Abstract
Purpose
To investigate the long‐term visual quality and stability of implantable collamer lens (ICL) and laser refractive surgery (LRS) for myopia.
Methods
This study comprised 52 eyes of 26 high‐myopia anisometropia patients who were suitable for surgical treatment. In each patient, the higher‐myopia eye was implanted with ICL and the lower‐myopia eye was treated with LRS. The patients were followed for 3 years. During that time period, uncorrected (UDVA) and corrected distance visual acuity (CDVA), refraction, wavefront aberration and visual quality were evaluated.
Results
The spherical equivalent refractive error changed from −14.11 ± 3.39 D preoperatively to −1.27 ± 1.05 D 3 years after ICL implantation and from −8.75 ± 2.76 D to −1.12 ± 1.30 D after LRS. The changes in refractive error from 1 month to 3 years were −0.52 and −0.77 D for the ICL and LRS groups, respectively. The safety indices (postoperative CDVA/preoperative CDVA) were 1.84 ± 1.00 and 1.32 ± 0.40, and the efficacy indices (postoperative UDVA/preoperative CDVA) were 1.40 ± 1.10 and 1.11 ± 0.44, respectively. The postoperative coma, spherical and total higher‐order aberrations in the ICL group were lower than those in the LRS group.
Conclusion
Both ICL implantation and LRS are safe and effective procedures for myopia with suitable indications, but ICL implantation is more stable. Fewer induced aberrations are gained after ICL implantation.
Keywords: high myopia, higher‐order aberration, implantable collamer lens, laser refractive surgery, visual quality
Introduction
High‐myopia anisometropia patients may have difficulty with fusion when wearing spectacles (Tomac 1998; Tomac & Birdal 2001). Contact lens and surgical options can correct the refractive error and avoid aniseikonia, and both options have advantages and disadvantages (Achiron et al. 1998; BenEzra et al. 2000; Khandekar et al. 2013; Hedayati et al. 2015; Carnt & Stapleton 2016; Eissa 2016). Surgical options include implantable collamer lens (ICL; STAAR Surgical, Monrovia, CA, USA) and corneal laser refractive surgery (LRS). Which surgical procedure will be chosen for the eyes of anisometropia patients mainly depends on the level of refractive error. Due to the limits of patients’ corneal conditions, LRS has been generally chosen for the correction of <12 D of myopia, and the outcomes are worse in high‐myopia cases compared to cases of low to moderate myopia. While ICL, a posterior‐chamber phakic intraocular lens, is the preferred technique for high myopia of >12 D. Previous studies (Sanders & Vukich 2003, 2006; Sanders 2007; Igarashi et al. 2009; Kamiya et al. 2012) have reported the grouping comparisons of ICL and laser‐assisted in situ keratomileusis (LASIK); the current study was objectively conducted to compare the outcomes of ICL implantation in one eye and LRS in the contralateral eye and investigate the long‐term visual quality and stability.
Materials and Methods
This study adhered to the Declaration of Helsinki and was approved by the Ethical Committee Review Board of Eye and ENT Hospital of Fudan University. Written informed consent was obtained from all patients after the possible consequences of the study were explained.
Fifty‐two eyes from 26 myopia anisometropia patients (six men and 20 women) were included in the study, with one eye implanted with an ICL while the contralateral eye underwent LRS at the Eye and ENT Hospital of Fudan University. All patients underwent preoperative routine ophthalmic examinations at the Refractive Surgery Center and met the surgical requirements. The inclusion criteria for this study included patients aged between 20 and 45 years, an anterior chamber depth of 2.80 mm or more, and an endothelial cell density >2000 cells/mm2. Patients were also required to have a reasonable expectation of surgical outcomes; no pre‐existing ocular pathology; no previous keratoconus, cataract or glaucoma; and no systemic disease.
The preoperative variables are summarized in Table 1. The mean follow up was 3.50 ± 0.76 (3–5) years. The mean age of the patients was 30.42 ± 7.41 (20–43) years. Among the patients, 14 eyes were underwent LASIK, 12 eyes underwent laser‐assisted subepithelial keratomileusis (LASEK), which LRS technique would be chosen for the eyes was a comprehensive evaluation daily depending on the level of refractive error, patients’ corneal conditions and patient's willingness because of different costs.
Table 1.
The preoperative statistics of the subjects in the ICL and LRS groups
| Variables | ICL group | LRS group | p value |
|---|---|---|---|
| Refractive error (D) | |||
| Spherical equivalent | −14.11 ± 3.39 (−8.38 to −19.63) | −8.75 ± 2.76 (−2.38 to −13.63) | <0.001a |
| Spherical | −13.22 ± 3.31 (−8.25 to −19.00) | −7.89 ± 2.69 (−1.25 to −13.00) | <0.001a |
| Cylindrical | −1.78 ± 1.35 (0 to −4.75) | −1.71 ± 1.12 (0 to −4.00) | 0.787 |
| Axial length (mm) | 28.61 ± 1.40 (26.42 to 31.32) | 26.65 ± 1.08 (24.24 to 28.59) | <0.001a |
| UDVA (logMAR) | 1.67 ± 0.37 (1.00 to 2.00) | 1.29 ± 0.36 (0.70 to 2.00) | <0.001a |
| CDVA (logMAR) | 0.29 ± 0.31 (0.00 to 1.30) | 0.10 ± 1.29 (−0.08 to 0.40) | <0.001a |
| Keratometric value (D) | |||
| Flat K | 43.15 ± 1.35 (40.6 to 46.0) | 43.15 ± 1.36 (40.5 to 45.3) | 0.571 |
| Steep K | 44.74 ± 1.82 (40.8 to 47.9) | 44.87 ± 1.57 (41.6 to 47.0) | 0.292 |
| IOP (mmHg) | 14.71 ± 2.78 (10.0 to 21.3) | 15.15 ± 2.84 (8.5 to 20.3) | 0.196 |
| CCT (mm) | 520.65 ± 30.90 (461 to 589) | 519.81 ± 30.11 (472 to 598) | 0.642 |
| ECD (cells/mm2) | 3321.84 ± 474.71 (2486 to 4466) | 3256.92 ± 483.60 (2617 to 4203) | 0.390 |
| HOAs | 0.31 ± 0.15 (0.10 to 0.71) | 0.31 ± 0.18 (0.10 to 0.99) | 0.958 |
CCT = central corneal thickness, CDVA = corrected distance visual acuity, D = dioptres, ECD = corneal endothelial cell density, HOAs = total higher‐order aberrations, ICL = implantable collamer lens, IOP = intraocular pressure, K = keratometry, LRS = laser refractive surgery, N = number of eyes, UDVA = uncorrected distance visual acuity.
