Abstract
Objectives
In this review, we aim at updating the available information on the improvement of the Hypericum perforatum L. (Hypericaceae) phytochemical profile and pharmacological properties via elicitation.
Key findings
Hypericum perforatum seedlings, shoots, roots, calli and cell suspension cultures were treated with diverse elicitors to induce the formation of secondary metabolites. The extracts of the elicitor‐treated plant material containing naphthodianthrones, phloroglucinols, xanthones, flavonoids and other new compounds were quantitatively analysed and tested for their bioactivities. While hypericins were mainly produced in H. perforatum cultures containing dark nodules, namely shoots and seedlings, other classes of compounds such as xanthones, phloroglucinols and flavonoids were formed in all types of cultures. The extracts obtained from elicitor‐treated samples generally possessed better bioactivities compared to the extract of control biomass.
Summary
Although elicitation is an excellent tool for the production of valuable secondary metabolites in H. perforatum cell and tissue cultures, its exploitation is still in its infancy mainly due to the lack of reproducibility and difficulties in scaling up biomass production.
Keywords: elicitation, Hypericum perforatum, in vitro cultures, nanoparticles, plant secondary metabolism
Introduction
Hypericum perforatum L. (Hypericaceae) known as St John's wort is an important medicinal plant. Extracts, infusions and decoctions have been used in the treatment of various ailments since ancient times. A number of pharmacological studies and clinical trials have shown that H. perforatum extracts possess an astounding array of pharmacological properties including antidepressant, anti‐inflammatory, antiviral, anticancer and antibacterial activities. In particular, H. perforatum extracts are as efficient as their drug counterparts like fluoxetine (Prozac), sertraline (Zoloft) and other leading antidepressant drugs in the treatment of depression. Nowadays, it is a widely used medicinal plant for the treatment of mild and moderate depression,1 prescribed for this indication in some EU countries, while sold in the United States as herbal supplement over the counter.2
In addition to its efficacy in the treatment of neurological disorders, studies suggest that this herb may be useful in treating cancer, inflammation‐related disorders and bacterial and viral diseases.3, 4, 5, 6, 7 These medicinal properties are related to the composition of the secondary metabolites present in the extract, particularly hypericins, hyperforins, flavonoids, xanthones and other valuable compounds.8 Phytochemical characterization of H. perforatum revealed the presence of various classes of compounds, including naphthodianthrones (hypericin and pseudohypericin), prenylated acylphloroglucinols (hyperforin and adhyperforin), flavonoids (quercetin, hyperoside, rutin, catechin and isoquercitrin), biflavones (amentoflavone, biapigenin), phenolic compounds (chlorogenic acid, tannic acid and caffeic acid), xanthones (1,3,6,7‐tetrahydroxyxanthone) and essential oil rich in sesquiterpenes9, 10, 11 (Figure 1).
Figure 1.
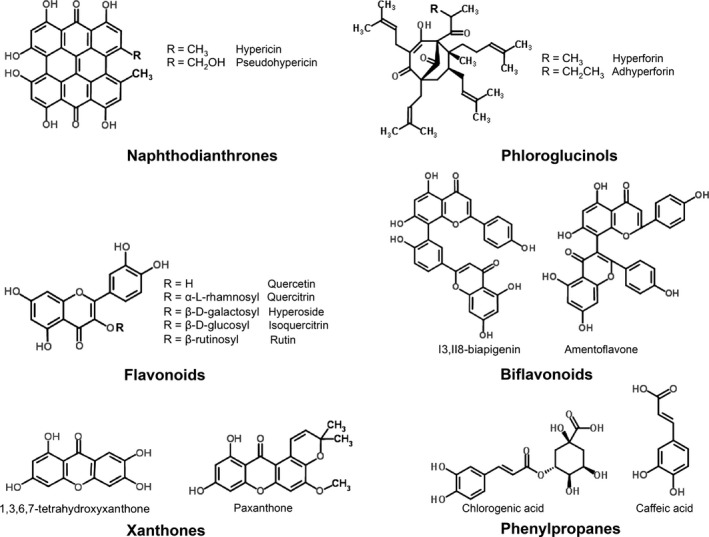
Major classes of secondary metabolites found in Hypericum perforatum.
Currently, H. perforatum is one of the top‐selling herbal medicines worldwide. Because of its reputed medicinal values, this species is included in the Pharmacopoeias of several countries including Europe and USA. According to the U.S. and European Pharmacopoeias, the crude drug consists of the dried flowering tops or the aerial parts of the plant. In order to meet the ever‐increasing demands of the pharmaceutical industry and to obtain quality biomass, H. perforatum is cultivated in many countries. Plants growing in the field conditions are generally exposed to biotic and abiotic challenges, which can affect the phytochemical composition. For example, H. perforatum plants obtained from different geographical regions, seasons, soil conditions significantly differ in their phytochemical profile.12, 13, 14 As the pharmacological potential of H. perforatum extracts is mainly determined by its phytochemical composition and ratios between important compounds, such changes may affect the treatment efficacy of the extracts.15, 16 In vitro cultures grown under controlled conditions can overcome these issues and have been emerged as an attractive alternative to field cultivation.17, 18
Today, enhancing the contents of important bioactive molecules and producing novel compounds are major aspects of H. perforatum biotechnology. Developments in plant cell culture systems and molecular biology offered many ways to improve the production of compounds such as cell line selection, cell immobilization, permeabilization, precursor feeding, product secretion, biotransformation, metabolic engineering, bioreactor engineering, synthetic biology and elicitation.19, 20, 21 Among all, elicitation emerges as an attractive strategy for enhancement of secondary metabolite production in plant species like H. perforatum, in which the application of metabolic engineering or synthetic biology tools remains difficult due to the lack of proficient transformation methods and genetic information about biosynthetic pathways.22 Hence, production of secondary metabolites via elicitation using various in vitro culture systems is of great interest in H. perforatum research.
Significant amounts of data on the manipulation of H. perforatum secondary metabolism via elicitation have been accumulated in the literature in the recent years. However, a consolidated account or a critical analysis of these published data is not available to the best of our knowledge. Here, we holistically review the available information on the elicitation of H. perforatum secondary metabolism, envisage the potential application of nanoparticles as elicitors and discuss how elicitation‐mediated improvement of the H. perforatum secondary metabolite profile may be exploited for drug discovery.
The basis of elicitation
Plants have to defend themselves against any threats posed by their environment, such as pathogen attack, herbivory, drought, salinity, exposure to UV radiation. Plants perceive such danger signals through their receptors and sensors and activate defence responses to counteract against these stresses.23, 24, 25, 26 The responses include secondary metabolism.27 For instance, plants recognize pathogen‐derived elicitors through receptors bound to the plasma membrane and activate, as a defence response, the production of low‐molecular‐weight antimicrobial compounds. While these phytoalexins are synthesized by and accumulated in plants only after exposure to pathogenic microbes, phytoanticipins are either pre‐existing or synthesized after the microbial attack solely from pre‐existing constituents of the plant.28 In addition to their role in plant defence response, these compounds often possess important pharmacological properties.29, 30 The ability of plants to counteract with biotic and abiotic stresses via mobilizing their secondary metabolism is the central dogma of elicitation.
