Abstract
This randomized, phase 1, single‐dose, crossover study (NCT02189304) compared the 12‐hour pharmacokinetic (PK) and safety profiles of budesonide/glycopyrronium/formoterol fumarate dihydrate metered dose inhaler (BGF MDI) 320/14.4/10 μg and budesonide/formoterol fumarate dihydrate (BFF) MDI 320/10 μg (both formulated using innovative co‐suspension delivery technology) to an active comparator (budesonide/formoterol fumarate dihydrate dry powder inhaler [BUD/FORM DPI] 320/9‐μg delivered dose) in healthy adults. The potential for PK interaction between glycopyrronium and budesonide/formoterol within BGF MDI was assessed. Of 72 subjects randomized, 59 completed treatment. Systemic budesonide exposure (primary objective) based on area under the plasma drug concentration‐time curve 0‐12 hours (AUC0‐12; % coefficient of variation) was 1598.38 (49.7), 1657.09 (50.4), and 1276.75 (70.4) pg·h/mL for BGF MDI, BFF MDI, and BUD/FORM DPI, respectively; and formoterol exposure (AUC0‐12 [% coefficient of variation]) was 39.16 (45.9), 39.53 (40.5), and 23.24 (59.2) pg·h/mL, respectively. BGF MDI and BFF MDI were bioequivalent for budesonide and formoterol. All treatments were well tolerated. While systemic exposure to budesonide and formoterol was higher for BGF MDI and BFF MDI than for BUD/FORM DPI, there were no appreciable differences in the incidence of pharmacologically predictable adverse events. This, coupled with the absence of PK interactions, suggests the BGF MDI safety profile will be comparable to BUD/FORM DPI.
Keywords: pharmacokinetics, metered dose inhaler, fixed‐dose combination, co‐suspension delivery technology, COPD
As a major cause of morbidity and the third leading cause of death worldwide in 2010, chronic obstructive pulmonary disease (COPD) exerts a significant health and economic burden on society.1, 2 The prevalence and impact of COPD is also projected to increase in the coming years.3 Pharmacologic treatment strategies for COPD center on the management of symptoms, the reduction in risk for (and resolution of) acute exacerbations, and the overall improvement of health status and exercise tolerance.4 Long‐acting bronchodilators, alone or in combination with inhaled corticosteroids (ICS), have been widely used for the treatment of COPD for many years. Triple therapy with an ICS, a long‐acting muscarinic antagonist (LAMA), and a long‐acting β2‐agonist (LABA) is recommended for symptomatic patients who have had, or are at risk for, exacerbations on dual therapies with LAMA/LABA or ICS/LABA.4, 5, 6 Triple therapy has been shown to improve lung function and health status and to reduce the frequency of moderate‐to‐severe exacerbations compared with ICS/LABA dual therapy7, 8, 9, 10 and LAMA monotherapy.11
As the complexity of COPD treatment increases, the practical benefits of combining all medications within a single inhaler have become apparent. Fixed‐dose combinations of different drugs in a single inhaler improves adherence and, thereby, may reduce morbidity and mortality in COPD12, 13, 14 and prevent the selective use or discontinuation of one or more of the components.
The budesonide/glycopyrronium/formoterol fumarate dihydrate metered dose inhaler (BGF MDI) is a fixed‐dose combination of ICS/LAMA/LABA in clinical development for the treatment of COPD. BGF MDI has been formulated using innovative co‐suspension delivery technology, which has been shown to provide consistent drug delivery with similar in vitro profiles, regardless whether a drug is administered alone or in combination with one or more other drugs.15, 16, 17
Glycopyrronium is a LAMA that binds to muscarinic receptors with high affinity and has a prolonged dissociation profile. Inhaled glycopyrronium causes long‐lasting bronchodilation in patients with COPD.18 The major metabolic pathway of glycopyrronium involves hydroxylation of the cyclopentyl ring and oxidation of the hydroxyl group in the mandelic acid residue.19 However, most (80%) of intravenously administered glycopyrronium is excreted unmetabolized in humans.19 In vivo studies of glycopyrronium metabolism have demonstrated rapid clearance (>90% in 5 minutes) from serum and secretion from urine (85% in 48 hours).19 Approximately 52.6% of the inhaled dose of glycopyrronium is absorbed through the lungs by a slow phase absorption process.20 Estimates of the mean terminal elimination half‐life (t½) of inhaled glycopyrronium vary, ranging from 13‐22 hours based on a noncompartmental analysis,21 to 50‐60 hours based on a population pharmacokinetic (PK) modeling approach.20
Formoterol fumarate is a LABA bronchodilator that binds to β2‐adrenergic receptors in bronchial smooth muscle tissue. Following oral inhalation, formoterol is rapidly absorbed into the plasma. Formoterol is metabolized by direct glucuronidation, and O‐demethylation by four cytochrome P450 (CYP) enzymes (CYP2D6, CYP2C9, CYP2C19, and CYP2A6). Formoterol is excreted in the urine (59%‐62%) and feces (32%‐34%), with approximately 10% excreted as unchanged formoterol. The mean t½ of formoterol is 10 hours.22
Budesonide is a synthetic glucocorticosteroid that is metabolized primarily by CYP3A4 into its 2 major metabolites, 16α‐hydroxyprednisolone and 6β‐hydroxybudesonide, which are primarily excreted in the urine.23, 24 As the metabolism of budesonide is primarily mediated by CYP3A4, CYP3A4 inhibitors may potentially affect the metabolism of budesonide.
In this phase 1, single‐dose, randomized, single‐center, crossover study, we compared the PK and safety profiles of BGF MDI and budesonide/formoterol fumarate dihydrate MDI (BFF MDI) with that of an active control (budesonide/formoterol fumarate [BUD/FORM], a dry powder inhaler [DPI] containing a fixed‐dose combination of budesonide and formoterol fumarate dihydrate [Symbicort Turbohaler; AstraZeneca; 160/4.5‐μg delivered dose; 200/6‐μg metered dose per inhalation]) that is approved for the treatment of COPD in many countries worldwide.