Values differed significantly between the two groups (p < 0.05). Results are expressed as mean ± SD (range).
Surgical procedures
All surgeries were performed by the same experienced surgeon (XW). Antibiotic medications (levofloxacin, Santen, Japan) and a non‐steroidal anti‐inflammatory drug (pranoprofen, Senju, Japan) were administered four times daily for 3 days.
ICL implantation
The implantation of ICL and the surgical procedure were the same as our previous studies (Chen et al. 2016a,b). The ICL model used in this study was ICL V4.
LASIK
The VisuMax femtosecond laser system (Carl Zeiss, Oberkochen, Germany) with a pulse of 125 nJ and a frequency of 500 kHz was used to create the corneal stroma flap. The intended diameter and flap thickness were 8.0 mm and 110 mm, respectively. The track and spot distances were set as 3.0 mm for the flap creation and 1.5 mm for turning the flap side cut. The pedicle of the flap was placed on the top. The MEL 80 excimer laser system (Carl Zeiss) was used for stromal ablation. The optical diameter was 6–6.5 mm, and the transition zone was 1.0 mm. Then, the flap was carefully repositioned over the stroma and washed with BSS solution.
LASEK
After the cornea was soaked by 20% alcohol for 12 seconds, a corneal flap was made with a corneal epithelial shovel. The MEL 80 excimer laser system was used for stromal ablation. The optical diameter was 6–6.5 mm. Then, the flap was carefully repositioned, and a contact lens (Aeuvue Oasys. Johnson & Johnson, New Brunswick, NJ, USA) was applied.
After surgery, the patients were given levofloxacin eye drops four times daily for 1 week, artificial tears four times daily for 1 month and 0.1% fluorometholone tapered off over 2 weeks for ICL implantation, 1 month for LASIK and 2 months for LASEK.
Follow‐up
The patients were followed for 3 years. During that time period, uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), manifest refractive error, axial length (IOL Master; Carl Zeiss), intraocular pressure (Tonemeterx‐10; Canon, Tokyo, Japan), corneal endothelial cell density (SP. 2000P; Topcon, Tokyo, Japan), corneal topography (Pentacam; Oculus, Wetzlar, Germany), Hartmann‐Shack wavefront aberration (WASCA; Carl Zeiss) and visual quality (OQAS II; Visiometrics, Terrassa, Spain) were evaluated.
Statistical analysis
All statistical analyses were performed using SPSS version 20.0 (SPSS Inc., IBM, Al Monk, NY, USA), and the results are expressed as mean ± SD. Preoperative parameters were analysed using the paired t‐test, and the postoperative p value was determined using single‐factor analysis of covariance. A p value <0.05 was considered statistically significant.
Results
Safety
All surgeries were uneventful, and no intraoperative complication was observed. The safety indices (postoperative CDVA/preoperative CDVA) at 1 month, 1 year and 3 years in the ICL group were 1.73 ± 0.88, 1.80 ± 0.89 and 1.84 ± 1.00, respectively, and they were 1.30 ± 0.40, 1.27 ± 0.38 and 1.32 ± 0.40 in the LRS group, respectively. The logMAR CDVA values at baseline, 1 month, 1 year and 3 years were 0.29 ± 0.31, 0.09 ± 0.20, 0.07 ± 0.19, and 0.06 ± 0.21, respectively, in the ICL group and 0.10 ± 1.29, −0.01 ± 0.08, 0.00 ± 0.06 and −0.01 ± 0.07, respectively, in the LRS group. At 3 years postoperatively, no patient lost 1 or more lines of CDVA, 19.23% and 15.38% of eyes gained 1 line, 23.08% and 11.54% of eyes gained 2 lines, 26.92% and 11.54% of eyes gained 2 or more lines of CDVA, and 30.77% and 61.54% of eyes did not change compared to baseline in the ICL group and LRS group, respectively. At baseline and 1 month, 1 year and 3 years postoperatively, 11.54%, 65.38%, 69.23% and 65.38% of eyes gained a CDVA of 20/20 in the ICL group, while 42.31%, 76.92%, 80.77% and 76.92% of eyes gained a CDVA of 20/20 in the LRS group, respectively. At baseline and 1 month, 1 year and 3 years postoperatively, 65.38%, 92.31%, 92.31% and 92.31% of eyes gained a CDVA of 20/40 in the ICL group, and 96.15%, 100.00%, 100.00% and 100.00% of eyes gained a CDVA of 20/40 in the LRS group, respectively (Fig. 1).
Figure 1.