An elicitor can be an environmental factor or a signal molecule that activates a signal‐transduction cascade, which mediates the expression of genes related to the biosynthesis of secondary metabolites in the biotechnological point of view. Elicitors are mainly classified into three categories based on their origin, namely biological, chemical and physical triggers. The biological elicitors are mainly components of microbial cell walls (chitin, chitosan and glucans) and carbohydrates such as poly‐ and oligosaccharides derived from plant cell walls (pectin, pectic acid, and cellulose). Poly‐ and oligosaccharides are the most studied signalling molecules for elicitation pathways because these compounds can effectively induce similar plant defence responses to pathogen invasion.31 Upon elicitation, a series of metabolic changes are systemically initiated throughout the plant to activate the plant's innate immune system32, 33 and also to prime the plant for stress challenge.34 Furthermore, the plant defence signalling compounds such as salicylic acid (SA), jasmonic acid (JA), methyl jasmonate (MeJA) and nitric oxide which mediate the defence response can also serve as elicitors and their ability to induce secondary metabolism is well‐documented.8, 35, 36, 37, 38, 39 Inorganic agents like heavy metals, metal ions and metal oxides can act as chemical elicitors of plant secondary metabolism.40, 41, 42 Physical components like cold shock, UV, ozone, osmotic and water stress also induce enzymatic activity and secondary metabolism.43
The mechanism of elicitation is vastly complex because of thousands of intertwined events. Additionally, all these events fluctuate with the origin, specificity and concentration of elicitors, stage of the growth cycle and nutritional uptake of plants, physiochemical environment of the interaction etc. Although it is difficult to propose a universal model for the elicitation mechanism, calcium flux, reactive oxygen species (ROS) burst and mitogen‐activated protein kinase (MAPK) phosphorylation are the initial events triggered in most of the elicitor–plant cell interactions.44 Later events like activation of signalling pathways and activation of transcription factors leading to the induction of plant secondary metabolism are also well‐documented.45, 46, 47
Signal recognition is mediated by receptors and elicitor‐binding sites present on the plasma membrane in response to elicitors, which activate the next cascade of events like ion fluxes, Ca2+ burst, cytoplasmic acidification, ROS burst, NADPH oxidase activation, G‐protein activation and MAPK phosphorylation.31 The initial plant response is the exchange of ions, for instance K+/Cl− effluxes and Ca2+/H+ influxes, in response to elicitors. Ca2+ influx is considered as the most important event because of its diverse involvement in physiological and cellular processes.48, 49 Ca2+ signals are elaborated through conformational changes in various Ca2+‐binding proteins such as calmodulin, calmodulin‐like proteins, calcium‐dependent kinases (CDPKs) and phospholipases as well as through secondary messengers like inositol 1,4,5‐ triphosphate (IP3) and diacylglycerol (DAG).50, 51, 52 Ca2+/Calmodulin‐mediated pathways are involved in many physiological responses of plants to stimuli. CDPKs have diverse roles in downstream signalling cascades and protein phosphorylation to coordinate cellular processes like regulation of the oxidative burst, hormonal signalling and gene expression.53 ROS generation is another important phenomenon in plant defence response, as is the effect of NADPH oxidase and other oxidases in plant cells, and even Ca2+ spiking is also responsible for ROS generation.49, 54, 55 Studies have shown the linked role of G‐proteins in stimulating ion channels, phospholipase A, phospholipase C and phospholipase D, ROS generation and cell death in plants.50, 56, 57 Activated G‐protein can stimulate the level of cAMP, IP3 and DAG, which triggers the target kinases PKA and PKC. These induced protein kinases cause phosphorylation of MAPKs, which results in gene expression leading to enzymatic reactions, which in turn reprogram the pathway of secondary metabolite production58 (Figure 2).
Figure 2.
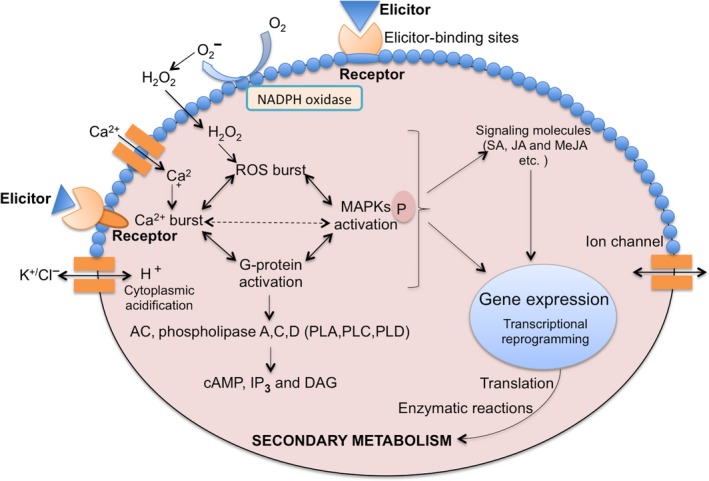
The molecular mechanism of elicitation: Recognition of elicitors by plasma membrane‐bound receptors results in ion fluxes, Ca2+ burst, cytoplasmic acidification, ROS burst, NADPH oxidase activation, G‐protein activation and mitogen‐activated protein kinase phosphorylation. It also activates downstream signalling pathway messengers like salicylic acid, jasmonic acid and methyl jasmonate. Messengers activate transcription factors and gene expression, which lead to reprogramming secondary metabolism. [Colour figure can be viewed at wileyonlinelibrary.com]
Manipulation of Hypericum perforatum Secondary Metabolism Via Elicitation
In general, the type of culture rather than the type of elicitor defines the compounds induced in H. perforatum upon elicitation. Hypericins are the most frequently elicitor‐induced compounds in seedlings and shoot cultures, probably due to the presence of hypericin nodules. On the other hand, cell suspensions, calli and root cultures mostly form flavonoids and xanthones.
In addition to the type of culture, several other factors affect the success of elicitation, which include the type of elicitor, concentration, incubation conditions and duration of elicitor treatment. Various biotic and abiotic elicitors tested for the manipulation of H. perforatum secondary metabolism are categorized in Figure 3. The important compounds induced by these elicitors in various types of H. perforatum cultures such as seedling, shoot, root, callus and cell suspension are listed in Table 1.
Figure 3.
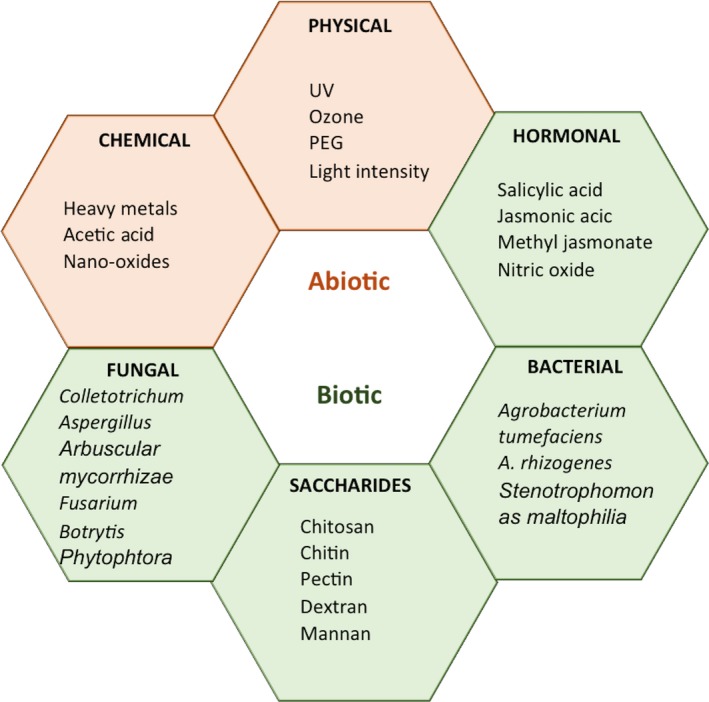
Elicitors of biotic and abiotic origin tested for the induction of secondary metabolites in Hypericum perforatum. [Colour figure can be viewed at wileyonlinelibrary.com]
Table 1.
