Abstract
Aim
To compare the efficacy and safety of once‐weekly dulaglutide with that of insulin glargine in combination with metformin and/or a sulphonylurea in mainly Asian patients with type 2 diabetes mellitus (T2DM).
Materials and Methods
In this 52‐week, randomized, parallel‐arm open‐label study, we enrolled patients aged ≥18 years with T2DM for at least 6 months and a glycated haemoglobin (HbA1c) concentration ≥53.0 mmol/mol (7.0%) and ≤96.7 mmol/mol (11.0%). The primary outcome was change in HbA1c from baseline to week 26 to determine non‐inferiority of dulaglutide 1.5 mg versus glargine.
Results
A total of 774 patients from China, South Korea, Mexico and Russia were randomly assigned (1:1:1) to dulaglutide 1.5 mg, dulaglutide 0.75 mg or glargine treatment groups. The patients' mean age was 55 years and the average T2DM duration was ~8 years. The least squares mean (SE) changes from baseline in HbA1c at 26 weeks were − 18.9 (0.73) mmol/mol (−1.73 [0.067]%) for dulaglutide 1.5 mg and −14.5 (0.73) mmol/mol (−1.33 [0.067]%) for dulaglutide 0.75 mg, compared with −12.7 (0.73) mmol/mol (−1.16 [0.067]%) for glargine. Statistical criteria for superiority were met with both dulaglutide 1.5 mg and dulaglutide 0.75 mg. More patients in the dulaglutide 1.5 and 0.75 mg groups achieved HbA1c target <53.0 mmol/mol (<7.0%) than in the glargine group at week 26 (P < 0.001 and P = 0.004, respectively). Body weight decreased with dulaglutide and increased with glargine. The incidence and rate of total hypoglycaemia were lower with dulaglutide versus glargine. Gastrointestinal adverse events, including diarrhoea and nausea, were the most frequently reported for patients taking dulaglutide.
Conclusions
Once‐weekly dulaglutide provides greater improvement in HbA1c, with weight loss and less hypoglycaemia, than once‐daily insulin glargine in a population of mainly Asian patients with T2DM who had failed to achieve optimal glycaemic control on metformin and/or a sulphonylurea.
Keywords: dulaglutide, GLP‐1 analogue, type 2 diabetes
1. INTRODUCTION
Type 2 diabetes mellitus (T2DM) is the most prevalent form of diabetes,1 and is a progressive disease characterized by a combination of varying degrees of insulin resistance and relative insulin secretory deficiency.2 Generally, patients with T2DM inadequately controlled on lifestyle measures and oral antihyperglycaemic medication (OAM) need further injectable therapy to attain optimal glycaemic control.3, 4 Once‐daily basal insulin is a frequent option to maintain glycaemic control, but is associated with adverse effects such as hypoglycaemia and weight gain. Glucagon‐like peptide‐1 (GLP‐1) receptor agonists stimulate insulin secretion in a glucose‐dependent manner and inhibit the release of glucagon, and evidence shows that they improve glucose control and are also associated with weight loss and lower risk of hypoglycaemia.5, 6, 7, 8, 9, 10
Dulaglutide is an approved long‐acting human GLP‐1 receptor agonist, administered as a once‐weekly subcutaneous injection for the treatment of T2DM.11, 12 Dulaglutide has been evaluated in several phase III trials known as the global AWARD (Assessment of Weekly AdministRation of LY2189265 [dulaglutide] in Diabetes) programme in mainly white and Hispanic/Latino patients with T2DM, showing that dulaglutide as monotherapy or in combination with other antidiabetic medication was effective, generally safe and well tolerated.8, 13, 14, 15, 16, 17, 18 In AWARD‐2, once‐weekly dulaglutide 1.5 mg demonstrated greater glycated haemoglobin (HbA1c) reduction and weight loss compared with daily insulin glargine in patients with T2DM treated with maximum tolerated doses of metformin and glimepiride. The safety profile showed a higher incidence of gastrointestinal adverse events (AEs) and a lower risk of hypoglycaemia compared with glargine without forced titration8; however, limited data exist comparing dulaglutide 1.5 mg with glargine in Asian patients with T2DM.19
The objective of the present study was to compare the efficacy and safety of once‐weekly dulaglutide with daily insulin glargine in mainly Asian patients with T2DM who were inadequately controlled on treatment with metformin and/or a sulphonylurea.
2. PATIENTS AND METHODS
2.1. Study design and patients
This 52‐week, randomized, parallel‐arm, open‐label (blinded to dulaglutide dose), active comparator non‐inferiority study consisted of three periods: a screening (2 weeks) and lead‐in period (2 weeks); a treatment period (52 weeks); and a safety follow‐up period (30 days; Figure 1A). The study was conducted at 45 sites in China, Russia, Mexico and South Korea.
Figure 1.
