Abstract
Background
Chronic pelvic pain, in particular dysmenorrhoea, is a significant yet unresolved healthcare problem in gynaecology. As interoceptive sensitivity and underlying neural mechanisms remain incompletely understood, this functional magnetic resonance imaging (fMRI) study assessed behavioural and neural responses to visceral stimuli in primary dysmenorrhoea (PMD).
Methods
Women with PMD (N = 19) without psychological comorbidity and healthy women (N = 20) were compared with respect to visceral sensory and pain thresholds, and to neural responses to individually calibrated mildly painful and painful rectal distensions implemented during scanning. Trial‐by‐trial ratings of perceived intensity were accomplished with visual analogue scales (VAS).
Results
Although women with dysmenorrhoea reported significantly higher chronic pain intensity and pain interference with daily life activities (p < 0.01, assessed with the West Haven‐Yale Multidimensional Pain Inventory), there were no differences between groups in visceral sensitivity and mean trial‐by‐trial VAS ratings were virtually identical. Analysis of neural responses revealed activation in brain regions previously shown to be involved in the processing of visceral stimuli with differences between painful and mildly painful stimulation, but no group differences were found even when using a liberal statistical threshold.
Conclusions
Dysmenorrhoea patients show unaltered perceptual and neural responses to experimental interoceptive stimuli. Despite limited sample size, these negative results argue against a generalized sensitization towards interoceptive stimuli in patients without psychological comorbidities. Future studies should clarify the role of psychosocial factors in central sensitization using more pain region‐specific models in larger and clinically more heterogeneous samples.
Significance
Despite higher chronic pain and pain interference with daily life activities, women with primary dysmenorrhoea do not differ from healthy women with respect to visceral sensitivity or neural processing of aversive interoceptive stimuli induced by rectal distensions. Generalized sensitization may be present only in subgroups with pronounced psychosocial or psychiatric disturbances.
1. INTRODUCTION
Dysmenorrhoea constitutes the most common gynaecological condition in female adolescents and women of reproductive age (Coco, 1999), with significant individual and societal consequences (Böttcher et al., 2014; Iacovides, Avidon, & Baker, 2015a; Leyendecker, Wildt, & Mall, 2009; Leyendecker et al., 2015). Pelvic pain is the hallmark symptom in both primary and secondary dysmenorrhoea. Whereas in secondary dysmenorrhoea, pain originates from several identifiable pathological conditions, especially endometriosis, in primary dysmenorrhoea (PDM), pain occurs in the absence of discernible pelvic pathology and is closely linked with menstruation with respect to onset, severity and duration. PDM has been classified as a chronic pelvic pain syndrome (CPP) (Baranowski, Lee, Price, & Hughes, 2014) and overlaps with other CPP including irritable bowel syndrome (IBS) (Olafsdottir, Gudjonsson, Jonsdottir, Björnsson, & Thjodleifsson, 2012; Zondervan et al., 2001). Although enhanced pain sensitivity and central sensitization have been proposed to play a crucial role in the pathophysiology of PDM (Iacovides et al., 2015a), visceral sensitivity and neural mechanisms involved in the central processing of interoceptive, visceral stimuli remain incompletely understood.
