Abstract
Objectives
The aim of this study was to assess the predictive value of PMA measurement for mortality.
Background
Current surgical risk stratification have limited predictive value in the transcatheter aortic valve implantation (TAVI) population. In TAVI workup, a CT scan is routinely performed but body composition is not analyzed. Psoas muscle area (PMA) reflects a patient's global muscle mass and accordingly PMA might serve as a quantifiable frailty measure.
Methods
Multi‐slice computed tomography scans (between 2010 and 2016) of 583 consecutive TAVI patients were reviewed. Patients were divided into equal sex‐specific tertiles (low, mid, and high) according to an indexed PMA. Hazard ratios (HR) and their confidence intervals (CI) were determined for cardiac and all‐cause mortality after TAVI.
Results
Low iPMA was associated with cardiac and all‐cause mortality in females. One‐year adjusted cardiac mortality HR in females for mid‐iPMA and high‐iPMA were 0.14 [95%CI, 0.05–0.45] and 0.40 [95%CI, 0.15–0.97], respectively. Similar effects were observed for 30‐day and 2‐years cardiac and all‐cause mortality. In females, adding iPMA to surgical risk scores improved the predictive value for 1‐year mortality. C‐statistics changed from 0.63 [CI = 0.54–0.73] to 0.67 [CI: 0.58–0.75] for EuroSCORE II and from 0.67 [CI: 0.59–0.77] to 0.72 [CI: 0.63–0.80] for STS‐PROM.
Conclusions
Particularly in females, low iPMA is independently associated with an higher all‐cause and cardiac mortality. Prospective studies should confirm whether PMA or other body composition parameters should be extracted automatically from CT‐scans to include in clinical decision making and outcome prediction for TAVI.
Keywords: transcatheter aortic valve replacement, sarcopenia, computed tomography, psoas muscle mass
1. INTRODUCTION
In the past decade transcatheter aortic valve implantation (TAVI) has evolved as a suitable treatment for patients with severe and symptomatic aortic valve stenosis (AS) with intermediate to high surgical risk.1, 2 For TAVI planning and device selection, a computed tomography (CT) is routinely performed and covers the complete thorax and abdomen. In addition to its current use, CT can also be used to measure body composition. One of the most prominent shortcomings of current risk stratification models is an objective measure for frailty, which is associated with a lower quality of life and a higher risk of death after TAVI.3, 4 Sarcopenia, which is defined as the loss of skeletal muscle mass and functioning due to ageing, is likely to reflect frailty,4, 5 covering the biological age as it reflects a state of declined functional capacity and increased vulnerability to disease, disability and death.6, 7 Psoas muscle area (PMA) is a validated surrogate for global muscle mass.8 The in vivo‐reference standard for measuring muscle mass, including PMA, is by CT scan.4 Accordingly, in this study we examined ne the predictive value of PMA assessed with CT for all‐cause and cardiac mortality at short‐ and long‐term in patients who underwent a TAVI.
2. METHODS
2.1. Subjects
All 651 consecutive patients with severe AS who were treated with a TAVI between January 2010 and January 2016 in the Academic Medical Center (AMC, Amsterdam, The Netherlands) were identified. All patients provided informed consent for the procedure. The institutional review board approved this study with a waiver. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
2.2. CT scanning protocol
The CT angiography scans were acquired per protocol during the workup for the TAVI procedure using a 64‐slice multi‐detector scanner (Brilliance, Philips Medical Systems, Cleveland, OH) and a dual source 2 × 192 slice multi‐detector scanner (Somatom Force, Siemens, Erlangen, Germany). For the 64‐slice scanner, the CT angiography scan parameters were fixed: kV 120 and mAs 200. For the dual‐source scanner, the CT angiography scan parameters were adjusted to patient characteristics derived from the topogram and timing bolus scan (care kV), reference kV 80 and quality reference mAs 178.
The contrast injection protocol and timing differed between the 64‐slice and dual‐source scanner. For both scanners intravenous contrast medium (300 mg Iodine/mL) and saline flush was injected at 5 mL/sec. For the 64‐slice scanner bolus tracking was used. The CT angiography scan was initiated after injection of contrast (120 mL) followed by a saline flush (40 mL) using a region of interest in the ascending aorta and using an attenuation threshold of 125 Hounsfield Units (HU). For the dual‐source scanner a timing bolus (contrast 10 mL, saline flush 40 mL) was used for timing the CT angiography acquisition (contrast 90 mL, saline flush 40 mL) using a region of interest in the ascending aorta.