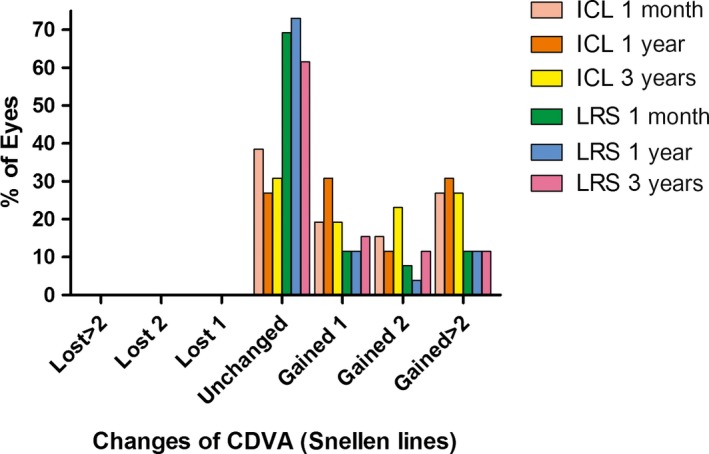
The percentage of eyes that gained/lost lines of corrected distance visual acuity at different time‐points of follow‐up after implantable collamer lens versus laser refractive surgery.
The endothelial cell densities at 3 years postoperatively were 2993.73 ± 499.45 and 3045.42 ± 395.55 cells/mm2 in the ICL and LRS groups, respectively. The 3‐year loss rates of the two groups were 9.01% and 6.49%, respectively, with no significant difference observed between the groups (χ = 0.00, p = 1.00). The vault of ICL changed from 516.54 ± 235.24 μm at 1 month to 431.54 ± 243.32 μm at 3 years postoperatively.
Efficacy
The efficacy indices (postoperative UDVA/preoperative CDVA) at 1 month, 1 year and 3 years were 1.41 ± 0.86, 1.54 ± 1.07 and 1.40 ± 1.10 in the ICL group, respectively, and 1.14 ± 0.37, 1.14 ± 0.37 and 1.11 ± 0.44 in the LRS group, respectively. The logMAR UDVA values at baseline, 1 month, 1 year and 3 years were 1.67 ± 0.37, 0.19 ± 0.27, 0.17 ± 0.27 and 0.22 ± 0.24 in the ICL group, respectively, and 1.29 ± 0.36, 0.06 ± 0.14, 0.06 ± 0.14 and 0.09 ± 0.22 in the LRS group, respectively. Additionally, 38.46%, 38.46% and 30.77% of eyes gained a UDVA of 20/20 in the ICL group at 1 month, 1 year and 3 years postoperatively, respectively, 53.85%, 50.00% and 50.00% of eyes gained a UDVA of 20/20 in the LRS group at 1 month, 1 year and 3 years postoperatively, respectively. At 1 month, 1 year and 3 years, 84.62%, 84.62% and 76.92% of eyes gained a UDVA of 20/40 in the ICL group, respectively, 96.15%, 96.15% and 88.46% of eyes gained a UDVA of 20/40 in the LRS group, respectively (Fig. 2).
Figure 2.
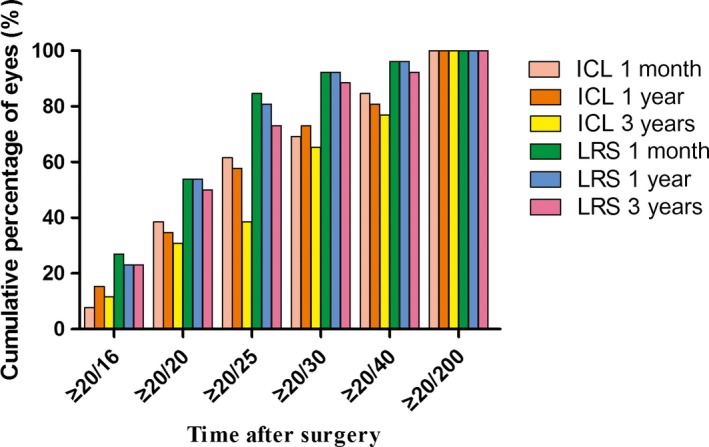
The cumulative percentage of uncorrected distance visual acuity at different time‐points after implantable collamer lens versus laser refractive surgery.
Stability
The manifest refractive spherical equivalent (MRSE) changed from −14.11 ± 3.39 D preoperatively to −0.75 ± 0.88 D at 1 month, −0.88 ± 0.99 D 1 year and −1.27 ± 1.05 D 3 years after ICL implantation (Fig. 3). A significant change in the MRSE of 0.52 ± 0.89 (−0.13 to 2.38) D was seen from 1 month to 3 years postoperatively (p = 0.007), with 42.31% eyes within ± 0.50 D, 61.54% within ± 1.00 D and 92.31% within ± 1.50 D. The spherical equivalent refractive error changed from −8.75 ± 2.76 D preoperatively to −0.35 ± 0.81 D at 1 month, −0.73 ± 1.03 D 1 year and −1.12 ± 1.30 D 3 years after LRS (Fig. 3). A significant change of 0.77 ± 0.89 (−0.25 to 3.13) D in the MRSE was seen from 1 month to 3 years postoperatively (p < 0.001), with 30.77% eyes within ± 0.50 D, 65.38% within ± 1.00 D and 80.77% within ± 1.50 D (Table 2, Fig. 3).
Figure 3.