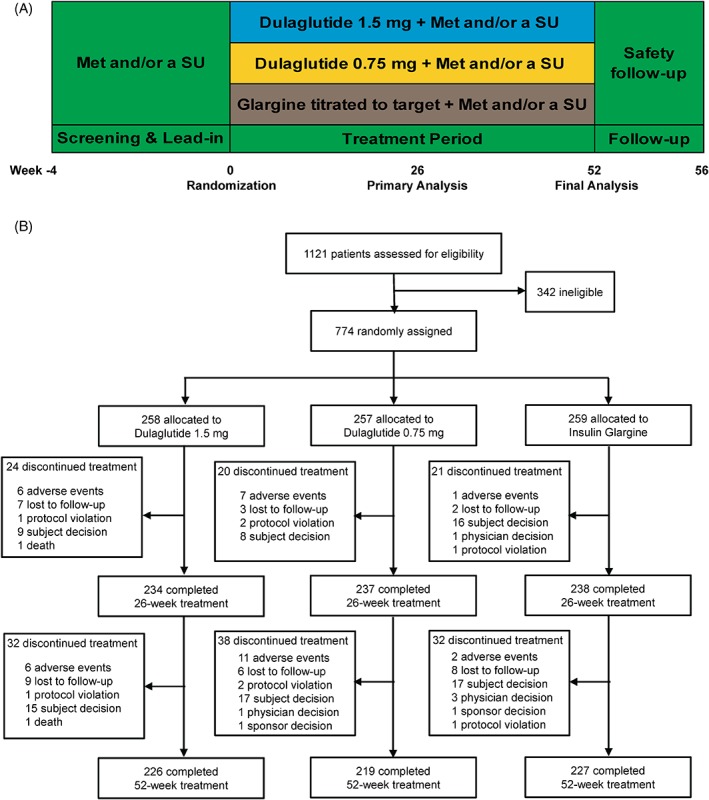
A, Study design and B, patient disposition. Abbreviations: Met, metformin and SU, sulphonylurea
Patients aged ≥18 years with a diagnosis of T2DM for at least 6 months, a body mass index ≥19.0 and ≤35.0 kg/m2 and HbA1c ≥53.0 mmol/mol (7.0%; considered inadequate glycaemic control) and ≤96.7 mmol/mol (11.0%), who had been taking metformin and/or a sulphonylurea for at least 3 months and were on a stable therapeutic dose (at least half the maximum dose according to the product information in the country of treatment) for at least 8 weeks before screening, were eligible. Patients were excluded from the study if they had type 1 diabetes, had a clinically significant gastric emptying abnormality, had a history of pancreatitis, had a serum calcitonin concentration ≥20 ng/L (or 5.83 pmol/L), were taking insulin treatment or had been treated with a GLP‐1 receptor agonist within 3 months before screening.
Institutional review boards provided written approval of the protocol, and patients provided written informed consent before any study‐related activities. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practices.20 The trial is registered with ClinicalTrials.gov (NCT01648582).
2.2. Randomization and masking
Eligible patients were randomized (1:1:1) to subcutaneously injected once‐weekly dulaglutide 1.5 mg or dulaglutide 0.75 mg, or once‐daily insulin glargine according to a computer‐generated random sequence using an interactive voice‐response system. Randomization was stratified by country, baseline HbA1c concentration (<8.5% or ≥8.5%, 69.4 mmol/mol) and type of baseline OAM regimen.
2.3. Procedures
During the study treatment period, patients in the two dulaglutide treatment groups received a fixed, double‐blind dose of dulaglutide (either 1.5 or 0.75 mg) once weekly as a subcutaneous injection. This study used an insulin glargine dosing algorithm that has previously been found to be safe and effective in Japanese patients in conjunction with glimepiride.21 Patients in the glargine group started their once‐daily subcutaneous injection before bedtime on the day of randomization. The initial dose of glargine was determined from the patient's average plasma‐equivalent fasting plasma glucose (FPG) from the previous 2 to 4 days. The initial dose of glargine was 6 IU/day, with an average FPG of ≥7.8 mmol/L and was reduced by 1 to 2 IU/d with an average FPG of <7.8 mmol/L at the investigator's discretion. The patients measured FPG every morning and were instructed to adjust insulin doses to achieve the target FPG range of 4.0 to 5.6 mmol/L (Table S1, Supporting Information). In each of three treatment groups, patients continued taking their usual OAM (metformin and/or a sulphonylurea) dose and regimen throughout the treatment period.
The primary efficacy measure was HbA1c change from baseline to week 26. Secondary efficacy measures included: change in HbA1c from baseline at week 52; percentage of patients achieving HbA1c <53.0 mmol/mol or ≤47.5 mmol/mol (<7.0% or ≤6.5%); fasting serum glucose (FSG); and seven‐point self‐monitored blood glucose (SMBG) profiles.
Safety assessments included: AEs; serious AEs (SAEs); hypoglycaemic episodes; serial collection of laboratory variables (e.g. calcitonin and pancreatic enzymes); vital signs; ECG results; and dulaglutide anti‐drug antibodies. All major adverse cardiovascular events (eg, myocardial infarction, hospitalization for unstable angina, hospitalization for heart failure, coronary interventions and cerebrovascular events) and potential events of pancreatitis were adjudicated by a committee of physicians external to Eli Lilly and Company. Laboratory analyses were performed at a central laboratory (Quintiles). Immunogenicity testing was performed by Wu Xi AppTec (Shanghai, China). Total hypoglycaemia was defined as a plasma‐equivalent blood glucose level of ≤3.9 mmol/L and/or signs and/or symptoms associated with hypoglycaemia. Documented symptomatic hypoglycaemia was defined as any time a patient experienced symptoms and/or signs associated with hypoglycaemia and had a plasma‐equivalent blood glucose level of 3.9 mmol/L. Severe hypoglycaemia was defined as an episode that required assistance from another person to actively administer therapy, as determined by the investigator.20
2.4. Statistical analyses
The study was designed with 90% power to show non‐inferiority of dulaglutide 1.5 mg versus glargine for change from baseline in HbA1c at the 26‐week primary endpoint, assuming a non‐inferiority margin of 0.4%, no true difference in HbA1c reduction between dulaglutide 1.5 mg and glargine, an SD of 1.3%, and a one‐sided α‐value of 0.025. This corresponds to a randomized 263 patients per treatment group, with an assumed drop‐out rate of 15%. Tree‐gatekeeping was used to control the family‐wise type 1 error rate at 0.025 (one‐sided) while assessing non‐inferiority/superiority of dulaglutide 1.5 mg versus glargine and non‐inferiority/superiority of dulaglutide 0.75 mg versus glargine for change from baseline in HbA1c at week 26.22 Two‐sided P values are reported throughout the manuscript so that each could be compared to 0.05 to assess significance while accounting for multiplicity adjustments.23
Efficacy analyses were based on a modified intention‐to‐treat (mITT) population, consisting of all randomized patients who had a baseline HbA1c measurement and at least one post‐baseline HbA1c measurement for the respective analysis period (week 26 and week 52) and received at least one dose of injectable study drug. Safety analyses were based on the as‐treated population (the safety analysis set) consisting of all randomized patients who received one dose of the study drug.