2.3. Psoas muscle assessment
The PMA was measured at vertebrae lumbar three (L3)8, 9 with a slice thickness of 3 mm. The slices were selected with Sante DICOM viewer (version 5.0.4, Santesoft, Athens, Greece) by one investigator (MSvM) using 3‐axial view of the axial, coronal, and saggital plane. Hence, the PMA was manually traced with Slice‐O‐Matic software (version 5.0, TomoVision, Montreal, Quebec, Canada) by a second investigator (YCJ), blinded for patient, procedural characteristics and outcomes. HU‐thresholds were used to distinguish between muscle tissue and other tissue types such as intramuscular adipose of fibrotic tissue. PMA was measured in a range of −29–150 HU, consistent with previous studies.8, 10 Pixels compliant with the HU thresholds were selected.
The tracing area within the HU thresholds in square centimeters was computed by summing up the selected tissue pixels. The measured PMA values were normalized for body surface area (BSA),11 resulting in an indexed PMA (iPMA). Patients were classified into equal sex‐specific tertiles according to iPMA (low, mid, and high) to compare thee group differences, with high PMA indicating more muscle mass.
PMA of 25 random patients were double measured for intraobserver agreement. The same slice was also measured by a second observer for interobserver agreement.
2.4. Data collection
Patient demographics, medical history, and procedural characteristics were collected from the prospective AMC TAVI registry.
2.5. Outcome
The primary outcome of the study was all‐cause and cardiac mortality. Complete follow‐up of mortality was collected through the Dutch national municipal population registry. Mortality rates were computed at 30 days, 1 year, and 2 years after TAVI. Cause of death was reported as cardiac or non‐cardiac and was actively retrieved at the general practitioner or nursing home physician. Patients without clearly described cause of death were reported as cardiac mortality, all according to VARC2 criteria.12
2.6. Statistical analysis
All analyses were conducted with R statistical software package (version 1.0.136; R Foundation for Statistical Computing, Vienna, Austria). Significance level was set to P < 0.05. Ninety‐five percent CI were obtained by logistic regression analysis.
Results for continuous variables were presented as the mean ± standard deviation or median (interquartile range [IQR]) as appropriate. Categorical variables were reported as a frequency and percentage.
Analyses were performed separately for males and females because of gender specific skeletal muscle mass differences.13 Simple and multivariate Cox regression was used to determine the relationship between PMA and mortality. The added value of normalized PMA was examined by multivariable logistic regression correcting for STS‐PROM and other confounders (age, peripheral artery disease, BMI, serum albumin, hemoglobin, chronic lung disease, estimated glomerular filtration rate, transfemoral/transthoracic access route).
3. RESULTS
3.1. Patient characteristics
Of the 651 patients undergoing a TAVI between January 2010 and January 2016, 583 were eligible for inclusion (Figure 1). The study population consisted of 264 males (45%) and 319 females (55%) with a median age of 83 [IQR: 78–86] and 83 [IQR: 79–86] respectively. Median STS‐PROM score in males was 4.4 [IQR: 2.9–6.3]% and 4.6 [IQR: 3.4–6.4]% in females (Table 1). A more detailed baseline characteristics table per tertile and sex is presented in the online supplementary material (Supporting Information Table S1).
Figure 1.
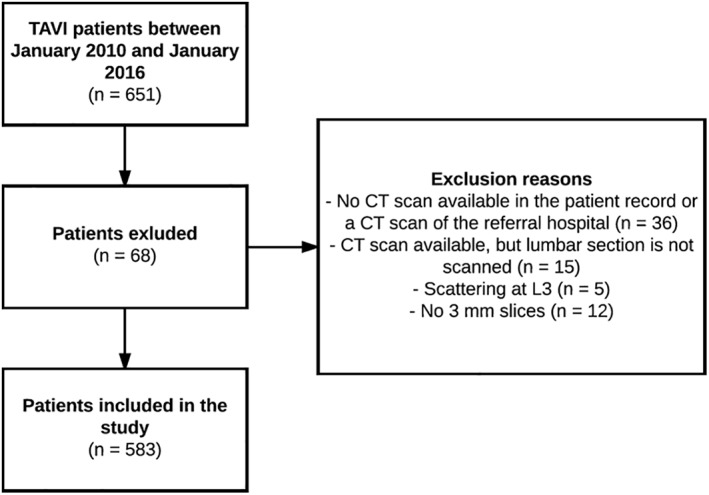
Study flow diagram
Table 1.
