Abstract
Aims
To describe the prevalence of major cardiovascular disease (CVD) and risk factor control in a contemporary population with Type 2 diabetes.
Methods
We used data from the national registry in Scotland, Scottish Care Information‐Diabetes, linked to hospital admissions. Using descriptive statistics and logistic regression we described associations of risk factors with CVD. CVD was defined based on diagnostic codes in primary or secondary care data for ischaemic heart disease, cerebrovascular disease peripheral arterial disease, heart failure, cardiac arrhythmia, hypertensive heart disease and revascularization procedures.
Results
Among 248 400 people with Type 2 diabetes with a median age of 67.5 years (IQR 58.2, 76.1) and median diabetes duration of 7.8 years (3.8, 13.0), 32% had prior CVD (35% of men, 29% of women). Median HbA1c overall was 55 mmol/mol (7.2%), median SBP was 132 mmHg, median total cholesterol was 4.1 mmol/l and mean BMI was 32 kg/m2 . Overall two‐thirds (65% of men, 68% of women) have two or more of the following CVD risk factor thresholds: HbA1c ≥ 53 mmol/mol (7%), SBP > 130 mmHg or DBP > 80 mmHg, total cholesterol ≥ 5 mmol/l or BMI ≥ 30 kg/m2, or were currently smoking. Overall 84% were taking anti‐hypertensives and 75% a statin. Use of metformin was common at 58%, but other diabetes drugs that reduce CVD were rarely used.
Conclusions
There continues to be a high prevalence of CVD among people with Type 2 diabetes and a high level of unmet need for risk factor control. This implies substantial scope for reducing the excess risk of CVD in diabetes through improved management of known risk factors.
What's new?
There have been substantial advances in the management and prevention of cardiovascular disease in diabetes. To understand where unmet needs lie, it is important to understand the current burden of cardiovascular disease and the levels of treated and untreated risk factors.
In this paper, we show that there continues to be a high prevalence of cardiovascular disease among people with Type 2 diabetes and a high level of unmet need for risk factor control. This implies substantial scope for reducing the excess risk of cardiovascular disease in diabetes through improved management of known risk factors.
What's new?
There have been substantial advances in the management and prevention of cardiovascular disease in diabetes. To understand where unmet needs lie, it is important to understand the current burden of cardiovascular disease and the levels of treated and untreated risk factors.
In this paper, we show that there continues to be a high prevalence of cardiovascular disease among people with Type 2 diabetes and a high level of unmet need for risk factor control. This implies substantial scope for reducing the excess risk of cardiovascular disease in diabetes through improved management of known risk factors.
Introduction
Total mortality rates are currently 40% higher in men and 50% higher in women with Type 2 diabetes mellitus compared with the background population 1. Cardiovascular disease (CVD) remains the leading cause of loss of life expectancy in Type 2 diabetes and rates remain elevated compared with those without diabetes. Ongoing elevations in risk have been reported in recent data from Scotland 2, Sweden and the USA 3, 4.
Key aspects of the prevention of primary and secondary CVD in diabetes include smoking prevention, weight control, blood pressure reduction, cholesterol lowering and glycaemic control. With regards to glycaemic control, evidence of vascular benefit has been demonstrated for metformin with respect to myocardial infarction and pioglitazone with respect to CVD; by contrast heart failure is increased 5, 6, 7. For newer drugs, major cardiovascular outcome trials have been conducted in recent years 8. In four drugs from two anti‐diabetes drug classes, sodium glucose co‐transporter 2 inhibitors (SGLT2i) and glucagon‐like peptide 1 receptor agonists (GLP‐1RA), evidence of reduced risk of major CVD events has been demonstrated. These four drugs are canagliflozin (SGLT2i), empagliflozin (SGLT2i), liraglutide (GLP‐1RA) and semaglutide (GLP‐1RA). Drug labels and clinical guidelines are being updated accordingly 9, 10, 11, 12, 13, 14, 15, 16, and it is hoped that the target of lowering HbA1c in Type 2 diabetes and the major goal of lowering the cause of excess death will be increasingly achieved. For medical service planning and to understand current standards of care, it is important to quantify the unmet treatment needs of people with Type 2 diabetes, including the prevalence of established CVD and current drug treatment regimens in a broad and representative Type 2 diabetes population. Accordingly, we aimed to establish the current prevalence of established CVD in a typical contemporary population with Type 2 diabetes. Further, we wanted to investigate the scope for CVD reduction through known risk factor control by identifying the proportion of people having two, three, four or more risk factors for CVD in the Scottish population with Type 2 diabetes. These data are timely given that the European Association for the Study of Diabetes/American Diabetes Association (EASD/ADA) are currently revising their joint recommendations on hyperglycaemia management and because these guidelines are apparently going to highlight ‘the need to consider the patient's important comorbidities, particularly cardiovascular disease or high cardiovascular risk, in selecting glucose‐lowering therapy’ 17.