The primary objective of this study was to examine the 12‐hour PK profile of budesonide following administration of 3 single‐inhaler, multicomponent drug formulations—BGF MDI 320/14.4/10 μg, BFF MDI 320/10 μg, and BUD/FORM DPI 320/9 μg—to healthy volunteers. Examination of the 12‐hour PK profile of formoterol was a secondary objective. In addition, the budesonide and formoterol PK profiles of BGF MDI and BFF MDI were compared in order to determine whether there was evidence for PK interactions between each component and glycopyrronium in BGF MDI. Safety and tolerability outcomes were evaluated across all treatments.
Subjects and Methods
Study Population
The study population comprised healthy male and female subjects (aged 18‐55 years) with a body weight ≥50 kg and a body mass index of 18.5‐32.0 kg/m2. Health status was confirmed by a thorough medical history and physical examination, electrocardiogram (ECG), vital signs, and clinical laboratory evaluation.
Subjects with clinically significant medical conditions (in the opinion of the investigator), including symptomatic prostatic hypertrophy, bladder neck obstruction, urinary retention, and inadequately treated glaucoma, or with a history of ECG abnormalities, were excluded from participation in the study, as were subjects with a history of smoking (within 3 months of screening) or substance‐related disorders (within 1 year of screening). Use of nicotine‐containing products within 3 months of screening or treatment with any prescription or nonprescription drug within 28 days or 5 half‐lives (whichever was longer) before the start of study treatment, was prohibited. Pregnant and nursing females were excluded from the study, and all subjects of child‐bearing potential or subjects with partners of child‐bearing potential were required to use appropriate contraception for the duration of the study. Subjects unable to use the MDI correctly, including coordinating actuation with inhalation, were also excluded.
Study Design and Treatments
This was a phase 1, single‐dose, randomized, double‐blind, 3‐period, 3‐treatment, Williams crossover design25 study conducted in healthy adult subjects at a single study site (Pharmaron Clinical Pharmacology Center [formerly SNBL Clinical Pharmacology Center]) in Baltimore, Maryland (ClinicalTrials.gov identifier: NCT02189304). The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines and applicable regulatory requirements. An institutional review board (IntegReview IRB, Austin, Texas) approved the protocol and informed consent form prior to initiation of the study. All subjects provided written informed consent before any protocol‐specific screening procedures were performed.
After a screening period of up to 27 days, eligible subjects were randomly assigned to 1 of 6 treatment sequences, each comprising a single dose (delivered as 2 actuations for all treatments) of BGF MDI 320/14.4/10‐μg ex‐actuator (equivalent to budesonide/glycopyrrolate/formoterol fumarate 320/18/9.6‐μg ex‐actuator), BFF MDI 320/10‐μg ex‐actuator (equivalent to budesonide/formoterol fumarate 320/9.6‐μg ex‐actuator), and BUD/FORM DPI (2 inhalations using the 200/6‐μg inhaler, corresponding to a total delivered dose of budesonide/formoterol fumarate dihydrate 320/9 μg).26 The BGF MDI and BFF MDI were identical in form and function, and this study was double blind, except for BUD/FORM DPI, which was provided open label. Qualified site staff were present at the time of dosing to ensure that the 2 actuations of the MDI and DPI devices were properly administered by the subjects. Subjects were required to wear a surgical mask for approximately 30 minutes before and 30 minutes after dosing to prevent cross‐contamination.
For each treatment period, subjects reported to the clinic on the day before dosing and were discharged on the dosing day after all scheduled assessments were completed. Each inpatient treatment period was separated by an outpatient washout period of 7‐14 days. Subjects were required to fast for at least 4 hours prior to collection of the first blood sample, 60 minutes before dosing. Meals during the dosing day of each treatment period were standardized after the 4‐hour postdose blood sample had been taken. There were no restrictions on clear fluid intake; however, subjects were not allowed to consume grapefruit or grapefruit juice throughout the study and were not allowed xanthine‐containing foods and beverages (eg, coffee, tea, chocolate, and cola) for at least 6 hours prior to each study visit and for the duration of each study visit.
Pharmacokinetic Evaluation
Whole blood samples (approximately 10 mL) were collected 30 minutes before dosing and at 2, 6, 20, and 40 minutes, and 1, 2, 4, 8, 10, and 12 hours after dosing for each treatment period. Plasma was harvested and stored at ≤–60°C. Budesonide, glycopyrronium, and formoterol plasma concentrations were determined by Tandem Laboratories (Salt Lake City, Utah) using high‐performance liquid chromatography tandem mass spectrometry methods validated for measuring these analytes in human plasma (tripotassium ethylenediaminetetraacetic acid; see the Supplementary material for a complete description of the method).
The following PK parameters were estimated for all treatments: maximum observed plasma concentration (Cmax), time to Cmax (tmax), apparent t½, area under the plasma drug concentration‐time curve (AUC) from 0 to the last measurable plasma concentration (AUC0‐t), AUC from 0 to 12 hours after dose (AUC0‐12), AUC from 0 extrapolated to infinity (AUC0‐∞), and apparent total body clearance (Cl/F).
Safety Evaluation
Safety was evaluated by adverse event (AE) reporting and findings from physical examination, vital signs (including blood pressure, heart rate, respiratory rate, and body temperature), clinical laboratory tests (including hematology, biochemistry, and urinalysis), and 12‐lead ECGs. Clinical assessments were conducted at screening, on the day of clinic admission, and up to 12 hours after dosing on each treatment day. The severity and relationship to study drug of all AEs was determined by the investigator.
Statistical Analyses
PK analyses were performed on the PK population, which included all subjects in the safety population with sufficient data to reliably calculate at least 1 PK parameter for BGF MDI, BFF MDI, or BUD/FORM DPI, and who did not have major protocol deviations. The safety population included all subjects who received at least 1 dose of any study medication.
The primary objective of this study was to compare the 12‐hour PK profiles of budesonide after a single dose of BGF MDI or BFF MDI with that after a single dose of BUD/FORM DPI in healthy subjects. Secondary PK objectives included a comparison of the 12‐hour PK profiles of formoterol after treatment with BGF MDI or BFF MDI with BUD/FORM DPI and a comparison of the safety and tolerability of BGF MDI and BFF MDI versus BUD/FORM DPI. The potential for a PK interaction between glycopyrronium and either budesonide or formoterol was also assessed by comparing the budesonide and formoterol 12‐hour PK profiles following treatment with BGF MDI and BFF MDI.
