Abstract
Objectives
This study introduces an initial evaluation of a novel High‐Intensity Focused Electromagnetic (HIFEM) technology. The primary goal is to quantify any effects the treatments may have on abdominal tissues, as well as to establish hypotheses for future research of this technology.
Methods
Twenty‐two patients received four abdominal treatments using the EMSCULPT device (BTL Industries Inc., Boston, MA). Anthropometric evaluations were recorded and digital photographs were taken at baseline, at 2 months, and at 6 months post‐treatments. The MRI without contrast determined by vertertebras T12 and S1 (FIESTA and FSPRG sequences) was used to measure dimensions in coronal cross‐sectional images of abdominal muscle and fatty tissues, in order to assess any anatomical changes induced by the application.
Results
Analysis of the same MRI slices verified by tissue artefacts showed a statistically significant (all P < 0.0001) average 18.6% reduction of adipose tissue thickness, 15.4% increase in rectus abdominis muscle thickness, and 10.4% reduction in rectus abdominus separation (diastasis recti) as measured from the medial border of the muscle 2 months post‐treatment. More significant improvements were observed in patients with BMI 18.5–24.9 (classified as “normal”). MRI data from 6‐month follow‐up suggest the changes can be preserved in longer term. Tape measurements showed on average 3.8 cm subumbilical circumference reduction. The weight of the subjects did not change significantly (average −0.5 lb; P > 0.05). No adverse events were reported.
Conclusions
MRI, considered as a highly precise diagnostic method, revealed simultaneous muscle growth, fat reduction and reduced abdominal separation at 2 months and at 6 months post treatments, unrelated with dieting. Further research should investigate the exact physiological processes which stand behind the tissue changes observed in this study. Lasers Surg. Med. 51:40–46, 2019. © 2018 The Authors. Lasers in Surgery and Medicine Published by Wiley Periodicals, Inc.
Keywords: diastasis recti, fat reduction, HIFEM, magnetic technology, muscle growth
INTRODUCTION
The popularity of non‐invasive body shaping procedures has been growing rapidly—the number of procedures performed in the US more than doubled between 2012 and 2016 1. Cryolipolysis, radiofrequency, low level laser therapy and focused ultrasound 2 are most widely used for treating patients’ fat bulges, and their efficacy has been demonstrated in multiple previous studies. Similar to every aesthetic procedure, these technologies have also certain limitations. All current non‐invasive fat removal treatments are based on thermal effects and as such, they may bring about various cold or heat related side effects. More importantly, all these modalities are designed to address only fat tissue.
Subcutaneous fat is an important factor affecting patient's body contours as it comprises approximately 25% 3 of human body composition. However, muscle tissue comprises even a larger portion of the human body composition (42% male/36% female 4) and depending on individual characteristics, the condition of patient's muscle can play either an equal or even more important role in defining the overall aesthetic appearance. Still, physical workout is currently the only generally available method for natural strengthening of one's muscles.
The use of magnetic stimulation has a proven track record when treating various medical indications, ranging from neurology 5, 6, 7, psychiatry 8, physiotherapy 9, 10, 11, 12, to treating urinary incontinence in women 13. Furthermore, due to the non‐thermal and non‐ionizing nature of the technology, its application is considered relatively safe 8. Even though the technology is highly effective, it is not as widely used as electrical stimulation 14.
This study brings an initial evaluation of a novel High‐Intensity Focused Electro‐Magnetic (HIFEM) technology applied to the abdominal area, in order to assess the physiological response in treated patients. The primary goal is to quantify any effects the treatments may have on abdominal tissues, as well as to establish hypotheses for future research of this technology. The outcomes of the study are expected to suggest if HIFEM can be potentially used as a new technology for non‐invasive body shaping treatments.