The changes from baseline in efficacy endpoints and body weight at weeks 26 and 52 were analysed using a mixed model with repeated measures (MMRM) with restricted maximum likelihood on MITT analysis set. The change from baseline value was the dependent variable; treatment, country, prestudy therapy, visit and the treatment‐by‐visit interaction were fixed effects, and baseline value was a covariate and patient was a random effect. The percentage of patients achieving HbA1c targets (with LOCF for the missing endpoint HbA1c) and of patients experiencing AEs or hypoglycaemia were analysed using Fisher's exact test. Hypoglycaemia rate was analysed using the generalized estimating equation method for negative binomial distribution. This generalized linear model used the count of hypoglycaemic episodes as response, prestudy therapy, country, treatment, visit and treatment‐by‐visit interaction as fixed effects and the logarithm of days between visits as an offsetting variable to account for unequal duration between visits and between patients. No multiplicity adjustment was made for other objectives, so the interpretation of the relevant P values should be made with caution.
No explicit imputation of missing post‐baseline HbA1c data was performed, as the MMRM implicitly adjusts for them. Percentage calculations included only patients with available data in the denominator, unless otherwise specified. Type 1 error was controlled for primary and gated secondary objectives. As per convention, a P value <0.05 was taken to indicate statistical significance, but should mostly be interpreted as descriptive. All adjusted means refer to least squares (LS) means. All analyses were performed in SAS version 9.3.
3. RESULTS
A total of 774 patients were randomly assigned to one of the three treatment groups. Six patients in the glargine group did not take the study drug (five patients decided to discontinue treatment and one patient did not meet the inclusion criteria). A total of 709 patients completed the 26‐week study period and 672 patients completed the study up to week 52. The number of patients who discontinued the study was similar across groups, and the most common reason for discontinuation was patient decision (Figure 1B). The baseline demographic and clinical characteristics of the 755 patients in the mITT population were similar among treatment groups (Table 1). In all the groups, most patients were Asian (83.6%). During the study treatment, patients were maintained on stable doses of their usual OAMs for up to 52 weeks. The mean daily dose (LOCF) of insulin glargine was 22.0 IU (0.293 IU/kg) at week 26 and 22.9 IU (0.303 IU/kg) at week 52 (Figure S1, Supporting Information).
Table 1.
Baseline patient characteristics
| Variable | Dulaglutide 1.5 mg n = 253 | Dulaglutide 0.75 mg n = 252 | Insulin glargine n = 250 | Total n = 755 |
|---|---|---|---|---|
| Age, years | 55.0 (9.6) | 54.5 (10.0) | 55.4 (9.2) | 55.0 (9.6) |
| Women, n (%) | 118 (46.6) | 109 (43.3) | 111 (44.4) | 338 (44.8) |
| Country, n (%) | ||||
| China | 200 (79.1) | 196 (77.8) | 195 (78.0) | 591 (78.3) |
| Korea | 13 (5.1) | 13 (5.2) | 14 (5.6) | 40 (5.3) |
| Mexico | 22 (8.7) | 24 (9.5) | 24 (9.6) | 70 (9.3) |
| Russia | 18 (7.1) | 19 (7.5) | 17 (6.8) | 54 (7.2) |
| Body mass index, kg/m2 | 26.6 (3.7) | 27.0 (3.8) | 26.7 (3.5) | 26.8 (3.7) |
| Weight, kg | 73.6 (13.0) | 74.6 (12.7) | 73.4 (13.1) | 73.9 (12.9) |
| Duration of diabetes, years | 7.9 (4.8) | 8.1 (5.3) | 8.4 (5.3) | 8.1 (5.1) |
| FSG, mmol/L | 9.57 (2.65) | 9.59 (2.41) | 9.69 (2.36) | 9.62 (2.47) |
| HbA1c, % | 8.5 (1.2) | 8.3 (1.1) | 8.3 (1.1) | 8.4 (1.1) |
| HbA1c, mmol/mol | 69.4 (13.1) | 67.2 (12.0) | 67.2 (12.0) | 68.3 (12.0) |
| OAM treatment, %a | ||||
| Metformin only | 41.9 | 40.0 | 40.2 | 40.7 |
| Sulphonylureas only | 11.5 | 12.4 | 11.2 | 11.7 |
| Metformin and sulphonylureas | 46.6 | 47.6 | 48.6 | 47.6 |
Abbreviations: FSG, fasting serum glucose; HbA1c, glycated haemoglobin; OAM, oral antihyperglycaemic medication.
Treatment used at time of study entry (screening). Data are n (%) or mean (SD).
3.1. Efficacy
The LS mean (SE) changes from baseline in HbA1c at the 26‐week primary endpoint were −18.9 (0.73) mmol/mol (−1.73 [0.067]%) for dulaglutide 1.5 mg and −14.5 (0.73) mmol/mol (−1.33 [0.067]%) for dulaglutide 0.75 mg, compared with −12.7 (0.73) mmol/mol (−1.16 [0.067]%) for glargine. Statistical criteria for superiority were met with both dulaglutide 1.5 mg (estimated treatment difference −6.23 mmol/mol, 95% CI −8.09 to −4.37 [−0.57%, 95% CI −0.74 to −0.40]; P < 0.001) and dulaglutide 0.75 mg (estimated treatment difference −1.97 mmol/mol, 95% CI −3.83 to −0.11 [−0.18%, 95% CI −0.35 to −0.01]; P = 0.037 [Figure 2A, Table S2]).
Figure 2.