Baseline characteristics of the TAVI cohort
| Total | Males | Females | |
|---|---|---|---|
| (n = 583) | (n = 264) | (n = 319) | |
| Age in years [IQR] | 82.6 [78.1–85.8] | 82.6 [77.9–85.9] | 82.6 [78.5–85.7] |
| BMI [IQR](kg/m2) (n = 582) | 26.7 [24.4–30.0] | 26.3 [24.2–28.7] | 27.1 [24.6–31.2] |
| BSA [IQR] (m2) (n = 582) | 1.9 [1.7–2.0] | 2.0 [1.8–2.1] | 1.8 [1.7–1.9] |
| NYHA classification, III–IV | 411 (70.5) | 185 (70.1) | 226 (70.8) |
| Comorbidities | |||
| Diabetes mellitus (n = 582) | 175 (30.0) | 87 (33.0) | 88 (27.6) |
| Hypertension (n = 582) | 487 (83.5) | 220 (83.3) | 267 (83.7) |
| Peripheral artery disease (n = 582) | 157 (26.9) | 94 (35.6) | 63 (19.7) |
| Atrial fibrillation (n = 582) | 232 (39.8) | 110 (41.7) | 122 (38.4) |
| Chronic lung disease, GOLD III–IV (n = 582) | 32 (5.5) | 15 (5.7) | 17 (5.3) |
| Poor mobility | 18 (3.1) | 5 (1.9) | 13 (4.1) |
| Previous PCI (n = 582) | 153 (26.2) | 90 (34.1) | 63 (19.7) |
| Previous CABG (n = 582) | 73 (12.5) | 57 (21.6) | 16 (5.0) |
| Previous stroke (n = 582) | 64 (11.0) | 23 (8.7) | 41 (12.9) |
| Previous aortic valve surgery (n = 582) | 9 (1.5) | 5 (1.9) | 4 (1.3) |
| Previous mitral valve surgery (n = 582) | 11 (1.9) | 6 (2.3) | 5 (1.6) |
| Previous TAVI | 2 (0.34) | 0 (0.0) | 2 (0.6) |
| Previous pacemaker implantation | 60 (10.3) | 35 (13.3) | 25 (7.8) |
| Laboratory | |||
| Hemoglobin level (mmol/L) (n = 580) | 7.9 [7.2–8.5] | 8.1 [7.1–8.9] | 7.8 [7.2–8.3] |
| Albumin level (g/L) (n = 480) | 42.0 [40.0–44.0] | 42.0 [39.0–44.0] | 42.0 [40.0–44.0] |
| eGFR (mL/min/1.73 m2) (n = 578) | 64.8 [50.3–79.4] | 64.8 [50.7–78.6] | 64.7 [49.7–80.7] |
| Surgical risk scores | |||
| STS score (%) | 4.6 [3.2–6.4] | 4.4 [2.9–6.3] | 4.6 [3.4–6.4] |
| EuroSCORE I (logistic) (n = 582) | 14.4 [9.6–21.2] | 15.4 [9.8–24.4] | 13.6 [9.5–19.2] |
| EuroSCORE II (%) | 4.2 [2.6–7.2] | 4.6 [2.6–8.1] | 3.9 [2.6–5.9] |
| Echocardiographic parameters | |||
| Left ventricular ejection fraction <40% (n = 582) | 108 (18.5) | 69 (26.1) | 39 (12.2) |
| Aortic valve area (cm2) (n = 540) | 0.8 [0.7–1.0] | 0.8 [0.7–1.0] | 0.8 [0.6–1.0] |
| Aortic valve peak gradient (mmHg) (n = 583) | 65.0 [52.0–81.0] | 65.0 [51.0–81.00] | 65.0 [52.0–82.5] |
Figures are medians [IQRs] or numbers (%).