MATERIALS AND METHODS
Study Population
Twenty‐two subjects (10 females and 12 males) participated in this prospective, multi‐center, non‐randomized, pilot study. The average age of the participants was 39.4 ± 10.2 with a mean BMI prior to the treatments of 25.7 ± 2.4 kg/m2. The exclusion criteria included pregnancy, breastfeeding, any medical condition contraindicating the application of an electromagnetic field, heart disorders, unhealed wound in abdominal area, and any concomitant medication known to cause bloating or affect weight. See Table 1 for the baseline demographic profile. Patients were not financially incentivized for either participation or completion of the study; an informed consent was obtained from all of them. The study was conducted in compliance with applicable ethical standards and used an IRB approved protocol.
Table 1.
Baseline Demographic Profile of the Subjects
| Count | % | |
|---|---|---|
| Age | ||
| <30 | 6 | 27 |
| 30–40 | 4 | 18 |
| 40–50 | 7 | 32 |
| >50 | 5 | 23 |
| BMI | ||
| <18.5 (Underweight) | 0 | 0 |
| 18.5–24.9 (Normal) | 8 | 36 |
| 25.0–29.9 (Overweight) | 13 | 59 |
| >30.0 (Obese) | 1 | 5 |
| Gender | ||
| Female | 10 | 45 |
| Male | 12 | 55 |
| Ethnicity | ||
| Caucasian | 22 | 100 |
| Deliveries (10 patients) | 1.6 a |
Average nr. of childbirths.
Study Design
Prior to the treatments, each subject was inquired about his/her physical activity habits and an approximate daily caloric intake was calculated in cooperation with a professional nutritionist. All patients were asked to maintain their routine diet and activity level without any modifications until study completion. Afterwards, patients received four treatments (spaced by 2–5 days) using a HIFEM technology device (EMSCULPT, BTL Industries, Boston, MA) as per the IRB‐approved protocol.
EMSCULPT Procedure
During the application, patients did not receive any anesthesia and were lying in a supine position. All procedures were applied to the abdomen and each session included exactly 30 minutes of continuous application. One applicator (see Fig. 1) was placed on the skin at the umbilical level. The center of the magnetic coil was placed exactly above the navel. The applicator was affixed by a disinfected fixation belt to minimize movements during the procedure. The stimulation intensity started at 0% and within 60 seconds to the treatment it was slowly increased by the operator until reaching patient's tolerance threshold. The tolerance threshold was continuously challenged during the course of the treatments. A dual feedback principle was applied, with the operator visually checking the intensity and homogeneity of the muscle contractions across the abdomen, as well as regularly asking the patient about feedback regarding the level of comfort and the balance of contractions between different abdominal areas.
Figure 1.

Scheme of the EMSCULPT applicator.
Evaluation Methodology
A complete evaluation of the patients was performed at baseline and 2 months after their last treatment, and included a brief medical history and examination, magnetic resonance imaging (MRI) scan, weight and waist circumference measurements, digital photography, and monitoring of any adverse events. Due to financial constraints, only four randomly selected patients were scheduled for a 6‐month follow‐up to gain an insight into the tendencies the result may have in the long term.
MRI scans were used to observe changes in abdominal fat and muscle tissues of the treated patients. The scanned body volume was defined by T12 and S1 vertebrae and the array coil system was set up in such a way to minimize any pressure on patient's torso. The images were acquired using the BH‐Ax‐T2‐FIESTA and BH‐Ax‐T1‐FSPGR sequences. For each patient, lateral subumbilical and epiumbilical slices of the same sequence and of the same bodily section were extracted in cooperation with a qualified radiologist (experienced in reading abdominal scans), and the thickness of subcutaneous adipose tissue as well as rectus abdominis were measured (InVesalius 3.1). The measurements were taken in multiple points which were laid out laterally in the range between patient's iliac crests. Direct umbilical area was excluded from evaluation due to absence of the muscle structure (linea alba) and adipose layer (the navel). Furthermore, the size of abdominal separation was measured from the same MRI slices.
Gulick II spring‐loaded tape assisted measurements were taken 5 cm below umbilicus; patients had the most distinctive fat bulges in this region prior to the treatments. Frontal and lateral digital photography was taken; a positioning mat was used to ensure consistency.