PK parameters were estimated from the budesonide, glycopyrronium, and formoterol plasma concentration data by noncompartmental analysis (NCA) using the software Phoenix WinNonlin (Certara, L.P., Princeton, New Jersey). Actual blood sample collection times relative to dosing were used for the NCA. Mean plots of plasma concentration time data used nominal sample collection times. Postdose samples with a plasma concentration below the lower limit of quantification were treated as missing in the NCA analysis; however, for mean and individual plasma concentration profile plots, they were assigned a value of zero. Predose samples with a concentration of budesonide, glycopyrronium, or formoterol below the lower limit of quantification were also set to zero.
Descriptive statistics of the PK parameters were summarized, by treatment, for the PK population. Budesonide and formoterol Cmax, AUC0‐12, AUC0‐t, and AUC0‐∞ for the BGF MDI and BFF MDI treatments were compared with BUD/FORM DPI using natural log‐transformed values and an analysis of variance (ANOVA) with fixed effects for treatment (1 degree of freedom), period, sequence, and subject within sequence. A separate ANOVA model was fitted for each pair of compared treatments, and only subjects who had data for both treatments (for the relevant PK parameter) for the respective analyte were included. The ratios of geometric least squares means (LSM) and the corresponding 90%CIs for each treatment comparison were determined by exponentiation of the mean differences between treatments and 90%CI on the logarithm scale. Bioequivalence between BGF MDI or BFF MDI and BUD/FORM DPI was determined by comparing the 90%CI for the geometric mean ratio to bounds of 80% and 125% for budesonide and formoterol for Cmax, AUC0‐12, AUC0‐t, and AUC0‐∞. Budesonide and formoterol Cmax and AUC0‐12 were similarly compared between BGF MDI and BFF MDI to evaluate the potential for a PK interaction.
A sample size of 72 subjects was planned for randomization into the study to provide approximately 90% and 80% probability to demonstrate bioequivalence of budesonide AUC0‐12 and Cmax, respectively, using a 90%CI for the geometric mean ratio and bounds of 80% to 125% for the comparison of BGF MDI or BFF MDI to BUD/FORM DPI, under assumptions of a 5% true difference and intrasubject coefficient of variation (CV%) values of 30% and 35% for AUC0‐12 and Cmax, respectively.
Results
Study Population
A total of 203 subjects were screened and 72 (35.5%) were randomly assigned to receive study treatment; of those, 59 (81.9%) completed the study. Thirteen subjects discontinued early from the study due to withdrawal of consent (7 subjects), protocol deviations (5 subjects), or loss to follow‐up (1 subject). Of the 72 randomly assigned subjects, 64 (88.9%) received BGF MDI 320/14.4/10 μg, 66 (91.7%) received BFF MDI 320/10 μg, and 66 (91.7%) received BUD/FORM DPI. All 72 subjects (100%) were included in the safety and PK populations.
The demographics and baseline characteristics of the randomized subjects are shown in Table 1. The median age of the overall study population was 32.0 years (range: 19‐55 years), and 52 subjects (72.2%) were male. The majority of study participants (86.1%) were black, representative of the local community around the single study site. Most (88.9%) had never smoked; 8 (11.1%) were former smokers. There were no clinically relevant differences among patients according to treatment group.
Table 1.
Subject Demographics and Characteristics (Safety Population)
| Parameter | BGF MDI 320/14.4/10 μg (n = 64) | BFF MDI 320/10 μg (n = 66) | BUD/FORM DPI 320/9 μg (n = 66) | Any Treatment (N = 72) |
|---|---|---|---|---|
| Median age, years (range) | 32.0 (19‐55) | 32.0 (19‐55) | 31.5 (19‐55) | 32.0 (19‐55) |
| Sex, % male | 68.8 | 71.2 | 71.2 | 72.2 |
| Race, % Black/White/Other | 85.9/12.5/1.6 | 84.8/13.6/1.5 | 87.9/10.6/1.5 | 86.1/12.5/1.4 |
| Smoking status, % never smoked | 87.5 | 89.4 | 89.4 | 88.9 |
| Mean weight, kg (SD) | 75.62 (11.32) | 75.56 (11.34) | 75.84 (11.50) | 76.10 (11.19) |
| Mean height, cm (SD) | 172.01 (8.94) | 172.30 (9.01) | 172.78 (9.37) | 172.75 (9.03) |
| Mean BMI, kg/m2 (SD) | 25.5 (3.4) | 25.4 (3.2) | 25.4 (3.2) | 25.5 (3.2) |
BFF, budesonide/formoterol fumarate dihydrate; BGF, budesonide/glycopyrronium/formoterol fumarate dihydrate; BMI, body mass index; BUD/FORM, budesonide/formoterol fumarate dihydrate; DPI, dry powder inhaler; MDI, metered dose inhaler; SD, standard deviation.
Comparison of Budesonide in BGF MDI, BFF MDI, and BUD/FORM DPI
The AUC profiles for budesonide showed an early peak followed by a monophasic decline in budesonide concentration (Figure 1A). The PK parameters for budesonide are presented in Table 2, and as geometric means in Supplementary Table 1. Mean AUC0‐12 and AUC0‐t were comparable for BGF MDI (1762 and 1763 pg·h/mL, respectively) and BFF MDI (both 1827 pg·h/mL), and were higher (both 1517 pg·h/mL) than for BUD/FORM DPI. Cmax ranged from 505‐595 pg/mL for the 3 treatments. The range of tmax was also similar for all 3 treatments (0.10‐4.00 hours, 0.03‐4.00 hours, and 0.03‐2.00 hours for BGF MDI, BFF MDI, and BUD/FORM DPI, respectively). The Cl/F of budesonide was higher with BUD/FORM DPI than with BGF MDI or BFF MDI. However, it should be noted that there was a larger CV% reported on the geometric means of the budesonide AUC PK parameters and Cmax for BUD/FORM DPI compared with both BGF MDI and BFF MDI (Supplementary Table 1).
Figure 1.
