Abstract
Roxadustat is a hypoxia‐inducible factor prolyl hydroxylase inhibitor in late‐stage clinical development for the treatment of anemia in chronic kidney disease. Spherical carbon adsorbent (SCA) is used in patients with chronic kidney disease and has been shown to impact absorption of certain concomitant drugs. Two phase 1, open‐label, randomized, crossover studies were conducted in healthy adult Japanese males to investigate the effect of food and SCA on the pharmacokinetics of a single oral dose of roxadustat. Subjects in the food effect study received a single dose of 100‐mg roxadustat under fed and fasted conditions. Subjects in the SCA/roxadustat drug‐drug interaction study received a single dose of 100‐mg roxadustat alone, concomitantly with SCA, and 1 and 2 hours before and after SCA to consider the real‐world clinical situation and assess any potential impact of a lag time on the pharmacokinetics of roxadustat. Primary outcomes for both studies were area under the concentration‐time curve from the time of dosing extrapolated to infinity and maximum concentration of drug in blood plasma. In the food effect study (N = 16), the geometric mean ratio (fed/fasted) and 90% confidence interval for area under the concentration‐time curve from the time of dosing extrapolated to infinity and maximum concentration of roxadustat were 94.44 (89.93‐99.18) and 79.88 (72.09‐88.52), respectively. In the SCA/roxadustat drug‐drug interaction study, all geometric mean ratios and 90% confidence intervals (roxadustat + SCA/roxadustat) were within the no‐effect boundaries of 80% and 125%. Roxadustat was generally well tolerated. The effect of food on the pharmacokinetics of roxadustat and the drug‐drug interaction between roxadustat and SCA do not appear to be clinically relevant and support the safe use of roxadustat under these conditions.
Keywords: drug‐drug interaction, food‐drug interaction, pharmacokinetics, roxadustat, spherical carbon adsorbent
Chronic kidney disease (CKD) is a condition characterized by long‐term decline in renal function that typically requires dialysis treatment in later stages and is often associated with other comorbidities such as hypertension, diabetes, and cardiovascular disease.1, 2 Anemia is a complication that often accompanies CKD, and is characterized by reduced hemoglobin levels, resulting, in part, from the inability of the failing kidneys to produce sufficient erythropoietin.1 The incidence of anemia among subjects with CKD increases with the severity of disease3 and is associated with an impaired quality of life.4
Roxadustat (ASP1517, FG‐4592, AZD9941) is an orally active, hypoxia‐inducible factor prolyl hydroxylase inhibitor5 that promotes erythropoiesis by increasing endogenous erythropoietin. Roxadustat has demonstrated safety and efficacy in phase 2 studies by increasing hemoglobin levels in subjects with anemia associated with CKD who are on6, 7, 8 or not on dialysis,9, 10 and is currently being investigated globally in phase 3 clinical studies. Roxadustat is rapidly absorbed after oral administration, reaches maximum plasma concentration within 2 hours, and is highly bound to albumin; the terminal elimination half‐life (t½) is approximately 12 hours in healthy subjects after a single dose.11 The primary elimination pathways are phase I oxidation (cytochrome P450 2C8) and phase II conjugation (glucuronidation via uridine diphosphate‐glucuronosyltransferase and glucosidation). The solubility of roxadustat is pH dependent and ranges from 0.001 mg/mL in a simulated gastric fluid (pH 1.2) to 3.7 mg/mL in a potassium phosphate buffer (pH 7.5). A previous study in healthy volunteers demonstrated that the pharmacokinetics (PK) of roxadustat is not impacted by food (data on file). Furthermore, a drug‐drug interaction (DDI) study revealed that roxadustat administered concomitantly with warfarin in healthy volunteers did not impact the maximum concentration (Cmax) or area under the concentration–time curve from the time of dosing extrapolated to infinity (AUCinf) of S‐ or R‐warfarin, but the time to reach maximum concentration (tmax) of R‐warfarin was delayed by ≈1 hour.12 In patients with moderate hepatic impairment, the AUCinf of roxadustat was increased by 23% and Cmax was decreased by 16%.11
Orally administered spherical carbon adsorbent (SCA, Kremezin Fine Granules 2g®; Kureha Corporation, Tokyo, Japan) is used to improve symptoms of uremia and to delay initiation of dialysis in subjects with CKD.13 SCA acts by adsorbing indole in the intestinal tract and excreting it through the feces, thereby reducing the concentration of uremic toxins in the systemic circulation.14 Previous studies have shown that SCA may decrease the blood concentration of certain drugs when administered concomitantly.15 In view of the future use of roxadustat for the treatment of anemia in CKD patients, it is important to determine whether exposure of roxadustat may be affected by various concomitant drugs. Additionally, no studies have been published reporting the impact of food on the exposure of roxadustat in Japanese subjects using the final commercial formulation of roxadustat, as recommended by the Japanese guideline Clinical Pharmacokinetic Studies of Pharmaceuticals.16 To address these data needs, 2 studies were conducted in Japan to assess (1) the effect of food on the PK of roxadustat and (2) the impact of SCA on the PK of roxadustat.
Methods
These studies were conducted in accordance with the clinical study protocol, Good Clinical Practice, International Conference on Harmonisation guidelines, applicable regulations and guidelines governing clinical study conduct, and the ethical principles of the Declaration of Helsinki. These studies were approved by an independent Institutional Review Board (food effect study, P‐One Clinic Institutional Review Board, Tokyo, Japan; SCA/roxadustat drug‐drug interaction [DDI] study, Hakata Clinic IRB, Fukuoka, Japan), and all subjects provided written informed consent.