All data collected prior to the treatments were compared with the follow‐up data; all results were tested for significance with a two‐sample paired t‐test. Descriptive data were presented as the mean and SD.
RESULTS
The Procedures
All 22 subjects completed the entire study. On average, 12.6 ± 2.5 days and 57.1 ± 8.6 days elapsed between the baseline and the last procedure, and between the last procedure and the follow‐up evaluation, respectively. Most patients tolerated stimulation intensities ranging between 90 and 100% already by the end of their first session or during their second session, depending on individual sensitivity. Minimum tolerable intensity was 74% (a patient with BMI 19.7), 17 out of 22 patients tolerated 100% intensity. Higher BMI patients tended to tolerate slightly higher intensity settings. No adverse events occurred. The only noticed side effect was mild muscle soreness 1 day after the first treatment reported by six patients; in all cases the soreness resolved itself within the next 24 hours. Overall the patients did not change their lifestyle or dietary intake significantly.
MRI Evaluation of Abdominal Tissues
The study average and individual patient changes in abdominal fat, abdominal muscle and diastasis are presented in Table 2 and Figure 2, respectively. On average a statistically significant improvement was observed in all three measurements when comparing the 2‐month follow‐up to the baseline—a reduction in adipose tissue thickness (−18.6%), an increase in rectus abdominis thickness (+15.4%) and a reduction in abdominal separation (−10.4%). In total 91 % (n = 20) of patients improved in all three facets simultaneously. The analysis did not show any non‐responding patients who would not have any changes in the tissue at all. No other structural changes in the tissues were observed.
Table 2.
Average Changes in Abdominal Tissues in Treated Subjects
| Measurement | Baseline | 2‐Month FU | Difference | P‐value |
|---|---|---|---|---|
| Muscle thickness [mm] | 11.1 ± 3.1 | 12.7 ± 3.3 | 1.6 ± 0.7 | P < 0.001 |
| Fat thickness [mm] | 23.6 ± 8.2 | 19.3 ± 7.6 | −4.3 ± 2.5 | P < 0.001 |
| Abdominal separation [mm] | 16.6 ± 7.2 | 14.9 ± 6.7 | −1.8 ± 1.5 | P < 0.001 |
| Waist circumference [cm] | 95.3 ± 6.6 | 91.5 ± 7.4 | −3.8 ± 2.1 | P < 0.001 |
| Weight [lb] | 175.8 ± 24.8 | 175.2 ± 24.3 | −0.5 ± 2.5 | P > 0.05 |
Figure 2.
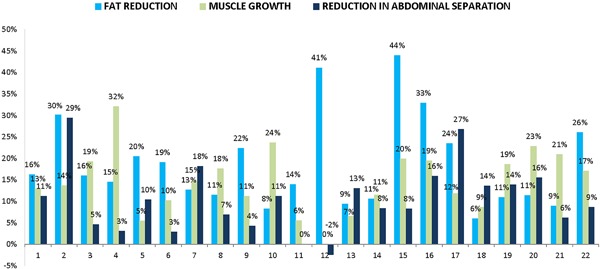
Changes (%) in abdominal tissues in individual patients. Reduction in subcutaneous adipose tissue thickness (light blue), growth in rectus abdominis thickness (dark blue), and reduction in abdominal separation (gray) are presented.
An increase in the abdominal muscle mass was observed in 95% (n = 21) of patients; one subject did not show any change. The muscle growth was relatively consistent, with majority of patients showing an increase in the range of 10–20% (see Fig. 3). The changes were calculated across both sides of the muscle; the difference in growth between the right and left rectus abdominis was insignificant. However, the distance (separation) between the left and right abdominal muscles decreased in 91% (n = 20) of patients; one patient did not show any change and for another patient the distance marginally increased (+0.26 mm or +2.4%). Contrary to our expectations, a subgroup of women who had previously been pregnant (n = 9) did not have higher values of abdominal separation before treatments (average 14.9 mm compared to 17.8 mm in other patients). They however did trend toward slightly greater proportional improvement (average reduction was 11.0% compared to 10.0% in the rest of the cohort). The percentage change in abdominal separation was independent of its severity (size) before treatments. Also, statistical analysis confirmed that the changes in muscle thickness and changes in abdominal separation were two highly independent effects (P > 0.05; correlation coefficient −0.31). MRI of subjects with major muscle growth thus did not necessarily reveal a major reduction in abdominal separation.