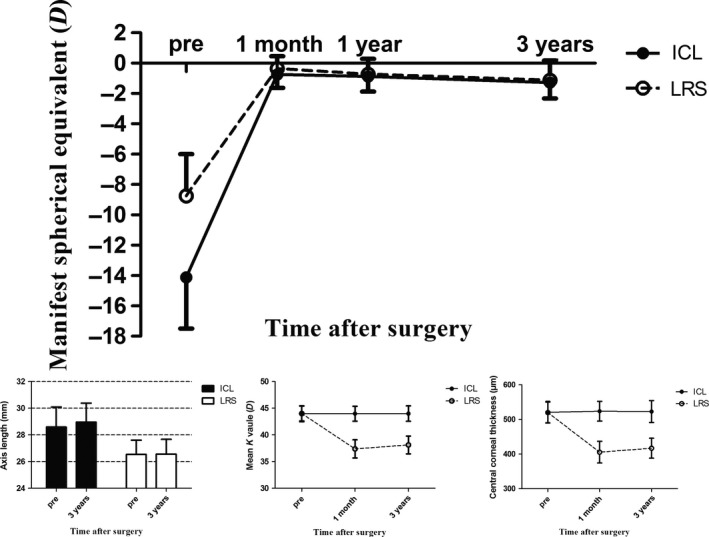
The manifest spherical equivalent, axis length, mean K value and central corneal thickness during follow‐up in the implantable collamer lens and laser refractive surgery groups for all subject eyes.
Table 2.
The spherical equivalent, axis length, mean K value and central corneal thickness during follow‐up in the ICL and LRS groups
| Time | Spherical equivalent (D) | Axis length (mm) | Mean K value (D) | Central corneal thickness (μm) | ||||
|---|---|---|---|---|---|---|---|---|
| ICL | LRS | ICL | LRS | ICL | LRS | ICL | LRS | |
| Pre | −14.11 ± 3.39 (−8.38 to −19.63) | −8.75 ± 2.76 (−2.38 to −13.63) | 28.61 ± 1.40 (26.42 to 31.32) | 26.54 ± 1.07 (24.24 to 28.59) | 43.97 ± 1.49 (44.10 to 46.70) | 44.01 ± 1.40 (44.15 to 46.10) | 520.65 ± 30.90 (461 to 589) | 519.81 ± 30.11 (472 to 589) |
| 1 month | −0.75 ± 0.88 (−3.00 to 0.82) | −0.35 ± 0.81 (−2.00 to 1.88) | − | − | 43.97 ± 1.40 (41.30 to 46.60) | 37.38 ± 1.71 (33.95 to 41.45) | 523.62 ± 28.54 (471 to 588) | 405.46 ± 31.42 (354 to 469) |
| 3 years | −1.27 ± 1.05 (−3.50 to 0.13) | −1.12 ± 1.30 (−4.38 to 0.63) | 28.97 ± 1.42 (26.51 to 32.41) | 26.56 ± 1.11 (24.23 to 28.66) | 43.99 ± 1.44 (41.20 to 46.95) | 38.12 ± 1.66 (34.95 to 41.90) | 522.69 ± 31.45 (461 to 606) | 416.73 ± 28.96 (373 to 474) |
| ▵ | 0.52 ± 0.89 (−0.13 to 2.38) | 0.77 ± 0.89 (−0.25 to 3.13) | 0.36 ± 0.56 (−0.10 to 2.67) | 0.02 ± 0.22 (−0.38 to 0.57) | 0.02 ± 0.16 (−0.35 to 0.35) | 0.74 ± 0.68 (−0.40 to 3.10) | −0.92 ± 10.06 (−21 to 19) | 11.27 ± 13.44 (−8 to 48) |
| p value | 0.007a | <0.001a | 0.003a | 0.721 | 0.513 | <0.001a | 0.644 | <0.001a |
For spherical equivalent, mean K value, and central corneal thickness, ▵ = the value at 3 years – the value at 1 month, for axis length, ▵ = the value of 3 years – the value at baseline.
ICL = implantable collamer lens, LRS = laser refractive surgery.
The changes differed significantly between 3 years and 1 month or baseline (p < 0.05). Results are expressed as the mean ± standard deviation (range).
A significant axial elongation of 0.36 ± 0.56 mm was seen from baseline to 3 years after ICL implantation (p = 0.003), while no significant difference was seen after LRS, with an axial change of 0.02 ± 0.22 mm (p = 0.721). However, a significant K value change of 0.74 ± 0.68 (−0.40 to 3.10) D and a central corneal thickness change of 11.27 ± 13.44 (−8 to 48) μm were seen after LRS (p < 0.001), while no significant difference was seen after ICL implantation, with a K value change of 0.02 ± 0.16 (−0.35 to 0.35) D and a central corneal thickness change of −0.92 ± 10.06 (−21 to 19) μm (p = 0.513, 0.644, respectively; Table 2, Fig. 3).
Wavefront aberrations
No significant changes were observed in the total higher‐order aberrations (HOAs) after ICL implantation, while significant increases in the HOAs were seen after LRS. In particular, 3 years after ICL implantation, the trefoil aberration was higher while the spherical aberration was lower than that preoperatively, and the coma, spherical and HOAs 3 years after LRS were higher compared to the baseline values (Fig. 4). There was no significant difference between the groups at baseline, and significant differences in the coma, trefoil, spherical and HOAs were observed between the groups 3 years after the surgeries (p = 0.044, <0.001, <0.001 and 0.004, respectively). The induced HOAs in the ICL group was lower than that in the LRS group (Table 3).
Figure 4.
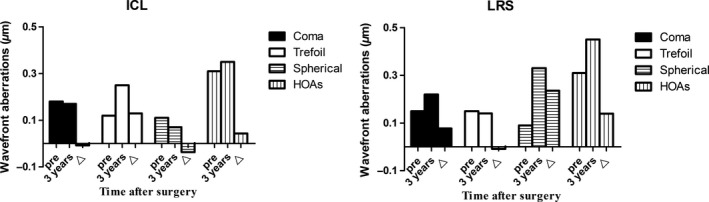
Changes in the aberration components of 6 mm diameters between time‐points after implantable collamer lens versus laser refractive surgery.