Methods
Data sources
The Scottish Care Information‐Diabetes (SCI‐Diabetes) is a register and database that covers almost all (> 99%) of those in Scotland with a diagnosis of diabetes. It has been described in detail previously 18. It captures data from clinical episodes and laboratory data in primary care, National Health Service (NHS) hospital diabetes clinics, community care and the national retinopathy screening programme. Using the unique health service identifier, it has been linked to hospital admissions data (Scottish Morbidity Record 01) and to death data from the National Records of Scotland. Availability of risk factor data was generally high, being ≥ 94% for HbA1c, total cholesterol, eGFR, smoking status, blood pressure and retinopathy status. Availability was lower for albuminuria (82%), BMI (80%), triglycerides (79%), weight (72%) and LDL cholesterol (56%). However, there was no difference in missingness for those with and without CVD.
Participants
We defined the study cohort here as all people who were alive, > 18 years of age and with a clinical diagnosis of Type 2 diabetes as of 1 January 2016.
Outcomes
To obtain information on CVD status we selected the Scottish Morbidity Records inpatient and day case procedure records (SMR01) which use the World Health Organization (WHO) International Classification of Disease version 10 (ICD‐10) for diagnosis (in earlier years version 9), and we use the Classification of Interventions and Procedures (OPCS‐4) 19. Each person's records were queried for a look‐back period of 10 years.
CVD history was defined as having codes for main cause of admission for any of chronic ischaemic heart disease (ICD‐10: I20–I25), cerebrovascular disease including transient ischaemic attack (ICD‐10: I60–I69 and G45), peripheral arterial disease (ICD‐10: I70.2 and I73.9), heart failure (III.0, I13 I50), cardiac arrhythmia (I48, I49), hypertensive heart disease (I13.0, I15.0) or procedure codes for revascularization procedures of coronary, carotid or lower limb arteries. Primary care records were also queried for corresponding Read codes for coronary heart disease (CHD), cerebrovascular disease and revascularization procedures, peripheral vascular disease and atrial fibrillation 20.
Other risk factor and covariate data were obtained from SCI‐Diabetes on retinopathy screening status, HbA1c, bodyweight, BMI, blood pressure, estimated GFR (eGFR), plasma total cholesterol, triglycerides and urinary albumin–creatinine ratio. The value for these variables nearest in time to 1 January 2016 was used, with a maximum look‐back period of 2 years. The most recent of the observational records in the 2‐year period was selected for each individual. Age was calculated at 1 January 2016.
Statistical analysis
Simple summary statistics were used to describe the prevalence and distributions of disease and risk factors. We counted the number of the following risk factor thresholds each person had: HbA1c ≥ 53 mmol/mol (7%), SBP > 130 mmHg or DBP > 80 mmHg, total cholesterol ≥ 5 mmol/l or BMI ≥ 30 kg/m2, or current smoking. For HbA1c and blood pressure, these thresholds were chosen to reflect typical clinical guideline intervention levels 5, 21; BMI and total cholesterol were chosen to reflect more extreme lack of control, because most people with Type 2 diabetes require interventions for BMI and warrant lipid‐lowering therapy. Because for some people, target BP is < 140/80 mmHg, we also report these data using this threshold. Logistic regression was used to quantify the differences in risk factors in those with and without prior CVD.
Results
Some 248 400 people with Type 2 diabetes were included. Almost a third (32%) of those with Type 2 diabetes had established CVD, the prevalence being 29% (31 635/108 259) in women and 35% in men (48 962/140 141) (Table 1). The prevalence rose from 15% (11 249/72 894) in those aged < 60 years to 53% (19 987/38 096) in those aged ≥ 80 years. In this cross‐sectional analysis, those with established CVD were older, more likely to be male and had longer diabetes duration (Table 1). Adjusted for age, sex and diabetes duration, those with CVD had significantly (P < 0.01) higher BMI, higher HbA1c, were more likely to have albuminuria and currently smoking, and had lower eGFR. Those with CVD had lower age, diabetes duration and sex‐adjusted achieved SBP (P < 0.01) and lower cholesterol levels (P < 0.01) compared with those without a prior history of CVD, on a background of significantly more anti‐hypertensive and statin therapy (both P < 0.01). Overall, 58% had a SBP > 130 mmHg or a DBP > 80 mmHg, with this prevalence being 54% in those with CVD and 60% in those without CVD. Overall, almost all (97%) of those with CVD were taking at least one anti‐hypertensive drug compared with 77% of those without CVD (Table 1). Of those with CVD, 2% were hypertensive and not prescribed any blood pressure drug, whereas among those without CVD, 11% were hypertensive and not taking any anti‐hypertensive drug (Fig. 1). Overall, 75% were currently prescribed a statin. Overall 23% (17% of those with CVD and 26% of those without) had a total cholesterol ≥ 5 mmol/l (Table 1). Among those with CVD, 6% had a total cholesterol ≥ 5 mmol/l and were not taking a statin. Among those without CVD, 11% had a total cholesterol ≥ 5 mmol/l and were not taking a statin (Fig. 1).