Figure 3.
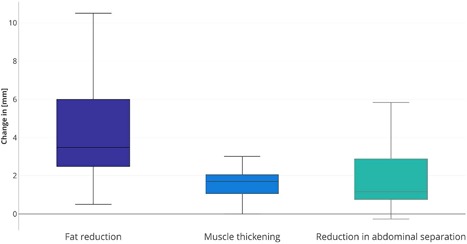
Plots show the median value, quartile values, as well as the maximum and minimum sample value with regards to changes in abdominal tissues of treated patients calculated from MRI scans. The changes represent a comparison between the baseline and the 2‐month follow‐up.
Measurements of the fat tissue revealed an opposite trend, with the average thickness decreasing in all patients. The reduction was slightly more variable than changes in the muscle (coefficient of var. 58.1%); this was primarily driven by two positive extremities. In total 82% (n = 18) of patients had the fat layer reduced by more than 10% at the follow‐up. More significant absolute changes were observed in subumbilical MRI cuts opposed to epiumbilical cuts.
For both the reduction in fat and reduction in abdominal separation, slightly more significant improvements were seen in patients with BMI classified as “normal” (18.5–24.9 kg/m2). Their subcutaneous fat mass decreased on average by 20.6%, and the size of diastasis decreased by 11.7%. For “overweight” patients (25.0–29.9 kg/m2) the same measurements averaged 18.1% and 10.0%, respectively.
6‐Month Data
Based on MRI evaluation, the muscle thickness continued to grow and the abdominal separation continued to shorten in all four randomly selected patients when compared to the 2‐month follow‐up. The average thickness in these patients evolved from 9.67 mm (baseline), to 11.38 mm (17.7% increase at 2 months), and on to 11.65 mm (20.5% increase at 6 months). The abdominal separation further improved from average of 12.95 mm (2 months) to 11.18 mm (6 months). In the same patients, the average thickness of subcutaneous fat was on average 3.03 mm lower (22.69 mm) at 6 months compared to the baseline (25.72 mm), see Figure 4.
Figure 4.
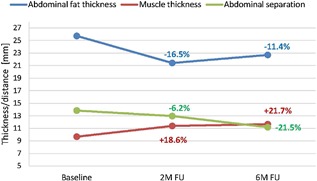
Average results of MRI evaluation at 6 months post‐treatments.
Other Evaluation
Compared to the baseline, the average subumbilical circumference of patients decreased by 3.8 ± 2.1 cm at the 2‐month follow‐up. The change was statistically independent of weight variations; the average weight remained stable. Digital photographs showed distinctive aesthetic improvements in all patients except for one. Examples of digital photographs linked with corresponding MRI images are shown in Figures 5 and 6.
Figure 5.
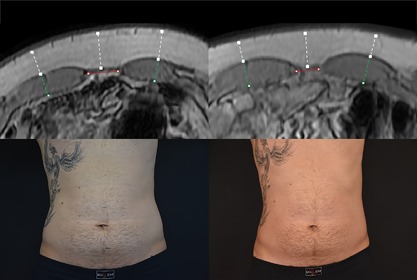
Magnetic resonance and digital images of Subject ID2 before (left) and 2 months post‐treatments (right). Male (30), BMI 24.8 kg/m2 (before) and 24.5 kg/m2 (2 months), weight −2.2 lb (−1.2 %), subcutaneous fat −30.3% (white markings), muscle thickness +13.7% (green markings), abdominal separation −24.9% (red markings), circumference −3 cm. Combination of the effects produced an overall visual improvement in patient's abdominal area.