Various types of Hypericum perforatum cultures and elicitors used in the induction of secondary metabolites
| Culture type | Elicitor | Compounds elicited | Reference |
|---|---|---|---|
| Cell suspension | Zinc nano‐oxide | Hypericin and hyperforin | 41 |
| Iron nano‐oxide | Hypericin and hyperforin | 41 | |
| Chitin | Phenolics, flavonols, flavanols, anthocyanins, hypericin and pseudohypericin | 66 | |
| Pectin | Phenolics, flavonols, flavanols, anthocyanins, hypericin and pseudohypericin | 66 | |
| Dextran | Phenolics, flavonols, flavanols, anthocyanins, hypericin and pseudohypericin | 66 | |
| Phytophthora cinnamoni fungal cell wall extract | None | 39 | |
| Colletotrichum gloeosporioides mycelium extract | Xanthones and flavonoids | 35 | |
| Aspergillus flavus mycelium extract | Phenolic compounds, flavanols, flavonols, anthocyanins and hypericins | 62 | |
| Fusarium oxysporum | Phenolics, flavonoids, anthocyanins, hypericin and pseudohypericin | 63 | |
| Phoma exigua | Phenolics, flavonoids, anthocyanins, hypericin and pseudohypericin | 63 | |
| Botrytis cinerea | Phenolics, flavonoids, anthocyanins, hypericin and pseudohypericin | 63 | |
| JA | Hypericins | 39 | |
| JA | Phenolic compounds, flavanols, flavonols, hypericin and pseudohypericin | 38 | |
| MeJA | Flavones | 35 | |
| MeJA | Flavonoids | 8 | |
| SA | Xanthones | 85 | |
| SA | None | 35 | |
| SA | None | 39 | |
| SA | Hypericins, pseudohypericins | 86 | |
| SA | Flavonoids | 8 | |
| MeJA + C. gloeosporioides | Xanthones | 35 | |
| SA + C. gloeosporioides | Xanthones | 35 | |
| Agrobacterium tumefaciens | Xanthones | 71 | |
| A. tumefaciens | Lignin and flavanoids | 72 | |
| A. tumefaciens | Phenolics, flavonols, flavanols and xanthones | 73 | |
| A. rhizogenes | Phenolics, flavonols, flavanols and xanthones | 73 | |
| Callus | SA | Hypericins, pseudohypericins | 86 |
| Root | MeJA | Hypericin | 84 |
| IBA | Hypericin | 84 | |
| Acetic acid | Xanthones | 91 | |
| Chitosan | Xanthones | 68 | |
| Chitosan | Xanthones | 68 | |
| Shoot | SA | Hypericins and pseudohypericins | 86 |
| Mannan | Pseudohypericin and hypericin | 59 | |
| β‐1,3‐glucan | Pseudohypericin | 59 | |
| Pectin | Pseudohypericin | 59 | |
| Yeast extract | None | 59 | |
| Chitin | None | 64 | |
| Pectin | Hypericin and pseudohypericin | 64 | |
| Dextran | Hypericin and pseudohypericin | 64 | |
| Saccharose | Hypericin and hyperforin | 69 | |
| Saccharose + PEG | Hypericin and hyperforin | 69 | |
| Saccharose + MeJA | Hypericin and hyperforin | 69 | |
| Saccharose + A. tumefaciens | Hypericin and hyperforin | 69 | |
| Stenotrophomonas maltophilia culture filtrate | Pseudohypericin | 70 | |
| Seedling | Chromium | Protopseudohypericin, hypericin and pseudohypericin | 40 |
| Nickel | None | 12 | |
| Rhizophagus intraradices | Hypericin and pseudohypericin | 60 | |
| S. maltophilia | Hypericin and pseudohypericin | 70 |
SA, salicylic acid; JA, jasmonic acid; MeJA, methyl jasmonate; PEG, polyethylene glycol.
Biotic elicitation
Fungi and extracts from fungal mycelia
In H. perforatum shoot cultures, yeast extract did not show any stimulatory effect on either hypericin or pseudohypericin production.59 The arbuscular mycorrhizal fungi (AMF) species Rhizophagus intraradices enhanced H. perforatum seedling growth and hypericin and pseudohypericin production, when supplied to the soil either alone or as mixture with AMF, namely Funneliformis constrictum, F. geosporum and F. mosseae.60
Hypericum perforatum cell suspension cultures treated with Colletotrichum gloeosporioides cell wall extract showed a significant increase in xanthone accumulation.35 Interestingly, this xanthone accumulation was increased 12‐fold when the cultures were primed with MeJA or SA, before adding the fungal extract. Aspergillus niger, Fussarium oxysporum and yeast extracts increased the accumulation of phenolic compounds and flavonoids in addition to the production of new constituents such as p‐hydroxybenzoic acid in the suspended cells of H. triquetrifolium.61 On the other hand, mycelium extract from Aspergillus flavus only stimulated the level of anthocyanins in H. perforatum cell suspensions.62
Interestingly, cell wall extracts from F. oxysporum, Phomaexigua and Botrytis cinerea showed rapid stimulation of naphthodianthrones (hypericin and pseudohypericin) in addition to flavonoids and anthocyanins in cell suspension cultures of H. perforatum.63 A. niger cell walls induced hypericin biosynthesis in H. perforatum cell suspension cultures.36
Polysaccharides
Oligosaccharides and polysaccharides, respectively, from fungal and plant cell walls are the most studied signalling molecules in elicitation pathways.31 In H. perforatum shoot cultures, mannan, β‐1,3‐glucan, pectin and yeast extract were studied for the production of naphthodianthrones.59 Although mannan stimulated the production of pseudohypericin and hypericin substantially, β‐1,3‐glucan and pectin showed a weak effect on pseudohypericin production but had no effect on hypericin production. While pectin and dextran enhanced the content of pseudohypericin and hypericin, chitin did not show any stimulatory effect in H. perforatum shoot cultures.64 Mannan and pectin were tested for the biosynthesis of hypericins in H. adenotrichum seedlings grown in vitro.65 Although both elicitors stimulated the biosynthesis of pseudohypericin and hypericin, the stimulatory potential of mannan was lower than that of pectin.