Table 3.
A comparison of different aberrations in the root–mean‐square values between the two groups for 6 mm diameters 3 years postoperatively
| Aberration components (μm) | ICL group | LRS group | p value |
|---|---|---|---|
| Coma | |||
| Pre | 0.18 ± 0.14 | 0.15 ± 0.10 | 0.279 |
| 3 years | 0.17 ± 0.10 | 0.22 ± 0.12 | 0.044a |
| ▵ (p) | −0.01 ± 0.14 (0.766) | 0.08 ± 0.15 (0.014) | 0.044a |
| Trefoil | |||
| Pre | 0.12 ± 0.08 | 0.15 ± 0.08 | 0.124 |
| 3 years | 0.25 ± 0.12 | 0.14 ± 0.07 | <0.001a |
| ▵ (p) | 0.13 ± 0.13 (<0.001) | −0.01 ± 0.09 (0.618) | <0.001a |
| Spherical | |||
| Pre | 0.11 ± 0.07 | 0.09 ± 0.07 | 0.300 |
| 3 years | 0.07 ± 0.05 | 0.33 ± 0.17 | <0.001a |
| ▵ (p) | −0.04 ± 0.09 (0.039) | 0.24 ± 0.17 (<0.001) | <0.001a |
| HOAs | |||
| Pre | 0.31 ± 0.15 | 0.31 ± 0.18 | 0.958 |
| 3 years | 0.35 ± 0.12 | 0.45 ± 0.11 | 0.004a |
| ▵ (p) | 0.04 ± 0.17 (0.218) | 0.14 ± 0.23 (0.005) | 0.004a |
HOAs = total higher‐order aberrations, ICL = implantable collamer lens, LRS = laser refractive surgery, RMS = root mean square, coma, RMS for and , trefoil, RMS for and , spherical, RMS for .
Values differed significantly between the groups (p < 0.05). The results are expressed as mean ± standard deviation (range).
Retinal image quality and intraocular scattering
Retinal image quality and intraocular scattering were objectively measured using a double‐pass optical quality analysis system (OQASII). Based on the data 3 years after surgeries, the objective scatter index (OSI) of eyes implanted with ICL was lower than that of eyes that underwent LRS, while the modulation transfer function cut‐off frequency (MTFcutoff), Strehl ratio (SR) and optical quality analysis system values (OV) at different contrasts (OV100%, OV20% and OV9%) in the ICL group were higher than those in the LRS group (Table 4).
Table 4.
A comparison of the retinal image quality and intraocular scattering between the two groups at 3 years postoperatively
| Parameters | ICL group | LRS group |
|---|---|---|
| OSI | 1.28 ± 0.60 (0.23–2.65) | 1.79 ± 0.94 (0.73–3.95) |
| MTFcutoff (cpd) | 34.70 ± 9.10 (19.89–51.87) | 27.48 ± 8.44 (8.86–41.56) |
| SR | 0.18 ± 0.55 (0.11–0.29) | 0.16 ± 0.04 (0.07–0.23) |
| OV100% | 1.15 ± 0.30 (0.67–1.70) | 0.90 ± 0.26 (0.35–1.37) |
| OV20% | 0.79 ± 0.27 (0.40–1.40) | 0.63 ± 0.18 (0.25–0.97) |
| OV9% | 0.44 ± 0.17 (0.20–0.80) | 0.37 ± 0.12 (0.15–0.57) |
cpd = cycles per degree, ICL = implantable collamer lens, LRS = laser refractive surgery, MTF cut‐off = modulation transfer function cut‐off frequency, OSI = objective scatter index, OV = optical quality analysis system values, SR = Strehl ratio in two dimensions.
The results are expressed as mean ± SD (range).
Adverse reactions
No infection, corneal dilation, pupillary block, pigmentary glaucoma, intraocular inflammation or increased postoperative intraocular pressure requiring medication were noted during the follow‐up period. Mild anterior capsule opacity was seen in one eye of one patient with a vault of 130 μm; however, no treatment was performed because the CDVA was 20/16.
Discussion
The different surgical methods for the correction of myopia among anisometropia patients are based on the principles of safety and efficacy. The long‐term visual quality and stability of different surgical methods should be evaluated. Both eyes of anisometropia patients underwent different surgical methods, which had the following specific benefits: the contralateral eye comparison more naturally assesses the objective outcomes between ICL and corneal LRS. In this study, we investigated the long‐term safety, efficacy, stability, characteristics of aberration and visual quality of anisometropia patients with one eye implanted with an ICL and the other eye treated with LRS.