Figure 6.
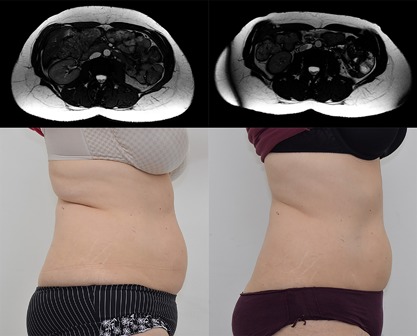
Magnetic resonance and digital images of Subject ID16 before (left) and 2 months post‐treatments (right). Female (52), BMI 25.1 kg/m2 (before) and 24.4 kg/m2 (2 months), weight −4.4 lb (−2.9%), subcutaneous fat −32.9%, muscle thickness +19.4 %, abdominal separation −15.9%, circumference −5.7 cm. Combination of the effects produced an overall visual improvement in patient's abdominal area.
DISCUSSISON
The findings from MRI scans presented herein have shown that application of the HIFEM technology on the abdomen can cause three different simultaneous changes in abdominal tissues non‐invasively. Visual improvement in patients’ appearance observed 2 months after their last treatment very much resemble the effects of non‐invasive heat or cold‐based fat reduction treatments combined with an extremely intensive physical workout.
The majority of therapeutic approaches aim at reducing the subcutaneous fat layer (either surgically or non‐invasively), yet none of the previous ones deal with strengthening of the muscular foundations. Currently, the only way to strengthen the core is a physical workout plan. The investigated device uses HIFEM technology to induce almost 20 thousand pulses in one 30‐minute session. Such frequency of nerve stimuli leads to supramaximal muscle contractions which are not achievable voluntarily. The muscle tissue is forced to adapt to this stress, resulting in muscle thickening. The principle of muscle hypertrophy and hyperplasia induced by intensive muscle contractions has already been proven in previous studies 15, 16, 17, 18, 19. The 6 month data suggest that the muscles continue to improve in longer term, both in terms of their overall mass and lateral separation, yet further investigation is necessary to better understand the exact physiology.
Research on high intensity muscle training has shown that a lipolytic reaction takes plan in fat tissue adjacent to the contracting muscle 20. The MRI scans presented herein show a reduction in adipose tissue not immediately after the treatments, but 2 months after the last procedure. A possible explanation for the lasting reduction in fat is that the lipolytic reaction is so intense, releasing large amount of free fatty acids (FFA) which intoxicate the adipocytes and trigger their death. This cell reaction has already been shown in multiple studies in other fields of medicine 21, 22, 23, 24. A recent histology study reported a significant increase in the apoptotic index of adipocytes after one HIFEM treatment on pigs (Weiss R, presented at ASLMS, Dallas TX, April 2018). Their observation was coupled with an increased presence of mRNA pro‐apoptotic markers in molecular biochemistry results, as well as with an increased concentration of FFA in blood serum. In addition, an increase of 91.7% (from 18.8 to 35.9) in the apoptotic index was calculated from 120 histological samples. This again suggests a potential relationship between FFA released after the muscle contractions and fat apoptosis, however this hypothesis requires further research as its validation was not the purpose of our study.
The third major observation, a reduction in abdominal separation, was rather variable. An 84.8% coefficient of variation shows that the response in patients differed significantly, from very little change to more dramatic reduction in the muscle distance. At the baseline, only one patient suffered from actual diastasis recti as per the medical definition (i.e., gap >2.7 cm) 25. Still 91% of subjects showed an improvement. This suggests that the application can not only help severely affected individuals, but is effective on most individuals regardless of their condition. This concept of reducing abdominal separation by using a magnetic field technology would deserve further investigation. In addition, there may be some role in prevention by intervention prior to reaching the medical definition of diastasis, although this would deserve further study as well.