Treatment with chitin, pectin and dextran improved production of phenylpropanoids (phenolics, flavanols, anthocyanins) in cell suspension cultures.66 Brasili et al.67, 68 studied the effect of chitosan treatment in H. perforatum adventitious root cultures. Chitosan increased the synthesis of epicatechin, xanthones and isoprenoids68 and a new xanthone, brasilixanthone B, was identified in treated root cultures.67
Bacteria
Elicitation of H. perforatum shoot cultures with a combination of saccharose and inactivated Agrobacterium tumefaciens promoted the hypericin and hyperforin production.69 Treatment of H. perforatum seedlings with Stenotrophomonas maltophilia increased the hypericin and pseudohypericin contents.70 These authors also reported that treating H. perforatum shoot cultures with a S. maltophilia culture filtrate induced only pseudohypericin production.70
Analysis of the methanolic extract of cell suspension cultures treated with A. tumefaciens revealed a 12‐fold increase in the total xanthone concentration and the emergence of many new xanthones.71 In addition, the contents of lignin and flavonoids (quercetin, quercetrin etc.) were also significantly increased in the cell wall fraction of phenolics after elicitation with A. tumefaciens.72 Similarly, improvement of phenolic, flavonol, flavanol and xanthone concentrations in response to A. tumefaciens and A. rhizogenes treatment was observed in cell suspension cultures.73 However, A. rhizogenes was less effective in the induction of secondary metabolism compared to A. tumefaciens.73 Induction of H. perforatum plant secondary metabolism by Agrobacterium might be attributed to components such as cold‐shock protein, flagellin, peptidoglycan and elongation factor‐Tu.74, 75, 76, 77, 78, 79, 80
Signalling compounds and plant growth regulators
Plant defence signalling compounds such as SA, MeJA, JA have been shown to modulate H. perforatum secondary metabolism. When treated with an analogue of MeJA, 2,3‐dihydroxypropyl jasmonate, hypericins and hyperforin production was increased in H. perforatum and H. sampsonii shoot cultures.81 Different combinations of plant growth regulators and signalling compounds (JA and SA) enhanced the accumulation of hypericins, pseudohypericin and hyperforin in shoot cultures of H. hirsutum and H. maculatum.82 The supplementation of plant growth regulators in culture medium variously affected naphthodianthrone accumulation in shoot cultures.83 This study reported that the presence of IBA actually decreased the concentration of hypericin and pseudohypericin although the naphthodianthrone production in plantlets was not influenced by IAA supplementation.
The MeJA concentration showed a negative correlation with biomass production and a positive correlation with hypericin production in adventitious root cultures grown in balloon‐type airlift bioreactors, in which an optimum hypericin content of 1.61 mg/g DW was achieved at 350 μm MeJA.84
The effect of signalling compounds on the induction of secondary metabolism in cell suspension cultures of H. perforatum is divergently reported between groups. Conceicao et al.35 reported that MeJA‐treated H. perforatum cell suspension cultures accumulated new flavonoids (flavones), whereas SA was unable to induce any change of secondary metabolism. Elicitation of cell cultures with 100 μmol/l MeJA on day 15 resulted in 2.7 times higher flavonoid production (280 mg/l) compared to control cultures.8 Another study reported that JA treatment significantly increased phenolic compounds, flavanols and flavonols, concomitantly reducing the anthocyanin content.38 A twofold increase in the xanthone, cadensin G and paxanthone contents was found in cell suspension cultures of H. perforatum following elicitation with SA.85
Walker et al.39 found that JA dramatically enhanced hypericin production in cell cultures incubated in the dark compared to cultures grown under photoperiodic conditions, although SA treatment could not induce hypericin production in cell suspensions. Contrarily, SA remarkably influenced the production of hypericin and pseudohypericin in H. perforatum cell suspension cultures as per the report of Gadzovska‐Simic et al.86
Abiotic elicitation
Physical factors
A few physical factors such as exposure to high light intensity, ozone, UV and osmotic stress have been tested for the induction of secondary metabolism in H. perforatum. The hyperforin, pseudohypericin and hypericin concentrations were altered in H. perforatum plants after UV‐B exposure during the vegetative growth.87 However, the level and length of UV‐B radiation negatively affected the hypericin concentration in leaves, although the flavonoid and tannin contents increased with enhanced levels of UV‐B exposure.88 Treatment with polyethylene glycol (PEG) increased the hypericin and pseudohypericin concentrations in cultured H. adenotrichum seedlings. However, this increase was dependent on the concentration of PEG and the treatment period, 10 g/l PEG for a 15‐day treatment being optimal.89 On the other hand, synthesis of hypericin and hyperforin in H. perforatum shoot cultures was not increased in the presence of 10 and 15 g/l PEG, although a lower PEG concentration stimulated the production of those compounds.69 Sucrose as an osmotic agent was shown to stimulate hypericins in cultured seedlings of H. adenotrichum with increasing concentrations until 45 g/l, followed by decline.89 Correspondingly, the presence of saccharose (10–30 g/l) influenced the production of hypericin and hyperforin in H. perforatum shoot cultures and a combination of PEG (1.25–5 g/l) and saccharose (10–30 g/l) synergistically increased the production of the above compounds.69
Hypericum perforatum cell suspension cultures accumulated anthocyanins and flavonoids with concomitant reduction of xanthones upon increasing the irradiance of cultures from 20 μmol/s per m2 to 60 μmol/s per m2.90 Exposure to ozone stimulated hypericin synthesis in H. perforatum cell suspension cultures, with the cell cultures at the exponential phase being more responsive than the ones at the lag and stationary phases.91
Chemical
Chromium treatment induced the production of protopseudohypericin, hypericin and pseudohypericin in H. perforatum seedlings, whereas nickel treatment did not show any stimulatory effect.40 In H. perforatum root cultures, acetic acid enhanced xanthone production.91
Treatment of H. perforarum cell suspension cultures with 100 ppb zinc and iron nano‐oxides promoted hypericin and hyperforin production, while higher concentrations of these nanomaterials negatively affected the production of these compounds.41
Pharmacological properties of Hypericum perforatum after elicitation
Although a number of studies report the elicitor‐induced modulation of the phytochemical profile of H. perforatum in vitro cultures, only a few of them actually tested the bioactivity of the treated cultures. The methanolic extract of H. perforatum cell suspension cultures challenged with A. tumefaciens was studied for various bioactivities. This extract showed a higher antioxidant potential, a greater capacity to prevent synaptosomal lipid peroxidation71 and improved antimicrobial activity against several human pathogenic bacteria.92 It also offered human HepG2 cells more protection against t‐BOOH‐induced oxidative damage, compared to control extract.93 Tocci et al.94 have shown that extracts of root tissue elicited with chitosan, O‐carboxymethyl chitosan and its derivatives showed antifungal activity against human fungal pathogens such as Candida spp., Cryptococcus neoformans and dermatophytes.