No patient had a CDVA loss 3 years after both ICL implantation and LRS; 19.23% and 15.38% of eyes gained 1 line, 23.08% and 11.54% of eyes gained 2 lines, 26.92% and 11.54% of eyes gained 2 more lines of CDVA and 30.77% and 61.54% of eyes did not change compared to baseline in the ICL group and LRS group, respectively. No infection, corneal dilation or other adverse reactions were observed, suggesting that both ICL implantation and corneal LRS are safe and reliable in cases with suitable indications (Sanders & Vukich 2003, 2006; Sanders 2007). In addition, the safety index of the ICL group in our study was higher than that of other studies (Igarashi et al. 2014; Moya et al. 2015). We attributed this result to the different subjects; the anisometropic myopia patients in our study were different from the common myopia patients included in other studies. The worse eyes with higher myopia characteristic of anisometropic patients had not been fully corrected preoperatively, nor were amblyopia eyes (Ying et al. 2013; Pascual et al. 2014). Furthermore, the visual image magnification rate and visual quality were improved after ICL implantation (Simader et al. 2004; Igarashi et al. 2009; Kamiya et al. 2012; Perez‐Vives et al. 2013). Therefore, the higher postoperative CDVA and lower preoperative CDVA of anisometropic myopia patients relative to common myopia patients may result in a higher safety index (postoperative CDVA/preoperative CDVA). The 3‐year endothelial cell density loss rate of the ICL group (9.01%) was slightly higher than that of the LRS group (6.49%), but no significant difference was observed between the groups, indicating that the effect of intraocular surgery on corneal endothelial cells was negligible. Previous studies (Dejaco‐Ruhswurm et al. 2002; Edelhauser et al. 2004) showed that the surgical procedure had an influence on endothelial cell loss in the early follow‐up period, but the ICL had no influence on the long‐term stability of endothelial cells. The endothelial cell density loss rate in this study was consistent with that of 8–9% observed in an American phase 3 clinical trial of ICL (Edelhauser et al. 2004).
The mean preoperative UDVA and CDVA of the ICL group were lower than those of the LRS group, and the percentages of UDVA and CDVA above 20/20 or 20/40 in the ICL group were also lower than those in the LRS group. To balance spectacles used by patients with high‐myopia anisometropia, the higher‐myopia eye cannot achieve sufficient correction, which causes anisometropic amblyopia; therefore, the eyes of the ICL group were somewhat amblyopic. The efficacy index of the ICL and LRS group were 1.41 ± 0.86, 1.14 ± 0.37 postoperatively at 1 month and 1.40 ± 1.10, 1.11 ± 0.44 at 3 years, respectively, suggesting that both ICL implantation and LRS are effective surgical methods. Laser refractive surgery (LRS) is the most commonly used effective surgery to correct myopia when corneal thickness and morphology are in the safe range. However, in the case of higher refractive error, the corneal thickness may not meet the need of magnitude of corneal ablation of the stroma, which may impair the biomechanical stability of the cornea. In these cases, ICL implantation has been proven to be safe and effective because it avoids corneal ablation and has a wide range correction of myopia (Sanders et al. 2003, 2004).
In our study, a significant change of −0.52 ± 0.89 D (p = 0.007) in the MRSE was seen from 1 month to 3 years postoperatively (p = 0.007) after ICL implantation and −0.77 ± 0.89 D (p < 0.001) after corneal LRS. We attributed the change in the ICL group to the significant axial elongation of 0.36 ± 0.56 mm. Meanwhile, the significant K value increase of 0.74 ± 0.68 D from 1 month to 3 years postoperatively, with a central corneal thickness change of 11.27 ± 13.44 μm, might have contributed to the MRSE changes after LRS. Implantable collamer lens (ICL) implantation was more stable than LRS for patients with high and extreme myopia, which is consistent with the results of previous studies (Sanders & Vukich 2003; Igarashi et al. 2009). In the LRS group, the mean preoperative spherical equivalent of the patients within a 1.00 D change in the MRSE was −6.89 ± 2.43 D, while that of the patients above a 1.00 D change in the MRSE was −10.23 ± 1.92 D, indicating that LRS was a stable option for the correction of low‐to‐moderate myopia and refractive regression might be observed in cases of high and extreme myopia. The regression in this study was up to −3.13 D after LRS, which suggests that ICL implantation was more stable in the long term compared to LRS for cases of high and extreme myopia, which should be considered during the selection of the surgical method (Sanders & Vukich 2003, 2006; Sanders 2007; Igarashi et al. 2009; Kamiya et al. 2012). The vault of the ICL group changed from 516.54 ± 235.24 μm at 1 month to 431.54 ± 243.32 μm 3 years postoperatively, indicating a decreasing trend of the vault in the long term, which is in accordance with the results of previous studies (Kamiya et al. 2009; Alfonso et al. 2010, 2012; Schmidinger et al. 2010).
No significant change in the HOA was seen 3 years after ICL implantation (p = 0.218), while a significant change was seen after LRS (p = 0.005). Additionally, the HOA of the ICL group was significantly lower than that of the LRS group 3 years postoperative (p = 0.002), which indicated that excellent visual quality could be gained after ICL implantation, although the eyes had high and extreme myopia preoperatively. Specifically, the coma and spherical aberration values 3 years after ICL implantation were significantly lower than those after LRS (p = 0.036 and p < 0.001, respectively), but the trefoil aberration was higher than that after LRS (p < 0.001). The postoperative spherical aberration of the ICL group decreased significantly (p = 0.039) and the trefoil aberration increased (p < 0.001) compared with baseline, while the postoperative coma and spherical aberration of the LRS group increased (p = 0.014 and <0.001, respectively) compared with baseline, indicating that the trefoil aberration had been inducted after ICL implantation and that coma and spherical aberration were inducted after LRS. Several scholars (Igarashi et al. 2009; Kamiya et al. 2012) have found that ICL implantation has a significantly lower induction rate of aberrations compared to LASIK. Implantable collamer lens (ICL) implantation was considered to induce fewer spherical aberrations and HOAs than LRS, possibly because it maintains the prolate shape of the cornea, regardless of the amount of myopic correction. The previous study of our group (Li et al. 2014) had found a significant association between the magnitude of decentration and induced horizontal coma. Therefore, in this study, decentration might be the cause to the postoperative coma increase observed in the LRS group. The trefoil aberration increase of the ICL group may be attributed to the 3.0 mm corneal incision. Lumping LASIK and LASEK patients together in the LRS group may not be wise; however, no significant difference was seen from our study population in preoperative and 3 years postoperative coma, trefoil, spherical and HOAs and the induced aberrations (p = 0.131, 0.988, 0.953, 0.233, respectively). Therefore, we believe that this study is clinically acceptable for comparisons of the postoperative induced aberrations of the ICL implantation and LRS techniques.
