project: expert consensus on the definition, classification and terminology of chronic prurigo. J Eur Acad Dermatol Venereol 2017. 10.1111/jdv.14570. [DOI] [PubMed] [Google Scholar]
- 2. Zeidler C, Stander S. The pathogenesis of prurigo nodularis–’Super‐Itch’ in exploration. Eur J Pain 2016; 20: 37–40. [DOI] [PubMed] [Google Scholar]
- 3. Vaidya DC, Schwartz RA. Prurigo nodularis: a benign dermatosis derived from a persistent pruritus. Acta Dermatovenerol Croat 2008; 16: 38–44. [PubMed] [Google Scholar]
- 4. Saco M, Cohen G. Prurigo nodularis: picking the right treatment. J Fam Pract 2015; 64: 221–226. [PubMed] [Google Scholar]
- 5. Lee MR, Shumack S. Prurigo nodularis: a review. Australas J Dermatol 2005; 46: 211–218. [DOI] [PubMed] [Google Scholar]
- 6. Iking A, Grundmann S, Chatzigeorgakidis E, Phan NQ, Klein D, Stander S. Prurigo as a symptom of atopic and non‐atopic diseases: aetiological survey in a consecutive cohort of 108 patients. J Eur Acad Dermatol Venereol 2013; 27: 550–557. [DOI] [PubMed] [Google Scholar]
- 7. Jorgensen KM, Egeberg A, Gislason GH, Skov L, Thyssen JP. Anxiety, depression and suicide in patients with prurigo nodularis. J Eur Acad Dermatol Venereol 2017; 31: e106–e107. [DOI] [PubMed] [Google Scholar]
- 8. Fostini AC, Girolomoni G, Tessari G. Prurigo nodularis: an update on etiopathogenesis and therapy. J Dermatol Treat 2013; 24: 458–462. [DOI] [PubMed] [Google Scholar]
- 9. Pereira MP, Stander S. Itch management: treatments under development. Curr Prob Dermatol 2016; 50: 71–76. [DOI] [PubMed] [Google Scholar]
- 10. Tsianakas A, Zeidler C, Stander S. Prurigo nodularis management. Curr Prob Dermatol 2016; 50: 94–101. [DOI] [PubMed] [Google Scholar]
- 11. European Academy of Dermatology and Venereology . History of the EADV. Available at https://www.eadv.org/history. Accessed 21 June 2017.
- 12. European Academy of Dermatology and Venereology . Meet the Task Force Pruritus. 2017. Available at http://www.pruritussymposium.de/taskforcepruritus.html. Accessed 21 June 2017.
- 13. Stander S, Zeidler C, Riepe C et al European EADV network on assessment of severity and burden of Pruritus (PruNet): first meeting on outcome tools. J Eur Acad Dermatol Venereol 2016; 30: 1144–1147. [DOI] [PubMed] [Google Scholar]
- 14. Lotti T, Buggiani G, Prignano F. Prurigo nodularis and lichen simplex chronicus. Dermatol Ther 2008; 21: 42–46. [DOI] [PubMed] [Google Scholar]
- 15. Weisshaar E, Szepietowski JC, Darsow U et al European guideline on chronic pruritus. Acta Derm Venereol 2012; 92: 563–581. [DOI] [PubMed] [Google Scholar]
- 16. Kini SP, DeLong LK, Veledar E, McKenzie‐Brown AM, Schaufele M, Chen SC. The impact of pruritus on quality of life: the skin equivalent of pain. Arch Dermatol 2011; 147: 1153–1156. [DOI] [PubMed] [Google Scholar]
- 17. Silverberg JI, Garg NK, Paller AS, Fishbein AB, Zee PC. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population‐based study. J Invest Dermatol 2015; 135: 56–66. [DOI] [PubMed] [Google Scholar]
- 18. Carr CW, Veledar E, Chen SC. Factors mediating the impact of chronic pruritus on quality of life. JAMA Dermatol 2014; 150: 613–620. [DOI] [PubMed] [Google Scholar]
- 19. Tey HL, Wallengren J, Yosipovitch G. Psychosomatic factors in pruritus. Clin Dermatol 2013; 31:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schneider G, Driesch G, Heuft G, Evers S, Luger TA, Stander S. Psychosomatic cofactors and psychiatric comorbidity in patients with chronic itch. Clin Exp Dermatol 2006; 31: 762–767. [DOI] [PubMed] [Google Scholar]
- 21. van Os‐Medendorp H, Eland‐de Kok PC, Grypdonck M, Bruijnzeel‐Koomen CA, Ros WJ. Prevalence and predictors of psychosocial morbidity in patients with chronic pruritic skin diseases. J Eur Acad Dermatol Venereol 2006; 20: 810–817. [DOI] [PubMed] [Google Scholar]
- 22. Erturk IE, Arican O, Omurlu IK, Sut N. Effect of the pruritus on the quality of life: a preliminary study. Ann Dermatol 2012; 24: 406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leader B, Carr CW, Chen SC. Pruritus epidemiology and quality of life. Handb Exp Pharmacol 2015; 226: 15–38. [DOI] [PubMed] [Google Scholar]
- 24. Lim VM, Maranda EL, Patel V, Simmons BJ, Jimenez JJ. A review of the efficacy of thalidomide and lenalidomide in the treatment of refractory prurigo nodularis. Dermatol Ther (Heidelb) 2016; 6: 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gencoglan G, Inanir I, Gunduz K. Therapeutic hotline: treatment of prurigo nodularis and lichen simplex chronicus with gabapentin. Dermatol Ther 2010; 23: 194–198. [DOI] [PubMed] [Google Scholar]
- 26. Mazza M, Guerriero G, Marano G, Janiri L, Bria P, Mazza S. Treatment of prurigo nodularis with pregabalin. J Clin Pharm Ther 2013; 38: 16–18. [DOI] [PubMed] [Google Scholar]
- 27. Solak Y, Biyik Z, Atalay H et al Pregabalin versus gabapentin in the treatment of neuropathic pruritus in maintenance haemodialysis patients: a prospective, crossover study. Nephrology 2012; 17: 710–717. [DOI] [PubMed] [Google Scholar]
- 28. Grundmann S, Stander S. Chronic pruritus: clinics and treatment. Ann Dermatol 2011; 23: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wallengren J. Prurigo: diagnosis and management. Am J Clin Dermatol 2004; 5: 85–95. [DOI] [PubMed] [Google Scholar]
- 30. Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med 2013; 368: 1625–1634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Survey Questions for the EADV and Task Force Pruritus.