Although the sample is not large enough for a detailed statistical analysis of fragmented sub‐groups, the data indicate that neither gender nor age affect the outcomes of the treatments. The fact that slightly more significant changes in abdominal tissues were observed in thinner rather than overweight patients can most likely be attributed to the intensity of the magnetic field which decreases with an increasing distance from the actual magnetic coil. For higher BMI patients, the distance between the coil and the motor neurons responsive to the current will tend to be much larger due to the interspacing fat deposits. Such patients might not achieve as intensive muscle contractions compared to normal BMI individuals. Despite the fact that inductive effects of HIFEM taper with distance, they can be felt from a distance of more than 7 cm from the actual applicator. Data from our study suggest that ideal candidates might be patients with less than an inch (2.5 cm) of a pinchable subcutaneous fat. Our 6‐month data suggest the tissue changes may last. However, due to the absence of any guidance in the literature on performing longer follow‐up studies, 4 to 6 months seem to be a reasonable time window for re‐invitation of patients to assess if any additional procedures may or may not be beneficial.
CONCLUSION
The aim of this study was not to establish conclusive evidence for the efficacy of the investigated device. To the best of our knowledge, no peer‐reviewed study has investigated a potential use of the HIFEM technology for non‐invasive body shaping. The data presented herein show an initial evaluation on 22 patients, and suggest possible physiological responses of the human body to the treatments. We may well conclude that the results have established a hypothesis of three simultaneous abdominal tissue effects induced as a direct result of the treatments, yet additional research is necessary to validate this in a larger controlled study, as well as in a histological study that would help further cast light on the exact mechanism of action that would explain our observations. If confirmed, the technology would represent a completely new approach to non‐invasive body shaping, bringing the additional muscle effects to the already established fat removal market.
[This article was modified on 16 October, 2018 after initial publication to correct the first author's affiliation and address.]
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and have disclosed the following: Brian Kinney MD is a medical advisor to BTL. Paula Lozanova MD has no conflicts to declare.
REFERENCES
- 1.The American Society for Aesthetic Plastic Surgery (2016). Cosmetic surgery national data bank statistics. http://www.surgery.org/sites/default/files/ASAPS‐Stats2016.pdf. Accessed April 25, 2017. [DOI] [PubMed]
- 2. Bernstein D, Farberg AS, Khorasani H, Kriegel D. Noninvasive body contouring: Literature review and summary of objective data. SKIN J Cutan Med 2017;1:18–31. [Google Scholar]
- 3. Muth ND. What are the guidelines for percentage of body fat loss. Am Counc Exerc ACE Ask Expert Blog 2009. https://www.acefitness.org/education-and-resources/lifestyle/blog/112/what-are-the-guidelines-for-percentage-of-body-fat-loss. [Google Scholar]
- 4. Wallis MC, Davies EA, Thalib L, Griffiths S. Pelvic static magnetic stimulation to control urinary incontinence in older women: A randomized controlled trial. Clin Med Res 2012;10:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ziemann U. Cortical threshold and excitability measurements In: Eisen A, editor. Handbook of Clinical Neurophysiology. vol. 4. Supplement C vols. Amsterdam, The Netherlands: Elsevier; 2004. pp 317–335. [Google Scholar]
- 6. Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 2000;527:633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Badawy RAB, Loetscher T, Macdonell RAL, Brodtmann A. Cortical excitability and neurology: Insights into the pathophysiology. Funct Neurol 2012;27:131–145. [PMC free article] [PubMed] [Google Scholar]