Halo may be complained in the early time after ICL implantation (Eom et al. 2017); however, the OSI, MTFcutoff and OV values of the ICL group were all better than those of the LRS group based on the numerical values in the long term, indicating that good optical visual quality could also be obtained after ICL implantation, although the preoperative spherical equivalent in the ICL group was significantly higher than that in the LRS group, with a worse CDVA than that in the LRS group.
There are several limitations to this study. First, we did not completely match the patients based on preoperative refraction, CDVA and axis length. However, as higher preoperative myopic refraction was often associated with poor safety, efficacy, predictability and stability, the postoperative clinical outcomes in the ICL group tended to bias the data in favour of the LRS group. Second, different surgical methods were included in the LRS group, but all LRS techniques had the same basic principle of laser corneal ablation and the characteristics of refractive regression: the spherical equivalent refractive error changed from −9.85 ± 2.26 D preoperatively to −0.38 ± 0.86 D at 1 month and −1.44 ± 1.53 D 3 years after LASIK. A significant change of −1.07 ± 1.00 D in the MRSE was see from 1 month to 3 years postoperatively (p = 0.01) with a significant K value change of 0.98 ± 0.71 D (p < 0.001) after LASIK; the spherical equivalent refractive error changed from −8.06 ± 2.73 D preoperatively to −0.37 ± 0.87 D at 1 month and −1.08 ± 1.18 D 3 years after LASEK. A significant change of −0.72 ± 0.88 D in the MRSE was seen from 1 month to 3 years postoperatively (p = 0.008) with a significant K value change of 0.66 ± 0.48 D (p = 0.003) after LASEK; no significant differences were seen in the changes of MRSE and K value between LASIK and LASEK. Additionally, the optical quality parameters of all eyes before surgery were not assessed because the double‐pass optical quality analysis system was not available 3 years ago. Interestingly, the postoperative optical quality parameters of the ICL group were not worse than those of the LRS group, although higher preoperative refraction with weaker CDVA was often relevant to poor optical quality (Miao et al. 2014). Thus, we believe that this study is clinically acceptable for comparisons of the postoperative clinical outcomes and visual performance of the ICL implantation and LRS techniques.
In summary, our comparative study demonstrates that ICL implantation and LRS techniques are both safe and effective procedures for myopia with suitable indications, but ICL implantation is more stable. Fewer‐induced aberrations are obtained after ICL implantation.
This work was supported by the Shanghai Shenkang Hospital Development Center (Grant No. SHDC12016207), and the Health and Family Planning Committee of Pudong New District of Shanghai (Grant No. PW2014D‐1).
Contributor Information
Xiaoying Wang, Email: doctxiaoyingwang@163.com.
Xingtao Zhou, Email: doctzhouxingtao@163.com.
References
- Achiron LR, Witkin NS, Ervin AM & Broocker G (1998): The effect of relative spectacle magnification on aniseikonia. J Am Optom Assoc 69: 591–599. [PubMed] [Google Scholar]
- Alfonso JF, Lisa C, Abdelhamid A, Fernandes P, Jorge J & Montés‐Mic RÓ (2010): Three‐year follow‐up of subjective vault following myopic implantable collamer lens implantation. Graefes Arch Clin Exp Ophthalmol 248: 1827–1835. [DOI] [PubMed] [Google Scholar]
- Alfonso JF, Fernandez‐Vega L, Lisa C, Fernandes P, Gonzalez‐Meijome J & Montes‐Mico R (2012): Long‐term evaluation of the central vault after phakic Collamer(R) lens (ICL) implantation using OCT. Graefes Arch Clin Exp Ophthalmol 250: 1807–1812. [DOI] [PubMed] [Google Scholar]
- BenEzra D, Cohen E & Karshai I (2000): Phakic posterior chamber intraocular lens for the correction of anisometropia and treatment of amblyopia. Am J Ophthalmol 130: 292–296. [DOI] [PubMed] [Google Scholar]
- Carnt N & Stapleton F (2016): Strategies for the prevention of contact lens‐related Acanthamoeba keratitis: a review. Ophthalmic Physiol Opt 36: 77–92. [DOI] [PubMed] [Google Scholar]
- Chen X, Miao H, Naidu RK, Wang X, Wang X & Zhou X (2016a): Comparison of early changes in and factors affecting vault following posterior chamber phakic implantable collamer lens implantation without and with a central hole (ICL V4 and ICL V4c). BMC Ophthalmol 16: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wang X, Zhang X, Chen Z & Zhou X (2016b): Implantable collamer lens for residual refractive error after corneal refractive surgery. Int J Ophthalmol 9: 1421–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejaco‐Ruhswurm I, Scholz U, Pieh S, Hanselmayer G, Lackner B, Italon C, Ploner M & Skorpik C (2002): Long‐term endothelial changes in phakic eyes with posterior chamber intraocular lenses. J Cataract Refract Surg 28: 1589–1593. [DOI] [PubMed] [Google Scholar]
- Edelhauser HF, Sanders DR, Azar R & Lamielle H (2004): Corneal endothelial assessment after ICL implantation. J Cataract Refract Surg 30: 576–583. [DOI] [PubMed] [Google Scholar]