Future Perspectives
Potential application of nanotechnology
In addition to several well‐known biotic and abiotic elicitors discussed above, nanoparticles are currently emerging as new class of elicitors. A few studies on the induction of secondary metabolites in response to nanoparticle treatment provide insights in the exploitation of these submicron size particles as elicitors of secondary metabolites. For instance, the artemisinin content was increased in a hairy root culture of Artemisia annua upon treatment with core–shell silver nanoparticles.95 In Calendula officinalis, the anthocyanin and flavonoid contents were decreased, whereas carotenoid and saponin contents were increased in response to silver nanoparticles, as revealed by classical colorimetric assays.96 In addition, multiwalled carbon nanotubes stimulated the biosynthesis of secondary metabolites and the antioxidant capacity in the medicinal plant Satureja khuzestanica grown in vitro.97 In the case of H. perforatum, one study has reported that zinc and iron nano‐oxides stimulate hypericin and hyperforin production in cell suspension cultures.41
In addition to the use of metallic nanoparticles as elicitors, signalling compounds such as JA, SA and MeJA can be encapsulated in biodegradable polymers as nanoparticles and can be exploited for sustained release of the signalling molecules into the culture medium, which might be useful in sustained production of secondary metabolites without affecting the growth of cultures. The possibility of using metallic nanoparticles as trappers of secondary metabolites from plants has been demonstrated in A. thaliana.98 The authors reported that anatase TiO2 nanoparticles (smaller than 20 nm) enter plant cells, conjugate enediol‐ and catechol group‐rich flavonoids in situ and exit plant cells as flavonoid‐nanoparticle conjugates. The compound adsorption capacities of nanoparticles may be further improved by functionalization. For instance, the adsorption capacity of SiO2 nanoparticles towards quercetin could be improved by TiO2 functionalization compared to non‐functionalized and decyl group‐functionalized SiO2 nanoparticles due to possible binding of quercetin to the metal oxide.99 This adsorption capacity increased linearly with surface coverage of TiO2 emphasizing the correlation between the functional surface and quercetin adsorption. This phenomenon may provide us an attractive opportunity to establish nanotrapping strategies for a wide variety of secondary metabolites in the near future.
Elicitation of Hypericum perforatum secondary metabolism: a road to drug discovery
Although manipulation of H. perforatum secondary metabolism via elicitation has been reported in numerous studies, very few authors have actually tested the pharmacological properties of induced compounds and extracts from treated cells.92, 93, 94 To promote the discovery of novel drugs, extracts from elicitor‐treated cells containing new compounds should be analysed and screened for a variety of bioactivities. Bioactivity‐guided fractionation may be used. The possible road to drug discovery after elicitation is summarized in the following Figure 4.
Figure 4.
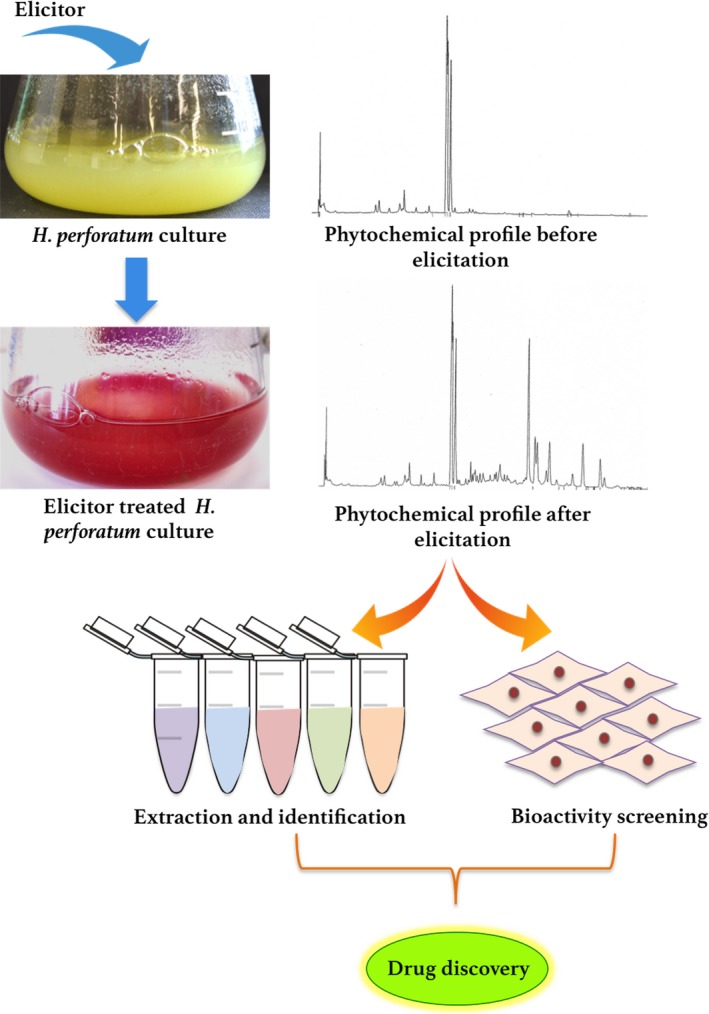
Scheme of possible exploitation of elicitation‐mediated changes in the Hypericum perforatum secondary metabolite profile for drug discovery via bioactivity‐guided fractionation. [Colour figure can be viewed at wileyonlinelibrary.com]
In spite of the exciting possibility of inducing diverse classes of bioactive compounds in H. perforatum cell and tissue cultures via elicitation, industrial exploitation of elicitation‐based changes in secondary metabolism and pharmacological properties is still in its infancy. The production of significant quantities of aseptic biomass for elicitation is the major concern. In this context, small‐scale bioreactors of in vitro cultures for the production of active compounds have been reported.18 Recently, adventitious root cultures in large‐scale bioreactors for the production of H. perforatum phytochemicals have been developed.84, 100 The choice of the correct culture vessels and the determination of exogenous signals needed for in vitro production of biomass and optimization of elicitation measures are essential factors for the further progress in this area.
Conclusion
Based on our analysis of the literature, it is evident that seedlings and in vitro cultures of H. perforatum are efficient systems to produce a wide variety of bioactive secondary metabolites via elicitation. The paradoxical results obtained between groups in terms of phytochemical profile, namely hypericin production after elicitation, argue for the complexity of culture maintenance, elicitation and analytical methods. For example, under normal conditions, hypericin is produced only in the presence of dark hypericin nodules. Several studies have confirmed a clear positive correlation between the presence of dark cell clusters and hypericin accumulation irrespective of the tissue, genotype, species etc.101, 102, 103, 104 Roots of H. perforatum plants lack these clusters, where no traces of hypericin were found.105 In spite of the absence of these nodules in roots and cell suspension cultures of H. perforatum, few groups have actually reported the formation of hypericins in these cultures after elicitation.38, 39, 41, 63, 66, 84, 86 Further investigation on the ability of H. perforatum cell and tissue cultures without these dark cell clusters to produce hypericin might offer novel clues on the hypericin biosynthetic pathway.
As the secondary metabolite elicitation potential varies between the types of cultures, elicitors, treatment conditions and other parameters, further research is needed to optimize the best and reproducible protocols. In this context, understanding the metabolic pathways leading to the production of specific secondary metabolites and their regulation is imperative. However, with the exception of the flavonoid pathway, complex biosynthetic pathways specifically evolved in Hypericum spp. are far from being understood. The lack of relevant information on the enzymes and genes involved as well as the transcription factors and master switches controlling these pathways is the major obstacle for developing efficient strategies for elicitation of H. perforatum secondary metabolism. Comparative transcriptomic, metabolomic and proteomic studies carried out on both control and elicitor‐treated cultures are expected to offer important clues.