Table 1.
Demographic characteristics and risk factor levels by cardiovascular disease (CVD) status in people with Type 2 diabetes
| Variable | Established CVD | No CVD | Total |
|---|---|---|---|
| Total included | 80 597 (32.45) | 167 803 (67.55) | 248 400 (100) |
| Gender | |||
| Male | 48 962 (60.4) | 91 179 (54.3) | 140 141 (56.4) |
| Female | 31 635 (39.3) | 76 624 (45.7) | 108 259 (43.6) |
| Age | |||
| < 60 | 11 249 (14.0) | 61 645 (36.7) | 72 894 (29.3) |
| 60–69 | 21 647 (26.9) | 50 246 (29.9) | 71 893 (28.9) |
| 70–79 | 27 714 (34.4) | 37 803 (22.5) | 65 517 (26.4) |
| 80+ | 19 987 (24.8) | 18 109 (10.8) | 38 096 (15.3) |
| Age at study day (years)* | 72.8 (65.1, 80.0) | 64.7 (55.6, 73.3) | 67.5 (58.2, 76.1) |
| Age at diabetes diagnosis (years)* | 61.9 (53.5, 69.7) | 56.0 (47.4, 64.6) | 58.0 (49.2, 66.5) |
| Diabetes duration (years)* | 9.5 (4.8, 15.0) | 7.1 (3.4, 12.0) | 7.8 (3.8, 13.0) |
| HbA1c (mmol/mol)* | 54 (47, 66) | 55 (47, 67) | 55 (47, 66) |
| HbA1c (%)* | 7.1 (6.5, 8.2) | 7.2 (6.5, 8.3) | 7.2 (6.5, 8.2) |
| Systolic BP (mmHg)* | 132 (122, 140) | 133 (124, 140) | 132 (124, 140) |
| Diastolic BP (mmHg)* | 73 (67, 80) | 77 (70, 80) | 76 (70, 80) |
| MDRD eGFR (ml min−1 1.73 m−2)* | 68.5 (53.0, 84.3) | 79.0 (65.2, 93.4) | 75.8 (61.0, 90.9) |
| CKDEpi eGFR (ml min−1 1.73 m−2)* | 70.1 (52.9, 85.9) | 83.6 (67.6, 95.8) | 79.7 (62.3, 93.1) |
| BMI (kg/m2)* | 30.6 (27.3, 34.6) | 31.3 (27.7, 35.8) | 31.1 (27.6, 35.4) |
| Weight (kg)* | 88.0 (76.0, 100.0) | 89.5 (77.3, 103.0) | 89.0 (77.0, 102.0) |
| Total cholesterol (mmol/l)* | 3.9 (3.3, 4.6) | 4.2 (3.6, 5.0) | 4.1 (3.5, 4.9) |
| LDL cholesterol (mmol/l)* | 1.9 (1.4, 2.4) | 2.1 (1.6, 2.7) | 2.0 (1.5, 2.6) |
| Triglycerides (mmol/l)* | 1.8 (1.3, 2.5) | 1.7 (1.2, 2.5) | 1.7 (1.3, 2.5) |
| Albuminuric status | |||
| Normoalbuminuric | 46 288 (69.3) | 111 602 (81.3) | 157 890 (77.4) |
| Microalbuminuric | 16 916 (25.3) | 22 805 (16.6) | 39 721 (19.5) |
| Macroalbuminuric | 3562 (5.3) | 2924 (2.1) | 6486 (3.2) |
| Renal status | |||
| CKD‐EPI CKD Stage 1 | 14 214 (18.1) | 60 156 (37.1) | 74 370 (30.9) |
| CKD‐EPI CKD Stage 2 | 37 090 (47.3) | 75 436 (46.5) | 112 526 (46.8) |
| CKD‐EPI CKD Stage 3 | 23 635 (30.2) | 24 524 (15.1) | 48 159 (20.0) |
| CKD‐EPI CKD Stage 4/5 | 3423 (4.4) | 2074 (1.3) | 5497 (2.3) |
| MDRD CKD Stage 1 | 14 112 (18.0) | 49 088 (30.3) | 63 200 (26.3) |
| MDRD CKD Stage 2 | 36 656 (46.8) | 84 104 (51.9) | 120 760 (50.2) |
| MDRD CKD Stage 3 | 24 609 (31.4) | 27 164 (16.7) | 51 773 (21.5) |
| MDRD CKD Stage 4/5 | 2985 (3.8) | 1834 (1.1) | 4819 (2.0) |
| eGFR < 60 ml min−1 1.73 m−2 | 28 402 (35.2) | 29 682 (17.7) | 58 084 (23.4) |
| Receiving renal replacement therapy | 946 (1.2) | 582 (0.3) | 1528 (0.6) |
| Retinopathy status | |||