- Eissa SA (2016): Management of pseudophakic myopic anisometropic amblyopia with piggyback Visian® implantable collamer lens. Acta Ophthalmol 95: 188–193. [DOI] [PubMed] [Google Scholar]
- Eom Y, Kim DW, Ryu D, Kim JH, Yang SK, Song JS, Kim SW & Kim HM (2017): Ring‐shaped dysphotopsia associated with posterior chamber phakic implantable collamer lenses with a central hole. Acta Ophthalmol 95: e170–e178. [DOI] [PubMed] [Google Scholar]
- Hedayati H, Ghaderpanah M, Rasoulinejad SA & Montazeri M (2015): Clinical presentation and antibiotic susceptibility of contact lens associated microbial keratitis. J Pathog 2015: 152767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi A, Kamiya K, Shimizu K & Komatsu M (2009): Visual performance after implantable collamer lens implantation and wavefront‐guided laser in situ keratomileusis for high myopia. Am J Ophthalmol 148: 164–170.e1. [DOI] [PubMed] [Google Scholar]
- Igarashi A, Shimizu K & Kamiya K (2014): Eight‐year follow‐up of posterior chamber phakic intraocular lens implantation for moderate to high myopia. Am J Ophthalmol 157: 532–539. [DOI] [PubMed] [Google Scholar]
- Kamiya K, Shimizu K & Kawamorita T (2009): Changes in vaulting and the effect on refraction after phakic posterior chamber intraocular lens implantation. J Cataract Refract Surg 35: 1582–1586. [DOI] [PubMed] [Google Scholar]
- Kamiya K, Igarashi A, Shimizu K, Matsumura K & Komatsu M (2012): Visual performance after posterior chamber phakic intraocular lens implantation and wavefront‐guided laser in situ keratomileusis for low to moderate myopia. Am J Ophthalmol 153: 1178–1186.e1. [DOI] [PubMed] [Google Scholar]
- Khandekar RB, Gogri UP & Al HS (2013): The impact of spectacle wear compliance on the visual function related quality of life of Omani students: a historical cohort study. Oman J Ophthalmol 6: 199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zhao J, Miao H, Shen Y, Sun L, Tian M, Wadium E & Zhou X (2014): Mild decentration measured by a Scheimpflug camera and its impact on visual quality following SMILE in the early learning curve. Invest Ophthalmol Vis Sci 55: 3886–3892. [DOI] [PubMed] [Google Scholar]
- Miao H, Tian M, He L, Zhao J, Mo X & Zhou X (2014): Objective optical quality and intraocular scattering in myopic adults. Invest Ophthalmol Vis Sci 55: 5582–5587. [DOI] [PubMed] [Google Scholar]
- Moya T, Javaloy J, Montes‐Mico R, Beltran J, Munoz G & Montalban R (2015): Implantable collamer lens for myopia: assessment 12 years after implantation. J Refract Surg 31: 548–556. [DOI] [PubMed] [Google Scholar]
- Pascual M, Huang J, Maguire MG et al. (2014): Risk factors for amblyopia in the vision in preschoolers study. Ophthalmology 121: 622–629.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez‐Vives C, Ferrer‐Blasco T, Dominguez‐Vicent A, Garcia‐Lazaro S & Montes‐Mico R (2013): Optical and visual quality of the visian implantable collamer lens using an adaptive‐optics visual simulator. Am J Ophthalmol 155: 499–507. [DOI] [PubMed] [Google Scholar]
- Sanders DR (2007): Matched population comparison of the visian implantable collamer lens and standard LASIK for myopia of ‐3.00 to ‐7.88 diopters. J Refract Surg 23: 537–553. [DOI] [PubMed] [Google Scholar]
- Sanders DR & Vukich JA (2003): Comparison of implantable contact lens and laser assisted in situ keratomileusis for moderate to high myopia. Cornea 22: 324–331. [DOI] [PubMed] [Google Scholar]
- Sanders D & Vukich JA (2006): Comparison of implantable collamer lens (ICL) and laser‐assisted in situ keratomileusis (LASIK) for low myopia. Cornea 25: 1139–1146. [DOI] [PubMed] [Google Scholar]
- Sanders DR, Vukich JA, Doney K & Gaston M (2003): U.S. Food and Drug Administration clinical trial of the Implantable Contact Lens for moderate to high myopia. Ophthalmology 110: 255–266. [DOI] [PubMed] [Google Scholar]
- Sanders DR, Doney K & Poco M (2004): United states food and drug administration clinical trial of the implantable collamer lens (ICL) for moderate to high myopia: three‐year follow‐up. Ophthalmology 111: 1683–1692. [DOI] [PubMed] [Google Scholar]
- Schmidinger G, Lackner B, Pieh S & Skorpik C (2010): Long‐term changes in posterior chamber phakic intraocular collamer lens vaulting in myopic patients. Ophthalmology 117: 1506–1511. [DOI] [PubMed] [Google Scholar]
- Simader CC, Schmidinger G, Lackner B, Franz C, Paikl D, Skorpik C & Pieh S (2004): Visual acuitiy after ICL implantation: comparison of clinical outcomes and calculations of image magnifications. Invest Ophthalmol Vis Sci 452: U14. [Google Scholar]
- Tomac S (1998): Anisometropia and binocularity. Ophthalmology 105: 1–2. [DOI] [PubMed] [Google Scholar]
- Tomac S & Birdal E (2001): Effects of anisometropia on binocularity. J Pediatr Ophthalmol Strabismus 38: 27–33. [DOI] [PubMed] [Google Scholar]
- Ying G, Huang J, Maguire MG, Quinn G, Kulp MT, Ciner E, Cyert L & Orel‐Bixler D (2013): Associations of anisometropia with unilateral amblyopia, interocular acuity difference, and stereoacuity in preschoolers. Ophthalmology 120: 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]