Current knowledge regarding pain sensitivity and central pain processing in PDM largely comes from studies that have implemented exteroceptive, somatic pain stimuli such as pressure, heat, ischaemic, electrical (Giamberardino, Berkley, Iezzi, de Bigontina, & Vecchiet, 1997) or laser‐evoked pain stimuli applied in different bodily regions (Iacovides et al., 2015a). Little is known about perceptual responses to interoceptive, visceral pain in PDM. Clinically relevant interoceptive pain models, especially controlled distension of the uterine cervix, rectum or colon, have very rarely been implemented in patients thus far (Arendt‐Nielsen, Madsen, Jarrell, Gregersen, & Drewes, 2014; Brinkert, Dimcevski, Arendt‐Nielsen, Drewes, & Wilder‐Smith, 2007) and have never been employed as part of a brain imaging study. Given marked differences between pain modalities in behavioural and neural processing in healthy women (Aziz et al., 2000; Dunckley et al., 2005; Koenen et al., 2017; Strigo, Duncan, Boivin, & Bushnell, 2003) and patients with IBS (Verne et al., 2003), and first evidence suggesting modality‐specific pain processing in PDM (Bajaj, Bajaj, Madsen, & Arendt‐Nielsen, 2002; Wei, Chao, Tu, Li et al., 2016), it is important to complement and extend existing knowledge about pain mechanisms in PDM specifically for the visceral modality. In this functional magnetic resonance imaging (fMRI), conducted during the phase of menstruation, we implemented a clinically relevant visceral pain model (i.e. rectal distensions) in order to address visceral sensitivity and behavioural and neural responses to individually calibrated mildly painful and painful aversive visceral stimuli in women with PDM and healthy controls. In addition to pain‐specific behavioural and neural measures, we also repeatedly assessed cognitive aspects relevant to pain perception, given accumulating knowledge about expectations as fundamental mechanisms underlying placebo and nocebo effects in the context of visceral pain (Elsenbruch & Enck, 2015; Elsenbruch & Labrenz, 2018). Finally, given the broad role of stress‐related factors in visceral pain modulation (for a recent review, see Elsenbruch & Enck, 2017) and knowledge regarding stress and particularly of the hypothalamic‐pituitary‐adrenal (HPA) axis in CPP and endometriosis (for review, see Brawn, Morotti, Zondervan, Becker, & Vincent, 2014), we analysed stress‐related measures based on ratings and blood samples, that is state anxiety, perceived arousal, cortisol and prolactin.
In this comprehensive study with behavioural, endocrine and neural aspects, our specific aims were to test the following hypotheses:
Given evidence suggesting enhanced sensitivity to distension of the sigmoid colon in PDM (Brinkert et al., 2007), we expected reduced thresholds for both first perception and pain induced by rectal distensions in women with PDM.
Based on data indicating temporal summation upon repeated distensions of the uterine cervix in PDM (Arendt‐Nielsen et al., 2014), we hypothesized a greater increase in trial‐by‐trial pain ratings in PDM upon repeated distensions in the scanner.
With respect to distension‐induced blood oxygen‐level‐dependent (BOLD) responses, we tested enhanced activation in sensory‐discriminative brain areas (e.g. thalamus, posterior insula, somatosensory cortex) as well as in regions encoding emotional arousal and cognitive pain aspects (e.g. anterior insula, cingulate cortex, amygdala, prefrontal cortex) in women with PDM. This hypothesis was based on altered neural processing of painful rectal distensions in patients with IBS (Mayer, Gupta, Kilpatrick, & Hong, 2015).
Finally, we explored the specificity to pain by comparing responses to mildly painful distensions, and additionally assessed pain anticipation, state anxiety and neuroendocrine mediators of the hypothalamic‐pituitary‐adrenal (HPA) axis in our study paradigm.
2. METHODS
2.1. Study population
A total of 23 women with primary dysmenorrhoea and 23 healthy women were recruited via word of mouth or advertisement at the Department of Gynecologic Endocrinology and Reproductive Medicine of the Medical University Innsbruck, Austria, between August 2013 and August 2015. All participants gave informed consent and were scheduled for the study during the phase of their menstruation (days 1–5, given feasibility considerations). The recruitment and screening process included an initial semistandardized telephone screening confirming the self‐reported presence of dysmenorrhoea, a personal interview with completion of standardized questionnaires and a gynaecological examination and ultrasound scan performed by a licensed gynaecologist (author B.B.) at the Department of Gynecologic Endocrinology and Reproductive Medicine of the Medical University Innsbruck, Austria. The psychosocial questionnaire battery included the Hospital Anxiety Depression Scale (HADS) for symptoms of anxiety and depression (Herrmann‐Lingen, Buss, & Snaith, 2011), the SF‐12 for health‐related quality of life (QoL; Morfeld, Kirchberger, & Bullinger, 2011) and the West Haven‐ Yale Multidimensional Pain Inventory (MPI; Flor, Rudy, Birbaumer, Streit, & Schugens, 1990).