| None | 55 588 (73.3) | 121 389 (77.4) | 176 977 (76.1) |
| Mild or moderate | 15 137 (19.9) | 27 293 (17.4) | 42 430 (18.2) |
| Pre‐proliferative or worse | 5159 (6.8) | 8065 (5.1) | 13 224 (5.7) |
| Current smoker | 14 371 (17.9) | 28 640 (17.1) | 43 011 (17.4) |
| HbA1c ≥ 53 mmol/mol | 43 890 (55.8) | 92 976 (57.3) | 136 866 (56.8) |
| HbA1c ≥ 58 mmol/mol | 32 185 (40.9) | 68 374 (42.1) | 100 559 (41.7) |
| SBP >130 mmHg or DBP >80 mmHg | 43 715 (54.2) | 100 228 (59.7) | 143 943 (57.9) |
| SBP >140 mmHg or DBP >80 mmHg | 23 183 (28.8) | 56 659 (33.8) | 79 842 (32.1) |
| Total cholesterol ≥ 5 mmol/l | 130 66 (16.9) | 40 708 (25.5) | 53 774 (22.7) |
| Currently prescribed a statin | 67 378 (83.6) | 119 381 (71.1) | 186 759 (75.2) |
| BMI ≥ 30 kg/m2 | 34 227 (42.5) | 81 190 (48.4) | 115 417 (46.5) |
| Currently on anti‐hypertensives | 77 936 (96.7) | 129 737 (77.3) | 207 673 (83.6) |
Values are given as N (%) unless indicated otherwise; *values are median (IQR). CKD, chronic kidney disease; CKD‐EPI, Chronic Kidney Disease Epidemiology.
Figure 1.
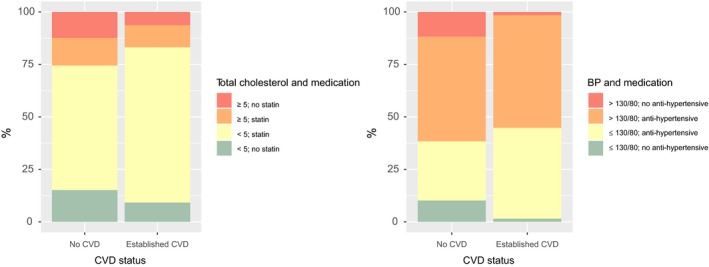
Statin and anti‐hypertensive use by risk factor threshold and cardiovascular disease (CVD) status.
We counted the number of the following risk factor thresholds exceeded by each person; HbA1c ≥ 53 mmol/mol, SBP > 130 mmHg or DBP > 80 mmHg, total cholesterol ≥ 5 mmol/l or BMI > 30 kg/m2, or current smoking. Overall, 65% of men and 68% of women with Type 2 diabetes have two or more of these risk factor thresholds (Table 2; Table S1). Among those with CVD, 62% had at least two risk factors; in those without CVD, the value was 69%. At all ages and in both sexes, the proportions exceeding these thresholds were high (Table 2; Table S1). In the oldest group, aged ≥ 80 years, the proportions were lowest at 45% of men and 52% of women exceeding two or more thresholds. This reflected significantly lower proportions exceeding the HbA1c and BMI thresholds, and less smoking compared with younger age groups (Fig. S1; P < 0.01 for comparison with the youngest group adjusted for sex and diabetes duration). There was more hypertension and more current anti‐hypertensive therapy in the older age group (P < 0.01 compared with the youngest age group). The proportions with total cholesterol ≥ 5 mmol/l fell from 35% in those aged < 50 years to 16% in those aged ≥ 80 years (P < 0.01 compared with those aged < 50 years). Statins were prescribed in 55% of those aged < 50 years, 80% in those aged 50–79 years and 70% in those aged ≥ 80 years. As expected, albuminuria and low eGFR were more common at older ages (data not shown). When a cut‐off of 140 mmHg rather than 130 mmHg was used for SBP the prevalence of those exceeding two or more thresholds was 57% overall.
Table 2.