Study Population
Healthy nonelderly adult male Japanese subjects, aged 20 to 45 years, with a body weight of 50 to 80 kg, and with a body mass index of 17.6 to 26.4 kg/m2, were enrolled in both studies. Subjects had to agree to use 2 highly effective forms of birth control and to not donate sperm for 84 days after the last study drug administration. Subjects who received any investigational drugs, medications, or supplements before hospital admission or who were previously treated with hypoxia‐inducible factor prolyl hydroxylase inhibitors were excluded from the study. Other exclusion criteria included any deviation from the normal range of blood pressure, pulse rate, body temperature, electrocardiogram, and laboratory tests; concurrent or previous drug allergy; hepatic, heart, respiratory, renal, or gastrointestinal disease; and excessive alcohol use or smoking.
Study Design
Food Effect Study
The primary objective of the food effect study was to evaluate the effect of food on the PK of a single oral dose of 100‐mg roxadustat. The secondary objective was to determine the safety of a single oral dose of 100‐mg roxadustat, under fed and fasted conditions. This was a phase 1, open‐label, randomized, 2‐sequence, crossover study (NCT02805374) consisting of 2 periods separated by ≥2 days washout, conducted at a single contract hospital in Japan (P‐One Clinic, Tokyo, Japan). Informed consent was provided, and a screening assessment was performed between day –30 and day –3. Subjects were admitted to the hospital on day –1, where they were randomized to 1 of 2 groups based on a sequence of fed/fasted conditions. The fasted condition preceding group was fasted during period 1 and fed during period 2, while the fed condition preceding group was fed during period 1 and fasted during period 2. On day 1 of period 1, subjects received a single oral dose of 100‐mg roxadustat under fasted (n = 8) or fed (n = 8) conditions and remained in the hospital for PK and safety assessments until discharge on day 4. After a ≥2‐day washout period at home, all subjects returned to the hospital for initiation of period 2, which followed the same procedure as period 1. Including PK and safety assessments and the ≥2‐day washout, the total time between roxadustat doses was a minimum of 6 days. A follow‐up visit for physical examination, laboratory tests, vital signs, and standard 12‐lead electrocardiogram occurred on day 7 of period 2.
Spherical Carbon Adsorbent/Roxadustat DDI Study
The primary objective of the SCA/roxadustat DDI study was to evaluate the effect of SCA on the PK of a single oral dose of 100‐mg roxadustat. The secondary objective was to determine the safety of a single oral dose of 100‐mg roxadustat, administered alone or in combination with SCA. The SCA/roxadustat DDI study was a phase 1, open‐label, randomized, crossover study (NCT02693613) conducted at a single contract hospital in Japan (Medical Co. LTA Sumida Hospital, Tokyo, Japan). This study was divided into 2 parts to reduce subject dropout due to longer study duration; subjects in part 1 of the study were prohibited from participating in part 2. Informed consent and screening assessments for all subjects were performed between day –30 and day –3, and subjects were admitted to the hospital on day –1 for parts 1 and 2.
Part 1 of the SCA/roxadustat DDI study was a 4 × 4 crossover (Williams design)17 study consisting of 4 sequential periods, each lasting 4 days, and each separated by ≥2‐day washout. On day –1 of period 1, subjects (n = 16) were randomized in equal proportions to 4 of 4 sequences (A‐D) of drug regimens (1 roxadustat alone and 3 SCA concomitant) (Table 1). All subjects received a single oral dose of 100‐mg roxadustat on day 1 of each period; in periods with SCA administration, subjects also received 2 g of SCA, 3 times daily (TID) (6 g/day) on days 1 and 2. Pharmacokinetic assessments were performed on days 1 through 4 of each period.
Table 1.
Kremezin/Roxadustat DDI Study Treatment Schedule (Part 1)
| Sequence | Period 1 | Period 2 | Period 3 | Period 4 |
|---|---|---|---|---|
| A (n = 4) | Kremezin concomitant 1 hour before roxadustat | Kremezin concomitant without a time lag | Roxadustat alone | Kremezin concomitant 1 hour after roxadustat |
| B (n = 4) | Roxadustat alone | Kremezin concomitant 1 hour before roxadustat | Kremezin concomitant 1 hour after roxadustat | Kremezin concomitant without a time lag |
| C (n = 4) | Kremezin concomitant without a time lag | Kremezin concomitant 1 hour after roxadustat | Kremezin concomitant 1 hour before roxadustat | Roxadustat alone |
| D (n = 4) | Kremezin concomitant 1 hour after roxadustat | Roxadustat alone | Kremezin concomitant without a time lag | Kremezin concomitant 1 hour before roxadustat |
DDI, drug‐drug interaction.
Part 2 of the SCA/roxadustat DDI study was a 6 × 3 crossover (Williams design)17 study consisting of 3 sequential periods, each lasting 4 days, and each separated by a ≥2‐days washout. On day –1 of Period 1, subjects (n = 18) were randomized in equal proportions to 1 of 6 sequences (E‐J) of drug regimens (1 roxadustat alone and 2 SCA concomitant) (Table 2). All subjects received a single oral dose of 100‐mg roxadustat on day 1 of each period; in periods with SCA administration, subjects also received 2 g of SCA TID (6 g/day) from day 1 to day 2. Pharmacokinetic assessments were performed on days 1 through 4 of each period.
Table 2.
Kremezin/Roxadustat DDI Study Treatment Schedule (Part 2)
| Sequence | Period 1 | Period 2 | Period 3 |
|---|---|---|---|
| E (n = 3) | Kremezin concomitant 2 hours before roxadustat | Roxadustat alone | Kremezin concomitant 2 hours after roxadustat |
| F (n = 3) | Kremezin concomitant 2 hours before roxadustat | Kremezin concomitant 2 hours after roxadustat | Roxadustat alone |
| G (n = 3) | Roxadustat alone | Kremezin concomitant 2 hours before roxadustat | Kremezin concomitant 2 hours after roxadustat |
| H (n = 3) | Roxadustat alone | Kremezin concomitant 2 hours after roxadustat | Kremezin concomitant 2 hours before roxadustat |
| I (n = 3) | Kremezin concomitant 2 hours after roxadustat | Kremezin concomitant 2 hours before roxadustat | Roxadustat alone |
| J (n = 3) | Kremezin concomitant 2 hours after roxadustat | Roxadustat alone | Kremezin concomitant 2 hours before roxadustat |
DDI, drug‐drug interaction.