Inclusion criteria for patients consisted of a typical history of primary dysmenorrhoea since menarche or shortly afterwards with severe menstrual pain. Severe menstrual pain on the day of the study was verified with a visual analogue scale (VAS, 0–100 mm). The cut‐off for sufficiently severe ongoing menstrual pain severity was the published cut‐off (i.e. a VAS > 54 mm; Collins, Moore, & McQuay, 1997), consistent with other neuroimaging studies in PDM patients (Vincent et al., 2011; Wei, Chao, Tu, Li et al., 2016; Wei, Chao, Tu, Lin et al., 2016). Patients with known secondary dysmenorrhoea (i.e. with a previously established histological diagnosis of endometriosis) were not included. To further verify the absence of menstrual pain on the study day in the healthy sample, the menstrual pain severity rating had to be below the published cut‐off (i.e. <54 mm) on the VAS (Collins et al., 1997). Patients were instructed to abstain from pain medications (e.g., NSAIDs) on the day of the study; compliance was confirmed on the study day based on self‐report.
General exclusion criteria for all participants were age <18 or >45 years, a body mass index (BMI) <18 or >30 kg/m2, breastfeeding, any known medical or psychological pathological condition (except dysmenorrhoea for the patient group), current medication use (except thyroid medication, occasional over‐the‐counter drugs for minor allergies, benign headaches, etc.), current clinically relevant anxiety or depression symptoms above the published cut‐off values on the Hospital Anxiety and Depression Scale (HADS; Herrmann‐Lingen et al., 2011), current gastrointestinal (GI) symptoms suggestive of an undiagnosed GI condition including IBS based on self‐report or symptoms suggestive of fibromyalgia. Any evidence of external and/or internal anorectal tissue damage upon digital anorectal examination (e.g. painful haemorrhoids which may interfere with rectal balloon placement), previous third or fourth degree of perianal tear, active anal fissure, evidence of structural brain abnormality upon structural MRI scan and regular MRI‐specific exclusion criteria (phobic anxiety, claustrophobia, etc.) were also exclusionary. Pregnancy was exclusionary, verified during gynaecological examination and again on the study day via urinary HCG analysis. Note that the use of hormonal contraceptives was not exclusionary for participation.
2.2. Experimental design and procedures
The study day was scheduled on days 1–5 of the menstrual cycle, confirmed by participant report of menstruation and hormonal analysis [luteinizing hormone (LH); follicle‐stimulating hormone (FSH), estradiol and progesterone]. Upon arrival, an indwelling cannula was applied, blood and urinary samples were taken and the rectal balloon catheter was placed. Rectal perceptual and pain thresholds were determined using a pressure‐controlled barostat device using established methodology (see below, Section 2.3). After a rest period of 10 min, a structural MRI scan was completed. The fMRI study implemented a block design consisting of six mildly painful rectal distensions followed by six painful distensions presented in fixed order. The duration of each distension was 16.8 s including inflation, plateau and deflation of the rectal balloon. Trial‐by‐trial ratings of perceived distension‐induced pain intensity were accomplished with visual analogue scales (VAS: 0–100 mm) using a MR‐compatible, hand‐held response system with keypads (NordicNeuroLab, Bergen, Norway). Immediately prior to and after MR scanning, additional blood samples were taken for assessment of serum cortisol and prolactin concentrations. State anxiety was assessed in parallel to blood draws using the state version of the Spielberger State Trait Anxiety Inventory (STAI‐S; Laux, Schaffner, & Glanzmann, 1981). In addition, expected pain intensity and current tension/arousal were assessed with VAS (0–100 mm, ends labelled “very little – very high” for expected pain intensity; “none – very much” for arousal).
2.3. Rectal distensions
Graded distensions of the rectum with a pressure‐controlled inflatable balloon system constitute an established and clinically relevant experimental model for interoceptive, visceral sensitivity. It has been applied in different patient populations, including IBS, to address perceptual responses to visceral stimuli and central visceral pain processing. The distension model allows the determination of sensory and discomfort/pain thresholds and (in the context of experimental studies) the controlled and finely tuned application of distensions inducing mild, intermediate or strong sensations of pain or discomfort. Given high interindividual variations in rectal sensory and pain thresholds in healthy volunteers (Elsenbruch et al., 2014), it is important to utilize individually calibrated distension pressures for repeated implementation of specific stimulus intensities in experimental studies where perceptual intensity is relevant, such as herein. As previously described (Benson et al., 2015; Elsenbruch, Rosenberger, Bingel et al., 2010; Elsenbruch, Rosenberger, Enck et al., 2010; Icenhour et al., 2017; Rosenberger et al., 2009), in all our studies, distension pressures for repeated stimulation in the scanner are individually titrated based on rectal sensory and pain thresholds.