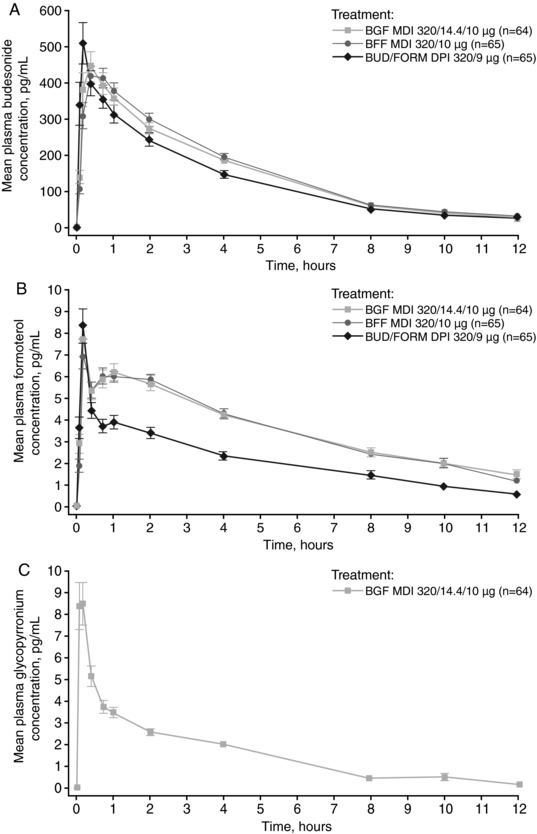
Arithmetic mean (±SE) plasma drug concentration‐time profile of (A) budesonide, (B) formoterol, and (C) glycopyrronium (PK population). BFF, budesonide/formoterol fumarate dihydrate; BGF, budesonide/glycopyrronium/formoterol fumarate dihydrate; BUD/FORM, budesonide/formoterol fumarate dihydrate; DPI, dry powder inhaler; MDI, metered dose inhaler; PK, pharmacokinetic; SE, standard error.
Table 2.
PK Parameters for Budesonide and Formoterol According to Treatmenta (PK Population)
| Parameter | BGF MDI 320/14.4/10 μg | BFF MDI 320/10 μg | BUD/FORM DPI 320/9 μg |
|---|---|---|---|
| AUC0‐12, pg·h/mL | |||
| Budesonide | 1762.47 (753.98), n = 64 | 1827.17 (738.71), n = 65 | 1516.86 (825.89), n = 65 |
| Formoterol | 42.86 (18.15), n = 60 | 42.34 (14.89), n = 62 | 26.73 (14.34), n = 58 |
| AUC0‐t, pg·h/mL | |||
| Budesonide | 1762.72 (754.16), n = 64 | 1826.72 (738.52), n = 65 | 1517.11 (826.15), n = 65 |
| Formoterol | 41.63 (19.46), n = 60 | 41.34 (15.63), n = 62 | 24.20 (16.05), n = 58 |
| Cmax, pg/mL | |||
| Budesonide | 528.91 (347.50), n = 64 | 505.48 (264.92), n = 65 | 595.11 (511.94), n = 65 |
| Formoterol | 9.36 (4.98), n = 60 | 8.34 (3.61), n = 62 | 8.39 (5.42), n = 58 |
| tmax, hoursa | |||
| Budesonide | 0.33 (0.10‐4.00), n = 64 | 0.67 (0.03‐4.00), n = 65 | 0.33 (0.03‐2.00), n = 65 |
| Formoterol | 0.67 (0.10‐12.00), n = 60 | 0.67 (0.10‐8.00), n = 62 | 0.10 (0.03‐2.00), n = 58 |
| t½, hours | |||
| Budesonide | 3.07 (0.42), n = 64 | 3.07 (0.64), n = 65 | 3.04 (0.47), n = 64 |
| Formoterol | 5.13 (2.23), n = 40 | 5.20 (2.51), n = 47 | 5.19 (1.78), n = 28 |
| Cl/F, L/hour | |||
| Budesonide | 207.34 (125.64), n = 64 | 203.54 (116.62), n = 64 | 361.83 (268.79), n = 64 |
| Formoterol | 204.77 (55.15), n = 17 | 184.83 (40.71), n = 21 | 251.90 (75.18), n = 10 |
AUC0‐12, area under the plasma drug concentration‐time curve from 0‐12 hours; AUC0‐t, area under the plasma drug concentration‐time curve up to the last measurable plasma concentration; BFF, budesonide/formoterol fumarate dihydrate; BGF, budesonide/glycopyrronium/formoterol fumarate dihydrate; BUD/FORM, budesonide/formoterol fumarate dihydrate; Cmax, maximum observed plasma concentration; Cl/F, apparent total body clearance; DPI, dry powder inhaler; MDI, metered dose inhaler; PK, pharmacokinetic; SD, standard deviation; t½, apparent terminal elimination half‐life; Tmax, time to maximum observed plasma concentration.
All pharmacokinetic values are expressed as the arithmetic mean (SD) with the exception of Tmax, which is expressed as the median value (min‐max).
Statistical comparison of budesonide PK parameters between BGF MDI and BUD/FORM DPI and between BFF MDI and BUD/FORM DPI are shown in Table 3. The AUC0‐12 and AUC0‐t geometric LSM ratios indicated that the AUCs were approximately 25% and 27% higher for BGF MDI and BFF MDI, respectively, compared with BUD/FORM DPI. Although the budesonide Cmax geometric LSM ratios were similar, the 90%CIs were just outside of the bioequivalence bounds of 80% and 125%, which was likely due to the high intrasubject CV% (84%‐92%) induced by BUD/FORM DPI.
Table 3.