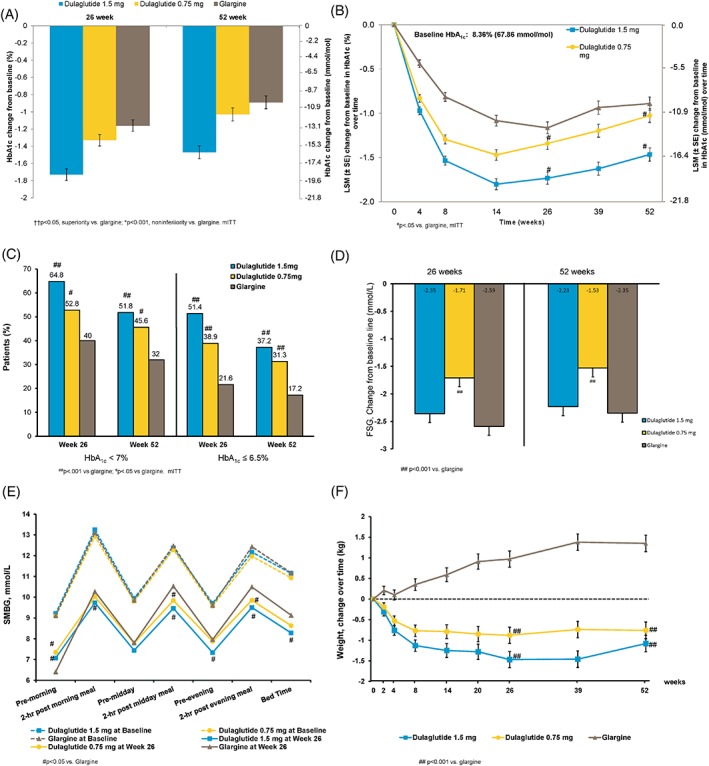
Efficacy variables. A, Least squares mean (LSM) ± SE changes in glycated haemoglobin (HbA1c) from baseline at 26 weeks and 52 weeks, mixed model with repeated measures (MMRM). B, LSM ± SE HbA1c values over time, MMRM. C, Percentage of patients achieving HbA1c targets in the modified intention‐to‐treat (mITT) population (LOCF). D, LSM ± SE fasting serum glucose (FSG) change from baseline. E, Baseline and 26‐week self‐monitored blood glucose (SMBG) profiles, dashed lines are baseline and solid lines are at 26 weeks, mean of actual value, SD not shown. F, Change in weight over time. ††P < 0.05, superiority vs. glargine; *P < 0.001, non‐inferiority vs. glargine; ##P < 0.001 vs. glargline; #P < 0.05 vs. glargine
The LS mean (SE) changes from baseline in HbA1c at week 52 were − 16.1 (0.83) mmol/mol (−1.47 [0.076]%) for dulaglutide 1.5 mg and −11.3 (0.83) mmol/mol (−1.03 [0.076]%) for dulaglutide 0.75 mg, compared with −9.7 (0.82) mmol/mol (−0.89 [0.075]%) for glargine. Statistical criteria for superiority were maintained with dulaglutide 1.5 mg (estimated treatment difference −6.23 mmol/mol, 95% CI −8.42 to −4.15 [−0.57%, 95% CI −0.77 to −0.38]; P < 0.001). Statistical criteria for non‐inferiority were met with dulaglutide 0.75 mg (estimated treatment difference −1.42 mmol/mol, 95% CI −3.61 to −0.66 [−0.13%, 95% CI −0.33 to 0.06]; P < 0.001 [Figure 2A]). All sensitivity analyses were consistent with these results. Figure 2B shows HbA1c values over time up to week 52 (Table S2).
The percentage of patients achieving the HbA1c target of <53.0 mmol/mol (<7.0%) at week 26 was significantly higher in both the dulaglutide 1.5‐mg and the dulaglutide 0.75‐mg groups (64.8% and 52.8%, respectively) compared with the glargine group (40.0%; P < 0.001 and P = 0.004, respectively). At the same time point, 51.4% and 38.9% of patients receiving dulaglutide 1.5 mg and dulaglutide 0.75 mg, respectively, achieved an HbA1c of ≤47.5 mmol/mol (≤6.5%) compared with 21.6% in the glargine arm (P < 0.001, both comparisons). The percentages of patients achieving HbA1c <53.0 mmol/mol and ≤47.5 mmol/mol (<7.0% and ≤6.5%) at week 52 in both dulaglutide doses were significantly higher than with glargine (Figure 2C).
The LS mean (SE) changes from baseline to week 26 in FSG were −2.35 (0.162) mmol/L, −1.71 (0.161) mmol/L and − 2.59 (0.161) mmol/L for dulaglutide 1.5 mg, dulaglutide 0.75 mg and glargine, respectively, with a greater decrease for glargine compared with dulaglutide 0.75 mg (P < 0.001). Similar FSG results were observed at week 52 (Figure 2D). At the primary endpoint of week 26, 17.6% of patients in the glargine treatment group achieved the FSG target of <5.6 mmol/L and 56.0% of patients had an FSG of <7.0 mmol/L.
Figure 2E shows the seven‐point SMBG profiles at baseline and week 26. The pre‐morning blood glucose value from SMBG profiles decreased more with glargine than with dulaglutide 1.5 mg and dulaglutide 0.75 mg (P < 0.001). The reduction from baseline for 2‐hour postprandial glucose (PPG) for all three meals and bedtime was significantly greater for dulaglutide 1.5 mg compared with glargine. Similar results were demonstrated at week 52 (Figure S2, Supporting Information).
The LS mean (SE) changes from baseline in body weight at 26‐week primary endpoint were − 1.47 (0.197) kg for dulaglutide 1.5 mg and − 0.88 (0.197) kg for dulaglutide 0.75 mg, compared with 0.97 (0.197) kg for glargine, with a significant difference between dulaglutide and glargine (P < 0.001, both comparisons). Similar results were demonstrated at week 52 (Figure 2F).
3.2. Safety
For overall safety, there was a higher incidence of treatment‐emergent AEs during 52 weeks in both dulaglutide doses than in glargine, primarily because of a higher incidence of gastrointestinal AEs. Gastrointestinal AEs, including diarrhoea and nausea, were the most frequently reported AEs for patients taking dulaglutide (Table 2); the incidence rates were observed to peak during the first 2 weeks of treatment and then declined and remained low until 52 weeks. One death (dulaglutide 1.5 mg, gunshot wound) occurred during the study and was assessed as not related to the study drug. A total of 48 patients (6.3%) experienced at least one SAE (24 [9.3%], 15 [5.8%], nine patients [3.6%] in the dulaglutide 1.5 mg, dulaglutide 0.75 mg, and glargine groups, respectively). A total of four SAEs were judged by the investigator as possibly related to dulaglutide (reflux oesophagitis in dulaglutide 0.75‐mg group, thyroid adenoma, transient ischaemic attach and pancreatic enzyme elevation in the dulaglutide 1.5‐mg group).