Number of risk factor thresholds exceeded by cardiovascular disease (CVD) status
| Number of risk factors | 0 | 1 | 2 | 3 | 4 | 5 | Total |
|---|---|---|---|---|---|---|---|
| Established and no CVD | |||||||
| All | 21 308 (8.6) | 62 376 (25.1) | 85 308 (34.3) | 59 609 (24.0) | 17 803 (7.2) | 1996 (0.8) | 248 400 (100) |
| Male | 12 605 (9.0) | 36 273 (25.9) | 48 695 (34.7) | 32 583 (23.3) | 9016 (6.4) | 969 (0.7) | 140 141 (56.4) |
| Female | 8703 (8.0) | 26 103 (24.1) | 36 613 (33.8) | 27 026 (25.0) | 8787 (8.1) | 1027 (0.9) | 108 259 (43.6) |
| Established CVD | |||||||
| All | 8231 (10.2) | 22 658 (28.1) | 27 866 (34.6) | 16 945 (21.0) | 4441 (5.5) | 456 (0.6) | 80 597 (100) |
| Male | 5152 (10.5) | 14 182 (29.0) | 17 026 (34.8) | 10 037 (20.5) | 2342 (4.8) | 223 (0.5) | 48 962 (60.7) |
| Female | 3079 (9.7) | 8476 (26.8) | 10 840 (34.3) | 6908 (21.8) | 2099 (6.6) | 233 (0.7) | 31 635 (39.3) |
| No CVD | |||||||
| All | 13 077 (7.8) | 39 718 (23.7) | 57 442 (34.2) | 42 664 (25.4) | 13 362 (8.0) | 1540 (0.9) | 167 803 (100) |
| Male | 7453 (8.2) | 22 091 (24.2) | 31 669 (34.7) | 22 546 (24.7) | 6674 (7.3) | 746 (0.8) | 91 179 (54.3) |
| Female | 5624 (7.3) | 17 627 (23.0) | 25 773 (33.6) | 20 118 (26.3) | 6688 (8.7) | 794 (1.0) | 76 624 (45.7) |
Values are given as N (%).
The following risk factor thresholds were used: SBP > 130 mmHg or DBP > 80 mmHg; HbA1c ≥ 53 mmol/mol (7%); BMI ≥ 30 kg/m2; total cholesterol ≥ 5 mmol/l; current smoker.
The number of diabetes drugs used was highest in the 50–70‐year age groups, falling off thereafter (Table 3; Table S2). The number of diabetes drugs used was similar in those with and without CVD, but the use of insulin was higher in those with CVD compared with those without. Metformin and pioglitazone use (99% of the thiazolidinedione/other category shown in Table 4 comprised pioglitazone) was slightly lower in those with than without CVD (Table 4). Overall just 2.4% of those with CVD and 2.9% of those without were on a GLP‐1 agonist and just 1.4% and 2.2% of those with and without CVD respectively were on an SGLT2 inhibitor.
Table 3.
Number of diabetes drugs by cardiovascular (CVD) status
| Number of diabetes drugs | 0 | 1 | 2 | 3+ | Total |
|---|---|---|---|---|---|
| Established and no CVD | |||||
| All | 75 648 (30.5) | 89 571 (36.1) | 57 464 (23.1) | 25 717 (10.4) | 248 400 (100) |
| Male | 40 244 (28.7) | 49 640 (35.4) | 33 844 (24.1) | 16 413 (11.7) | 140 141 (56.4) |
| Female | 35 404 (32.7) | 39 931 (36.9) | 23 620 (21.8) | 9304 (8.6) | 108 259 (43.6) |
| Established CVD | |||||
| All | 24 605 (30.5) | 29 625 (36.8) | 18 693 (23.2) | 7674 (9.5) | 80 597 (100) |
| Male | 13 956 (28.5) | 17 654 (36.1) | 11 957 (24.4) | 5395 (11.0) | 48 962 (60.7) |
| Female | 10 649 (33.7) | 11 971 (37.8) | 6736 (21.3) | 2279 (7.2) | 31 635 (39.3) |
| No CVD | |||||
| All | 51 043 (30.4) | 59 946 (35.7) | 38 771 (23.1) | 18 043 (10.8) | 167 803 (100) |
| Male | 26 288 (28.8) | 31 986 (35.1) | 21 887 (24.0) | 11 018 (12.1) | 91 179 (54.3) |
| Female | 24 755 (32.3) | 27 960 (36.5) | 16 884 (22.0) | 7025 (9.2) | 76 624 (45.7) |
Values are given as N (%).
Table 4.
Use of diabetes drugs by cardiovascular disease (CVD) status
| Drug | Established CVD | No CVD | Total |
|---|---|---|---|
| Insulin | 12 203 (15.1) | 14 842 (8.8) | 27 045 (10.9) |
| Metformin | 42 641 (52.9) | 100 178 (59.7) | 142 819 (57.5) |
| Sulphonylurea | 21 842 (27.1) | 43 476 (25.9) | 65 318 (26.3) |
| DPP‐4 inhibitor | 8024 (10.0) | 17 054 (10.2) | 25 078 (10.1) |
| GLP‐1 agonist | 1952 (2.4) | 4866 (2.9) | 6818(2.7) |
| SGLT2 inhibitor | 1158 (1.4) | 3741 (2.2) | 4899(2.0) |
| TZD/other | 2852 (3.5) | 9076 (5.4) | 11 928 (4.8) |
Values are given as N (%). DPP‐4, dipeptidyl peptidase‐4; GLP‐1, glucagon‐like peptide 1; SGLT2, sodium glucose co‐transporter 2; TZD, thiazolidinediones.