Comparison of Budesonide and Formoterol PK Parameters for BGF MDI or BFF MDI versus BUD/FORM DPI (PK Population)
| Geometric LSM | Treatment Comparisonsa | ||||
|---|---|---|---|---|---|
| BGF MDI 320/14.4/10 μg vs BUD/FORM DPI 320/9 μg | BGF MDI 320/14.4/10 μg | BUD/FORM DPI 320/9 μg | Geometric LSM Ratio (%) | 90%CI | Intrasubject CV%b |
| Budesonide | |||||
| n | 64 | 65 | – | – | – |
| AUC0‐12, pg·h/mL | 1618.79 | 1293.75 | 125.12 | 106.81‐146.58 | 55.4 |
| AUC0‐t, pg·h/mL | 1618.96 | 1293.87 | 125.12 | 106.81‐146.58 | 55.4 |
| AUC0‐∞, pg·h/mL | 1757.19 | 1397.58c | 125.73 | 107.52‐147.02 | 54.0 |
| Cmax, pg/mL | 426.27 | 416.58 | 102.32 | 80.50‐130.07 | 92.2 |
| Formoterol | |||||
| n | 60 | 58 | – | – | – |
| AUC0‐12, pg·h/mL | 39.21 | 23.70 | 165.45 | 141.47‐193.49 | 49.2 |
| AUC0‐t, pg·h/mL | 36.53 | 18.68 | 195.55 | 156.21‐244.80 | 74.9 |
| Cmax, pg/mL | 8.44 | 6.73 | 125.45 | 101.97‐154.34 | 67.9 |
| BFF MDI 320/10 μg vs BUD/FORM DPI 320/9 μg | BFF MDI 320/10 μg | BUD/FORM DPI 320/9 μg | Geometric LSM Ratio (%) | 90%CI | Intrasubject CV% b |
|---|---|---|---|---|---|
| Budesonide | |||||
| n | 65 | 65 | – | – | – |
| AUC0‐12, pg·h/mL | 1650.19 | 1296.94 | 127.24 | 109.86‐147.37 | 50.6 |
| AUC0‐t, pg·h/mL | 1649.95 | 1297.12 | 127.20 | 109.83‐147.32 | 50.6 |
| AUC0‐∞, pg·h/mL | 1789.85c | 1404.59c | 127.43 | 109.94‐147.70 | 50.0 |
| Cmax, pg/mL | 432.41 | 416.49 | 103.82 | 82.95‐129.95 | 83.9 |
| Formoterol | |||||
| n | 62 | 58 | – | – | – |
| AUC0‐12, pg·h/mL | 39.32 | 23.23 | 169.24 | 145.75‐196.52 | 47.6 |
| AUC0‐t, pg·h/mL | 37.77 | 18.57 | 203.36 | 163.24‐253.34 | 74.5 |
| Cmax, pg/mL | 7.87 | 6.55 | 120.19 | 97.83‐147.66 | 68.8 |
ANOVA, analysis of variance; AUC0‐12, area under the plasma drug concentration‐time curve from 0‐12 hours; AUC0‐t, area under the plasma drug concentration‐time curve up to the last measurable concentration; AUC0‐∞, area under the plasma drug concentration‐time curve from zero extrapolated to infinity; BFF, budesonide/formoterol fumarate dihydrate; BGF, budesonide/glycopyrronium/formoterol fumarate dihydrate; BUD/FORM, budesonide/formoterol fumarate dihydrate; CI, confidence interval; Cmax, maximum observed plasma concentration; CV%, coefficient of variation; DPI, dry powder inhaler; LSM, least squares mean; MDI, metered dose inhaler; MSE, mean square error; PK, pharmacokinetic.
Ratio (expressed as percentage) of exponentiated mean difference of log‐transformed PK parameter. Confidence interval from ANOVA model with fixed effects for treatment (1 degree of freedom), period, sequence, and subject within sequence. A statistical comparison of AUC0‐∞ was not performed for formoterol, as this parameter was only estimable in a low proportion of subjects.
100*√[exp(MSE) ‐ 1].
n = 64.
Comparison of Formoterol in BGF MDI, BFF MDI, and BUD/FORM DPI
The AUC profiles of formoterol for all 3 treatments were similar in shape and showed an early high peak followed by a low, broader peak and a monophasic decline (Figure 1B). AUC0‐12 and AUC0‐t were comparable for BGF MDI and BFF MDI, both of which were higher than for BUD/FORM DPI (Table 2; Supplementary Table 1). In addition, tmax was achieved more rapidly with BUD/FORM DPI (0.1 hours) compared with BGF MDI and BFF MDI (both 0.67 hours). Of note, there were appreciably larger CV%s reported on the geometric means for formoterol PK parameters for BUD/FORM DPI than for either BGF MDI or BFF MDI (Supplementary Table 1).
Statistical comparison of formoterol PK parameters between BGF MDI and BUD/FORM DPI and between BFF MDI and BUD/FORM DPI are shown in Table 3. The geometric LSM ratios for AUC0‐12 and AUC0‐t indicated that exposure to formoterol with BGF MDI and BFF MDI was 65% and 69% higher, respectively (AUC0‐12), and 96% and 103% higher, respectively (AUC0‐t), compared with BUD/FORM DPI. In addition, Cmax was increased by 25% and 20% with BGF MDI and BFF MDI, respectively, compared with BUD/FORM DPI. The 90%CIs for formoterol Cmax, AUC0‐12, and AUC0‐t geometric LSM ratios all fell outside of the bioequivalence bounds of 80% and 125%, indicating that BGF MDI and BFF MDI were not bioequivalent with BUD/FORM DPI with respect to the formoterol component.
Pharmacokinetic Interaction
The plasma concentration‐time profile for glycopyrronium following BGF MDI treatment is shown in Figure 1C. The glycopyrronium geometric mean Cmax and AUC0‐12 values (CV%) were 7.36 pg/mL (88.1) and 19.73 pg·h/mL (48.8) respectively (N = 53). The relative bioavailability of budesonide in BGF MDI versus BFF MDI, based on Cmax and AUC0‐12, is shown in Figure 2A. The geometric LSM ratio was approximately 100% for both PK parameters, and the 90%CIs for both geometric LSM ratios were within the prespecified bounds of 80% and 125%, indicating that the 2 formulations were bioequivalent for the budesonide component and that there was no drug‐drug interaction between glycopyrronium and budesonide. The observed intrasubject CV%s for these budesonide PK parameters were 26% (AUC0‐12) and 40% (Cmax), which were considerably lower than those observed for the comparisons relative to BUD/FORM DPI. Comparison of the same PK parameters for formoterol showed that the 90%CIs for the geometric LSM ratios were also within the 80% and 125% bounds, indicating that BGF MDI and BFF MDI were also bioequivalent for formoterol (Figure 2B). Overall, these results indicate that there was no drug‐drug interaction between glycopyrronium and formoterol when combined in BGF MDI.
Figure 2.