Declarations
Acknowledgements
This work has received funding from the National Science Center, Poland (2016/21/B/NZ9/01980). PS and GF are supported by European Union's 7th Framework Programme for research, technological development and demonstration under grant agreement no 621321 and co‐financed by funds allocated for education through project no W26/7.PR/2015 [GA 3413/7.PR/2015/2] for the years 2015–2019.
References
- 1. Oliveira AI et al Neuroprotective activity of Hypericum perforatum and its major components. Front Plant Sci 2016; 7: 1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shelton RC. St John's wort (Hypericum perforatum) in major depression. J Clin Psychiatry 2009; 70(Suppl 5): 23–27. [DOI] [PubMed] [Google Scholar]
- 3. Franchi GG et al Composition and antioxidant activity in vitro of different St. Johns wort (Hypericum perforatum L.) extracts. J Med Plants Res 2011; 5: 4349–4353. [Google Scholar]
- 4. Klemow KM et al 11 Medical Attributes of St. John's Wort (Hypericum perforatum). Lester Packer, PhD. 2011: 211. [PubMed]
- 5. Silva BA et al Phytochemical and antioxidant characterization of Hypericum perforatum alcoholic extracts. Food Chem 2005; 90: 157–167. [Google Scholar]
- 6. Silva BA et al St. John's wort (Hypericum perforatum) extracts and isolated phenolic compounds are effective antioxidants in several in vitro models of oxidative stress. Food Chem 2008; 110: 611–619. [Google Scholar]
- 7. Olivo M et al New frontier in hypericin‐mediated diagnosis of cancer with current optical technologies. Ann Biomed Eng 2012; 40: 460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang J et al Enhanced production of flavonoids by methyl jasmonate elicitation in cell suspension culture of Hypericum perforatum . Bioresour Bioprocess 2015; 2: 1–9. [Google Scholar]
- 9. Tatsis EC et al Identification of the major constituents of Hypericum perforatum by LC/SPE/NMR and/or LC/MS. Phytochemistry 2007; 68: 383–393. [DOI] [PubMed] [Google Scholar]
- 10. Guedes AP et al Hypericum sp.: essential oil composition and biological activities. Phytochem Rev 2012; 11: 127–152. [Google Scholar]
- 11. Bruni R, Sacchetti G. Factors affecting polyphenol biosynthesis in wild and field grown St. John's wort (Hypericum perforatum L. Hypericaceae/Guttiferae). Molecules 2009; 14: 682–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murch SJ et al Nickel contamination affects growth and secondary metabolite composition of St. John's wort (Hypericum perforatum L.). Environ Exp Bot 2003; 49: 251–257. [Google Scholar]
- 13. Bruni R et al Herbal drug quality and phytochemical composition of Hypericum perforatum L. affected by ash yellows phytoplasma infection. J Agric Food Chem 2005; 53: 964–968. [DOI] [PubMed] [Google Scholar]
- 14. Southwell IA, Bourke CA. Seasonal variation in hypericin content of Hypericum perforatum L. (St. John's Wort). Phytochemistry 2001; 56: 437–441. [DOI] [PubMed] [Google Scholar]
- 15. Marrelli M et al Hypericum perforatum: influences of the habitat on chemical composition, photo‐induced cytotoxicity, and antiradical activity. Pharm Biol 2014; 52: 909–918. [DOI] [PubMed] [Google Scholar]
- 16. Božin B et al Impact of origin and biological source on chemical composition, anticholinesterase and antioxidant properties of some St. John's wort species (Hypericum spp., Hypericaceae) from the Central Balkans. Molecules 2013; 18: 11733–11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murch SJ, Saxena PK. St. John's wort (Hypericum perforatum L.): challenges and strategies for production of chemically‐consistent plants. Can J Plant Sci 2006; 86: 765–771. [Google Scholar]
- 18. Zobayed SMA et al In vitro production and chemical characterization of St. John's wort (Hypericum perforatum L. cv ‘New Stem’). Plant Sci 2004; 166: 333–340. [Google Scholar]
- 19. Rao SR, Ravishankar G. Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv 2002; 20: 101–153. [DOI] [PubMed] [Google Scholar]
- 20. Wilson SA, Roberts SC. Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules. Plant Biotechnol J 2012; 10: 249–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Trosset J‐Y, Carbonell P. Synthetic biology for pharmaceutical drug discovery. Drug Des Devel Ther 2015; 9: 6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hou W et al A perspective on Hypericum perforatum genetic transformation. Front Plant Sci 2016; 7: 879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ulm R, Jenkins GI. Q&A: how do plants sense and respond to UV‐B radiation? BMC Biol 2015; 13: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu L et al Go in for the kill: how plants deploy effector‐triggered immunity to combat pathogens. Virulence 2014; 5: 710–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu J, Baldwin IT. New insights into plant responses to the attack from insect herbivores. Annu Rev Genet 2010; 44: 1–24. [DOI] [PubMed] [Google Scholar]
- 26. Osakabe Y et al Sensing the environment: key roles of membrane‐localized kinases in plant perception and response to abiotic stress. J Exp Bot 2013; 64: 445–458. [DOI] [PubMed] [Google Scholar]
- 27. Dixon RA, Paiva NL. Stress‐induced phenylpropanoid metabolism. Plant Cell 1995; 7: 1085–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. VanEtten HD et al Two classes of plant antibiotics: phytoalexins versus “phytoanticipins”. Plant Cell 1994; 6: 1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. González‐Lamothe R et al Plant antimicrobial agents and their effects on plant and human pathogens. Int J Mol Sci 2009; 10: 3400–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park S et al Glyceollins, one of the phytoalexins derived from soybeans under fungal stress, enhance insulin sensitivity and exert insulinotropic actions. J Agric Food Chem 2010; 58: 1551–1557. [DOI] [PubMed] [Google Scholar]
- 31. Zhao J et al Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 2005; 23: 283–333. [DOI] [PubMed] [Google Scholar]
- 32. Jones JD, Dangl JL. The plant immune system. Nature 2006; 444: 323–329. [DOI] [PubMed] [Google Scholar]
- 33. Van Wees SC et al Plant immune responses triggered by beneficial microbes. Curr Opin Plant Biol 2008; 11: 443–448. [DOI] [PubMed] [Google Scholar]
- 34. Conrath U. Molecular aspects of defence priming. Trends Plant Sci 2011; 16: 524–531. [DOI] [PubMed] [Google Scholar]
- 35. Conceicao LF et al Induction of phenolic compounds in Hypericum perforatum L. cells by Colletotrichum gloeosporioides elicitation. Phytochemistry 2006; 67: 149–155. [DOI] [PubMed] [Google Scholar]
- 36. Xu M‐J et al Nitric oxide mediates the fungal elicitor‐induced hypericin production of Hypericum perforatum cell suspension cultures through a jasmonic‐acid‐dependent signal pathway. Plant Physiol 2005; 139: 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sivakumar G, Paek K. Methyl jasmonate induce enhanced production of soluble biophenols in Panax ginseng adventitious roots from commercial scale bioreactors. Chem Nat Compd 2005; 41: 669–673. [Google Scholar]