- 8. Rossi S, Hallett M, Rossini PM, Pascual‐Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 2009;120:2008–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bogataj U, Gros N, Kljajić M, Aćimović R, Maležič M. The rehabilitation of gait in patients with hemiplegia: A comparison between conventional therapy and multichannel functional electrical stimulation therapy. Phys Ther 1995;75:490–502. [DOI] [PubMed] [Google Scholar]
- 10. Currier DP, Mann R. Muscular strength development by electrical stimulation in healthy individuals. Phys Ther 1983;63:915–921. [DOI] [PubMed] [Google Scholar]
- 11. Han T‐R, Shin H‐I, Kim I‐S. Magnetic stimulation of the quadriceps femoris muscle: Comparison of pain with electrical stimulation. Am J Phys Med Rehabil 2006;85:593–599. [DOI] [PubMed] [Google Scholar]
- 12. Abulhasan JF, Rumble YLD, Morgan ER, Slatter WH, Grey MJ. Peripheral electrical and magnetic stimulation to augment resistance training. J Funct Morphol Kinesiol 2016;1:328–342. [Google Scholar]
- 13. Galloway NTM, El‐Galley RES, Sand PK, Appell RA, Russell HW, Carlan SJ. Extracorporeal magnetic innervation therapy for stress urinary incontinence. Urology 1999;53:1108–1111. [DOI] [PubMed] [Google Scholar]
- 14. Szecsi J, Schiller M, Straube A, Gerling D. A comparison of functional electrical and magnetic stimulation for propelled cycling of paretic patients. Arch Phys Med Rehabil 2009;90:564–570. [DOI] [PubMed] [Google Scholar]
- 15. Charette SL, et al. Muscle hypertrophy response to resistance training in older women. J Appl Physiol 1991;70:1912–1916. [DOI] [PubMed] [Google Scholar]
- 16. Schoenfeld BJ. The mechanisms of muscle hypertrophy and their application to resistance training. J Strength Cond Res 2010;24:2857–2872. [DOI] [PubMed] [Google Scholar]
- 17. Seynnes OR, de Boer M, Narici MV. Early skeletal muscle hypertrophy and architectural changes in response to high‐intensity resistance training. J Appl Physiol 2007;102:368–373. [DOI] [PubMed] [Google Scholar]
- 18. Alway SE, Grumbt WH, Gonyea WJ, Stray‐Gundersen J. Contrasts in muscle and myofibers of elite male and female bodybuilders. J Appl Physiol Bethesda Md 1985 1989;67:24–31. [DOI] [PubMed] [Google Scholar]
- 19. Marconnet P, Komi P. Muscular Function in exercise and training. 3rd International symposium on biological sciences in sport, nice, October/November 1986. Med Sport Sci 1987;26:67–89. [Google Scholar]
- 20. Stallknecht B, Dela F, Helge JW. Are blood flow and lipolysis in subcutaneous adipose tissue influenced by contractions in adjacent muscles in humans? Am J Physiol Endocrinol Metab 2007;292:E394–E399. [DOI] [PubMed] [Google Scholar]
- 21. Hardy S, El‐Assaad W, Przybytkowski E, Joly E, Prentki M, Langelier Y. Saturated fatty acid‐induced apoptosis in MDA‐MB‐231 breast cancer cells. A role for cardiolipin. J Biol Chem 2003;278:31861–31870. [DOI] [PubMed] [Google Scholar]
- 22. Gunduz F, Aboulnasr FM, Chandra PK, et al. Free fatty acids induce ER stress and block antiviral activity of interferon alpha against hepatitis C virus in cell culture. Virol J 2012;9:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo W, Wong S, Xie W, Lei T, Luo Z. Palmitate modulates intracellular signaling, induces endoplasmic reticulum stress, and causes apoptosis in mouse 3T3‐L1 and rat primary preadipocytes. Am J Physiol Endocrinol Metab 2007;293:E576–E586. [DOI] [PubMed] [Google Scholar]
- 24. Zhang Y, Xue R, Zhang Z, Yang X, Shi H. Palmitic and linoleic acids induce ER stress and apoptosis in hepatoma cells. Lipids Health Dis 2012;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Benjamin DR, Van de Water ATM, Peiris CL. Effects of exercise on diastasis of the rectus abdominis muscle in the antenatal and postnatal periods: A systematic review. Physiotherapy 2014;100:1–8. [DOI] [PubMed] [Google Scholar]