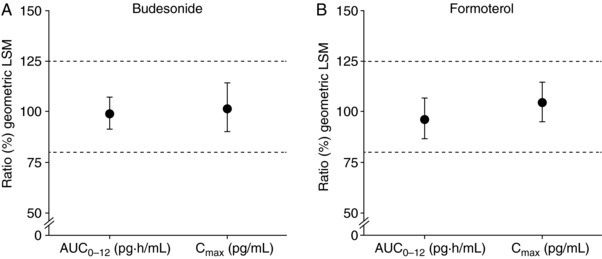
Relative bioequivalence of (A) budesonide and (B) formoterol in BGF MDI and BFF MDI (ratio of geometric least squares means [90%CI] for AUC0‐12 and Cmax; PK population). AUC0‐12, area under the plasma drug concentration‐time curve from 0‐12 hours; BFF, budesonide/formoterol fumarate dihydrate; BGF, budesonide/glycopyrronium/formoterol fumarate dihydrate; CI, confidence interval; Cmax, maximum observed plasma concentration; LSM, least squares mean; MDI, metered dose inhaler; PK, pharmacokinetic.
Safety
Overall, 24 subjects (33.3%) reported a total of 32 treatment‐emergent AEs (TEAEs) during the study. Of these, 22 TEAEs reported by 18 subjects (25%) were considered related to study drug (Table 4). All of the drug‐related TEAEs were mild in severity; there were no serious AEs, and no TEAE led to early withdrawal from the study. The incidence of individual TEAEs reported in ≥2 subjects is shown in Table 4.
Table 4.
Summary of TEAEs (Safety Population)
| Parameter | BGF MDI 320/14.4/10 μg (n = 64) | BFF MDI 320/10 μg (n = 66) | BUD/FORM DPI 320/9 μg (n = 66) | All Subjects (N = 72) |
|---|---|---|---|---|
| Subjects with at least 1 TEAE, n (%) | 8 (12.5) | 13 (19.7) | 9 (13.6) | 24 (33.3) |
| Subjects with TEAE related to study drug, n (%)a | 7 (10.9) | 9 (13.6) | 5 (7.6) | 18 (25.0) |
| TEAEs occurring in ≥2 subjects, n (%) | ||||
| Headache | 3 (4.7) | 0 | 1 (1.5) | 4 (5.6) |
| Hypertension | 0 | 2 (3.0) | 2 (3.0) | 4 (5.6) |
| Systolic hypertension | 0 | 2 (3.0) | 0 | 2 (2.8) |
| Blood pressure (diastolic) decreased | 1 (1.6) | 0 | 1 (1.5) | 2 (2.8) |
| Dizziness | 1 (1.6) | 0 | 1 (1.5) | 2 (2.8) |
| Hypokalemia | 0 | 2 (3.0) | 0 | 2 (2.8) |
BFF, budesonide/formoterol fumarate dihydrate; BGF, budesonide/glycopyrronium/formoterol fumarate dihydrate; BUD/FORM, budesonide/formoterol fumarate dihydrate; DPI, dry powder inhaler; MDI, metered dose inhaler; TEAE, treatment‐emergent adverse event.
Related = possibly, probably, or definitely (in the opinion of the investigator).
No clinically significant changes over time or differences among the treatments in clinical laboratory results, vital signs, or ECGs were observed during the study. Mean values for hematology and clinical chemistry were generally within the normal range. Two subjects had low postbaseline potassium concentrations after administration of BFF MDI (2.5 and 3.1 mmol/L) that were reported as hypokalemia. Both events resolved on the same day without treatment.
Discussion
The primary objective of this phase 1, randomized, crossover study was to compare the budesonide 12‐hour PK profiles of single doses of BGF MDI 320/14.4/10 μg with BFF MDI 320/10 μg and an active control, BUD/FORM DPI (320/9‐μg delivered dose), in healthy volunteers. Comparison of the budesonide PK parameters showed that Cmax values were similar for BGF MDI, BFF MDI, and BUD/FORM DPI, and AUCs were approximately 25% and 27% higher for BGF MDI and BFF MDI, respectively, compared with BUD/FORM DPI. Moreover, comparison of the formoterol PK parameters showed that Cmax was approximately 20%‐25% higher and total systemic exposure as assessed by AUC was approximately 65%‐103% higher with BGF MDI and BFF MDI compared with BUD/FORM DPI. This may be partly due to the higher formoterol dose (10 μg) in BGF MDI and BFF MDI than in BUD/FORM DPI (9 μg).
While the precise explanation for the lower Cmax and AUC values for budesonide and formoterol for BUD/FORM DPI relative to BGF MDI and BFF MDI is not known, it is important to note that the values in our study were approximately 2‐ to 6‐fold lower than those reported by another study with BUD/FORM DPI (1 inhalation using the 400/12‐μg metered dose).27 The fact that the DPI, unlike an MDI device, is an inspiratory flow‐dependent device, may have contributed to the high CV% and the lower Cmax and AUC parameters with BUD/FORM DPI observed in our study for budesonide and formoterol.
Different inhaler devices require different handling and inhalation techniques; MDIs require coordinated inhalation and device activation, whereas DPIs are breath‐actuated.28 Proper training in the handling and correct operation of both types of device is crucial. Poor inhalation technique, a common issue across different inhaler types,29 results in variable dose delivery and, consequently, may have led to variability in measured PK parameters. It is notable that greater variability in PK parameters was observed with the DPI compared with the MDI, suggesting that the co‐suspension delivery technology MDI achieved a more consistent dose delivery than did DPI in this study or that inhalation technique was more consistent with the MDI than with the DPI.
Although there were PK differences between BGF MDI and BFF MDI relative to BUD/FORM DPI, all treatments were well tolerated and there was no evidence that the differences in exposure had any effect on safety or tolerability. Moreover, there were no appreciable differences among the treatments in the incidence of pharmacologically predictable AEs associated with inhaled bronchodilators, such as palpitations or tremor, and only minor changes in ECGs were observed with any of the treatments. Although only single doses were administered in the present study, the safety profile of BUD/FORM DPI is well established,30, 31, 32 and the safety profile of doses of budesonide and formoterol considerably higher than those used in this study has been well documented.33, 34, 35, 36, 37
No PK interactions, whether due to formulation or drug‐drug interactions, were observed when glycopyrronium was formulated with budesonide and formoterol in the BGF MDI fixed‐dose combination using co‐suspension delivery technology, based on the equivalence of PK parameters of budesonide and formoterol between BGF MDI and BFF MDI. Similarly, a phase 1 study comparing PK parameters of BGF MDI with glycopyrrolate/formoterol fumarate MDI (Bevespi Aerosphere; AstraZeneca) found no evidence of within‐combination PK drug‐drug interactions when budesonide was formulated with glycopyrronium and formoterol, based on the equivalence of the PK parameters of glycopyrronium and formoterol between BGF MDI and glycopyrrolate/formoterol fumarate MDI using the same co‐suspension delivery technology platform.38 The absence of any drug‐drug interactions in the current study among the individual components of BGF MDI incorporated into the triple fixed‐dose combination is aligned with the consistent drug delivery seen across mono and dual bronchodilator MDI formulations incorporating co‐suspension delivery technology.17
The study was conducted in healthy volunteers rather than patients with COPD, which is typical for phase 1 studies and similar to other PK studies of budesonide/formoterol fixed‐dose combinations.27, 39, 40 A limitation of the study was the collection of blood samples for analysis of PK parameters only up to 12‐hours after dosing.