- 38. Gadzovska S et al Jasmonic acid elicitation of Hypericum perforatum L. cell suspensions and effects on the production of phenylpropanoids and naphtodianthrones. Plant Cell Tissue Organ Cult 2007; 89: 1–13. [Google Scholar]
- 39. Walker TS et al Jasmonic acid‐induced hypericin production in cell suspension cultures of Hypericum perforatum L. (St. John's wort). Phytochemistry 2002; 60: 289–293. [DOI] [PubMed] [Google Scholar]
- 40. Tirillini B et al Induction of hypericins in Hypericum perforatum in response to chromium. Fitoterapia 2006; 77: 164–170. [DOI] [PubMed] [Google Scholar]
- 41. Sharafi E et al Improvement of hypericin and hyperforin production using zinc and iron nano‐oxides as elicitors in cell suspension culture of St John's wort (Hypericum perforatum L.). J Med Plants By‐Prod 2013; 2: 177–184. [Google Scholar]
- 42. Ramirez‐Estrada K et al Elicitation, an effective strategy for the biotechnological production of bioactive high‐added value compounds in plant cell factories. Molecules 2016; 21: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ramakrishna A, Ravishankar GA. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 2011; 6: 1720–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seybold H et al Ca2+ signalling in plant immune response: from pattern recognition receptors to Ca2+ decoding mechanisms. New Phytol 2014; 204: 782–790. [DOI] [PubMed] [Google Scholar]
- 45. Schluttenhofer C, Yuan L. Regulation of specialized metabolism by WRKY transcription factors. Plant Physiol 2015; 167: 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Naoumkina MA et al Elicitor‐induced transcription factors for metabolic reprogramming of secondary metabolism in Medicago truncatula . BMC Plant Biol 2008; 8: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maeda K et al DcMYB1 acts as a transcriptional activator of the carrot phenylalanine ammonia‐lyase gene (DcPAL1) in response to elicitor treatment, UV‐B irradiation and the dilution effect. Plant Mol Biol 2005; 59: 739–752. [DOI] [PubMed] [Google Scholar]
- 48. Trewavas AJ, Malhó R. Ca2+ signalling in plant cells: the big network!. Curr Opin Plant Biol 1998; 1: 428–433. [DOI] [PubMed] [Google Scholar]
- 49. White PJ, Broadley MR. Calcium in plants. Ann Bot 2003; 92: 487–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Meijer HJ, Munnik T. Phospholipid‐based signaling in plants. Annu Rev Plant Biol 2003; 54: 265–306. [DOI] [PubMed] [Google Scholar]
- 51. Wang X. Phospholipase D in hormonal and stress signaling. Curr Opin Plant Biol 2002; 5: 408–414. [DOI] [PubMed] [Google Scholar]
- 52. Bigeard J et al Signaling mechanisms in pattern‐triggered immunity (PTI). Mol Plant 2015; 8: 521–539. [DOI] [PubMed] [Google Scholar]
- 53. Boudsocq M, Sheen J. CDPKs in immune and stress signaling. Trends Plant Sci 2013; 18: 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bolwell GP, Wojtaszek P. Mechanisms for the generation of reactive oxygen species in plant defence – a broad perspective. Physiol Mol Plant Pathol 1997; 51: 347–366. [Google Scholar]
- 55. Zhao J et al Elicitor‐induced indole alkaloid biosynthesis in Catharanthus roseus cell cultures is related to Ca 2+ influx and the oxidative burst. Plant Sci 2001; 161: 423–431. [Google Scholar]
- 56. Yang Z. Small GTPases versatile signaling switches in plants. Plant Cell 2002; 14(Suppl 1): S375–S388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Roos W et al A redox‐dependent, G‐protein‐coupled phospholipase A of the plasma membrane is involved in the elicitation of alkaloid biosynthesis in Eschscholtzia californica . Biochim Biophys Acta 1999; 1448: 390–402. [DOI] [PubMed] [Google Scholar]
- 58. Vasconsuelo A, Boland R. Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci 2007; 172: 861–875. [Google Scholar]
- 59. Kirakosyan A et al Stimulation of the production of hypericins by mannan in Hypericum perforatum shoot cultures. Phytochemistry 2000; 53: 345–348. [DOI] [PubMed] [Google Scholar]
- 60. Zubek S et al Hypericin and pseudohypericin concentrations of a valuable medicinal plant Hypericum perforatum L. are enhanced by arbuscular mycorrhizal fungi. Mycorrhiza 2012; 22: 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Azeez H, Ibrahim K. Effect of biotic elicitors on secondary metabolite production in cell suspensions of Hypericum triquetrifolium Turra. Bull Univ Agric Sci Vet Med Cluj‐Napoca Horticul 2013; 70: 26–33. [Google Scholar]
- 62. Gadzovska‐Simic S et al Secondary metabolite production in Hypericum perforatum L. cell suspensions upon elicitation with fungal mycelia from Aspergillus flavus . Arch Biol Sci 2012; 64: 113–121. [Google Scholar]
- 63. Simic SG et al Fungal elicitor‐mediated enhancement in phenylpropanoid and naphtodianthrone contents of Hypericum perforatum L. cell cultures. Plant Cell Tissue Organ Cult 2015; 122: 213–226. [Google Scholar]
- 64. Gadzovska Simic S et al Effects of polysaccharide elicitors on secondary metabolite production and antioxidant response in Hypericum perforatum L. shoot cultures. ScientificWorldJournal 2014; 2014: 609649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yamaner Ö et al Stimulation of the production of hypericins in in vitro seedlings of Hypericum adenotrichum by some biotic elicitors. Turk J Botany 2013; 37: 153–159. [Google Scholar]
- 66. Simic SG et al Polysaccharide elicitors enhance phenylpropanoid and naphtodianthrone production in cell suspension cultures of Hypericum perforatum . Plant Cell Tissue Organ Cult 2015; 122: 649–663. [Google Scholar]
- 67. Brasili E et al Metabolic profile and root development of Hypericum perforatum L. in vitro roots under stress conditions due to chitosan treatment and culture time. Front Plant Sci 2016; 7: 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Brasili E et al A non‐targeted metabolomics approach to evaluate the effects of biomass growth and chitosan elicitation on primary and secondary metabolism of Hypericum perforatum in vitro roots. Metabolomics 2014; 10: 1186–1196. [Google Scholar]
- 69. Pavlik M et al Hypericin and hyperforin production in St. John's wort in vitro culture: influence of saccharose, polyethylene glycol, methyl jasmonate, and Agrobacterium tumefaciens . J Agric Food Chem 2007; 55: 6147–6153. [DOI] [PubMed] [Google Scholar]
- 70. Mañero FJG et al Elicitation of secondary metabolism in Hypericum perforatum by rhizosphere bacteria and derived elicitors in seedlings and shoot cultures. Pharma Biol 2012; 50: 1201–1209. [DOI] [PubMed] [Google Scholar]
- 71. Franklin G et al Xanthone biosynthesis in Hypericum perforatum cells provides antioxidant and antimicrobial protection upon biotic stress. Phytochemistry 2009; 70: 60–68. [DOI] [PubMed] [Google Scholar]