Abbreviations: BMI, body mass index (kg/m2); BSA, body surface area (m2); CABG, coronary artery bypass grafting; eGFR, estimated glomerular filtration rate; EuroSCORE, European system for cardiac operative risk evaluation; GOLD, global initiative for chronic obstructive lung disease; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; STS, society of thoracic surgeons; TAVI, transcatheter aortic valve implantation.
Figure 2.
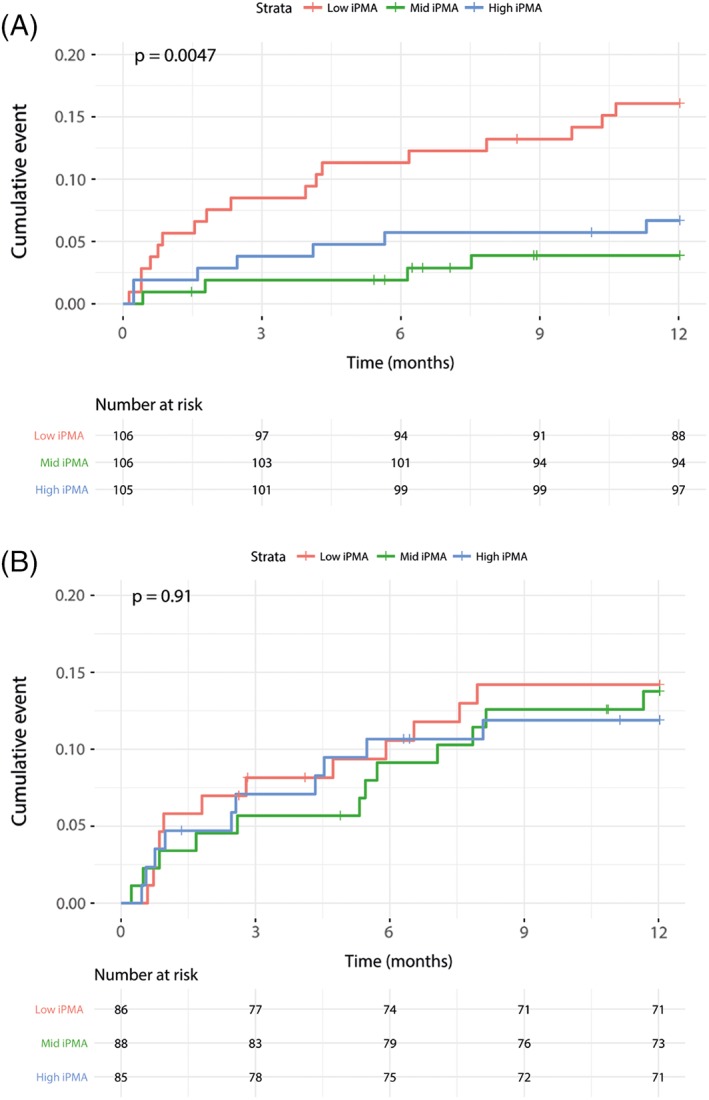
Kaplan–Meier cardiac mortality, 1 year follow‐up, stratified by indexed psoas muscle area (iPMA). A, Females. Cardiac mortality excluding procedural death (<72 hr); lowest tertile indexed psoas muscle area (iPMA) = red, mid iPMA = green, high iPMA = blue. Pairwise log‐rank testing using Benjamin‐Hochberg correction for multiple testing: Low‐mid (P = 0.010), low‐high (P = 0.049), and mid‐high (P = 0.359). B, Males. Cardiac mortality excluding procedural death (<72 hr); Lowest tertile indexed Psoas Muscle Area (iPMA) = red, Mid iPMA = green, High iPMA = Blue. Pairwise log‐rank testing using Benjamin‐Hochberg correction for multiple testing: Low‐Mid (P = 0.9), Low‐High (P = 0.9), Mid‐High (P = 0.9)
The median iPMA in males was 9.1 [IQR: 7.9–10.8] cm2 and 7.0 [IQR: 6.1–8.2] cm2 in females, respectively. The intraclass correlation coefficient for intraobserver variation was 0.98 and for interobserver variation 0.97 indicating near perfect reliability of PMA measurement.
All‐cause mortality at 30 days, 1 year, and 2 years in females was 3%, 13%, and 19%, respectively. In males, the all‐cause mortality rate was 6%, 19%, and 29%, respectively.