Although systemic exposure to both budesonide and formoterol was higher for BGF MDI and BFF MDI than for BUD/FORM DPI, these differences may have been exaggerated due to the lower‐than‐expected observations for BUD/FORM DPI. Despite these PK differences, there were no appreciable differences in the incidence of pharmacologically predictable AEs among the treatments. This, together with the absence of any PK interactions (whether drug‐drug or formulation related) between glycopyrronium and budesonide or formoterol, suggests that the safety profile of BGF MDI will likely be comparable to that of BUD/FORM DPI. This hypothesis will be evaluated further in phase 3 studies of the ICS/LAMA/LABA fixed‐dose combination BGF MDI using innovative co‐suspension delivery technology in patients with COPD.
Supporting information
Supplementary Methods
Acknowledgments
This study was supported by Pearl – a member of the AstraZeneca group. The authors would like to thank Chad Orevillo (former employee of Pearl) for his valuable contributions to the study. The authors would also like to thank Tandem Labs, who analyzed the blood samples. Medical writing support, under the direction of the authors, was provided by Carol McNair, PhD, of CMC CONNECT, a division of Complete Medical Communications Ltd, Glasgow, UK, and Janet Dawson, on behalf of CMC, Macclesfield, UK, which was funded by AstraZeneca, Cambridge, UK in accordance with Good Publication Practice (GPP3) guidelines.41
Declaration of Conflicting Interests and Financial Disclosure
This study was supported by Pearl – a member of the AstraZeneca group. A.M. and P.D. are employees of Pearl – a member of the AstraZeneca Group. P.DeP. is an employee of Pharmaron, formerly Shin Nippon Biomedical Laboratories Clinical Pharmacology Center, Inc. (SNBL CPC). S.S. is an employee of AstraZeneca. C.R. is an employee of AstraZeneca and Pearl – a member of the AstraZeneca Group.
References
- 1. European Respiratory Society . European Lung White Book. 2013. http://www.erswhitebook.org/. Accessed December 14, 2017.
- 2. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Chronic obstructive pulmonary disease (COPD) Fact sheet. 2017. http://www.who.int/mediacentre/factsheets/fs315/en/. Accessed June 13, 2017.
- 4. Global Initiative for Chronic Obstructive Lung Disease . Global Strategy for the Diagnosis, Management and Prevention of COPD. 2018. http://www.goldcopd.org. Accessed November 15, 2017.
- 5. Calverley P, Vlies B. A rational approach to single, dual and triple therapy in COPD. Respirology. 2016;21(4):581–589. [DOI] [PubMed] [Google Scholar]
- 6. National Institute for Health and Care Excellence . Chronic obstructive pulmonary disease in over 16s: diagnosis and management (CG101). 2010. https://www.nice.org.uk/guidance/cg101/resources/chronic-obstructive-pulmonary-disease-in-over-16s-diagnosis-and-management-pdf-35109323931589. Accessed June 13, 2017. [PubMed]
- 7. Frith PA, Thompson PJ, Ratnavadivel R, et al. Glycopyrronium once‐daily significantly improves lung function and health status when combined with salmeterol/fluticasone in patients with COPD: the GLISTEN study–a randomised controlled trial. Thorax. 2015;70(6):519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Siler TM, Kerwin E, Singletary K, Brooks J, Church A. Efficacy and safety of umeclidinium added to fluticasone propionate/salmeterol in patients with COPD: results of two randomized, double‐blind studies. COPD. 2016;13(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singh D, Papi A, Corradi M, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long‐acting beta2‐agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double‐blind, parallel group, randomised controlled trial. Lancet. 2016;388(10048):963–973. [DOI] [PubMed] [Google Scholar]
- 10. Lipson DA, Barnacle H, Birk R, et al. FULFIL trial: once‐daily triple therapy for patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196(4):438–446. [DOI] [PubMed] [Google Scholar]
- 11. Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long‐acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double‐blind, parallel group, randomised controlled trial. Lancet. 2017;389(10082):1919–1929. [DOI] [PubMed] [Google Scholar]
- 12. Vestbo J, Anderson JA, Calverley PM, et al. Adherence to inhaled therapy, mortality and hospital admission in COPD. Thorax. 2009;64(11):939–943. [DOI] [PubMed] [Google Scholar]
- 13. Yu AP, Guérin A, Ponce de Leon D, et al. Therapy persistence and adherence in patients with chronic obstructive pulmonary disease: multiple versus single long‐acting maintenance inhalers. J Med Econ. 2011;14(4):486–496. [DOI] [PubMed] [Google Scholar]
- 14. Yu AP, Guérin A, Ponce de Leon D, et al. Clinical and economic outcomes of multiple versus single long‐acting inhalers in COPD. Respir Med. 2011;105(12):1861–1871. [DOI] [PubMed] [Google Scholar]
- 15. Lechuga‐Ballesteros D, Noga B, Vehring R, Cummings RH, Dwivedi SK. Novel cosuspension metered‐dose inhalers for the combination therapy of chronic obstructive pulmonary disease and asthma. Future Med Chem. 2011;3(13):1703–1718. [DOI] [PubMed] [Google Scholar]
- 16. Vehring R, Lechuga‐Ballesteros D, Joshi V, Noga B, Dwivedi SK. Cosuspensions of microcrystals and engineered microparticles for uniform and efficient delivery of respiratory therapeutics from pressurized metered dose inhalers. Langmuir. 2012;28(42):15015–15023. [DOI] [PubMed] [Google Scholar]