Table 2.
Safety assessments
| Variable | Week 26 | Week 52 | ||||
|---|---|---|---|---|---|---|
| Dulaglutide | Glargine | Dulaglutide | Glargine | |||
| 1.5 mg | 0.75 mg | 1.5 mg | 0.75 mg | |||
| n = 258 | n = 257 | n = 253 | n = 258 | n = 257 | n = 253 | |
| Death, n (%) | 1 (0.4) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 0 (0.0) | 0 (0.0) |
| SAEs, n (%) | 14 (5.4) | 7 (2.7) | 5 (2.0) | 24 (9.3) | 15 (5.8) | 9 (3.6) |
| Treatment‐emergent AEs: patients with ≥1, n (%) | 157 (60.9) | 151 (58.8) | 122 (48.2) | 174 (67.4) | 177 (68.9) | 149 (58.9) |
| Gastrointestinal AEs: ≥5% patients, n (%) | 81 (31.4) | 67 (26.1) | 20 (7.9) | 82 (31.8) | 72 (28.0) | 22 (8.7) |
| Diarrhoea | 41 (15.9) | 24 (9.3) | 6 (2.4) | 42 (16.3) | 28 (10.9) | 7 (2.8) |
| Nausea | 26 (10.1) | 14 (5.4) | 2 (0.8) | 26 (10.1) | 14 (5.4) | 2 (0.8) |
| Abdominal distention | 18 (7.0) | 13 (5.1) | 0 (0.0) | 18 (7.0) | 13 (5.1) | 0 (0.0) |
| Vomiting | 15 (5.8) | 4 (1.6) | 2 (0.8) | 16 (6.2) | 6 (2.3) | 2 (0.8) |
| Decreased appetite | 19 (7.4) | 14 (5.4) | 0 (0.0) | 19 (7.4) | 14 (5.4) | 0 (0.0) |
| Infections and infestations, n (%) | 38 (14.7) | 32 (12.5) | 40 (15.8) | 54 (20.9) | 52 (20.2) | 57 (22.5) |
| Nasopharyngitis | 13 (5.0) | 14 (5.4) | 17 (6.7) | 18 (7.0) | 16 (6.2) | 22 (8.7) |
| Total hypoglycaemic incidence, n (%) | 50 (19.4) | 43 (16.7) | 75 (29.6) | 58 (22.5) | 51 (19.8) | 88 (34.8) |
| Mean (SD) total hypoglycaemia rate, events/patient/year | 1.27 (4.485) | 0.98 (4.202) | 2.13 (6.724) | 0.89 (3.777) | 0.80 (3.914) | 1.92 (6.983) |
| Nocturnal hypoglycaemia incidence, n (%) | 16 (6.2) | 10 (3.9) | 28 (11.1) | 18 (7.0) | 11 (4.3) | 35 (13.8) |
| Mean (SD) nocturnal hypoglycaemia rate, events/patient/year | 0.19 (0.904) | 0.13 (0.762) | 0.38 (1.444) | 0.11 (0.488) | 0.10 (0.551) | 0.31 (1.261) |
| Severe hypoglycaemia, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mean (SD) pancreatic enzymes, units/L | ||||||
| Lipase: baseline | 39.47 (20.765) | 41.81 (25.492) | 41.46 (22.347) | 39.47 (20.765) | 41.81 (25.492) | 41.46 (22.347) |
| Lipase Δ | 11.00 (34.134) | 10.67 (39.989) | −2.74 (21.818) | 10.76 (27.360) | 9.64 (33.715) | −3.66 (20.484) |
| Total amylase: baseline | 57.28 (21.000) | 59.70 (23.881) | 60.56 (26.618) | 57.28 (21.000) | 59.70 (23.881) | 60.56 (26.618) |
| Total amylase Δ | 7.50 (18.755) | 7.54 (22.388) | −0.37 (18.196) | 7.82 (16.250) | 6.42 (20.675) | 0.64 (18.696) |
| Pancreatic amylase: baseline | 25.31 (12.761) | 26.91 (15.568) | 26.76 (14.662) | 25.31 (12.761) | 26.91 (15.568) | 26.76 (14.662) |
| Pancreatic amylase Δ | 5.83 (14.607) | 5.14 (19.360) | −0.21 (11.287) | 5.48 (12.449) | 4.05 (15.490) | −0.49 (12.756) |
| Pancreatic enzymes: patients with >3 ULNa, n (%) | ||||||
| Lipase: baseline | 1 (0.4) | 3 (1.2) | 0 (0.0) | 1 (0.4) | 3 (1.2) | 0 (0.0) |
| Lipase | 3 (1.2) | 3 (1.2) | 1 (0.4) | 4 (1.6) | 4 (1.6) | 0 (0.0) |
| Total amylase: baseline | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total amylase | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pancreatic amylase: baseline | 0 (0.0) | 1 (0.4) | 1 (0.4) | 0 (0.0) | 1 (0.4) | 1 (0.4) |
| Pancreatic amylase | 0 (0.0) | 1 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Median calcitonin, pmol/L | ||||||
| Calcitionin: baseline | 0.29 | 0.29 | 0.29 | 0.29 | 0.29 | 0.29 |
| Calcitionin | 0.29 | 0.29 | 0.29 | 0.29 | 0.29 | 0.29 |
| Mean (SD) vital signs | ||||||
| Sitting blood pressure, mm Hg | ||||||
| Systolic: baseline | 130.79 (13.033) | 129.36 (12.642) | 131.42 (14.313) | 130.79 (13.033) | 129.36 (12.642) | 131.42 (14.313) |
| Systolic Δ | −5.53 (12.037) | −2.77 (11.920) | −2.22 (12.743) | −2.18 (11.134) | −0.61 (12.081) | −0.25 (11.717) |
| Diastolic: baseline | 78.31 (8.419) | 77.87 (9.161) | 79.15 (8.690) | 78.31 (8.419) | 77.87 (9.161) | 79.15 (8.690) |
| Diastolic Δ | −1.58 (7.842) | −0.92 (7.898) | −1.61 (8.417) | −0.19 (7.542) | 0.44 (7.582) | −1.13 (8.810) |
| Sitting pulse rate: baseline, bpm | 75.25 (10.033) | 76.09 (9.662) | 75.91 (9.761) | 75.25 (10.033) | 76.09 (9.662) | 75.91 (9.761) |
| Pulse rate Δ | 4.63 (8.681) | 3.26 (8.611) | −0.03 (8.816) | 4.18 (8.336) | 3.18 (8.917) | 0.07 (8.205) |
| Treatment‐emergent dulaglutide anti‐drug antibodyb, n (%) | NA | NA | NA | 10 (3.9) | 11 (4.3) | NA |
| Neutralizing dulaglutide | NA | NA | NA | 2 (0.8) | 4 (1.6) | NA |
| Neutralizing native‐sequence GLP‐1 | NA | NA | NA | 0 (0.0) | 0 (0.0) | NA |
Abbreviations: AE, adverse event; GLP‐1, glucagon‐like peptide‐1; SAE, serious adverse event; ULN, upper limit of normal.
Δ = change from baseline at week 26 or week 52.
Patients with at least 1 value >3 × ULN during the time period assessed.
Anti‐drug antibody was measured up to safety follow‐up period.
During the first 26 weeks, the incidence of total hypoglycaemia was lower for dulaglutide 1.5 mg and dulaglutide 0.75 mg than for glargine. The rate of total hypoglycaemia was also reported in patients with dulaglutide 1.5 mg and dulaglutide 0.75 mg (1.27 and 0.98 events/patient/year, respectively), and those receiving glargine (2.13 events/patient/year). For the patients taking sulphonylureas (with or without metformin) up to 26 weeks, the rate of hypoglycaemia was 1.59, 1.61 and 3.34 events/patient/year for dulaglutide 1.5 mg, dulaglutide 0.75 mg and glargine, respectively. It was higher for glargine compared with dulaglutide 1.5 mg (P = 0.007) or dulaglutide 0.75 mg (P = 0.008). The incidence and rate of nocturnal hypoglycaemia were significantly lower with dulaglutide versus glargine at week 52. (Table 2, Tables S2 and S3, Supporting Information.) No events of severe hypoglycaemia were reported during the study period.
Mean increases in pancreatic enzymes from baseline to week 52 were greater in the dulaglutide groups than in the glargine group. The incidence of clinically relevant increases in lipase (≥3 × upper limit of normal) was numerically higher with dulaglutide 1.5 mg (n = 4) and 0.75 mg (n = 4) than with glargine (n = 0). No patients had values of ≥3 × upper limit of normal for total or pancreatic amylase during the study (Table 2). No pancreatitis events were confirmed upon adjudication. No cases of pancreatic cancer were reported during the study.
In the dulaglutide groups, 21 patients (4.1%) developed treatment‐emergent dulaglutide anti‐drug antibodies (Table 2), and one patient in the dulaglutide 0.75 mg group experienced mild injection site reaction.
Dulaglutide 1.5 and 0.75 mg were associated with decreases in mean systolic blood pressure of ~5.53 mm Hg and 2.77 mm Hg, respectively, at week 26, compared with a small decrease of 2.22 mm Hg in the glargine group; however, no clinically relevant changes in systolic blood pressure among the three groups were observed by week 52. No differences were observed among groups for change in diastolic blood pressure at week 26 or week 52. The mean pulse rate increased in both dulaglutide groups compared with the glargine group (Table 2). There were no changes in the mean calcitonin values throughout the study in any of the treatment arms.
4. DISCUSSION
In the present study, once‐weekly dulaglutide 1.5 or 0.75 mg led to significant and clinically meaningful improvements in HbA1c compared with glargine at the 26‐week primary endpoint in mainly Asian patients with T2DM inadequately controlled on metformin and/or sulphonylureas, along with modest weight loss and lower risk of hypoglycaemia. Greater improvements in glycaemic control with dulaglutide 1.5 or 0.75 mg were also evident in the statistically significantly higher percentage of patients who achieved HbA1c targets of <53.0 mmol/mol and ≤47.5 mmol/mol (<7.0% and ≤6.5%) than with glargine at weeks 26 and 52.
It was reported that PPG excursions play an important role in overall glycaemic control. Although limitations exist when comparing studies, the present study seemed to include higher mean PPG and greater PPG excursions at baseline (Figure 2E) compared with the AWARD‐2 study.8 For example, the morning 2‐hour PPG and glucose excursions in the present study at randomization were ~13 and ~4 mmol/L, compared with ~11 and ~2 mmol/L in the AWARD‐2 study. The differences in PPG and PPG excursions between Asian and Western patients with T2DM were not only frequently reported in a number of clinical trials,8, 24, 25 but were also consistent with conclusions drawn from several landmark epidemiological surveys that newly diagnosed Asian patients with T2DM have higher PPG and postprandial excursions compared with Western patients.26, 27, 28
At week 26 and week 52, the pre‐morning blood glucose values obtained from seven‐point SMBG profiles showed significant and clinically meaningful reductions in both the dulaglutide groups and the glargine group. Reductions with glargine were significantly greater than with both dulaglutide doses, while the decreases in pre‐midday and pre‐evening meal among the three arms were generally similar. With regard to PPG changes, both dulaglutide 1.5 mg and dulaglutide 0.75 mg had a greater effect than glargine. Notably, dulaglutide 1.5 mg achieved significantly greater reductions than glargine at all three 2‐hour PPG assessments at week 26 and week 52 (Table S4, Supporting Information). Based on the dual effects on fasting glucose and PPG, greater reductions in postprandial excursions were observed in both dulaglutide doses compared with glargine, especially in morning 2‐hour excursion and midday 2‐hour excursion (Table S5, Supporting Information). In comparison with the AWARD‐2 study, the present study showed greater improvements in PPG and PPG excursion; this may have been attributable to the higher baseline PPG in Asian patients and multiple mechanisms of dulaglutide to improve PPG levels, including effect on glucose‐dependent first‐ and second‐phase insulin secretion, delay in gastric emptying and decreasing appetite. Associated with the improvements in FPG and PPG, both dulaglutide 1.5 and 0.75 mg resulted in a greater HbA1c change compared with glargine at the primary endpoint in the present study.
The safety profile of dulaglutide in the present study was consistent with the global AWARD programme. Patients treated with dulaglutide had a lower observed incidence of hypoglycaemia compared with those treated with glargine. It is widely accepted that GLP‐1 receptor agonists do not typically increase the risk of hypoglycaemia when used alone, but the risk of hypoglycaemia increases when it is used in combination with secretagogues or insulin, so reduction of the doses of the latter are usually recommended.
Both doses of dulaglutide were well tolerated for 52 weeks in predominantly Asian patients, consistent with the AWARD studies in mainly white and Hispanic/Latino patients.8, 13, 14, 15, 16, 17, 18 The most common AEs associated with dulaglutide treatment were gastrointestinal, consistent with the safety profile of the GLP‐1 receptor agonist class.17 These gastrointestinal events led to discontinuation from the study in a small proportion of patients. Small elevations in pancreatic enzymes within the normal range were observed over time. This finding is also consistent with elevations observed with other GLP‐1 receptor agonists.8, 13, 14, 15, 16, 17 No pancreatitis was confirmed upon adjudication, in agreement with the notion that there is limited clinical value in routine pancreatic enzyme testing in asymptomatic patients.29 Consistent with reports for other GLP‐1 receptor agonists and dulaglutide studies, the median serum calcitonin levels had no change after 52 weeks of treatment. It is not likely that administration of dulaglutide will increase the risk of thyroid cancer in humans based on accumulated non‐clinical and clinical data from the dulaglutide and GLP‐1RA class.16, 30, 31 An increase in pulse rate was observed with dulaglutide and was similar to changes observed within the GLP‐1 receptor agonist class.8, 13, 14, 15, 16, 17, 18 In the present study, dulaglutide treatment was associated with a low incidence (4.1%) of treatment‐emergent dulaglutide anti‐drug antibodies. This is consistent with the pooled data from nine phase II and phase III dulaglutide trials.32
The main limitation of the present study was its open‐label design, which could have affected physicians, and patients' behaviour; however, using a double‐blind design would have been difficult because glargine requires titration throughout the study period. It is possible that a more stringent titration of glargine achieving a lower mean FPG would have led to a greater decrease in HbA1c than that observed. Nevertheless, the data may actually reflect the most likely concerns in clinical practice when making decisions on insulin titration, such as the fear of hypoglycaemia and weight gain, especially in the absence of forced titration.
In conclusion, in mainly Asian patients with T2DM who fail to achieve optimal glycaemic control on metformin and/or a sulphonylurea, treatment with once‐weekly dulaglutide 0.75 or 1.5 mg, simultaneously addressing both fasting glucose and PPG, resulted in clinically meaningful improvement in glycaemic control associated with moderate body weight loss and a lower risk of hypoglycaemia compared with glargine.
CONFLICTS OF INTEREST
L.G., F.W. and J.Y. are employees of Eli Lilly and Company. P.L. was an employee of Eli Lilly and Company at the time of manuscript preparation.
Author contributions
W.W., L.N.R., E.F., K.H.S. and B.T. contributed to the study design, data collection and critical revision of the paper. L.G., F.W. and P.L. contributed to the data management, analysis, interpretation and critical revision of the paper. J.Y. contributed to the study design, data interpretation and critical revision of the paper.
Supporting information
Figure S1. Mean insulin glargine daily dose during the study, baseline to week 52, safety analysis population.
Figure S2. Baseline and 52‐week SMBG profiles.
Table S1. Insulin glargine dosing algorithm.
Table S2. Analysis of HbA1c (%) at week 26 and week 52.
Table S3. Summary of change from baseline in combined SMBG values.
Table S4. Summary of daily mean and excursions in combined SMBG values.
Table S5. Summary of HOMA2‐%B and HOMA2‐%S using LOCF at baseline to week 26 and week 52.
Table S6. Hypoglycemia summary at week 26.
Table S7. Hypoglycemia summary at week 52.
ACKNOWLEDGMENTS
We thank all the participants, investigators and trial‐site staff who were involved in the conduct of the study. We also thank David Bradley Woodward, Luis Emilio Garcia, Zvonko Milicevic, Li Shen and Ying Lou (Eli Lilly and Company) for their critical review of the draft manuscript, and Wan Qi Zhao, Fei Li and Fang Wei Yang (Eli Lilly and Company) for medical writing and editorial assistance.
Wang W, Nevárez L, Filippova E, et al. Efficacy and safety of once‐weekly dulaglutide versus insulin glargine in mainly Asian patients with type 2 diabetes mellitus on metformin and/or a sulphonylurea: A 52‐week open‐label, randomized phase III trial. Diabetes Obes Metab. 2019;21:234–243. 10.1111/dom.13506
Funding information This study was funded by Eli Lilly and Company.
REFERENCES
- 1. International Diabetes Federation . IDF Diabetes Atlas. 8th ed. Brussels, Belgium: International Diabetes Federation; 2017. http://www.diabetesatlas.org. [Google Scholar]
- 2. DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88:787‐835. ix. [DOI] [PubMed] [Google Scholar]
- 3. Weng J, Ji L, Jia W, et al. Standards of care for type 2 diabetes in China. Diabetes Metab Res Rev. 2016;32:442‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Standards of medical care in diabetes–2015: summary of revisions. Diabetes Care. 2015; 38(suppl 1):S4. [DOI] [PubMed] [Google Scholar]
- 5. Zinman B, Philis‐Tsimikas A, Cariou B, et al. Insulin degludec versus insulin glargine in insulin‐naive patients with type 2 diabetes: a 1‐year, randomized, treat‐to‐target trial (BEGIN Once Long). Diabetes Care. 2012;35:2464‐2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Riddle MC. The treat‐to‐target trial and related studies. Endocr Pract. 2006;12(suppl 1):71‐79. [DOI] [PubMed] [Google Scholar]
- 7. Yki‐Järvinen H, Kauppinen‐Mäkelin R, Tiikkainen M, et al. Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia. 2006;49:442‐451. [DOI] [PubMed] [Google Scholar]
- 8. Giorgino F, Benroubi M, Sun JH, Zimmermann AG, Pechtner V. Efficacy and safety of once‐weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD‐2). Diabetes Care. 2015;38:2241‐2249. [DOI] [PubMed] [Google Scholar]
- 9. Weissman PN, Carr MC, Ye J, et al. HARMONY 4: randomised clinical trial comparing once‐weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57:2475‐2484. [DOI] [PubMed] [Google Scholar]
- 10. Diamant M, Van Gaal L, Stranks S, et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION‐3): an open‐label randomised trial. Lancet. 2010;375:2234‐2243. [DOI] [PubMed] [Google Scholar]
- 11.Eli Lilly and Company. Trulicity [Prescribing Information]. Indianapolis ILU, LLC 2014. http://pi.lilly.com/us/trulicity-uspi.pdf. Accessed March 23, 2015.
- 12.Trulicity [Summary of Product Characteristics]. Houten TNELaChweeeeijcpmhmh.
- 13. Wysham C, Blevins T, Arakaki R, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD‐1). Diabetes Care. 2014;37:2159‐2167. [DOI] [PubMed] [Google Scholar]
- 14. Umpierrez G, Tofé Povedano S, Pérez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD‐3). Diabetes Care. 2014;37:2168‐2176. [DOI] [PubMed] [Google Scholar]
- 15. Blonde L, Jendle J, Gross J, et al. Once‐weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD‐4): a randomised, open‐label, phase 3, non‐inferiority study. Lancet. 2015;385:2057‐2066. [DOI] [PubMed] [Google Scholar]
- 16. Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD‐5). Diabetes Care. 2014;37:2149‐2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dungan KM, Povedano ST, Forst T, et al. Once‐weekly dulaglutide versus once‐daily liraglutide in metformin‐treated patients with type 2 diabetes (AWARD‐6): a randomised, open‐label, phase 3, non‐inferiority trial. Lancet. 2014;384:1349‐1357. [DOI] [PubMed] [Google Scholar]
- 18. Dungan KM, Raz I, Skrivanek Z, Sealls W, Fahrbach JL. Achieving the composite endpoint of glycated haemoglobin <7.0%, no weight gain and no hypoglycaemia in the once‐weekly dulaglutide AWARD programme. Diabetes Obes Metab. 2016;18:49‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Araki E, Inagaki N, Tanizawa Y, Oura T, Takeuchi M, Imaoka T. Efficacy and safety of once‐weekly dulaglutide in combination with sulphonylurea and/or biguanide compared with once‐daily insulin glargine in Japanese patients with type 2 diabetes: a randomized, open‐label, phase III, non‐inferiority study. Diabetes Obes Metab. 2015;17:994‐1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Medical Association Declaration of Helsinki . Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925‐926. [PubMed] [Google Scholar]
- 21. Kawamori R, Eliaschewitz FG, Takayama H, Hayashida CY. Efficacy of insulin glargine and glimepiride in controlling blood glucose of ethnic Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2008;79:97‐102. [DOI] [PubMed] [Google Scholar]
- 22. Dmitrienko A, Tamhane AC, Wiens BL. General multistage gatekeeping procedures. Biom J. 2008;50:667‐677. [DOI] [PubMed] [Google Scholar]
- 23. Westfall PH, Young SS. Resampling‐Based Multiple Testing: Examples and Methodsforp‐Value Adjustment. New York: John Wiley & Sons, Inc; 1993. [Google Scholar]
- 24. Pan CY, Sinnassamy P, Chung KD, Kim KW, Group LSI . Insulin glargine versus NPH insulin therapy in Asian type 2 diabetes patients. Diabetes Res Clin Pract. 2007;76:111‐118. [DOI] [PubMed] [Google Scholar]
- 25. Buse JB, Wolffenbuttel BH, Herman WH, et al. The DURAbility of Basal versus Lispro mix 75/25 insulin Efficacy (DURABLE) trial: comparing the durability of lispro mix 75/25 and glargine. Diabetes Care. 2011;34:249‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang SH, Dou KF, Song WJ. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:2425‐2426. author reply 6. [PubMed] [Google Scholar]
- 27. Shi Z. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:2425 author reply 6. [DOI] [PubMed] [Google Scholar]
- 28. Pang C, Bao YQ, Wang C, Lu JX, Jia WP, Xiang KS. Relationship between the level of fasting plasma glucose and beta cell functions in Chinese with or without diabetes. Chin Med J (Engl). 2008;121:2119‐2123. [PubMed] [Google Scholar]
- 29. Nauck MA, Frossard JL, Barkin JS, et al. Assessment of pancreas safety in the development program of once‐weekly GLP‐1 receptor agonist dulaglutide. Diabetes Care. 2017;40:647‐654. [DOI] [PubMed] [Google Scholar]
- 30. Sherman SI, Kloos RT, Tuttle RM, et al. No calcitonin change in a person taking dulaglutide diagnosed with pre‐existing medullary thyroid cancer. Diabet Med. 2018;35:381‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vahle JL, Byrd RA, Blackbourne JL, et al. Effects of dulaglutide on thyroid C cells and serum calcitonin in male monkeys. Endocrinology. 2015;156:2409‐2416. [DOI] [PubMed] [Google Scholar]
- 32. Milicevic Z, Anglin G, Harper K, et al. Low incidence of anti‐drug antibodies in patients with type 2 diabetes treated with once‐weekly glucagon‐like peptide‐1 receptor agonist dulaglutide. Diabetes Obes Metab. 2016;18:533‐536. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Mean insulin glargine daily dose during the study, baseline to week 52, safety analysis population.
Figure S2. Baseline and 52‐week SMBG profiles.
Table S1. Insulin glargine dosing algorithm.
Table S2. Analysis of HbA1c (%) at week 26 and week 52.
Table S3. Summary of change from baseline in combined SMBG values.
Table S4. Summary of daily mean and excursions in combined SMBG values.
Table S5. Summary of HOMA2‐%B and HOMA2‐%S using LOCF at baseline to week 26 and week 52.
Table S6. Hypoglycemia summary at week 26.
Table S7. Hypoglycemia summary at week 52.