Discussion
In this study, we find a high prevalence of established CVD in people with Type 2 diabetes, with a third of those with diabetes having had prior CVD. In both those with and those without a history of CVD there remain high levels of unmet need with regard to risk factor control. Overall, 65% of men and 68% of women with Type 2 diabetes have two or more of these risk factor thresholds. At present, HbA1c targets < 58 mmol/l are set for most people with Type 2 diabetes and targets < 53 mmol/l, where safely achievable, are set in US and UK guidelines 5. However, as shown here, just over half achieve the 58 mmol/l target. Approximately one‐third of people already receive two or more diabetes drugs, but these HbA1c levels suggest that there is scope, at least in some, for intensification of therapy or switching to different drug classes where existing therapy is not achieving targets. Metformin and pioglitazone, for which there are data showing a reduction in CVD, were slightly less commonly used in those with CVD than in those without, and the prevalence of use of pioglitazone was very low at < 5%. Of note, the least utilized anti‐diabetes drug classes for people with Type 2 diabetes in Scotland at the time of the study (2016) were the SGLT2i and GLP‐1 agonists. Because anti‐diabetes drugs in these classes have been shown to reduce CVD risk and CVD mortality, and this is now influencing guidelines, the pattern should change in the future. In the guidelines, BP targets are set at 130–140 mmHg for SBP and 80–90 mmHg for DBP, depending on CVD history. However, fewer than half are achieving the target of < 130/80 mmHg and two‐thirds the target of < 140/80 mmHg 5, 6, 7. This is despite the vast majority of people with Type 2 diabetes being on anti‐hypertensive therapy. So, recognizing the need for treatment is not the challenge, rather there may be scope for further intensification where this is tolerated. Similarly, most people are receiving statin therapy, yet total cholesterol levels are high at a mean of 4.1 mmol/l, with 21% exceeding 5 mmol/l. This also suggests scope for more intensive lipid‐lowering therapy where this is tolerated. Guidelines also suggest intervention for obesity, but more than half of the people in this study remain obese. Furthermore, a sizeable proportion are current smokers, suggesting scope for more intensive support for smoking cessation. The proportions exceeding risk factor thresholds for HbA1c, BMI, total cholesterol and smoking were lowest in those with an attained age of 80 years or more. This could reflect a survivor effect, or that some who develop diabetes in later age have less‐aggressive diabetes phenotypes, as recently discussed 22. We cannot discern which of these phenomena may underlie this lower risk factor prevalence at older ages, but we note that in contrast to anti‐hypertensive usage the intensity of diabetes drug use and statin fell in the older age bands, indicating treatment rates do not explain the HbA1c and lower cholesterol. We cannot comment with these data on the appropriateness of these lower treatment rates at older ages, but they may reflect the emphasis in national guidelines that diabetes care be tailored to the person's individual needs ‘taking into account their personal preferences, comorbidities, risks from polypharmacy, and their ability to benefit from long‐term interventions because of reduced life expectancy’ 5.
That further scope for narrowing the gap in life expectancy associated with Type 2 diabetes may be achievable by more intensified clinical management of this disease is emphasized by a recent analysis from the USA, which found that those with diabetes who met modest treatment goals for HbA1c, non‐HDL cholesterol and blood pressure had a 37% lower mortality than those who did not 23. In that analysis, almost half of those with Type 2 diabetes had HbA1c > 53 mmol/mol (7%) and 14% had an HbA1c of ≥ 75 mmol/mol (9%). In a recent survey from Denmark of people with Type 2 diabetes being cared for in primary care, the risk factor data are overall very similar to these data from Scotland 24. Specifically, mean age was 4 years higher (72 years), smoking prevalence was similar (17%), mean HbA1c was slightly (3 mmol/mol) lower, SBP was 2 mmHg lower, DBP was the same, and total cholesterol was just 0.1 mmol/l lower, whereas rates of lipid‐lowering drug and anti‐hypertensive use were very similar. The main difference in current diabetes drugs use was that in Denmark fewer people (8% compared with 26%) were using sulphonylureas and more (19% compared with 11%) were on insulin therapy. Compared with a summary from the US NHANES survey data for 2014 for all types of diabetes combined, the risk factor levels are broadly similar, with mean BMI being ~ 3 kg/m2 higher in the USA, blood pressure ~ 9 mmHg lower and total cholesterol ~ 0.7 mmol/l higher in their 2014 survey compared with these data from Scotland 25. Surprisingly, reported use of anti‐hypertensive (58%) and lipid‐lowering drugs (50%) was much lower in that study than in Scotland. In NHANES, 57% were achieving an HbA1c < 53 mmol/mol (7%) compared with 43% in Scotland. There were 15% current smokers compared with 17% in Scotland 25.