3.2. Primary outcome
Cox‐regression HR for iPMA group and mortality, adjusted for STS‐score and baseline characteristics for all‐cause and cardiac mortality are presented in Table 2.
Table 2.
Adjusted and unadjusted HRs
| A. For all‐cause mortality after exclusion of procedural mortality (mortality < 72 hr) | ||||
|---|---|---|---|---|
| Adjusted for STS‐PROM | Adjusted for baseline characteristicsa | |||
| Males | Females | Males | Females | |
| 1 year | ||||
| Low | Reference | Reference | Reference | Reference |
| Mid | 1.00 [0.49–2.06] | 0.54 [0.26–1.14] | 0.90 [0.51–2.43] | 0.44 [0.20–0.95] |
| High | 1.07 [0.51–2.24] | 0.41 [0.18–0.95] | 0.95 [0.49–2.26] | 0.40 [0.17–0.97] |
| 2 years | ||||
| Low | Reference | Reference | Reference | Reference |
| Mid | 1.00 0.58–1.72] | 0.61 [0.33–1.12] | 0.95 [0.54–1.69] | 0.54 [0.29–1.02] |
| High | 0.76 [0.42–1.38] | 0.51[0.27–0.99] | 0.73 [0.40–1.34] | 0.51 [0.26–1.02] |
| B. For cardiac mortality | ||||
| 1 year | ||||
| Low | Reference | Reference | Reference | Reference |
| Mid | 1.02 [0.46–2.29] | 0.19 [0.06–0.56] | 1.24 [0.50–3.08] | 0.14 [0.05–0.45] |
| High | 0.99 [0.42–2.32] | 0.38 [0.16–0.92] | 0.96 [0.39–2.36] | 0.38 [0.15–0.97] |
| 2 years | ||||
| Low | Reference | Reference | Reference | Reference |
| Mid | 1.10 [0.61–2.01] | 0.27 [0.12–0.65] | 0.93 [0.56–2.03] | 0.22 [0.09–0.53] |
| High | 0.68 [0.34–1.38] | 0.48 [0.23–0.99] | 1.55 [0.31–1.32] | 0.50 [0.23–1.08] |
Adjusted for: STS‐PROM, peripheral artery disease, baseline albumin, baseline hemoglobin, baseline estimated glomerular filtration rate, chronic obstructive lung disease, poor mobility, transfemoral/transthoracic route
In females, there was a significant protective effect of higher iPMA levels compared to the lowest tertile, especially for 1 year and 2 years cardiac mortality (Table 2). After adjustment for confounders the effect remained significant for 1 year all‐cause mortality for mid‐iPMA and high‐iPMA (HR = 0.14, CI = 0.05–0.45; HR = 0.38, CI = 0.16–0.99), respectively, and at 2 years all‐cause mortality only for mid‐iPMA; HR = 0.22, CI = 0.09–0.53. Analysis of the highest tertile of iPMA showed a trend towards better outcomes, but this did not reach statistically significant results for 30‐day and 2 years follow‐up; HR = 0.32 (CI = 0.05–1.91), HR = 0.50 (CI = 0.23–1.08), respectively. Similar results were observed for all‐cause mortality. Kaplan–Meier survival plots and log rank test (Figure) demonstrated a significant difference between iPMA groups in females (P = 0.0047) on cardiac mortality, but not in males (P = 0.53). All‐cause and cardiac mortality for the entire population is presented in Supporting Information Table S2.
3.3. Comparison with STS‐PROM and EuroSCORE II
In females, addition of the iPMA to current risk stratification models showed a modest improvement in the ability to predict 1‐year mortality. For the EuroSCORE II C‐statistic increased from 0.63 (CI = 0.54–0.73) to 0.67 (CI = 0.58–0.75) and for the STS‐PROM from 0.67 (CI = 0.59–0.77) to 0.72 (CI = 0.63–0.80). Calibration (Hosmer‐Lemeshow test statistic) in females at 1 year improved for the STS‐PROM (Chi‐squared 2.8; df = 4, P = 0.59–0.8; df = 4, P = 0.94) after addition of PMA. Calibration of the EuroSCORE II fell slightly (Chi‐squared 2.6; df = 4, P = 0.62–3.1; df = 4, P = 0.54) after addition of iPMA.
4. DISCUSSION
In this study, iPMA in females was predictive for all‐cause and cardiac mortality at 1 year and 2 years, but not at 30 days. Moreover, in females, addition of the iPMA showed a modest improvement in discrimination for both EuroSCORE II and STS‐PROM at 1 year. In males, we did not find a relationship between iPMA and mortality. A possible explanation of the difference between male and female is the higher baseline of iPMA for males compared to females, which might serve as a larger reserve before having negative outcome of a low iPMA. The lowest PMA values were only present in females. The reservoir function of skeletal muscle mass for proteins and amino acids may be an explanation for the relation between vulnerability and skeletal muscle mass.14 Prospective studies should confirm if interventions to enhance the reservoir function of muscle mass, for example, by exercise training or additional nutrition, would also lead to better outcomes.
Measuring iPMA is a part of determining sarcopenia. Sarcopenia is a component of frailty, which includes a degenerative loss of skeletal muscle mass and strength, possibly leading to adverse outcomes such as functional decline and death.4 A CT scan prior to TAVI is routinely performed and PMA measures can be easily obtained. Furthermore, PMA can be measured with high reliability.15, 16 In our study, we had nearly perfect intra‐observer and intra‐observer agreement.
4.1. Comparable literature
In multiple non‐cardiac populations an association between PMA and all‐cause mortality was demonstrated including in patients undergoing, for example, aortic aneurysm repair,16 pneumonectomy,17 and cancer surgery.18, 19 All of them found a relation between low PMA and increased mortality rates, but gender specific reporting was lacking.
4.2. Mortality
Previous studies investigating the association between PMA and mortality in TAVI patients revealed inconsistent results due to different time of follow‐up and various methods of PMA measurement. To our knowledge, currently four studies investigated the relation between PMA and all‐cause mortality in patients undergoing TAVI.10, 15, 20, 21 The results of these studies are somewhat inconclusive. Firstly, Garg et al. found no significant association between PMA and 1 year mortality.20 In contrast, Saji et al. found a significant association at 6 month.10, 15, 20 Another study focused on both psoas volume and area and found the same result that the lowest tertile had a significant higher mortality compared to the other tertiles.21 Mamane et al. have the only study investigating males and females separately. Similarly to this study, they found a significant difference in females, but it is unclear on what time interval.10 Our current study includes to our knowledge, the largest study population of all currently available studies on this topic. All‐cause mortality rate at 6 months was higher in other populations than in our study (21% vs. 10%), but can be ascribed to an earlier inclusion period with a corresponding higher mortality rate. Mortality rates were more comparable with our study sample at 30 days (5.9% vs. 5.4%) and 1 year (15.1% vs. 16.2%).
4.3. Assessment of sarcopenia
Comparing results between studies remains difficult due to the inconsistency of definition in iPMA with a variety in measurement methods.
First, various studies use different lumbar vertebrae, namely L317, 20 and L4.10, 15 Mourtzakis et al.22 investigated the relationship between measuring PMA and whole body muscle mass at L3. They found a strong relationship between muscle mass at L3 and whole body muscle mass. Further standardization enables easier comparison of studies to objectify sarcopenia and frailty and extrapolating results to other patient populations.
Second, there are differences in measuring PMA in the selected slices. Mamane et al.10 and Saji et al.15 both used density thresholds of −30–150 HU, comparable with our method. Mamane et al.10 used a threshold brush tool. Garg et al.20 outlined the psoas muscles (left and right) and computed the surface area based on the outline. By measuring the surface area on basis of the outline, these pixels are measured as muscle mass while the area consists of a combination of muscle mass, fat and connective tissue.23 In our cohort, we detected considerable differences in the composition of the psoas muscle. A part of the patients had less and/or small pieces that were not selected by the used HU range, while other patients had much larger and/or more unselected pieces. It is known that during the ageing process, an increase in intramuscular adipose tissue and loss of muscle mass occurs. A smaller area and a changed composition may lead to a decline in strength and muscle quality.23
Third, there are no validated cut‐off values to define sarcopenia, but are merely based on the distribution in the studied population. Cruz‐Jentoft et al.4 reported cut‐off values for measuring muscle mass by determining all skeletal muscle masses in a whole cross sectional slice. Two studies classified as low, mid, and high,10, 15 and one study only low and high.20 Our study has a much larger sample size than the aforementioned studies and used the same low, mid and high groups, which allows for more reliable and stable estimates of the effect of iPMA on mortality.
4.4. Study limitations
Despite the large sample size with negligible loss to follow‐up and complete data for most variables, this study has some limitations. First, this study was limited by its retrospective single center design with corresponding limitations. Second, because of the retrospective design it was not possible to investigate other aspects of sarcopenia or frailty including muscle strength (e.g., handgrip strength) and muscle performance (e.g., gait speed)4 or factors influencing outcome such as antiaggregant/anticoagulant therapy.24
4.5. Future perspective
We explored a possible variable to predict outcome after TAVI, which is easy to measure, without any extra tests for the patient. As new technologies emerge in the field in medical image analysis, quantification of body composition and muscle mass becomes more automated. Making use of more information contained in the routinely acquired CT‐scan would enhance decision making and provide a more personalized outcome prediction. In this study, we manually analyzed the psoas muscle in a single slice. With automatic segmentation the usability and clinical implementation becomes more feasible. Future studies should focus on valid iPMA cut‐offs for risk stratification and 3D volumetric segmentation opposed to the current 2D muscle area measurements in a single slice. A prospective study to evaluate the additional value of PMA measurement in TAVI work‐up, its relation to muscle strength and determine clinically relevant stratification could give a more definitive conclusion regarding the predictive value of PMA on outcome prediction after TAVI. Besides mortality, measures such as functional class or length‐of‐stay are of interest in future research.
5. CONCLUSIONS
In our study, a low iPMA is an independent predictor for cardiac and all‐cause mortality at 1 year and 2 years follow‐up in females. We conclude that the PMA measurements from routine preoperative imaging are likely of added value as objective tool in the prediction of mortality risks. We believe further studies should focus on automated and refined image analysis method to make body composition information easily available.
CONFLICT OF INTEREST
J. Baan receives a research grant from Edwards Lifesciences and is proctor for Edwards Lifesciences. The other authors report no relationships that could be construed as a conflict of interest.
Supporting information
Table S1. Baseline characteristics
Table S2. Outcomes
ACKNOWLEDGMENTS
The authors thank Paul F.C. Groot and Jan H.H. Wolters for their support in image retrieval and preparation for analysis.
van Mourik MS, Janmaat YC, van Kesteren F, et al. CT determined psoas muscle area predicts mortality in women undergoing transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2019;93:E248–E254. 10.1002/ccd.27823
The authors takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
REFERENCES
- 1. Praz F, Siontis GC, Verma S, Windecker S, Juni P. Latest evidence on transcatheter aortic valve implantation vs. surgical aortic valve replacement for the treatment of aortic stenosis in high and intermediate‐risk patients. Curr Opin Cardiol 2017;32(2):117–122. [DOI] [PubMed] [Google Scholar]
- 2. Sardar P, Kundu A, Chatterjee S, Feldman D, Owan T, Nairooz R, Feldman T, Abbott JD, Elmariah S. TCT‐756 Transcatheter versus surgical aortic‐valve replacement in intermediate‐risk patients: Evidence from a meta‐analysis. J Am Coll Cardiol 2016;68(18S):B305. [DOI] [PubMed] [Google Scholar]
- 3. Green P, Arnold SV, Cohen DJ, Kirtane AJ, Kodali SK, et al. Relation of frailty to outcomes after transcatheter aortic valve replacement (from the PARTNER trial). Am J Cardiol 2015;116(2):264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European working group on sarcopenia in older people. Age Ageing 2010;39(4):412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dodds RM, Sayer AA. Sarcopenia, frailty and mortality: The evidence is growing. Age Ageing 2016;45(5):570–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Finn M, Green P. Transcatheter aortic valve implantation in the elderly: Who to refer? Prog Cardiovasc Dis 2014;57(2):215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mitnitski A, Collerton J, Martin‐Ruiz C, Jagger C, von Zglinicki T, Rockwood K, Kirkwood TB. Age‐related frailty and its association with biological markers of ageing. BMC Med 2015;13:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boutin RD, Yao L, Canter RJ, Lenchik L. Sarcopenia: Current concepts and imaging implications. AJR Am J Roentgenol 2015;205(3):W255–266. [DOI] [PubMed] [Google Scholar]
- 9. Schweitzer L, Geisler C, Pourhassan M, Braun W, Gluer CC, Bosy‐Westphal A, Muller MJ. What is the best reference site for a single MRI slice to assess whole‐body skeletal muscle and adipose tissue volumes in healthy adults? Am J Clin Nutr 2015;102(1):58–65. [DOI] [PubMed] [Google Scholar]
- 10. Mamane S, Mullie L, Piazza N, Martucci G, Morais J, Vigano A, Levental M, Nelson K, Lange R, Afilalo J. Psoas muscle area and all‐cause mortality after Transcatheter aortic valve replacement: The Montreal‐Munich study. Can J Cardiol 2016;32(2):177–182. [DOI] [PubMed] [Google Scholar]
- 11. Mosteller RD. Simplified calculation of body‐surface area. N Engl J Med 1987;317(17):1098. [DOI] [PubMed] [Google Scholar]
- 12. Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The valve academic research Consortium‐2 consensus document. J Thorac Cardiovasc Surg 2013;145(1):6–23. [DOI] [PubMed] [Google Scholar]
- 13. Alizadehkhaiyat O, Hawkes DH, Kemp GJ, Howard A, Frostick SP. Muscle strength and its relationship with skeletal muscle mass indices as determined by segmental bio‐impedance analysis. Eur J Appl Physiol 2014;114(1):177–185. [DOI] [PubMed] [Google Scholar]
- 14. Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr 2006;84(3):475–482. [DOI] [PubMed] [Google Scholar]
- 15. Saji M, Lim DS, Ragosta M, LaPar DJ, Downs E, Ghanta RK, Kern JA, Dent JM, Ailawadi G. Usefulness of psoas muscle area to predict mortality in patients undergoing Transcatheter aortic valve replacement. Am J Cardiol 2016;118(2):251–257. [DOI] [PubMed] [Google Scholar]
- 16. Drudi LM, Phung K, Ades M, Zuckerman J, Mullie L, Steinmetz OK, Obrand DI, Afilalo J. Psoas muscle area predicts all‐cause mortality after endovascular and open aortic aneurysm repair. Eur J Vasc Endovasc Surg 2016;52(6):764–769. [DOI] [PubMed] [Google Scholar]
- 17. Hervochon R, Bobbio A, Guinet C, Mansuet‐Lupo A, Rabbat A, Regnard JF, Roche N, Damotte D, Iannelli A, Alifano M. Body mass index and Total psoas area affect outcomes in patients undergoing pneumonectomy for cancer. Ann Thorac Surg 2017;103(1):287–295. [DOI] [PubMed] [Google Scholar]
- 18. Park SY, Yoon JK, Lee SJ, Haam S, Jung J. Prognostic value of preoperative total psoas muscle area on long‐term outcome in surgically treated oesophageal cancer patients. Interact Cardiovasc Thorac Surg 2017;24(1):13–19. [DOI] [PubMed] [Google Scholar]
- 19. Boer BC, de Graaff F, Brusse‐Keizer M, Bouman DE, Slump CH, Slee‐Valentijn M, Klaase JM. Skeletal muscle mass and quality as risk factors for postoperative outcome after open colon resection for cancer. Int J Colorectal Dis 2016;31(6):1117–1124. [DOI] [PubMed] [Google Scholar]
- 20. Garg L, Agrawal S, Pew T, Hanzel GS, Abbas AE, Gallagher MJ, Shannon FL, Hanson ID. Psoas muscle area as a predictor of outcomes in Transcatheter aortic valve implantation. Am J Cardiol 2017;119(3):457–460. [DOI] [PubMed] [Google Scholar]
- 21. Kleczynski P, Tokarek T, Dziewierz A, Sorysz D, Bagienski M, Rzeszutko L, Dudek D. Usefulness of psoas muscle area and volume and frailty scoring to predict outcomes after Transcatheter aortic valve implantation. Am J Cardiol 2018;122:135–140. [DOI] [PubMed] [Google Scholar]
- 22. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33(5):997–1006. [DOI] [PubMed] [Google Scholar]
- 23. Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr 2009;90(6):1579–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. D'Ascenzo F, Benedetto U, Bianco M, Conrotto F, Moretti C, et al. Which is the best antiaggregant or anticoagulant therapy after TAVI? A propensity‐matched analysis from the ITER registry. The management of DAPT after TAVI. EuroIntervention 2017;13(12):e1392–e1400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics
Table S2. Outcomes