- 72. Singh RK et al Lignin and flavonoid content increases in Hypericum perforatum cell wall after Agrobacterium tumefaciens co‐cultivation. Planta Med 2014; 80: 1388. [Google Scholar]
- 73. Tusevski O et al Agrobacterium enhances xanthone production in Hypericum perforatum cell suspensions. Plant Growth Regul 2015; 76: 199–210. [Google Scholar]
- 74. Felix G, Boller T. Molecular sensing of bacteria in plants – the highly conserved RNA‐binding motif RNP‐1 of bacterial cold shock proteins is recognized as an elicitor signal in tobacco. J Biol Chem 2003; 278: 6201–6208. [DOI] [PubMed] [Google Scholar]
- 75. Felix G et al Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 1999; 18: 265–276. [DOI] [PubMed] [Google Scholar]
- 76. Kunze G et al The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell 2004; 16: 3496–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zipfel C et al Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 2004; 428: 764–767. [DOI] [PubMed] [Google Scholar]
- 78. Zipfel C et al Perception of the bacterial PAMP EF‐Tu by the receptor EFR restricts Agrobacterium‐mediated transformation. Cell 2006; 125: 749–760. [DOI] [PubMed] [Google Scholar]
- 79. Erbs G et al Peptidoglycan and muropeptides from pathogens Agrobacterium and Xanthomonas elicit plant innate immunity: structure and activity. Chem Biol 2008; 15: 438–448. [DOI] [PubMed] [Google Scholar]
- 80. Bauer Z et al Sensitivity of different ecotypes and mutants of Arabidopsis thaliana toward the bacterial elicitor flagellin correlates with the presence of receptor‐binding sites. J Biol Chem 2001; 276: 45669–45676. [DOI] [PubMed] [Google Scholar]
- 81. Liu X‐N et al Effects of cytokinins and elicitors on the production of hypericins and hyperforin metabolites in Hypericum sampsonii and Hypericum perforatum . Plant Growth Regul 2007; 53: 207–214. [Google Scholar]
- 82. Coste A et al Effects of plant growth regulators and elicitors on production of secondary metabolites in shoot cultures of Hypericum hirsutum and Hypericum maculatum . Plant Cell Tissue Organ Cult 2011; 106: 279–288. [Google Scholar]
- 83. Gadzovska S et al Identification and quantification of hypericin and pseudohypericin in different Hypericum perforatum L. in vitro cultures. Plant Physiol Biochem 2005; 43: 591–601. [DOI] [PubMed] [Google Scholar]
- 84. Wu S‐Q et al Several factors affecting hypericin production of Hypericum perforatum during adventitious root culture in airlift bioreactors. Acta Physiol Plant 2014; 36: 975–981. [Google Scholar]
- 85. Zubrická D et al Xanthones from roots, hairy roots and cell suspension cultures of selected Hypericum species and their antifungal activity against Candida albicans . Plant Cell Rep 2015; 34: 1953–1962. [DOI] [PubMed] [Google Scholar]
- 86. Gadzovska S et al The influence of salicylic acid elicitation of shoots, callus, and cell suspension cultures on production of naphtodianthrones and phenylpropanoids in Hypericum perforatum L. Plant Cell Tissue Organ Cult 2013; 113: 25–39. [Google Scholar]
- 87. Brechner ML et al Effects of UV‐B on secondary metabolites of St. John's wort (Hypericum perforatum L.) grown in controlled environments. Photochem Photobiol 2011; 87: 680–684. [DOI] [PubMed] [Google Scholar]
- 88. Germ M et al Flavonoid, tannin and hypericin concentrations in the leaves of St. John's wort (Hypericum perforatum L.) are affected by UV‐B radiation levels. Food Chem 2010; 122: 471–474. [Google Scholar]
- 89. Yamaner O, Erdag B. Effects of sucrose and polyethylene glycol on hypericins content in Hypericum adenotrichum . EurAsian J Biosci 2013; 7: 101–110. [Google Scholar]
- 90. Franklin G, Dias A. Hypericum perforatum cells accumulate anthocyanins and flavonoids at the expense of xanthone biosynthesis during light adaptation PlantEngine 1 (COST Action FA 1006‐ Plant Metabolic Engineering for high value products, 1st Annual Conference), 2011 (Nov 17–18) Murcia, Spain 2011.
- 91. Valletta A et al Acetic acid acts as an elicitor exerting a chitosan‐like effect on xanthone biosynthesis in Hypericum perforatum L. root cultures. Plant Cell Rep 2016; 35: 1009–1020. [DOI] [PubMed] [Google Scholar]
- 92. Singh RK et al Agrobacterium‐elicited Hypericum perforatum cell methanolic extract shows antibacterial activity against human pathogens. Planta Med 2014; 80: 1424–1425. [Google Scholar]
- 93. Carvalho AC et al Methanolic extract of Hypericum perforatum cells elicited with Agrobacterium tumefaciens provides protection against oxidative stress induced in human HepG2 cells. Ind Crops Prod 2014; 59: 177–183. [Google Scholar]
- 94. Tocci N et al Bioassay‐guided fractionation of extracts from Hypericum perforatum in vitro roots treated with carboxymethylchitosans and determination of antifungal activity against human fungal pathogens. Plant Physiol Biochem 2013; 70: 342–347. [DOI] [PubMed] [Google Scholar]
- 95. Zhang B et al Stimulation of artemisinin production in Artemisia annua hairy roots by Ag‐SiO2 core‐shell nanoparticles. Curr Nanosci 2013; 9: 363–370. [Google Scholar]
- 96. Ghanati F, Bakhtiarian S. Effect of methyl jasmonate and silver nanoparticles on production of secondary metabolites by Calendula officinalis L (Asteraceae). Trop J Pharm Res 2014; 13: 1783–1789. [Google Scholar]
- 97. Ghorbanpour M, Hadian J. Multi‐walled carbon nanotubes stimulate callus induction, secondary metabolites biosynthesis and antioxidant capacity in medicinal plant Satureja khuzestanica grown in vitro. Carbon 2015; 94: 749–759. [Google Scholar]
- 98. Kurepa J et al Direct isolation of flavonoids from plants using ultra‐small anatase TiO(2) nanoparticles. Plant J 2014; 77: 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Schlipf DM et al Flavonoid adsorption and stability on titania‐functionalized silica nanoparticles. Colloids Surf A 2015; 478: 15–21. [Google Scholar]
- 100. Cui X‐H et al Pilot‐scale culture of Hypericum perforatum L. adventitious roots in airlift bioreactors for the production of bioactive compounds. Appl Biochem Biotechnol 2014; 174: 784–792. [DOI] [PubMed] [Google Scholar]
- 101. Onelli E et al Ultrastructural studies on the developing secretory nodules of Hypericum perforatum . Flora Morphol Distrib Funct Ecol Plants 2002; 197: 92–102. [Google Scholar]
- 102. Piovan A et al Detection of hypericins in the “red glands” of Hypericum elodes by ESI–MS/MS. Phytochemistry 2004; 65: 411–414. [DOI] [PubMed] [Google Scholar]
- 103. Zobayed S et al Plant–environment interactions: accumulation of hypericin in dark glands of Hypericum perforatum . Ann Bot 2006; 98: 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kornfeld A et al The production of hypericins in two selected Hypericum perforatum shoot cultures is related to differences in black gland structure. Plant Physiol Biochem 2007; 45: 24–32. [DOI] [PubMed] [Google Scholar]
- 105. Gaid M et al Hyperforin production in Hypericum perforatum root cultures. J Biotechnol 2016; 222: 47–55. [DOI] [PubMed] [Google Scholar]