- 17. Doty A, Schroeder J, Vang K, et al. Drug delivery from an innovative LAMA/LABA co‐suspension delivery technology fixed‐dose combination MDI: evidence of consistency, robustness, and reliability. AAPS PharmSciTech. 2018;19(2):837–844. [DOI] [PubMed] [Google Scholar]
- 18. Tzelepis G, Komanapolli S, Tyler D, Vega D, Fulambarker A. Comparison of nebulized glycopyrrolate and metaproterenol in chronic obstructive pulmonary disease. Eur Respir J. 1996;9(1):100‐103. [DOI] [PubMed] [Google Scholar]
- 19. Mirakhur RK, Dundee JW. Glycopyrrolate: pharmacology and clinical use. Anaesthesia. 1983;38(12):1195–1204. [DOI] [PubMed] [Google Scholar]
- 20. Bartels C, Looby M, Sechaud R, Kaiser G. Determination of the pharmacokinetics of glycopyrronium in the lung using a population pharmacokinetic modelling approach. Br J Clin Pharmacol. 2013;76(6):868–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sechaud R, Renard D, Zhang‐Auberson L, Motte SL, Drollmann A, Kaiser G. Pharmacokinetics of multiple inhaled NVA237 doses in patients with chronic obstructive pulmonary disease (COPD). Int J Clin Pharmacol Ther. 2012;50(2):118–128. [DOI] [PubMed] [Google Scholar]
- 22. Cada DJ, Levien T, Baker DE. Formoterol fumarate inhalation powder. Hosp Pharm. 2001;36(7):753–767. [Google Scholar]
- 23. Jonsson G, Astrom A, Andersson P. Budesonide is metabolized by cytochrome P450 3A (CYP3A) enzymes in human liver. Drug Metab Dispos. 1995;23(1):137–142. [PubMed] [Google Scholar]
- 24. Szefler SJ. Pharmacodynamics and pharmacokinetics of budesonide: a new nebulized corticosteroid. J Allergy Clin Immunol. 1999;104(4 Pt 2):175–183. [DOI] [PubMed] [Google Scholar]
- 25. Williams EJ. Experimental designs balanced for the estimation of residual effects of treatments. Aust J Sci Res B. 1949;2(2):149–168. [Google Scholar]
- 26. AstraZeneca UK Limited . Symbicort Turbohaler 200/6 Inhalation Powder. Summary of Product Characteristics. 2017. https://www.medicines.org.uk/emc/medicine/4821. Accessed February 22, 2018.
- 27. Weisfeld L, Shu Y, Shah TP. Bioequivalence of budesonide plus formoterol (BF) Spiromax® and BF Turbohaler® (with and without charcoal block) in healthy volunteers. Int J Clin Pharmacol Ther. 2015;53(7):593–602. [DOI] [PubMed] [Google Scholar]
- 28. Laube BL, Janssens HM, de Jongh FHC, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. 2011;37(6):1308–1331. [DOI] [PubMed] [Google Scholar]
- 29. Molimard M, Raherison C, Lignot S, et al. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real‐life assessment of 2935 patients. Eur Respir J. 2017;49(2):1601794. [DOI] [PubMed] [Google Scholar]
- 30. Rosenhall L, Elvstrand A, Tilling B, et al. One‐year safety and efficacy of budesonide/formoterol in a single inhaler (Symbicort Turbuhaler) for the treatment of asthma. Respir Med. 2003;97(6):702–708. [DOI] [PubMed] [Google Scholar]
- 31. Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21(1):74–81. [DOI] [PubMed] [Google Scholar]
- 32. Morice AH, Peterson S, Beckman O, Osmanliev D. Therapeutic comparison of a new budesonide/formoterol pMDI with budesonide pMDI and budesonide/formoterol DPI in asthma. Int J Clin Pract. 2007;61(11):1874–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aalbers R, Ayres J, Backer V, et al. Formoterol in patients with chronic obstructive pulmonary disease: a randomized, controlled, 3‐month trial. Eur Respir J. 2002;19(5):936–943. [DOI] [PubMed] [Google Scholar]
- 34. Haahtela T, Järvinen M, Kava T, et al. Comparison of a beta 2‐agonist, terbutaline, with an inhaled corticosteroid, budesonide, in newly detected asthma. N Engl J Med. 1991;325(6):388–392. [DOI] [PubMed] [Google Scholar]
- 35. Campbell SC, Criner GJ, Levine BE, et al. Cardiac safety of formoterol 12μg twice daily in patients with chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2007;20(5):571–579. [DOI] [PubMed] [Google Scholar]
- 36. Rosenkranz B, Rouzier R, Kruse M, et al. Safety and tolerability of high‐dose formoterol (via Aerolizer) and salbutamol in patients with chronic obstructive pulmonary disease. Respir Med. 2006;100(4):666–672. [DOI] [PubMed] [Google Scholar]
- 37. Reisner C, Fabbri LM, Kerwin EM, et al. A randomized, seven‐day study to assess the efficacy and safety of a glycopyrrolate/formoterol fumarate fixed‐dose combination metered dose inhaler using novel Co‐Suspension™ Delivery Technology in patients with moderate‐to‐very severe chronic obstructive pulmonary disease. Respir Res. 2017;18(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Darken P, DePetrillo P, Reisner C, Rose ES, Dorinsky P. The pharmacokinetics of three doses of budesonide/glycopyrronium/formoterol fumarate dihydrate metered dose inhaler compared with active controls: a phase I randomized, single‐dose, crossover study in healthy adults. Pulm Pharmacol Ther. 2018;50:11–18. [DOI] [PubMed] [Google Scholar]
- 39. Lähelmä S, Sairanen U, Haikarainen J, et al. Equivalent lung dose and systemic exposure of budesonide/formoterol combination via Easyhaler and Turbuhaler. J Aerosol Med Pulm Drug Deliv. 2015;28(6):462–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eklund A, Tronde A, Johannes‐Hellberg I, Gillen M, Borgström L. Pharmacokinetics of budesonide and formoterol administered via a series of single‐drug and combination inhalers: four open‐label, randomized, crossover studies in healthy adults. Biopharm Drug Dispos. 2008;29(7):382–395. [DOI] [PubMed] [Google Scholar]
- 41. Battisti WP, Wager E, Baltzer L, et al. Good publication practice for communicating company‐sponsored medical research: GPP3. Ann Intern Med. 2015;163(6):461–464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods


