Abstract
Sleep spindle activity in infants supports their formation of generalized memories during sleep, indicating that specific sleep processes affect the consolidation of memories early in life. Characteristics of sleep spindles depend on the infant's developmental state and are known to be associated with trait‐like factors such as intelligence. It is, however, largely unknown which state‐like factors affect sleep spindles in infancy. By varying infants’ wake experience in a within‐subject design, here we provide evidence for a learning‐ and memory‐dependent modulation of infant spindle activity. In a lexical‐semantic learning session before a nap, 14‐ to 16‐month‐old infants were exposed to unknown words as labels for exemplars of unknown object categories. In a memory test on the next day, generalization to novel category exemplars was tested. In a nonlearning control session preceding a nap on another day, the same infants heard known words as labels for exemplars of already known categories. Central–parietal fast sleep spindles increased after the encoding of unknown object–word pairings compared to known pairings, evidencing that an infant's spindle activity varies depending on its prior knowledge for newly encoded information. Correlations suggest that enhanced spindle activity was particularly triggered, when similar unknown pairings were not generalized immediately during encoding. The spindle increase triggered by previously not generalized object–word pairings, moreover, boosted the formation of generalized memories for these pairings. Overall, the results provide first evidence for a fine‐tuned regulation of infant sleep quality according to current consolidation requirements, which improves the infant long‐term memory for new experiences.
Keywords: consolidation, generalization, learning, memory, sleep, sleep spindles
RESEARCH HIGHLIGHTS.
Characteristics of infant daytime naps depend on previous wake experience.
Infant's extensive encoding of unknown stimuli triggers extra spindle activity.
Infant's encoding‐related spindle increase supports generalization of memories.
Spindle‐dependent generalizations are retained in infant memory till the next day.
1. INTRODUCTION
Sleep supports the formation of long‐term memory. During the last two decades, certain components of the sleep architecture have been identified as part of the neural processes that result in the sleep‐dependent consolidation of memories. In adults, an increasing number of studies show that sleep spindles are correlated with improvements in subsequent memory performance (e.g., Clemens, Fabo, & Halasz, 2005; Gais, Mölle, Helms, & Born, 2002; Lustenberger, Wehrle, Tüshaus, Achermann, & Huber, 2015; Schabus et al., 2004, 2008; Tamminen, Payne, Stickgold, Wamsley, & Gaskell, 2010). Sleep spindles are transient oscillations at a frequency of 11–15 Hz with a duration of at least 0.5 s and an initially waxing and then waning amplitude (De Gennaro & Ferrara, 2003). They appear in NonREM sleep (NonREM for “non‐rapid eye movement”) and are most prominent in sleep stage 2. Beyond their supposed role in maintaining sleep, spindles are thought to be involved in the reactivation of recent memories during sleep and to be mainly responsible for the sleep‐dependent plasticity in the neocortex (Bergmann, Mölle, Diedrichs, Born, & Siebner, 2012; Latchoumane, Ngo, Born, & Shin, 2017; Mölle, Marshall, Gais, & Born, 2002; Niethard, Burgalossi, & Born, 2017; Rasch & Born, 2013; Rosanova & Ulrich, 2005; Steriade, 1999).
Sleep spindles first emerge within the second month of life and are consistently observed in infants of the ninth postnatal week. During early ontogeny, characteristics of sleep spindles undergo rapid developmental changes, such as an increase in spindle density from 1.5 to 3 months, which is followed by a relatively long period of individually stable spindle density (Louis, Zhang, Revol, Debilly, & Challamel, 1992). While frontal spindles are particularly thought to reflect aspects of brain maturation, central and parietal spindles are found to be more stable during development (Scholle, Zwacka, & Scholle, 2007; Shinomiya, Nagata, Takahashi, & Masumura, 1999).
Sleep is the predominant state in infants, and its importance for early development is unchallenged (for a recent review, see Grigg‐Damberger, 2017). Longitudinal research has shown, for instance, that sleep maturation predicts memory development. In particular, the individual ratio of daytime sleep to nighttime sleep is negatively related to an infants’ later language outcome (Dionne et al., 2011). However, despite the fact that daytime sleep decreases with development, the growth in vocabulary in a certain period increases with the frequency of daytime naps (Horváth & Plunkett, 2016), a finding that points to the timely consolidation of daytime experience during sleep.
Indeed, experimental studies have provided evidence that sleep supports the retention and reorganization of memories even in infancy (Friedrich, Wilhelm, Born, & Friederici, 2015; Friedrich, Wilhelm, Mölle, Born, & Friederici, 2017; Gómez, Bootzin, & Nadel, 2006; Horváth, Hannon, Ujma, Gombos, & Plunkett, 2018; Horváth, Liu, & Plunkett, 2016; Horváth, Myers, Foster, & Plunkett, 2015; Hupbach, Gomez, Bootzin, & Nadel, 2009; Konrad, Herbert, Schneider, Lorek, & Seehagen, 2015; Konrad, Herbert, Schneider, & Seehagen, 2016; Seehagen, Konrad, Herbert, & Schneider, 2015; Simon et al., 2017). For the consolidation of early lexical–semantic memories, a benefit of sleep has been demonstrated by analyzing the looking behavior in 16‐month‐olds (Horváth et al., 2015, 2016) and by measuring event‐related potentials (ERPs) in groups of 6‐ to 8‐ and 9‐ to 16‐month‐olds (Friedrich et al., 2015, 2017). In the ERP studies, the generalization of new object–word pairings to previously experienced similar object–word pairings was indicated by the so‐called N400 component that reflects a semantic processing stage (Kutas & Federmeier, 2011; Kutas & Hillyard, 1980) and is taken as evidence for the presence of lexical–semantic memories in infants and toddlers (Friedrich & Friederici, 2005a, 2005b, 2008; Junge, Cutler, & Hagoort, 2012; Parise & Csibra, 2012; Rämä, Sirri, & Serres, 2013; Von Koss Torkildsen, Syversen, Simonsen, Moen, & Lindgren, 2007; Von Koss Torkildsen et al., 2007b). In line with the looking preference to target objects in the behavioral study on lexical–semantic generalization (Horváth et al., 2016), the N400 generalization effect emerged first in the memory test, and only when infants slept after the encoding session (Friedrich et al., 2015, 2017). In these studies, thus, infants had generalized the newly encoded memories off‐line during the postencoding nap, and not immediately during encoding.
The strength of the generalization effect in the memory test of the ERP study was, moreover, related to the amount of fast sleep spindles over central and parietal brain regions, which evidences the involvement of infant sleep spindles in the sleep‐dependent generalization of early memories. Given the overall age range of 10 months in these studies, the relation between sleep spindles and memory generalization appears to be independent of developmental trends in spindle characteristics. But then, the question arises why some infants generate higher spindle activity and are able to generalize new experiences better than others. One possible reason is that the capability to generate sleep spindles represents a physiological index of intelligence (Fogel & Smith, 2011). Sleep spindles are relatively stable in an individual and their trait‐like characteristics are related to an individual's perceptual, cognitive, and learning abilities (Bódizs et al., 2005; Fogel, Nader, Cote, & Smith, 2007; Schabus et al., 2006, 2008). Individual abilities as reflected in spindle characteristics may affect stimulus processing already in infancy, as it appeared to be the case for visual habituation in 3‐month‐olds (Horváth et al., 2018). In the study with 6‐ to 8‐month‐old infants, however, not only spindle activity itself, but also its individually normalized local increase over the relevant central–parietal regions with reference to remaining regions was related to the generalization of the category–word pairings (Friedrich et al., 2017), which suggests that trait‐like differences in spindle activity do not fully explain the spindle‐related improvement in memory in these studies.
In adults, sleep spindles are also state‐dependent, since they vary with the current consolidation requirements. Spindle density, in particular, increases after learning when compared to a nonlearning control task (Gais et al., 2002; Mölle, Eschenko, Gais, Sara, & Born, 2009). Schabus and colleagues found that this learning‐related spindle increase affects the subsequent memory performance independent of individual intellectual abilities (Schabus et al., 2008). Overall, the pattern of findings in adults points to a reciprocal relationship between sleep and memory: not only do current sleep spindles enhance the consolidation of recently encoded memories, but also is the amount of current spindle activity enhanced by the encoding of new memories.
In the present study we asked whether this fine‐tuned regulation of sleep spindle activity in response to consolidation requirements is functional already in infancy. By applying a within‐subject design to 14‐ to 16‐months‐old infants we tested, whether the massed exposure to new category–word pairings increases infant sleep spindles in a subsequent nap, and if so, whether this encoding‐related spindle increase is related to the infant's memory on the next day.
2. METHODS
2.1. Participants
The experimental design was applied to 47 monolingual infants from ~14 to 16 months of age. Of these, 30 infants (mean age 469 days ± 30 days, 15 female) contributed to the final analyses. Data from 17 infants were excluded from analyses because of too few artifact‐free trials in one of the experimental conditions (n = 7), due to very noisy event‐related potential (ERP) responses (n = 3), due to lack of interest in the visual stimuli (n = 2), because infants did not fall asleep after the experimental session (n = 2), or due to technical problems (n = 3). When analyzing the data of the learning session, three infants were additionally excluded due to their low number of artifact‐free trials and resulting noisy ERPs. All parents gave informed consent before participation. The study was approved by the ethics committee of the Humboldt University of Berlin.
Infants of the present study varied in their socio‐economic background, with about half of the parents having a university (or equivalent) degree, and half a lower professional qualification. All infants were born in the 37th to 42nd week of pregnancy with a birth weight ranging from 2,480 g to 4,230 g (3,518 ± 493 g). They had no known visual or hearing deficits and no major sleep problems. As typical for the investigated age group, all infants were habitual nappers. According to parental reports, they usually napped between 1 and 3 hr (2 ± 0.64 hr) during the day, and slept 9–12 hr (11 ± 1.11 hr) during the night.
2.2. Procedure
In a within‐subject‐design, infants participated in three laboratory sessions, each taking place on a different day (Figure 1). An additional task was applied after the control nap, but not reported here. In the learning task on the first day, infants heard new words while seeing exemplars of unknown object categories. In the memory test session on the following day, generalization to novel category exemplars was tested. In the nonlearning control session on a third day (about a week later), infants heard known names for exemplars of already known object categories. In each experimental session, infants were exposed to 128 individual object–word pairs. The three sessions lasted each for 7 min.
Figure 1.
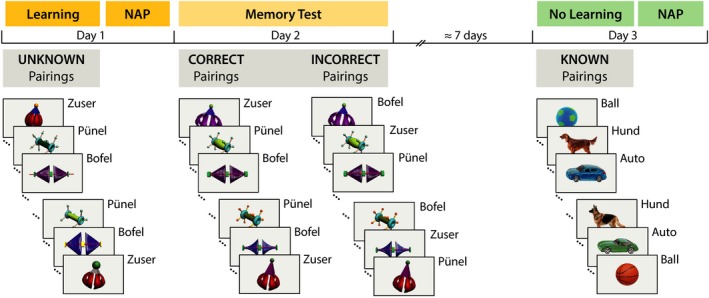
Experimental design. In the learning session on the first day, infants heard unknown pseudowords as names for exemplars of unknown similarity‐based object categories. In the memory test on the following day, generalization was tested by presenting novel category exemplars in both correct and incorrect pairings, that is, in same category–word pairings as in the learning session or in different pairings. In the nonlearning control session about a week later, infants heard known words as names for exemplars of known categories. Subsequent to the learning and control sessions infants napped. (For a detailed description, see Section 1)
In the learning session, 64 exemplars belonging to eight initially unknown similarity‐based categories (eight exemplars per category) were presented once together with a pseudoword as category name in the consistent pairing condition. In order to assess immediate generalization of the object–word pairings while controlling for repetition effects, additional eight objects and eight words were presented each eight times, but not consistently paired, such that the formation of stable object–word pairings was prevented.
In the memory test on the next day, each four novel exemplars of a category were presented in order to test for the presence of generalized memories of the category–word pairings. In the correct pairing condition, categories and words were paired as in the learning session on the previous day. In the incorrect pairing condition, the same exemplars and the same words as in the correct pairing condition were presented, but in different pairings that violated the category–word pairings of the learning session. Each individual pairing was presented once. For the case that infants did not show generalized memories, after the presentation of novel exemplars, four old exemplars of each category were presented with the correct and incorrect words in order to assess learning in the test phase compared to initial learning. Since generalized memory turned out to be present in the first half of the test phase, memory and new learning may have interfered in the second half, therefore we did not include these data in the analyses.
In the nonlearning control session, 64 exemplars belonging to eight basic‐level categories (eight exemplars per category) were each presented twice together with their correct word label. Categories were known to be acquired very early in infancy, the word labels were (in German): Auto (car), Ball (ball), Hund (dog), Eimer (pail), Keks (cookie), Löffel (spoon), Schuh (shoe), Vogel (bird). Infant's comprehension of these words was assessed by parental ratings. On average, infants comprehended seven of the eight words.
During the experimental sessions, infants sat on the mother's or father's lap in a sound‐attenuated room. In each trial a colored picture of a single object appeared on the screen for 3,200 ms. After an interval of 800 ms postpicture onset, the German indefinite article ein (masculine/neuter) was presented to direct the children's attention to the acoustically presented word that followed the article presentation after 900 ms.
For both the learning and the nonlearning sessions, infants were scheduled at a time when they were expected to take a nap within the next hour. In 26 of 30 infants, the learning and nonlearning tasks were applied before noon. On average, the learning session ended at 10:54 (SD 1:18) and the nonlearning session at 10:48 (SD 1:12). After the learning and the nonlearning sessions, infants were prepared for polysomnographic recordings (5–10 min) and laid down in a baby crib or in their pram. When necessary, infants were held by their parent until they fell asleep. Also if needed, infants were fed or diapered before laying down for sleep. Sleep onset latency from the end of preparation (M ± SD: 24.8 ± 24.2 min) did not significantly differ between the naps following the learning and nonlearning sessions (t 29 = −1.033, p = 0.310). After the learning session, infants slept for 63.1 ± 23.7 min, and after the nonlearning session, for 55.7 ± 19.8 min. Total sleep time did not significantly differ between the naps (t 29 = 1.517, p = 0.140).
2.3. Stimuli
Visual stimuli were colored illustrations of single objects (Figure 1). In the learning session, eight exemplars of each of eight different similarity‐based object categories were presented. In the memory test, four additional exemplars of each category were presented. For the nonlearning session, pictures of eight different exemplars for each of the selected eight categories were chosen.
In the nonlearning session, eight words naming the known basic‐level categories were used as auditory stimuli. In the learning session, eight disyllabic pseudowords were taken as names for the new categories. Pseudowords were phonotactically legal in German, were stressed on the first syllable, had a consonant–vowel onset, and had typical masculine or neuter endings. All auditory stimuli were spoken slowly by a female speaker, digitized at a rate of 44.1 kHz, and presented through loudspeaker with moderate intensity.
2.4. Sleep recordings and sleep spindle analyses
Infants’ sleep was recorded using a portable amplifier (SOMNOscreen EEG 10–20, Somnomedics, Kist, Germany). EEG recordings were obtained with electrodes attached at F3, FZ, F4, C3, C4, P3, PZ, P4, left, and right mastoids, referenced to CZ (positions according to the International 10–20 system), filtered between 0.03 and 35 Hz, and sampled at 256 Hz. Electrooculographic and electromyographic recordings were bipolar from electrodes close to the eyes and at the chin, respectively. Off‐line, EEG signals were rereferenced to the average potential at left and right mastoid electrodes. EEG recordings were visually scored according to standard criteria (Grigg‐Damberger et al., 2007; Rechtschaffen & Kales, 1968). For each nap, total sleep time (TST) and the time spent in the different sleep stages (1, 2, slow wave sleep, and REM sleep) were determined.
Periods of arousal were excluded and power spectral analysis of the EEG signal was performed using fast Fourier transformation for the remaining periods of NonREM sleep. The spectra were calculated for successive 8‐s (2,048 data points) artifact‐free intervals using a Hanning window to taper the data. Average power was calculated first over all bins in the frequency range of interest; then averages were calculated for the succeeding 8‐s intervals.
For the detection of discrete sleep spindles, the EEG of all artifact‐free NonREM epochs was low‐pass filtered (32 Hz) and down‐sampled (128 Hz). The spindle detection algorithm and criteria were adopted from Mölle et al. (2009). First, for each subject, the individual spindle peak frequency was identified in the NonREM sleep power spectra of all channels (learning: 14.12 ± 1.07 Hz, nonlearning: 14.08 ± 1.10 Hz, across all subjects and channels; t 29 = 0.875, p = 0.389 for the comparison of learning vs. nonlearning). The EEG signal was then filtered with a band‐pass width of 3 Hz centered on the detected individual peak frequency. A root mean‐square (RMS) representation of the filtered signal was calculated using a sliding window of 0.2 s with a step size of one sample. Additional smoothing was performed with a sliding‐window average of 0.2 s size and one sample point step size. Time frames were considered as spindle intervals if the RMS signal during NonREM sleep exceeded a threshold of 1.5 standard deviations of the filtered signal (learning: 5.90 ± 1.36 μV, nonlearning: 6.09 ± 1.23 μV, across all subjects and channels; t 29 = 1.183, p = 0.246 for learning vs. nonlearning) in an individual channel of a subject for 0.5–5 s and if the largest value within the frame was greater than 2.5 standard deviations of the filtered signal (learning: 9.84 ± 2.26 μV, nonlearning: 10.15 ± 2.04 μV; t 29 = 1.183, p = 0.246 for learning vs. nonlearning). Two succeeding spindles were counted as one spindle when the interval between the end of the first spindle and the beginning of the second spindle was shorter than 0.5 s and the resulting spindle was not longer than 5 s.
In the previous studies, memory generalization was particularly related to sleep spindles over central and parietal brain regions (Friedrich et al., 2015, 2017). In order to increase statistical power, here, we analyzed the mean spindle measures across all central and parietal channels. Analyses included spindle number, spindle density (spindles per 30 s NonREM sleep), peak‐to‐peak amplitude, and length. When testing the difference between the postlearning and the nonlearning control nap for the four spindle parameters, the significance level was Bonferroni‐adjusted to 0.0125.
2.5. ERP data acquisition and analyses
Infant memory was assessed by event‐related potential (ERP) responses to the word stimuli. The EEG was recorded with a stationary amplifier (REFA, TMS International, Oldenzaal, Netherlands) at 21 electrode sites and digitized online at 500 Hz. Off‐line, the EEG was rereferenced to the average potential recorded from left and right mastoid electrodes and filtered between 0.5 and 20 Hz (−3 dB cut‐off frequencies of 0.62 and 19.88 Hz). Trials with potential fluctuations exceeding a standard deviation of 80 μV within a sliding window of 500 ms at any electrode site were rejected.
ERPs were analyzed time‐locked to word onset. For each condition, epochs of 1,200 ms from word onset were averaged. A minimum of 10 artifact‐free trials for each condition (correct, incorrect pairing), were required for an individual average to be included in further analyses. On average, 19 (SD = 6) trials contributed to an ERP condition. Trial numbers did not differ between conditions (t 29 = 1.570, p > 0.127).
For the statistical analyses of the ERP data, lateral electrode sites were combined into regions‐of‐interests (ROIs). The averaged ERPs at F7, F3, and T7 formed the left fronto‐temporal region (LFT); at F8, F4, and T8 the right fronto‐temporal region (RFT); at FC3, C3, and CP5 the left central region (LC); at FC4, C4, and CP6 the right central region (RC); at P3, P7, and O1 the left parieto‐occipital region (LPO) and at P4, P8, and O2 the right parieto‐occipital region (RPO).
Memory effects were evaluated by ANOVAs with the within‐subject factors Pairing (correct vs. incorrect), Hemisphere (left vs. right), and Region (fronto‐temporal, central, parieto‐occipital), which were performed for the ERP mean amplitudes within two time windows (200–600 ms, 600–1,000 ms). For midline sites, analog ANOVAs were performed with Pairing and Region (FZ, CZ, PZ). To assess the impact of the learning‐related increase in spindle activity on the infants’ memory, the increases in spindle number and spindle density were defined by the individual differences in spindle number/density between postlearning and control nap and included as covariates into repeated measure ANCOVAs. In all AN(C)OVAs degrees of freedoms were adjusted according to Greenhouse–Geisser whenever they were >1.
Subsequent to interactions of Pairing with Spindle increase, the spatial maximum of the memory effect was tested for significance by one‐sample t‐test and taken for the correlation analysis (using Pearson's correlation coefficient) between spindle increase and memory performance. For the correlations of spindle increase with the two ERP memory effects, the significance level was adjusted to 0.025. In order to further qualify the impact of spindle increase on the infants’ memory, the ERP memory effects were tested separately in subgroups defined by a median split due to the individual's spindle increase. These subgroups did not significantly differ in age (t 28 = −1.037, p = 0.308), comprehension of words in the control condition (t 28 = 0.349, p = 0.730), attention during encoding as indicated by the number of artifact‐free trials (t 28 = −0.306, p = 0.763), nor in the typical amount of sleep during the day (t 28 = 1.214, p = 0.235) and night (t 28 = −1.748, p = 0.091). In order to test for differences in immediate generalization during the learning phase, Spindle group was included as a between‐subject factor into an ANOVA with the within‐subject factors Pairing (consistent vs. inconsistent), Hemisphere, and Region as well as into the respective midline ANOVA with Pairing and Region.
3. RESULTS
Table 1 provides a summary of the sleep architecture during the nap after the learning task and during the nap after the nonlearning control session. During the postlearning nap, infants spent slightly more time in NonREM sleep stage 2 than during the control nap (mean increase = 5.9 min, SD = 16.1, t 29 = 2.023, p = 0.052). Moreover, both the number and the density of central–parietal fast sleep spindles were higher in the postlearning nap after the presentation of unknown category–word pairings than in the control nap after the presentation of known pairings (number: t 29 = 2.686, p = 0.012, density: t 29 = 2.676, p = 0.012; Figure 2b, lower panels). The increase in spindle number was mainly due to the increase in sleep stage 2 (r = 0.836, p < 0.0001; Figure 2c left), whereas the increase in spindle density was unrelated to changes in stage 2 sleep time (r = 0.054, p = 0.777; Figure 2c right). Mean frontal spindles did not significantly differ between learning and nonlearning conditions (number: t 29 = 1.831, p = 0.077; density: t 29 = −0.196, p = 0.867; Figure 2b, upper panel). Also spindle amplitude and spindle length were not affected by the learning task (|t29| < 1.570, p > 0.127; Figure 2a).
Table 1.
Sleep characteristics during the nap after the learning task and during the nap after the nonlearning control session. stage 2 sleep, slow wave sleep, REM sleep, and TST in minutes, spindle peak frequency in Hz, and spindle density in number per 30 s
| Postlearning nap | Nonlearning control nap | |||
|---|---|---|---|---|
| M | SD | M | SD | |
| Stage 2 sleep | 31.93 | 14.64 | 25.99 | 11.40 |
| Slow wave sleep | 20.30 | 8.63 | 20.27 | 10.80 |
| REM sleep | 0.78 | 2.75 | 0.00 | 0.00 |
| TST | 63.08 | 23.74 | 55.67 | 19.85 |
| Spindle peak frequency | 14.12 | 1.07 | 14.08 | 1.10 |
| Spindle number frontal | 117.80 | 53.53 | 100.83 | 44.10 |
| Spindle number central–parietal | 100.44 | 46.37 | 79.62 | 35.87 |
| Spindle density frontal | 1.19 | 0.24 | 1.20 | 0.20 |
| Spindle density central–parietal | 1.03 | 0.24 | 0.96 | 0.24 |
Figure 2.
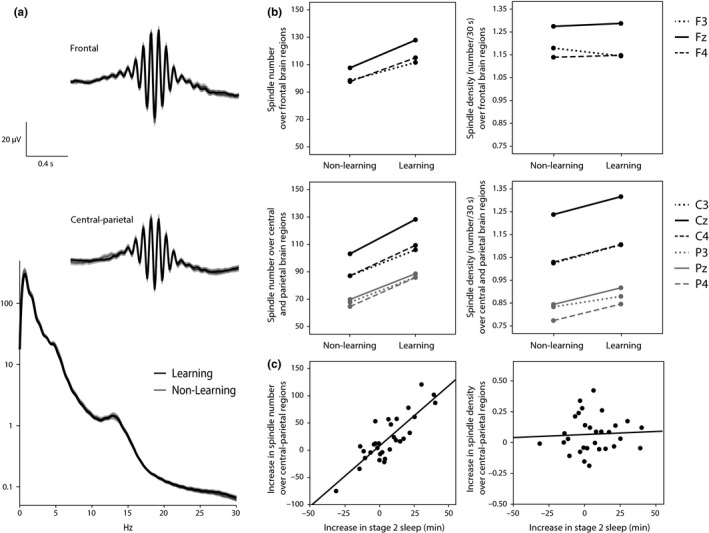
Sleep spindle activity and its increase after learning. (a) EEG power spectra during NonREM sleep (at CZ) and (above) sleep spindles averaged across frontal (F3, FZ, and F4) and across central–parietal (C3, CZ, C4, P3, PZ, and P4) brain regions for the nap after the learning session and the control nap after the nonlearning session (mean ± SEM). (b) Spindle numbers (left panels) and spindle density (right panels) in nonlearning control nap and postlearning nap for spindles over frontal (upper panels) and central–parietal (lower panels) cortex. Learning‐induced increases in spindle number and density were significant only for central–parietal spindles but not for frontal spindles. (c) Correlation between the learning‐induced increase in time spent in stage 2 NonREM sleep and the learning‐induced increase in spindle number (left: r = 0.836, p < 0.0001) and spindle density (right: r = 0.054, p = 0.777). Learning‐induced increases are determined by the individual infant's difference in respective parameters between the postlearning nap and the nonlearning control nap
Neither the absolute duration of stage 2 sleep, absolute spindle number, absolute spindle density nor their observed increases after the learning session with reference to the nonlearning control nap were correlated with the infants’ age (|r| < 0.167, p = 0.378). Gender had no effect on the spindle increase (comparison between girls and boys for increase in spindle number: t 28 = 1.301, p = 0.204, for increase in spindle density: t 28 = 0.050, p = 0.961). As an estimation of an infant's attention, we used the number of artifact‐free trials, which is typically higher in attentive than inattentive infants. The increase in spindle density was not related to estimated attention during the learning session (r = 0.101, p = 0.624) or the memory test (r = −2.56, p = 0.172). There was, however, a trend for a positive relation between attention during learning and the increase in the number of central–parietal spindles (r = 0.372, p = 0.061), which was even stronger, when controlling for the increase in stage 2 sleep (partial correlation: r = 0.414, p = 0.040). A similar relation was not present for attention during the memory test (r = 0.140, p = 0.461).
The individual increase in spindle density during the nap after the encoding of new category–word pairings significantly affected an infant's brain response in the memory test 1 day later (Figure 3). Overall, words presented in correct object–word pairings elicited a more negative‐going ERP response over the left hemisphere in the early time window (200–600 ms) than words in incorrect pairings (Pairing × Hemisphere F 1,29 = 12.569, p = 0.001, left: t 29 = 2.822, p = 0.009; Figure 3a). This ERP shift with a maximum over the left fronto‐temporal region (t 29 = 2.424, p = 0.022) is known as the infant N200–500 word form priming effect (e.g., Friedrich & Friederici, 2004, 2005a, 2005b; Von Koss Torkildsen et al., 2007, 2007b; Von Koss Torkildsen et al., 2008; for a review, see Friedrich, 2017). Its occurrence in response to pairings with novel category exemplars indicates that generalized memories of the categories were formed and linked with the appropriate words.
Figure 3.
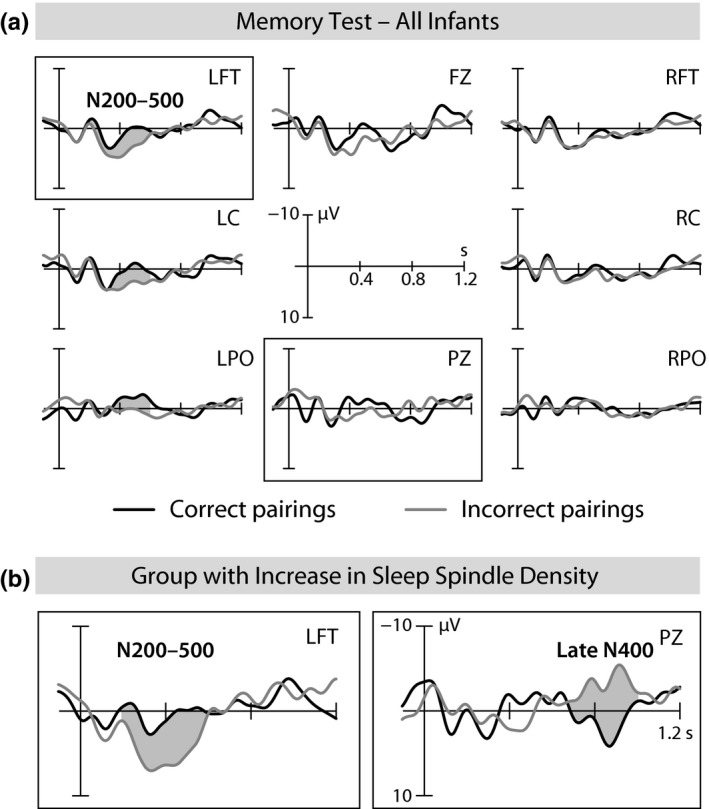
ERPs of the memory test on the day after encoding. (a) The ERP responses to the same words in correct pairings and incorrect pairings averaged across all infants. Negativity is plotted upward. (b) Early N200–500 memory effect (over the left fronto‐temporal region) and late N400 memory effect (at PZ) in the infants with strong spindle increase during the postencoding nap
After controlling for the learning‐related spindle increase, the overall N200–500 effect was clearly diminished (Pairing × Hemisphere F 1,27 = 4.594, p = 0.041), since the memory effect was modulated by the increase in spindle density (introduced as a covariate in the analyses: Pairing × Spindle density increase: F 1,27 = 7.957, p = 0.009, Pairing × Region × Spindle density increase: F 2,54 = 3.802, p = 0.047, midline: Pairing × Spindle density increase: F 1,27 = 4.609, p = 0.041, Pairing × Region × Spindle density increase: F 2,54 = 4.964, p = 0.011). The increase in spindle number as an additional covariate had no significant effect on this early‐latency component. The correlation between the maximum of the N200–500 memory effect over the left fronto‐temporal region and the learning‐induced increase in spindle density amounted to r = 0.478 (p = 0.008). A median split based on the strength of the individual infant's increase in central–parietal spindle density revealed that the left fronto‐temporal N200–500 memory effect was present only in the group with strong density increases (t 14 = 3.845, p = 0.002, Figure 3b left), and not in the group with weak increases or decreases in spindle density (t 14 = 0.116, p = 0.910; group difference: t 28 = 2.451, p = 0.021; Figure 4b, upper panel).
Figure 4.
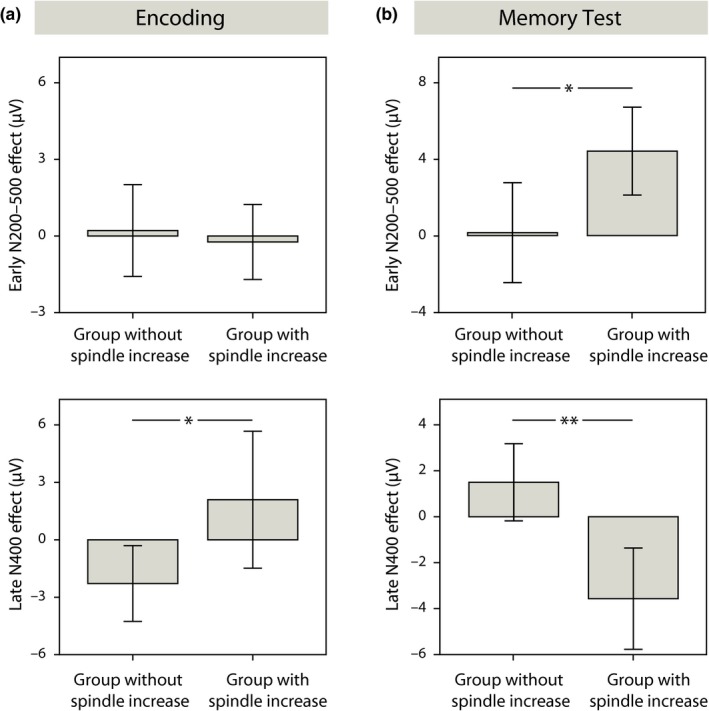
Comparison between the generalization effects of the spindle subgroups. Mean ERP responses with error bars (±2 SEM) in the subgroup with substantial learning‐related increase in spindle density (above the median of the whole group) and in the subgroup without substantial spindle density increase (below the median), (a) during encoding and (b) during the memory test. Upper panels: early N200–500 effect, lower panels: late N400 effect. *p < 0.05, **p < 0.01
The ERP response in the later time window (600–1,000 ms) did not differ between correct and incorrect pairings in the total group of infants, indicating that, overall, no additional effect appeared. However, a memory effect emerged when considering the modulating effect of the learning‐related increase in sleep spindles during the postlearning nap (Pairing × Spindle density increase: F 1,27 = 4.251, p = 0.049, Pairing × Hemisphere × Region × Spindle density increase F 2,54 = 4.358, p = 0.020, Pairing × Region × Spindle number increase F 2,54 = 4.104, p = 0.032; midline: Pairing × Spindle density increase F 1,27 = 5.495, p = 0.027). While the correlation of the increase in spindle number with the ERP difference between incorrect and correct pairings failed to meet significance when correcting for multiple comparisons (r = −0.361, p = 0.050 for LPO), the increase in spindle density was correlated with the ERP difference over the midparietal (PZ: r = −0.568, p = 0.001; Figure 5b) and the left parieto‐occipital (r = −0.470, p = 0.009) brain regions.
Figure 5.
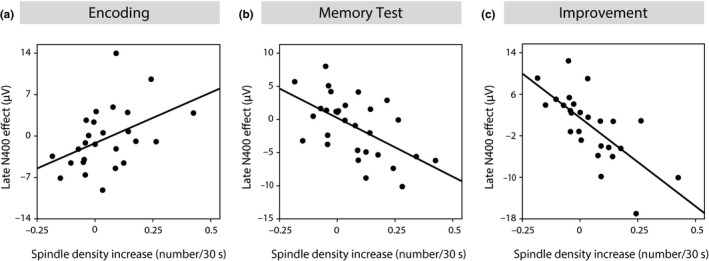
The relations between spindle density increase and generalization. (a) Relation between the increase in central–parietal spindle density and the N400 effect of immediate generalization during the second half of the learning phase (r = 0.444, p = 0.023). Due to the negative potential shift of the N400, the positive sign of the correlation coefficient reflects a negative dependency. (b) Positive relation between the increase in central–parietal spindle density and the negative N400 generalization effect in the memory test (r = −0.568, p = 0.001). (c) Positive relation between the increase in central–parietal spindle density and the increase in the N400 generalization effect from learning to memory test (r = −0.707, p = 0.00005)
After the median split of the total group based on the central–parietal spindle density increase, the group with strong increases exhibited a parietal memory effect (PZ: t 14 = −3.233, p = 0.006, Figure 3b right), but the group with weak increases or decreases did not (t 14 = 1.787, p = 0.096, group difference: t 28 = 3.655, p = 0.001; Figure 4b, lower panel). Although occurring at a quite late latency, both the polarity and the spatial distribution of this potential shift resemble those of the infant N400 effect that is seen to reflect a semantic word processing stage (e.g., Friedrich & Friederici, 2004, 2005a, 2005b; Von Koss Torkildsen, Syversen, Simonsen, Moen, & Lindgren, 2007, 2007b; Parise & Csibra, 2012; Borgström, von Koss Torkildsen, & Lindgren, 2015; for a review, see Friedrich, 2017). Its occurrence in response to novel category exemplars suggests that infants with strong increases in sleep spindle density have formed generalized lexical–semantic memories of the category–word pairings, that is, they have acquired and retained meanings for the previously unknown words (Friedrich et al., 2015).
To specify, whether infants of the group with strong increases in spindle density have formed the generalized representations of the category–word pairings immediately during encoding or subsequently during their nap, we analyzed the data of the second half of the learning session. In the overall group, there was neither a left fronto‐temporal N200–500 effect (t 15 = 0.041, p = 0.968) nor a parietal (PZ: t 25 = −0.817, p = 0.422) or a central (CZ: t 25 = −0.422, p = 0.676) N400 effect. Also, the generalization effects of the memory test were not correlated with their corresponding ERP differences during encoding (N200–500: r = 0.097, p = 0.638, N400: r = 0.010, p = 0.962), which suggests that the presence of generalized memories in the test phase after the nap did not simply depend on the formation of generalized memories during the learning session. However, infants of the spindle subgroups appeared to differ in their encoding (midline: Pairing × Spindle Group: F 1,24 = 2.965, p = 0.098). In the group with weak increases or decreases in spindle density, a late N400 effect was present over the central brain region (CZ: t 14 = −2.307, p = 0.037), showing that at least some of the infants in this group had generalized the object–word pairings already during the learning session. In contrast, in the group with strong increases in spindle density, the effect was missing (t 10 = 1.171, p = 0.269, group difference: t 24 = −2.288, p = 0.031; Figure 4a, lower panel). These findings are quite opposite to the hypothesis that the N400 generalization effect in the memory test on the next day was caused by immediate generalization during encoding. In particular, they strongly speak for the notion that infants with high increases in spindle density have generalized newly encoded memories during their postencoding nap.
So far, the increase in spindle density could also be explained by the novelty of the learning situation or by any other confounding variable that is unspecific to the newly encoded information. However, the observed learning‐induced increase in central–parietal spindle density was not only correlated with the left fronto‐temporal N200–500 effect and the parietal N400 effect of the test phase as neural indexes of generalized memories on the next day, but also with the central N400 effect of the learning session as a specific neural marker of immediate generalization prior to the nap (CZ: r = 0.444, p = 0.023). Importantly, the relation between the increase in spindle density with the N400 generalization effect during encoding was inversed compared to its relation with the N400 generalization effect of the memory test (Figure 5a, b). This means, the lower the immediate generalization effect during encoding, the stronger the spindle density increase during the postencoding nap; and the stronger the spindle density increase during the nap, the higher the generalization effect in the memory test on the next day. These inverse relations were jointly reflected in the high correlation between spindle density increase and the increase in generalization from encoding to memory test (i.e., the difference between the N400 effect of the memory test and that of the learning session), which amounted to −0.707 (p = 0.00005; Figure 5c).
The strong effect of the spindle‐related generalization during the nap even masked an effect of immediate generalization on later memory. When controlling for spindle increase in a partial correlation analysis, the generalization effect in the memory test was correlated with immediate generalization during encoding too (r = 0.402, p = 0.046), which implies that a certain part of the immediately generalized memory was still retained till the next day. Nevertheless, multivariate regression analysis (R = 0.635, F 2,23 = 7.764, p = 0.003) showed that spindle increase predicts later generalization much stronger (β = −0.705, p = 0.0007) than immediate generalization does (β = 0.379, p = 0.046). Thus, generalization in the memory test on the next day was mainly based on generalized memories formed during the postencoding nap and only to a distinctly lower degree on memories generalized immediate during encoding.
In order to figure out, how an infant's current state of language development has affected its individual responses, we further analyzed subgroups based on infants’ comprehension abilities as reported by parental ratings. Infants, who already comprehended all of the assessed words, formed the high‐comprehension group (N = 15), while infants, who did not yet comprehend all words, were assigned to the low‐comprehension group (N = 15). Comprehension groups particularly differed in their encoding of the similar object–word pairings in the learning session. While the low‐comprehension group did not show any effect of generalization during learning, the late central N400 effect was present in the high comprehension group (CZ: t 11 = −2.607, p = 0.024; group difference: t 24 = 2.418, p = 0.024; Figure 6a), indicating immediate generalization in these more advanced infants. Despite this initial encoding advantage, due to the strong effect of spindle‐related improvement in generalization, comprehension groups no longer differed in their memory effects on the next day (N200–500: t 29 = 0.310, p = 0.759, N400: t 29 = 0.248, p = 0.806; Figure 6b).
Figure 6.
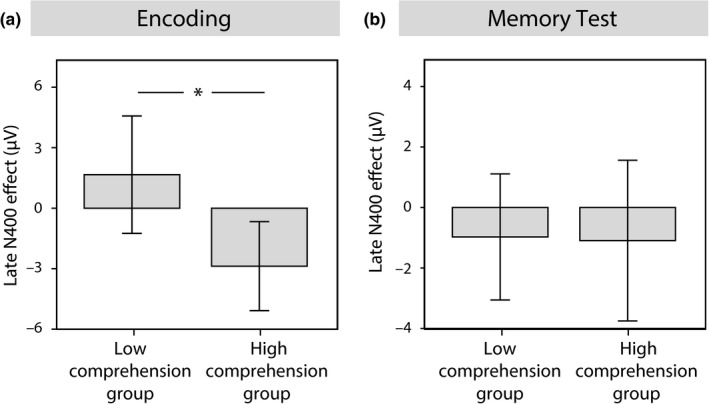
Comparison between the N400 generalization effect of the comprehension subgroups. Mean late N400 effect with error bars (±2 SEM) in the subgroup with lower word comprehension and in the subgroup with higher word comprehension, (a) during encoding and (b) during the memory test. *p < 0.05
4. DISCUSSION
By varying infants’ wake learning experience immediately before a nap, here, we provide first evidence for a learning‐induced modification of infant sleep. In particular, central–parietal fast sleep spindles, previously reported to be related to infants’ off‐line formation of generalized memories (Friedrich et al., 2015, 2017), increased in 14‐ to 16‐month‐old infants after exposing them to a large amount of unknown information.
Importantly, the visual and auditory stimuli presented in the learning and nonlearning sessions were of comparable complexity. Also, even though infants had already experience with the categories and words employed in the nonlearning session, the presented individual exemplars were novel in both sessions. Thus, the increase in spindle density after the learning session was neither caused by the pure massed presentation of visual and auditory information nor by the novelty of the specific exemplars. The crucial contrast was the presence or absence of lexical–semantic representations for the category–word pairings in long‐term memory. In the nonlearning session, in which novel exemplars of known categories were presented together with known words, infants could assign them to their existing lexical–semantic knowledge. In this session, infants may have encoded specific perceptual and episodic‐like memories, which may have been consolidated during the postencoding nap, but there was no need to build new generalized memories. In contrast, in the learning session, in which infants were exposed to novel exemplars of unknown categories paired with unknown words, they could not refer them to existing lexical–semantic representations in memory. The great amount of unreferred transient information encoded in this session, the similarity between certain exemplars, and the pairings of similar exemplars each with the same word might have induced the need for generalization. Apparently, this kind of consolidation pressure in the present study prompted the infants’ brain to generate further sleep spindles—an interpretation that is strongly supported by the specific relation between the generalization effect of the learning session and the increase in central–parietal fast sleep spindles. In those infants, who generalized immediately during encoding, the consolidation pressure was lower, consequently, their increase in sleep spindles was lower than in infants, who did not generalize immediately. Even though we cannot fully rule out the possibility that unspecific factors such as the order of the experimental sessions or the presentation of inconsistent pairings have triggered some additional spindle activity, their potential effect would not explain the here observed specific link of spindle increase to the kind of encoding during prior learning.
Like in adults (Gais et al., 2002; Mölle et al., 2009), infants’ enhancement of spindle activity manifested itself primarily as an increase in spindle density, which was independent of the time the infant spent in stage 2 sleep. Also the total number of spindles increased, partly with the duration of sleep stage 2, and partly with attention during the learning session as an index of the overall amount of newly encoded information. The state‐dependent increase of sleep spindles observed here in 14‐ to 16‐month‐olds evidences that the mechanisms regulating sleep spindle activity according to the current consolidation requirements are already functional early in the second year of life. Thus, individual differences in spindle generation do not only depend on brain maturation and trait‐like characteristics, but also reflect state‐like differences induced by the different encoding of recent wake experience and the different knowledge for the newly encoded information.
Not only did the extensive encoding of unknown information trigger an extra amount of infant spindle activity, this extra amount of spindle activity was also involved in consolidating the newly encoded information. On the day after encoding, robust ERP memory effects were present only in the infants with high learning‐induced increases in spindle density, suggesting that only these infants had built generalized memories that were strong enough to be retained till the next day. This result replicates and specifies previous findings showing that sleep spindles benefit the sleep‐dependent generalization of infant memories (Friedrich et al., 2015, 2017). In particular, it provides first‐time evidence for the impact of specific sleep characteristics during a daytime nap on infant memory 1 day later.
The reciprocal relation between sleep spindles and memory observed in the 14‐ to 16‐month‐old infants of the present ERP study parallels findings of a behavioral study with nonverbal material in 4‐year‐olds (Kurdziel, Duclos, & Spencer, 2013). As the relation between immediate recall performance and sleep spindle density during the postencoding nap in the 4‐year‐olds, here, spindle density increase was correlated negatively with the generalization effect at the end of the learning session (i.e., positively with the negative‐polarity effect). And the same spindle measure was correlated positively with the enhancement of generalization during the subsequent nap (i.e., negatively with the negative‐polarity effect), similar as it was the case for the benefit in the memory performance of 4‐year‐olds. These parallel findings suggest that the impact of sleep spindles on memory consolidation is not limited to language learning only. Also, the here observed fine‐tuned regulation of sleep spindles in response to the actual consolidation pressure might be effective within a wide age range during development.
In the present study, moreover, the reciprocal dependencies of sleep spindles and memory generalization particularly affected the performance of the subgroups defined by the infants’ comprehension abilities. Compared to infants with lower word comprehension, infants with high word comprehension displayed an advantage in their immediate generalization during the learning session. Because, however, the later generalization effect was much stronger based on the spindle‐related generalization during the nap than on the retention of immediately generalized memories, and immediate generalization was inversely associated with the subsequent increase in spindle density, 1 day later, infants of the high‐comprehension group did no longer profit from their initial encoding advantage. Also, because the increase in spindle density in response to missing immediate generalization enhanced the sleep‐dependent formation of generalized memories, infants with lower comprehension abilities compensated their missing immediate generalization by the formation of new generalized memories during the subsequent nap. This finding provides first experimental evidence that a specific modification in the characteristics of a daytime nap enables infants to overcome weak initial learning and to catch up with peers who encode better and sleep as well after encoding.
5. CONCLUSIONS
In the present study we show that the amount of central‐parietal fast sleep spindles in 14‐ to 16 month‐old infants depends on their existing knowledge for the information encoded before a nap. The observed increase in sleep spindle activity was particularly triggered by missing generalization of a large amount of new information, which points to an encoding‐ and memory‐dependent adjustment of spindle generation in the infant brain. It suggests that, from early infancy on, sleep spindles are partly generated on demand, that is, whenever novel memories need to be formed for information hold in temporary memory. This adjustment of infant spindle activity according to current consolidation requirements appears to be a mechanism that boosts memory development effectively. Whether the observed encoding‐depending increase in spindle activity indeed represents an active recruitment of resources caused by the consolidation pressure or whether it is rather a by‐product of the reorganization of memories during sleep remains to be solved by future studies.
AUTHOR CONTRIBUTIONS
Conceptualization, M.F. and J.B.; Analysis, M.F. and M.M.; Writing—Original Draft, M.F.; Writing—Review & Editing, M.M., A.D.F., and J.B.; Resources, M.F. and A.D.F.; Funding Acquisition, M.F.
ACKNOWLEDGEMENTS
We thank all families who participated in this study. Special thanks to Christina Rügen for recording the infant ERP data, to Kerstin Strelow‐Morgenstern for scoring the infant sleep data, and to Franziska Illner for recruiting participants. The study was supported by grants from the Deutsche Forschungsgemeinschaft to M. F. (FR 1336/2‐1, FR 1336/2‐2).
Friedrich M, Mölle M, Friederici AD, Born J. The reciprocal relation between sleep and memory in infancy: Memory‐dependent adjustment of sleep spindles and spindle‐dependent improvement of memories. Dev Sci. 2019;22:e12743 10.1111/desc.12743
REFERENCES
- Bergmann, T. O. , Mölle, M. , Diedrichs, J. , Born, J. , & Siebner, H. R. (2012). Sleep spindle‐related reactivation of category‐specific cortical regions after learning face‐scene associations. NeuroImage, 59(3), 2733–2742. [DOI] [PubMed] [Google Scholar]
- Bódizs, R. , Kis, T. , Lázár, A. S. , Havrán, L. , Rigó, P. , Clemens, Z. , & Halász, P. (2005). Prediction of general mental ability based on neural oscillation measures of sleep. Journal of Sleep Research, 14(3), 285–292. [DOI] [PubMed] [Google Scholar]
- Borgström, K. , von Koss Torkildsen, J. , & Lindgren, M. (2015). Event‐related potentials during word mapping to object shape predict toddlers’ vocabulary size. Frontiers in Psychology, 6, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens, Z. , Fabo, D. , & Halasz, P. (2005). Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience, 132(2), 529–535. [DOI] [PubMed] [Google Scholar]
- De Gennaro, L. , & Ferrara, M. (2003). Sleep spindles: An overview. Sleep Medicine, 7(5), 423–440. [DOI] [PubMed] [Google Scholar]
- Dionne, G. , Touchette, E. , Forget‐Dubois, N. , Petit, D. , Tremblay, R. E. , Montplaisir, J. Y. , & Boivin, M. (2011). Associations between sleep‐wake consolidation and language development in early childhood: A longitudinal twin study. Sleep, 34(8), 987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel, S. M. , Nader, R. , Cote, K. A. , & Smith, C. T. (2007). Sleep spindles and learning potential. Behavioral Neuroscience, 121(1), 1. [DOI] [PubMed] [Google Scholar]
- Fogel, S. M. , & Smith, C. T. (2011). The function of the sleep spindle: A physiological index of intelligence and a mechanism for sleep‐dependent memory consolidation. Neuroscience & Biobehavioral Reviews, 35(5), 1154–1165. [DOI] [PubMed] [Google Scholar]
- Friedrich, M. (2017). ERP indices of word learning: What do they reflect and what do they tell us about the neural representations of early words? In: Westermann Gert. & Mani Nivedita. (Eds.), Early word learning, series current issues in developmental psychology (pp. 123–137), London, Taylor & Francis Group. [Google Scholar]
- Friedrich, M. , & Friederici, A. D. (2004). N400‐like semantic incongruity effect in 19‐month‐olds: Processing known words in picture contexts. Journal of Cognitive Neuroscience, 16(8), 1465–1477. [DOI] [PubMed] [Google Scholar]
- Friedrich, M. , & Friederici, A. D. (2005a). Lexical priming and semantic integration reflected in the ERP of 14‐month‐olds. NeuroReport, 16(6), 653–656. [DOI] [PubMed] [Google Scholar]
- Friedrich, M. , & Friederici, A. D. (2005b). Phonotactic knowledge and lexical‐semantic processing in one‐year‐olds: Brain responses to words and nonsense words in picture contexts. Journal of Cognitive Neuroscience, 17(11), 1785–1802. [DOI] [PubMed] [Google Scholar]
- Friedrich, M. , & Friederici, A. D. (2008). Neurophysiological correlates of online word learning in 14‐month‐old infants. NeuroReport, 19(18), 1757–1762. [DOI] [PubMed] [Google Scholar]
- Friedrich, M. , Wilhelm, I. , Born, J. , & Friederici, A. D. (2015). Generalization of word meanings during infant sleep. Nature Communications, 6, 6004 10.1038/ncomms7004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich, M. , Wilhelm, I. , Mölle, M. , Born, J. , & Friederici, A. D. (2017). The sleeping infant brain anticipates development. Current Biology, 27, 1–7. 10.1016/j.cub.2017.06.070 [DOI] [PubMed] [Google Scholar]
- Gais, S. , Mölle, M. , Helms, K. , & Born, J. (2002). Learning‐dependent increases in sleep spindle density. Journal of Neuroscience, 22(15), 6830–6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez, R. L. , Bootzin, R. R. , & Nadel, L. (2006). Naps promote abstraction in language‐learning infants. Psychological Science, 17(8), 670–674. [DOI] [PubMed] [Google Scholar]
- Grigg‐Damberger, M. M. (2017). Ontogeny of sleep and its functions in infancy, childhood, and adolescence In Nevšímalová S., Bruni O. (Ed.), Sleep disorders in children (pp. 3–29). Cham: Springer. [Google Scholar]
- Grigg‐Damberger, M. , Gozal, D. , Marcus, C. L. , Quan, S. F. , Rosen, C. L. , Chervin, R. D. , … Iber, C. (2007). The visual scoring of sleep and arousal in infants and children. Journal of Clinical Sleep Medicine, 3(2), 201–240. [PubMed] [Google Scholar]
- Horváth, K. , Hannon, B. , Ujma, P. P. , Gombos, F. , & Plunkett, K. (2018). Memory in 3‐month‐old infants benefits from a short nap. Developmental Science, 21(3), e12587. [DOI] [PubMed] [Google Scholar]
- Horváth, K. , Liu, S. , & Plunkett, K. (2016). A daytime nap facilitates generalization of word meanings in young toddlers. Sleep, 39(1), 203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth, K. , Myers, K. , Foster, R. , & Plunkett, K. (2015). Napping facilitates word learning in early lexical development. Journal of Sleep Research, 24(5), 503–509. [DOI] [PubMed] [Google Scholar]
- Horváth, K. , & Plunkett, K. (2016). Frequent daytime naps predict vocabulary growth in early childhood. Journal of Child Psychology and Psychiatry, 57(9), 1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupbach, A. , Gomez, R. L. , Bootzin, R. R. , & Nadel, L. (2009). Nap‐dependent learning in infants. Developmental Science, 12(6), 1007–1012. [DOI] [PubMed] [Google Scholar]
- Junge, C. , Cutler, A. , & Hagoort, P. (2012). Electrophysiological evidence of early word learning. Neuropsychologia, 50(14), 3702–3712. [DOI] [PubMed] [Google Scholar]
- Konrad, C. , Herbert, J. S. , Schneider, S. , Lorek, S. , & Seehagen, S. (2015). Sleep after learning enhances flexibility of memory retrieval in 12‐month‐old infants. Developmental Psychobiology, 57(7), 872–873. [DOI] [PubMed] [Google Scholar]
- Konrad, C. , Herbert, J. S. , Schneider, S. , & Seehagen, S. (2016). Gist extraction and sleep in 12‐month‐old infants. Neurobiology of Learning and Memory, 134, 216–220. [DOI] [PubMed] [Google Scholar]
- Kurdziel, L. , Duclos, K. , & Spencer, R. M. (2013). Sleep spindles in midday naps enhance learning in preschool children. Proceedings of the National Academy of Sciences of the USA, 110(43), 17267–17272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas, M. , & Federmeier, K. D. (2011). Thirty years and counting: Finding meaning in the N400 component of the event‐related brain potential (ERP). Annual Review of Psychology, 62, 621–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas, M. , & Hillyard, S. A. (1980). Reading senseless sentences: Brain potentials reflect semantic incongruity. Science, 207(4427), 203–205. [DOI] [PubMed] [Google Scholar]
- Latchoumane, C. F. V. , Ngo, H. V. V. , Born, J. , & Shin, H. S. (2017). Thalamic spindles promote memory formation during sleep through triple phase‐locking of cortical, thalamic, and hippocampal rhythms. Neuron, 95(2), 424–435. [DOI] [PubMed] [Google Scholar]
- Louis, J. , Zhang, J. X. , Revol, M. , Debilly, G. , & Challamel, M. J. (1992). Ontogenesis of nocturnal organization of sleep spindles: A longitudinal study during the first 6 months of life. Electroencephalography and Clinical Neurophysiology, 83(5), 289–296. [DOI] [PubMed] [Google Scholar]
- Lustenberger, C. , Wehrle, F. , Tüshaus, L. , Achermann, P. , & Huber, R. (2015). The multidimensional aspects of sleep spindles and their relationship to word‐pair memory consolidation. Sleep, 38(7), 1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölle, M. , Eschenko, O. , Gais, S. , Sara, S. J. , & Born, J. (2009). The influence of learning on sleep slow oscillations and associated spindles and ripples in humans and rats. European Journal of Neuroscience, 29(5), 1071–1081. [DOI] [PubMed] [Google Scholar]
- Mölle, M. , Marshall, L. , Gais, S. , & Born, J. (2002). Grouping of spindle activity during slow oscillations in human non‐rapid eye movement sleep. Journal of Neuroscience, 22(24), 10941–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethard, N. , Burgalossi, A. , & Born, J. (2017). Plasticity during sleep is linked to specific regulation of cortical circuit activity. Frontiers in Neural Circuits, 11, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parise, E. , & Csibra, G. (2012). Electrophysiological evidence for the understanding of maternal speech by 9‐month‐old infants. Psychological Science, 23(7), 728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rämä, P. , Sirri, L. , & Serres, J. (2013). Development of lexical–semantic language system: N400 priming effect for spoken words in 18‐and 24‐month old children. Brain and Language, 125(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Rasch, B. , & Born, J. (2013). About sleep's role in memory. Physiological Reviews, 93(2), 681–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtschaffen, A. , & Kales, A. (1968). A manual of standardized, techniques and scoring system for sleep stages of human sleep. Los Angeles, CA: Brain Information Service, Brain Research Institute, University of California at Los Angeles. [Google Scholar]
- Rosanova, M. , & Ulrich, D. (2005). Pattern‐specific associative long‐term potentiation induced by a sleep spindle‐related spike train. Journal of Neuroscience, 25(41), 9398–9405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabus, M. , Gruber, G. , Parapatics, S. , Sauter, C. , Klösch, G. , Anderer, P. , & Zeitlhofer, J. (2004). Sleep spindles and their significance for declarative memory consolidation. Sleep, 27(8), 1479–1485. [DOI] [PubMed] [Google Scholar]
- Schabus, M. , Hödlmoser, K. , Gruber, G. , Sauter, C. , Anderer, P. , Klösch, G. , … Zeitlhofer, J. (2006). Sleep spindle‐related activity in the human EEG and its relation to general cognitive and learning abilities. European Journal of Neuroscience, 23(7), 1738–1746. [DOI] [PubMed] [Google Scholar]
- Schabus, M. , Hoedlmoser, K. , Pecherstorfer, T. , Anderer, P. , Gruber, G. , Parapatics, S. , … Zeitlhofer, J. (2008). Interindividual sleep spindle differences and their relation to learning‐related enhancements. Brain Research, 1191, 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholle, S. , Zwacka, G. , & Scholle, H. C. (2007). Sleep spindle evolution from infancy to adolescence. Clinical Neurophysiology, 118(7), 1525–1531. [DOI] [PubMed] [Google Scholar]
- Seehagen, S. , Konrad, C. , Herbert, J. S. , & Schneider, S. (2015). Timely sleep facilitates declarative memory consolidation in infants. Proceedings of the National Academy of Sciences, 112(5), 1625–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomiya, S. , Nagata, K. , Takahashi, K. , & Masumura, T. (1999). Development of sleep spindles in young children and adolescents. Clinical Electroencephalography, 30(2), 39–43. [DOI] [PubMed] [Google Scholar]
- Simon, K. N. , Werchan, D. , Goldstein, M. R. , Sweeney, L. , Bootzin, R. R. , Nadel, L. , & Gómez, R. L. (2017). Sleep confers a benefit for retention of statistical language learning in 6.5 month old infants. Brain and Language, 167, 3–12. [DOI] [PubMed] [Google Scholar]
- Steriade, M. (1999). Coherent oscillations and short‐term plasticity in corticothalamic networks. Trends in Neurosciences, 22(8), 337–345. [DOI] [PubMed] [Google Scholar]
- Tamminen, J. , Payne, J. D. , Stickgold, R. , Wamsley, E. J. , & Gaskell, M. G. (2010). Sleep spindle activity is associated with the integration of new memories and existing knowledge. Journal of Neuroscience, 30(43), 14356–14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Koss Torkildsen, J. V. K. , Svangstu, J. M. , Hansen, H. F. , Smith, L. , Simonsen, H. G. , Moen, I. , & Lindgren, M. (2008). Productive vocabulary size predicts event‐related potential correlates of fast mapping in 20‐month‐olds. Journal of Cognitive Neuroscience, 20(7), 1266–1282. [DOI] [PubMed] [Google Scholar]
- Von Koss Torkildsen, J. , Syversen, G. , Simonsen, H. G. , Moen, I. , & Lindgren, M. (2007). Brain responses to lexical‐semantic priming in children at‐risk for dyslexia. Brain and Language, 102(3), 243–261. [DOI] [PubMed] [Google Scholar]
- Von Koss Torkildsen, J. , Syversen, G. , Simonsen, H. G. , Moen, I. , & Lindgren, M. (2007. b). Electrophysiological correlates of auditory semantic priming in 24‐month‐olds. Journal of Neurolinguistics, 20(4), 332–351. [Google Scholar]