Parts 1 and 2 occurred sequentially, and results for both parts were analyzed at the end of the study. The timing of SCA administration relative to roxadustat dosing was evaluated at 3 intervals (0, ±1, and ±2 hours) in order to consider the real‐world clinical situation and assess any potential impact of a lag time on the PK of roxadustat.
A crossover design was selected for both studies to compare the PK of roxadustat among different dosing conditions within the same subject. Particularly, the Williams design was selected because it is a balanced design and reduces carryover effect and symmetry order of dosing. The use of multiple sequences was chosen because it reduces the duration of the study and minimizes the chance of patient withdrawal.
For both studies, a dose of 100‐mg roxadustat was selected because it is within the therapeutic dose range. The starting doses currently being evaluated in phase 3 studies of patients with CKD not on dialysis range from 50 mg to 100 mg 3 times weekly and can be up‐ or down‐titrated to a range of 20 mg to 300 mg 3 times weekly. A ≥2‐day washout between periods was selected to ensure full clearance of roxadustat. Including PK and safety assessments and the ≥2‐day washout, the total time between roxadustat doses was a minimum of 6 days, which is >6 times t½ of roxadustat.
Study Drug Administration
Food Effect Study
Roxadustat was provided as 100‐mg tablets and administered with 150 mL of water under fasted or fed conditions. For administration under fasted conditions, subjects fasted from 22:00 on day –1 and received roxadustat without breakfast on day 1. For administration under the fed condition, subjects fasted from 22:00 on day –1 until the morning of day 1, where they consumed a high‐fat breakfast (≥900 kcal containing 35.7 g of protein, 47.0 g of lipids, 80.7 g of carbohydrates, 85 mg of magnesium, 480 mg of phosphate, 184 mg of calcium, and 2.3 mg of iron) within 20 minutes, followed by a single dose of 100‐mg oral roxadustat within 10 minutes of meal consumption. For both groups, food intake was prohibited for 4 hours after roxadustat dosing, and water was prohibited for 1 hour before and after dosing.
Spherical Carbon Adsorbent/Roxadustat DDI Study
For the roxadustat alone regimen, roxadustat was provided as 100‐mg tablets administered with 200 mL of water in the morning on day 1 of each period, under fasted conditions. For the SCA concomitant regimens, SCA was supplied as fine granules in 2‐g packages, and administered with 200 mL of water TID (6 g/day), from the morning of day 1 to the evening of day 2 during each period. On the morning of day 1, the dose of 100‐mg roxadustat was administered concomitantly with the morning dose of 2‐g SCA; food intake was prohibited for ≥10 hours before and ≥4 hours after roxadustat + SCA dosing. For all other doses of SCA, food was prohibited for ≥2 hours after dosing. The dose of 2‐g SCA TID used in this study is in accordance with the dose regimen reported in the package insert of SCA (for adults, 6 g/day divided into 3 doses of 2 g administered orally). The 2‐day duration of SCA dosing was chosen to account for any potential enterohepatic circulation of roxadustat. The fasted condition for dosing and the schedule of meals around dosing was chosen to limit any potential interaction between SCA and food and to evaluate the interaction of SCA and roxadustat during the absorption phase under the condition of a maximum effect of SCA.
Sample Collection and Analysis
Food Effect Study
Blood sampling for roxadustat PK assessments occurred before dosing and 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 24, 36, 48, 60, and 72 hours after dosing. All spontaneous urinations were collected throughout the hospital admission and attempted urine samples were collected before dosing, and at 4, 8, 12, 24, 48, and 72 hours after dosing. The primary plasma roxadustat PK outcomes were AUCinf and Cmax; secondary plasma roxadustat PK outcomes were AUC from the time of dosing to the last measurable concentration (AUClast), apparent total systemic clearance after single or multiple extravascular dosing, t½, and tmax. Urine PK parameters were calculated and included the amount of unchanged drug excreted into the urine up to the last measurable sample, percentage of drug dose excreted into the urine up to the last measurable sample, and renal clearance.
Spherical Carbon Adsorbent/Roxadustat DDI Study
Blood sampling for roxadustat PK assessments occurred before dosing and 0.5, 1, 2, 3, 5, 6, 8, 12, 16, 24, 36, 48, 60, and 72 hours after dosing. The primary plasma roxadustat PK outcomes were AUCinf and Cmax; secondary plasma roxadustat PK outcomes were AUClast, apparent total systemic clearance after single or multiple extra‐vascular dosing, t½, and tmax.
For both studies, plasma concentrations of roxadustat were measured using a validated liquid chromatography–tandem mass spectrometry method at SRL Inc. (Kanagawa, Japan). Roxadustat was isolated from plasma by liquid‐liquid extraction using tert‐butyl methyl ether. Stable isotope‐labeled roxadustat was used as the internal standard. The extracts were analyzed by liquid chromatography–tandem mass spectrometry using isocratic elution, with 45% of 0.1% formic acid in water and 55% of 0.1% formic acid in acetonitrile, on a SunFireTM C18, 2.1 × 100 mm column, using AB Sciex API4000 with a turbo ion spray interface in positive ion mode. The method was validated over a range of 1 to 1000 ng/mL. Intra‐ and interprecision (coefficient of variation) values ranged from 0.9% to 4.4%. Intra‐ and interassay accuracy values ranged from 0.1% to 10.7%. The limit of quantification for roxadustat was 1 ng/mL using 0.05 mL of plasma. Pharmacokinetic parameters in both studies were calculated from plasma concentrations of roxadustat and actual times from dosing using Phoenix WinNonlin® version 6.3 software.
Safety and Tolerability Assessments
Safety and tolerability for both studies were assessed by the occurrence of treatment‐emergent adverse events (TEAEs; frequency, outcome, seriousness, severity, and relationship to the study drug), physical examination results, laboratory tests (hematology, biochemistry, and urinalysis), vital signs (supine blood pressure, supine pulse rate, and axillary body temperature), and standard 12‐lead electrocardiogram.
Statistical Analysis
For both studies, the pharmacokinetic analysis set (PKAS) included all subjects who received roxadustat and provided at least 1 measurable PK parameter; the safety analysis set included all subjects who received at least 1 dose of the study drug (roxadustat for the food effect study; roxadustat or SCA for the SCA/roxadustat DDI study). To assess the effect of food on the PK of roxadustat, the natural log‐transformed plasma Cmax, AUCinf, and AUClast for roxadustat were analyzed using a linear mixed‐effects model to provide the geometric least square mean ratios (% GMRs) and 90% confidence intervals (CIs) of fed to fasted conditions. To assess the effect of SCA on the PK of roxadustat, the same analysis was applied to Cmax and AUCinf to provide the GMRs and 90%CIs of roxadustat in combination with SCA and roxadustat alone for each dosing condition separately for parts 1 and 2.
For both studies, the GMR (fed/fasted; and roxadustat + SCA/roxadustat) was assumed to be 95% for AUCinf and Cmax, and the within‐subject variation was assumed to be 16% based on results from a phase 1 study (data on file). The sample size was calculated based on the condition that the effect of food or SCA could be determined to be absent if the 90%CI of GMR for AUCinf and Cmax was within the no‐effect boundaries of 80% and 125%. For the food effect study, a sample size of 14 subjects was required to provide 80% power to demonstrate the absence of effect of food on the PK of roxadustat. For each part of the SCA/roxadustat DDI study, a sample size of 12 subjects was required to provide 80% power to demonstrate the absence of effect of SCA on the PK of roxadustat. To compensate for potential dropouts, 16 subjects were enrolled in the food effect study; 16 and 18 subjects were enrolled in part 1 and part 2, respectively, of the SCA/roxadustat DDI study. Plasma and urine concentrations of roxadustat and PK parameters were reported using descriptive statistics: number of observation (n), arithmetic mean, standard deviation, coefficient of variation (% coefficient of variation), minimum, median, and maximum. Noncompartmental methods were used for PKAS to calculate PK parameters of plasma and urine roxadustat. Statistical analysis was conducted by Astellas (SAS® Drug Development v4.5 and SAS v9.4; SAS Institute, Charlotte, North Carolina).
Results
Subject Disposition and Demographic Characteristics
Food Effect Study
Of the 62 subjects who provided informed consent, 16 subjects were randomized to the fasted condition preceding group (n = 8) or fed condition preceding group (n = 8). Two subjects discontinued the study after receiving the study drug during period 1: 1 withdrawal by subject in the fasted condition preceding group, and 1 withdrawal due to TEAEs (elevated blood creatine phosphokinase, lactate dehydrogenase, and aspartate aminotransferase and presence of blood in urine) in the fed condition preceding group. All 16 subjects were included in the safety analysis set and PKAS populations. The mean age was 28.9 years (range, 22‐39 years) and the mean body mass index was 20.76 kg/m2 (range, 17.7‐23.8 kg/m2). Subject demographics and baseline characteristics are presented in Table 3. Other than the 2 subjects who discontinued the study, all subjects received the study drug according to the protocol.
Table 3.
Subject Demographics and Baseline Characteristics (Safety Analysis Set)
| Parameter | Food Effect Study (N = 16) | Kremezin/Roxadustat DDI Study Part 1 (N = 16) | Kremezin/Roxadustat DDI Study Part 2 (N = 18) |
|---|---|---|---|
| Age, y | |||
| Mean (SD) | 28.9 (5.4) | 29.3 (7.8) | 28.7 (7.3) |
| Median (range) | 27.0 (22‐39) | 28.0 (20‐44) | 29.0 (20‐44) |
| Weight, kg | |||
| Mean (SD) | 60.76 (7.15) | 64.18 (7.52) | 63.52 (5.60) |
| Median (range) | 60.25 (52.1‐75.6) | 62.55 (53.6‐79.5) | 62.15 (54.6‐75.8) |
| Height, cm | |||
| Mean (SD) | 170.73 (6.54) | 173.17 (4.97) | 171.19 (6.90) |
| Median (range) | 171.50 (159.8‐180.1) | 173.60 (163.2‐182.2) | 171.25 (153.2‐182.3) |
| BMI, kg/m2 | |||
| Mean (SD) | 20.76 (1.79) | 21.33 (2.13) | 21.62 (1.49) |
| Median (range) | 20.45 (17.7‐23.8) | 21.20 (17.9‐25.3) | 21.75 (19.2‐24.6) |
BMI, body mass index; DDI, drug‐drug interaction; SD, standard deviation.
Spherical Carbon Adsorbent/Roxadustat DDI Study
Of the 88 subjects who provided informed consent, 4 were randomized to each sequence A through D in part 1 (n = 16), and 3 were randomized to each sequence E through J in part 2 (n = 18). No subjects discontinued the study during part 1, but 1 subject from part 2 (sequence I) discontinued the study after treatment in period 1 due to a TEAE (nasopharyngitis). All randomized subjects were included in the safety analysis set and PKAS. The mean age was 29.3 and 28.7 years, and the mean body mass index was 21.33 and 21.62 kg/m2 in part 1 and part 2, respectively. Subject demographics and baseline characteristics are summarized in Table 3. Other than the 1 subject who discontinued the study during part 2, all subjects received the study drug according to the protocol.
Pharmacokinetic Analyses
Food Effect Study
The mean plasma concentration‐time profiles of roxadustat were similar between fasted and fed conditions (Figure 1). The only notable differences in the plasma PK parameters were a lower mean Cmax (8.46 μg/mL vs 10.60 μg/mL) and longer median tmax (3.0 vs 2.0 hours) under fed conditions (Table 4). The GMRs (fed/fasted) and 90%CIs for AUCinf and AUClast indicated that the food effect decreased AUC only minimally. The GMR (fed/fasted) for Cmax corresponded to an approximate 20% reduction under fed conditions. The urinary excretion of unchanged roxadustat was low, and the urine PK parameters were similar under fasted or fed conditions (Table 5).
Figure 1.
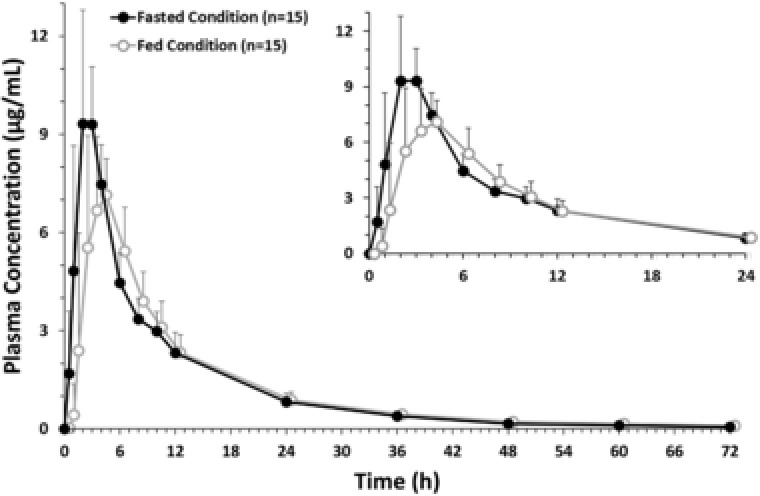
Mean plasma concentration profile of roxadustat (pharmacokinetic analysis set, food effect study). Data are presented as arithmetic mean ± standard deviation. All roxadustat levels that were below the level of quantification were set to zero. Inset displays roxadustat concentration from 0 hours to 24 hours for enhanced clarity.
Table 4.
Plasma Pharmacokinetic Parameters for Roxadustat (PKAS, Food Effect Study, N = 15)
| AUClast | AUCinf | Cmax | CL/F | tmax | t½ | |
|---|---|---|---|---|---|---|
| Parameter | (μg · h/mL) | (μg · h/mL) | (μg/mL) | (L/h) | (h) | (h) |
| Fasted condition | ||||||
| Mean (SD) | 87.60 (19.30) | 88.70 (19.40) | 10.60 (2.54) | 1.18 (0.255) | 13.1 (4.89) | |
| Median | 85.50 | 87.50 | 10.40 | 1.14 | 2.0 | 12.3 |
| Range | 56.20‐125.0 | 56.60‐126.0 | 6.61‐17.40 | 0.796‐1.77 | 1.0‐4.0 | 7.58‐28.7 |
| %CV | 22.0 | 21.9 | 24.0 | 21.6 | 37.5 | |
| Fed condition | ||||||
| Mean (SD) | 82.50 (14.70) | 83.80 (15.0) | 8.46 (1.84) | 1.23 (0.242) | 12.3 (3.43) | |
| Median | 85.50 | 88.10 | 7.82 | 1.13 | 3.0 | 11.5 |
| Range | 54.50‐109.0 | 55.60‐110.0 | 6.11‐12.10 | 0.912‐1.80 | 1.0‐4.0 | 8.33‐18.7 |
| %CV | 17.8 | 17.9 | 21.7 | 19.6 | 27.8 | |
| GMR (fed/fasted), % | 94.11 | 94.44 | 79.88 | |||
| 90%CI, % | 89.67‐98.78 | 89.93‐99.18 | 72.09‐88.52 | |||
AUCinf, area under the concentration‐time curve from the time of dosing extrapolated to time infinity; AUClast, area under the concentration‐time curve from the time of dosing to the last measurable concentration; CI, confidence interval; Cmax, maximum concentration; CL/F, apparent total systemic clearance after single or multiple extravascular dosing; CV, coefficient of variation; GMR, geometric mean ratio; PKAS, pharmacokinetic analysis set; SD, standard deviation; tmax, time of the maximum concentration; t½, terminal elimination half‐life.
Table 5.
Urine Pharmacokinetic Parameters of Roxadustat (PKAS, Food Effect Study, N = 15)
| Aelast | Aelast | CLR | |
|---|---|---|---|
| Parameter | (mg) | (%) | (L/h) |
| Fasted condition | |||
| Mean (SD) | 1.363 (0.654) | 1.36 (0.65) | 0.0161 (0.00874) |
| Median | 1.084 | 1.08 | 0.0132 |
| Range | 0.612‐2.689 | 0.61‐2.69 | 0.00812‐0.0384 |
| %CV | 48.0 | 48.0 | 54.4 |
| Fed condition | |||
| Mean (SD) | 1.194 (0.439) | 1.19 (0.44) | 0.0147 (0.00533) |
| Median | 1.137 | 1.14 | 0.0128 |
| Range | 0.609‐2.150 | 0.61‐2.15 | 0.00861‐0.0251 |
| %CV | 36.8 | 36.8 | 36.3 |
Aelast, amount of unchanged drug excreted into urine up to the last measurable sample; CLR, renal clearance; CV, coefficient of variation; SD, standard deviation.
Spherical Carbon Adsorbent/Roxadustat DDI Study
The mean plasma concentration‐time profiles of roxadustat in the absence and presence of SCA were nearly identical (Figure 2), with Cmax reached within 2 to 3 hours after dose for all dosing conditions in part 1 and part 2. The GMRs (SCA concomitant/roxadustat alone) and 90%CIs for AUCinf and Cmax showed that the effect of SCA on the PK of roxadustat was minimal. Administration of SCA without a time lag or 1 hour before or after roxadustat reduced the mean AUCinf of roxadustat by 9% to 10% and the mean Cmax of roxadustat by 7% to 11% compared with roxadustat administered alone. Administration of SCA 2 hours before or after roxadustat reduced the mean AUCinf of roxadustat by 6% to 7% and the mean Cmax of roxadustat by approximately 5% compared with roxadustat administered alone. The median tmax ranged between 2.0 and 3.0 hours, and the mean t½ ranged between 9.6 and 11.6 hours; neither were notably impacted by SCA (Table 6).
Figure 2.
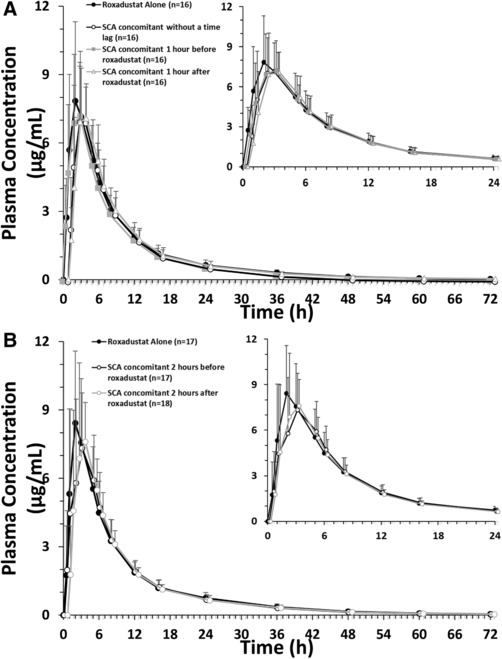
Mean plasma concentration profile of roxadustat (pharmacokinetic analysis set, SCA/roxadustat drug‐drug interaction study) for subjects in part 1 (A) and part 2 (B). Data are presented as arithmetic mean ± standard deviation. All roxadustat levels that were below the level of quantification were set to zero. Inset displays roxadustat concentration from 0 hours to 24 hours for enhanced clarity. SCA, spherical carbon adsorbent.
Table 6.
Pharmacokinetic Parameters for Roxadustat (PKAS)
| Part 1 | AUCinf (μg · h/mL) | Cmax (μg/mL) | CL/F (L/h) | tmax (h) | t½ (h) |
|---|---|---|---|---|---|
| Roxadustat alone, N = 16 | |||||
| Mean (SD) | 73.97 (16.51) | 9.45 (1.99) | 1.42 (0.352) | 10.85 (2.49) | |
| %CV | 22.3 | 21.0 | 24.8 | 22.9 | |
| Median (range) | 74.41 (46.42‐99.83) | 9.55 (5.75‐13.95) | 1.35 (1.00‐2.15) | 2.0 (1.0‐5.0) | 9.76 (8.31‐15.84) |
| Roxadustat + Kremezin (without a time lag), N = 16 | |||||
| Mean (SD) | 66.54 (14.02) | 8.38 (1.53) | 1.57 (0.321) | 9.61 (1.45) | |
| %CV | 21.1 | 18.3 | 20.5 | 15.1 | |
| Median (range) | 62.42 (47.67‐92.18) | 8.67 (5.41‐10.71) | 1.60 (1.08‐2.10) | 2.5 (1.0‐5.0) | 9.56 (7.21‐12.44) |
| GMR, % | 90.3 | 89.0 | |||
| 90%CI | 86.1‐94.8 | 81.7‐97.0 | |||
| Roxadustat + Kremezin (1 h before roxadustat), N = 16 | |||||
| Mean (SD) | 67.46 (15.61) | 8.71 (1.61) | 1.56 (0.368) | 10.11 (1.741) | |
| %CV | 23.1 | 18.5 | 23.6 | 17.2 | |
| Median (range) | 67.02 (43.00‐92.50) | 8.38 (6.20‐11.78) | 1.49 (1.08‐2.33) | 2.5 (1.0‐5.0) | 9.89 (7.75‐13.62) |
| GMR, % | 91.1 | 92.6 | |||
| 90%CI | 86.8‐95.7 | 85.0‐100.9 | |||
| Roxadustat + Kremezin (1 h after roxadustat), N = 16 | |||||
| Mean (SD) | 66.65 (12.93) | 8.66 (1.08) | 1.55 (0.303) | 9.91 (1.352) | |
| %CV | 19.4 | 12.4 | 19.5 | 13.7 | |
| Median (range) | 65.35 (46.47‐93.10) | 8.45 (7.15‐10.81) | 1.53 (1.07‐2.15) | 2.0 (1.0‐5.0) | 9.95 (7.37‐13.29) |
| GMR, % | 90.8 | 92.9 | |||
| 90%CI | 86.5‐95.3 | 85.2‐101.2 | |||
| Part 2 | AUCinf (μg · h/mL) | Cmax (μg/mL) | CL/F (L/h) | tmax (h) | t1/2 (h) |
|---|---|---|---|---|---|
| Roxadustat alone, N = 17 | |||||
| Mean (SD) | 78.07 (13.89) | 9.94 (1.10) | 1.32 (0.252) | ‐ | 10.96 (2.87) |
| %CV | 17.8 | 11.1 | 19.1 | ‐ | 26.2 |
| Median (range) | 78.96 (51.86‐104.17) | 10.00 (8.21‐11.56) | 1.27 (0.96‐1.93) | 2.0 (1.0‐5.0) | 10.12 (8.04‐18.28) |
| Roxadustat + Kremezin (2 h before roxadustat), N = 17 | |||||
| Mean (SD) | 73.91 (14.68) | 9.56 (2.03) | 1.41 (0.307) | 11.57 (4.17) | |
| %CV | 19.9 | 21.2 | |||
| Median (range) | 72.94 (45.41‐106.48) | 9.12 (7.03‐13.60) | 21.8 | 36.0 | |
| GMR, % | 94.5 | 95.3 | 1.37 (0.94‐2.20) | 3.0 (1.0‐5.0) | 10.66 (7.47‐24.17) |
| 90%CI | 88.5‐100.9 | 86.5‐105.1 | |||
| Roxadustat + Kremezin (2 h after roxadustat), N = 18 | |||||
| Mean (SD) | 72.39 (10.30) | 9.50 (2.01) | 1.41 (0.202) | 10.57 (2.43) | |
| %CV | 14.2 | 21.2 | 14.4 | 23.0 | |
| Median (range) | 72.68 (54.68‐91.65) | 9.55 (5.48‐13.62) | 1.38 (1.09‐1.83) | 2.03 (1.0‐5.0) | 10.72 (7.56‐15.59) |
| GMR, % | 92.7 | 94.6 | |||
| 90%CI, % | 86.9‐99.0 | 85.9‐104.2 | |||
AUCinf, area under the concentration‐time curve from the time of dosing extrapolated to time infinity; CI, confidence interval; Cmax, maximum concentration; CL/F, apparent total systemic clearance after single or multiple extravascular dosing; CV, coefficient of variation; GMR, geometric mean ratio; PKAS, pharmacokinetic analysis set; SD, standard deviation; tmax, time of the maximum concentration; t½, terminal elimination half‐life.
Safety
Food Effect Study
No TEAEs occurred under the fasted condition. Under the fed condition, one subject (6.7%) reported TEAEs that caused study discontinuation, including elevations in creatine phosphokinase and lactate dehydrogenase and presence of blood in urine, that were considered mild to moderate in severity; and elevation in aspartate aminotransferase that was reported as a serious TEAE. These events occurred on day 7 of period 1, 2 days after the subject conducted physical work at a warehouse for 8 hours, and the investigator considered the TEAEs a consequence of the physical activity and not related to the study drug. This subject had recovered from the event by the follow‐up visit on day 20.
Spherical Carbon Adsorbent/Roxadustat DDI Study
Nasopharyngitis, the only TEAE reported in this study, occurred in 1 of 16 subjects who received roxadustat alone during part 1, and in 3 of 18 subjects who received SCA 2 hours before (n = 1) and 2 hours after (n = 2) roxadustat during part 2. One subject who received SCA 2 hours after roxadustat discontinued the study due to nasopharyngitis. All TEAEs reported throughout the study were mild or moderate in severity and not related to the study drug.
Discussion
This report describes the findings of 2 studies that evaluated the effect of food and SCA on the PK, safety, and tolerability of a single oral dose of 100‐mg roxadustat in healthy nonelderly adult male Japanese subjects. The PK of roxadustat did not change significantly when roxadustat was administered under fed or fasted conditions, except for an ≈20% reduction of Cmax and a delay in tmax under fed conditions. Furthermore, the urine PK of roxadustat did not differ between fed or fasted conditions. The GMRs (fed/fasted) and 90%CIs for AUCinf and AUClast were both within the no‐effect boundaries of 80% and 125%, indicating absence of a food effect on these parameters. Considering that roxadustat shows good solubility at neutral pH, it is unlikely that the decrease observed in the Cmax under fed versus fasted conditions is caused by a reduced dissolution rate of roxadustat; however, it could have been a result of delayed gastric emptying. Generally speaking, a reduced gastric emptying rate associated with a fed state may delay the absorption of roxadustat and, in turn, impact these parameters.
When SCA was administered with roxadustat without a time lag or 1 hour before or after administration of roxadustat, mean AUCinf and Cmax of roxadustat were reduced by 9% to 10% and 7% to 11%, respectively. Administration of SCA 2 hours before or after roxadustat reduced the mean AUCinf by 6% to 7% and Cmax by approximately 5% compared with administration of roxadustat alone. Roxadustat was rapidly absorbed and reached Cmax in ≈2 hours. When SCA was administered 1 or 2 hours after roxadustat, the tmax was unchanged. When SCA was administered with no lag time or 1 or 2 hours before roxadustat, tmax of roxadustat ranged between 2.5 and 3 hours. Overall, coadministration of 2‐g SCA TID with a single oral dose of 100‐mg roxadustat in healthy nonelderly adult male subjects did not have a clinically relevant effect on roxadustat exposure when roxadustat and SCA were coadministered simultaneously or with a time lag.
A previous DDI study demonstrated that when 0.25 mg of triazolam was administered with SCA 2 g, the AUC and Cmax of triazolam were reduced by 41% and 33%, respectively.15 SCA can adsorb substances with a molecular weight of 100 to 1000 in vitro18; the molecular weight of roxadustat is 352.34, which theoretically supports the potential DDI between these 2 agents. Despite this potential for DDI, the current study showed no clinically relevant interaction between SCA and roxadustat. The lack of DDI observed in this study may be partly a result of a short residence time of roxadustat in the gastrointestinal tract, resulting from the high permeability and rapid absorption of roxadustat, limiting the interaction between SCA and roxadustat. Moreover, the interaction between SCA and roxadustat occurs in the absorption phase, which would not be expected to differ between healthy subjects and patients with CKD. These considerations suggest that the data reported in this study could be safely extrapolated to CKD patients. Furthermore, the PK of roxadustat, when administered alone, reported in this study is consistent with that reported in a previous study of healthy subjects.12
In both studies, a single oral dose of 100‐mg roxadustat administered alone or in combination with food or SCA was considered safe and well tolerated, regardless of dosing condition. No deaths occurred in either study. One subject in the food effect study reported a serious TEAE of increased aspartate aminotransferase on day 7 of period 1, which the investigator considered to be caused by physical activity conducted by the subject 2 days prior to the event and was not considered related to the study drug. The subject had recovered at the follow‐up visit on day 20.
Overall, this study demonstrated that the effect of food on the PK of roxadustat and DDI between roxadustat and SCA do not appear to be clinically relevant and supports the safe use of roxadustat under these conditions.
Acknowledgments
The authors thank Kota Kato, MSc, for assistance with the bioanalysis of roxadustat. Medical writing and editorial assistance was provided by SuccinctChoice Medical Communications (Chicago, IL) and funded by Astellas Pharma Inc.
Declaration of Conflicting Interests
Roxadustat is being developed by Astellas Pharma, FibroGen, and AstraZeneca. M.K., T.S., A.T., M.U., and Y.N. are employees of Astellas. K.F. and R.Y. declare no conflict of interest.
Funding
This study was sponsored and monitored by Astellas Pharma, Inc.
References
- 1. Covic A, Jackson J, Hadfield A, Pike J, Siriopol D. Real‐world impact of cardiovascular disease and anemia on quality of life and productivity in patients with non–dialysis‐dependent chronic kidney disease. Adv Ther. 2017;34(7):1662–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fraser SD, Roderick PJ, May CR, et al. The burden of comorbidity in people with chronic kidney disease stage 3: a cohort study. BMC Nephrol. 2015;16:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One. 2014;9(1):e84943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eriksson D, Goldsmith D, Teitsson S, Jackson J, van Nooten F. Cross‐sectional survey in CKD patients across Europe describing the association between quality of life and anaemia. BMC Nephrol. 2016;17(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Becker K, Saad M. A new approach to the management of anemia in CKD patients: a review on roxadustat. Adv Ther. 2017;34(4):848–853. [DOI] [PubMed] [Google Scholar]
- 6. Chen N, Qian J, Chen J, et al. Phase 2 studies of oral hypoxia‐inducible factor prolyl hydroxylase inhibitor FG‐4592 for treatment of anemia in China. Nephrol Dial Transplant. 2017;32(8):1373–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Provenzano R, Besarab A, Wright S, et al. Roxadustat (FG‐4592) versus epoetin alfa for anemia in patients receiving maintenance hemodialysis: a phase 2, randomized, 6‐ to 19‐week, open‐label, active‐comparator, dose‐ranging, safety and exploratory efficacy study. Am J Kidney Dis. 2016;67(6):912–924. [DOI] [PubMed] [Google Scholar]
- 8. Besarab A, Chernyavskaya E, Motylev I, et al. Roxadustat (FG‐4592): correction of anemia in incident dialysis patients. J Am Soc Nephrol. 2016;27(4):1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Besarab A, Provenzano R, Hertel J, et al. Randomized placebo‐controlled dose‐ranging and pharmacodynamics study of roxadustat (FG‐4592) to treat anemia in nondialysis‐dependent chronic kidney disease (NDD‐CKD) patients. Nephrol Dial Transplant. 2015;30(10):1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Provenzano R, Besarab A, Sun CH, et al. Oral hypoxia‐inducible factor prolyl hydroxylase inhibitor roxadustat (FG‐4592) for the treatment of anemia in patients with CKD. Clin J Am Soc Nephrol. 2016;11(6):982–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Groenendaal‐van de Meent D, Adel MD, Noukens J, et al. Effect of moderate hepatic impairment on the pharmacokinetics and pharmacodynamics of roxadustat, an oral hypoxia‐inducible factor prolyl hydroxylase inhibitor. Clin Drug Investig. 2016;36(9):743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Groenendaal‐van de Meent D, den Adel M, Rijnders S, et al. The hypoxia‐inducible factor prolyl‐hydroxylase inhibitor roxadustat (FG‐4592) and warfarin in healthy volunteers: a pharmacokinetic and pharmacodynamic drug‐drug interaction study. Clin Ther. 2016;38(4):918–928. [DOI] [PubMed] [Google Scholar]
- 13. Schulman G, Berl T, Beck GJ, et al. The effects of AST‐120 on chronic kidney disease progression in the United States of America: a post hoc subgroup analysis of randomized controlled trials. BMC Nephrol. 2016;17(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cha RH, Kang SW, Park CW, et al. Sustained uremic toxin control improves renal and cardiovascular outcomes in patients with advanced renal dysfunction: post‐hoc analysis of the Kremezin Study against renal disease progression in Korea. Kidney Res Clin Pract. 2017;36(1):68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kotegawa T, Tsutsumi K, Imai H, Ohashi K. Drug‐drug interactions of the oral spherical activated charcoal Kremezin. Jpn J Clin Pharmacol Ther. 2013;44(2):77–84. [Google Scholar]
- 16. Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour, and Welfare . Guidance on clinical pharmacokinetic studies of pharmaceuticals. Notification No. 796; June 1, 2001.
- 17. Williams EJ. Experimental designs balanced for the estimation of residual effects of treatments. Aust J Sci Res. 1949;2(2):149–168. [Google Scholar]
- 18. Honda Y, Nakano M. Studies of pharmacological interactions of AST‐120 capsule 200 with drugs. Clin Rep. 1994;28:2873–2881. [Google Scholar]