Our definition of CVD was wide, capturing not just CHD and cerebrovascular disease, but also cardiac arrhythmia, peripheral arterial disease and related revascularizations. Restricting the definition to prior CHD or cerebrovascular disease or coronary revascularization procedures or heart failure would yield a prevalence of 28%. On the other hand, if we define CVD based on the entry criteria in the LEADER trial, then 44% of our population met this definition 12. Clearly, the burden of established CVD is large under any definition used.
Our data are of substantial interest in understanding what percentage of people with diabetes have unmet need for CVD prevention therapies. In particular, although new diabetes drugs are being licensed based on efficacy for HbA1c and lack of harm for CVD, there is now evidence of cardiovascular benefit for some. New diabetes therapies that have shown benefit with regard to CVD reduction offer scope for simultaneous improvement in HbA1c while lowering CVD risk. For the SGLT2 inhibitor empagliflozin the hazard ratio (HR) for CVD was 0.86 (95% CI 0.74 to 0.99) 9 and for canagliflozin it was 0.86 (0.75 to 0.97) 10. For the GLP‐1 agonist semaglutide vs. placebo the HR was 0.74 (0.58 to 0.95) for CVD 11 and for liraglutide vs. placebo the HR was 0.87 (0.78 to 0.97) 12; for lixisenatide vs. placebo the HR was 1.02 (0.89 to 1.17) 13. Trials of the DPP‐4 inhibitors sitagliptin 14, alogliptin 15 and saxagliptin 16 have not shown these reductions. Further large cardiovascular outcome trials will report in the next 2 years 8, 26. These data from 2016 predate changes in guidelines and licencing for many of the new diabetes drugs and show very low rates of use of diabetes drugs with evidence of CVD prevention other than metformin.
This study aimed to give a brief contemporary snapshot of Type 2 diabetes and its management. Limitations are that for some risk factors data were incomplete. However, the similarity in missingness between CVD status groups and the fact that the sample size with available data is large in all strata, means that imputation is not needed, and our estimates have precision and are free of missing data bias or confounding. Another limitation is that we do not have data on why those with elevated risk factors seem to be insufficiently treated based on their risk factors – i.e. whether this is under‐prescribing, non‐adherence or side‐effect issues. A key aspect of our study is that the sample is large and representative of the Scottish population.
Conclusion
We found that there continues to be a high prevalence (32%) of CVD among people with diabetes and a high level of unmet need with regard to risk factor control. This implies substantial scope for further reduction in the relative risk of CVD associated with diabetes through adherence to healthy lifestyles and use of drugs that reduce CVD and control of known risk factors, including but not restricted to HbA1c.
Funding sources
This study was funded by Novo Nordisk A/S. Novo Nordisk provided a medical and scientific accuracy review of the final draft for submission. Emina Mocevic and Ulrik Haagen Panton are employed at Novo Nordisk A/S, Søborg, Denmark. Programming support for the Scottish Diabetes Research Network is provided by the Chief Scientist Office Scotland.
Competing interests
HMC received research support, travel expenses and honorarium and is also a member of the advisory panels and speaker's bureaus for Sanofi Aventis, Regeneron, and Eli Lilly. HMC is a member of the Advisory Panel and receives institutional fees from Novartis Pharmaceuticals. HMC receives or has recently received research support from Roche Pharmaceuticals, Pfizer Inc., Boehringer Ingelheim and AstraZeneca LP. HMC receives research support, travel expenses and is on the Steering Committee for Novo Nordisk. HMC is a shareholder of Roche Pharmaceuticals and Bayer. HMC has received speaker fees from Pfizer. NS reports personal fees from Boehringer Ingelheim, NovoNordisk, Eli Lilly, Janssen, Sanofi and Amgen. He has received research grants from Boehringer Ingelheim. RJM is on the advisory board for Novo Nordisk and the advisory board and honoraria for educational lectures for Sanofi. SW attended a Novo Nordisk conference and received associated expenses from Novo Nordisk. Emina Mocevic and Ulrik Haagen Panton are employed at Novo Nordisk A/S, Søborg, Denmark. All other authors declare no conflicts of interest.
Supporting information
Figure S1 Risk factors by age.
Table S1 Number of risk factor thresholds exceeded by CVD status.
Table S2 Number of diabetes drugs by CVD status.
Acknowledgements
This study was funded by Novo Nordisk A/S. Novo Nordisk provided a medical and scientific accuracy review of the final draft for submission. Emina Mocevic and Ulrik Haagen Panton are employed at Novo Nordisk A/S, Søborg, Denmark. Programming support for the Scottish Diabetes Research Network is provided by the Chief Scientist Office Scotland.
Diabet. Med. 36: 718–725 (2019)
The copyright line for this article was changed on 23 November 2018 after original online publication.
References
- 1. Read SH, Kerssens JJ, McAllister DA, Colhoun HM, Fischbacher CM, Lindsay RS et al Trends in type 2 diabetes incidence and mortality in Scotland between 2004 and 2013. Diabetologia 2016; 59: 2106–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Read SH, McAllister DA, Colhoun HM, Farran B, Fischbacher C, Kerssens JJ et al Incident ischaemic stroke and Type 2 diabetes: trends in incidence and case fatality in Scotland 2004–2013. Diabet Med 2018; 35: 99–106. [DOI] [PubMed] [Google Scholar]
- 3. Rawshani A, Rawshani A, Gudbjörnsdottir S. Mortality and cardiovascular disease in Type 1 and Type 2 diabetes. N Engl J Med 2017; 377: 300–301. [DOI] [PubMed] [Google Scholar]
- 4. Burrows NR, Li Y, Gregg EW, Geiss LS. Declining rates of hospitalization for selected cardiovascular disease conditions among adults aged ≥35 years with diagnosed diabetes, U.S., 1998–2014. Diabetes Care 2018; 41: 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Institute of Health and Care Excellence (NICE) . Type 2 Diabetes in Adults: Management. NG28. Available from https://www.nice.org.uk/guidance/ng28 Last accessed 28 February 2018.
- 6. Scottish Intercollegiate Guidelines Network (SIGN) . Pharmacological Management of Glycaemic Control in People with Type 2 Diabetes. Available from http://www.sign.ac.uk/assets/sign154.pdf Last accessed 17 September 2018.
- 7. Scottish Intercollegiate Guidelines Network SIGN) . Management of Diabetes: A National Clinical Guideline. Edinburgh: SIGN, 2010. [Google Scholar]
- 8. Schnell O, Rydén L, Standl E. Ceriello A; D&CVD EASD Study Group. Updates on cardiovascular outcome trials in diabetes. Cardiovasc Diabetol 2017; 16: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S et al Empagliflozin, cardiovascular outcomes, and mortality in Type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 10. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N et al Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 11. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA et al Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834–1844. [DOI] [PubMed] [Google Scholar]
- 12. Marso SP, Daniels GH, Brown‐Frandsen K, Kristensen P, Mann JFE, Nauck MA et al Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV et al Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373: 2247–2257. [DOI] [PubMed] [Google Scholar]
- 14. Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J et al Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 373: 232–242. [DOI] [PubMed] [Google Scholar]
- 15. White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL et al Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369: 1327–1335. [DOI] [PubMed] [Google Scholar]
- 16. Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B et al Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369: 1317–1326. [DOI] [PubMed] [Google Scholar]
- 17. ADA Daily . Scientific Sessions attendees to get first look at revised consensus report on hyperglycemia management in type 2 diabetes. ADA Daily News. Available at https://www.adadaily.org/scientific-sessions-attendees-to-get-first-look-at-revised-consensus-report-on-hyperglycemia-management-in-type-2-diabetes/ Last accessed 6 August 2018. [Google Scholar]
- 18. Colhoun HM, Livingstone SJ, Looker HC, Morris AD, Wild SH, Lindsay RS et al Hospitalised hip fracture risk with rosiglitazone and pioglitazone use compared with other glucose‐lowering drugs. Diabetologia 2012; 55: 2929–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. NHS Digital . Terminology and Classifications. Available at https://digital.nhs.uk/article/290/Terminology-and-Classifications Last accessed 28 February 2018.
- 20. NHS Digital . Read Codes Available at https://digital.nhs.uk/article/1104/Read-Codes Last accessed 28 February 2018.
- 21. American Diabetes Association. 9. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes–2018. Diabetes Care 2018; 41(Suppl 1): S86–S104. [DOI] [PubMed] [Google Scholar]
- 22. Steinarsson AO, Rawshani A, Gudbjörnsdottir S, Franzén S, Svensson A‐M, Sattar N. Short‐term progression of cardiometabolic risk factors in relation to age at type 2 diabetes diagnosis: a longitudinal observational study of 100,606 individuals from the Swedish National Diabetes Register. Diabetologia 2018; 61: 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saydah SH, Gregg EW, Kahn HS, Ali MK. Mortality associated with less intense risk‐factor control among adults with diabetes in the United States. Prim Care Diabetes 2018; 12: 3–12. [DOI] [PubMed] [Google Scholar]
- 24. Rungby J, Schou M, Warrer P, Ytte L, Andersen GS. Prevalence of cardiovascular disease and evaluation of standard of care in type 2 diabetes: a nationwide study in primary care. Cardiovasc Endocrinol 2017; 6: 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun X, Du T. Trends in cardiovascular risk factors among U.S. men and women with and without diabetes, 1988‐2014. BMC Public Health 2017; 17: 893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P et al Design and baseline characteristics of participants in the Researching cardiovascular Events with a Weekly INcretin in Diabetes (REWIND) trial on the cardiovascular effects of dulaglutide. Diabetes Obes Metab 2018; 20: 42–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Risk factors by age.
Table S1 Number of risk factor thresholds exceeded by CVD status.
Table S2 Number of diabetes drugs by CVD status.