Herein, we used a pressure‐controlled barostat system (modified ISOBAR 3 device, G and J electronics, Ontario, Canada). The barostat device was kept outside of the scanner suite and was connected to the rectal balloon by a 3‐m‐long polyethylene tube (3 mm outer diameter, 1.8 mm inner diameter). The catheter‐affixed polyethylene bag is of cylindrical shape and 10 cm in length. It is positioned in the rectum, 5 cm from the anal verge. Fully inflated, it has a diameter of 8 cm and a maximum volume of 500 ml; it is infinitely compliant up to its distensible limit. The thresholding procedure was accomplished prior to scanning. It consists of a double‐random staircase distention protocol with random pressure increments ranging between 2 and 10 mmHg. The limit of maximal distension pressure applied was set at 50 mmHg for safety reasons. Participants rated each distension on a Likert‐type scale (“1” = no pain perception, “2” = doubtful perception, “3” = sure perception, “4” = little discomfort, “5” = severe discomfort, “6” = not tolerable discomfort/pain). The threshold for first pain perception (sensory threshold) is defined at the pressure at which the rating changes from “2” to “3”; the pain threshold at the pressure where the rating changes from “5” to “6.”
For implementation of repeated mildly painful and painful distensions in the scanner, two individually calibrated stimulation intensities were chosen based on these thresholds, as previously accomplished (e.g. Elsenbruch, Rosenberger, Bingel et al., 2010; Elsenbruch, Rosenberger, Enck et al., 2010). The pressure for mildly painful distensions was the pressure at which a rating of “3” was reached; the pressure for painful distensions was the pain threshold pressure minus 2 mmHg for safety reasons.
2.4. MR imaging and statistical analysis
All MR images were acquired using a 3 T MR of the Neuroimaging Research Core Facility (Verio, Siemens, Erlangen, Germany) with a standard multichannel head coil. A 3D FLASH sequence (TR 10 ms, TE 4.5 ms, flip angle 30°, FOV 240 mm, matrix 512, slice thickness 1.0 mm) was used. Blood oxygen‐level‐dependent (BOLD) contrast images were acquired using an echo‐planar technique (TR 3000 ms, TE 25 ms, flip angle 90°, FOV 240 mm, and matrix 128) with 34 transversal slices angulated in the direction of the corpus callosum with a thickness of 3 mm and a 0.3‐mm slice gap. Prior to statistical analysis, images were realigned to the mean image, normalized to a standard EPI‐template as implemented in SPM 12 software (Wellcome Department of Cognitive Neurology, London, UK) and finally smoothed with an isotropic Gaussian kernel of 8 mm. Note that we re‐analysed all data with a smoothing kernel of 5 mm, with no appreciable effects on the results (data not shown). To correct for low frequency drifts, a temporal high‐pass filter with a cut‐off set at 128 s was implemented and serial autocorrelations were considered by means of an autoregressive model first‐order correction.
The statistical analysis was performed with a general linear model approach as implemented in SPM. For each participant, a first‐level model with the regressors mildly painful distensions, painful distensions, and ratings was estimated. The design matrix was obtained by convolving the delta function of the event onsets with a boxcar function based on the canonical hemodynamic response function. The motion parameters were entered as regressors of no interest. Specific effects were tested with appropriate linear contrasts on the different conditions, resulting in a t‐statistic for each voxel. First‐level contrast images for the conditions mildly painful and painful stimulation from both groups of participants were entered into a second‐level random effects analysis. A 2 × 2 flexible factorial model with the factors group (patients, controls) and condition (mildly painful, painful) was estimated.
In an initial analysis aiming to identify BOLD responses specific to visceral pain induced by rectal distensions (i.e. painful vs. mildly painful distensions), an initial threshold of p < 0.05 on voxel level, family‐wise error (FWE)‐corrected, was used, reporting only clusters with more than 10 voxels. This comparably conservative threshold was used to separate the activation maxima in this contrast. In the analysis conducted to test group differences (i.e. PDM > controls and vice versa), a more liberal initial threshold of p < 0.001, uncorrected, was applied, reporting only clusters with more than 50 voxels. Additional region of interest (ROI) analyses were conducted for the locations that we previously reported as more activated in IBS patients compared to healthy controls in a study using a very similar study protocol involving mildly painful and painful distensions (Elsenbruch, Rosenberger, Bingel et al., 2010). These were the two ROIs at 28 62 ‐4 and at ‐36 18 1. For each ROI, a sphere of 12 mm was centred around the peak coordinates and a small volume correction (p < 0.05) was calculated on the group by condition interaction contrast.
2.5. Endocrine measures
For verification of menstrual cycle phase, concentrations of LH, FSH, estradiol and progesterone in serum or plasma were assessed using commercially available kits for an electrochemiluminescence immunoassay (ECLIA) (Roche Diagnostics, Indianapolis, USA) and analysis in a Siemens Immulite2000 immunoassay system (Erlangen, Germany). As hormones of the stress response, cortisol and prolactin concentrations were measured using commercially available kits for an electrochemiluminescence immunoassay (ECLIA) from Roche (analysis with Roche Cobas8000, Roche Diagnostics) for cortisol and from Siemens for prolactin, analysed with a Siemens Immulite2000 analysing system (Erlangen, Germany). The inter‐ and intra‐assay coefficients of variation for the relevant range of the mentioned values were below 10%.
2.6. Statistical analysis of non‐fMRI data
All statistical analyses were conducted using SPSS version 22.0 (IBM Corporation, Armonk, NY, USA). The groups were characterized and compared with respect to sociodemographic and psychological variables using chi‐square tests or t tests where appropriate. For repeated measures, analysis of variance (ANOVA) with trial/time point as repeated factor and group as between factor were computed. Greenhouse–Geisser correction was applied if the sphericity assumption was violated (based on results of Mauchly test). In case of significant main or interaction effects, post hoc testing was accomplished using t tests. All results are reported as mean ± standard error of the mean (SEM) unless indicated otherwise.
2.7. Ethical approval
Ethics approval was granted by the ethics committee of the Medical University Innsbruck (protocol number AN 4940 321/4.16).
3. RESULTS
3.1. Sociodemographic and psychological characteristics
Due to motion artefacts, technical problems and handling errors with the response box, seven participants (four patients, three healthy controls) were excluded from data analysis, resulting in a final sample consisting of N = 19 patients and N = 20 healthy controls. The patient group was slightly older compared to the control group (p < 0.05) and had a comparable body mass index (Table 1). Anxiety and depressions scores were comparable and well below the published cut‐offs for clinically relevant symptoms. Both psychological and physical quality‐of‐life scores revealed no group differences. On the other hand, women with dysmenorrhoea reported significantly higher chronic pain severity and interference of chronic pain with daily life activities (both p < 0.01, Table 1). Seven patients and 11 healthy women reported using hormonal contraception.
Table 1.
Sociodemographic and psychosocial characteristics
| Patients (n = 19) | Healthy controls (n = 20) | p * | |
|---|---|---|---|
| Age (years) | 28.6 ± 1.4 | 24.8 ± 0.7 | 0.02 |
| Body mass index (kg/m2) | 22.7 ± 0.9 | 21.2 ± 0.5 | 0.15 |
| Physical quality of life (SF‐12) | 53.4 ± 1.9 | 56.4 ± 0.6 | 0.15 |
| Psychological quality of life (SF‐12) | 48.0 ± 2.1 | 51.0 ± 2.3 | 0.33 |
| Anxiety symptoms (HADS) | 4.9 ± 0.7 | 5.4 ± 0.6 | 0.62 |
| Depression symptoms (HADS) | 1.7 ± 0.5 | 2.5 ± 0.6 | 0.32 |
| Chronic pain severity (MPI) | 1.9 ± 0.4 | 0.3 ± 0.1 | <0.001 |
| Interference (MPI) | 2.1 ± 0.4 | 0.3 ± 0.1 | <0.01 |
| Negative mood (MPI) | 2.9 ± 0.2 | 2.7 ± 0.1 | 0.30 |
| Support (MPI) | 2.6 ± 0.4 | 1.7 ± 0.4 | 0.15 |
| Self‐control (MPI) | 4.0 ± 0.3 | 4.3 ± 0.4 | 0.44 |
HADS: Hospital Anxiety and Depression Scale; MPI: West Haven‐Yale Multidimensional Pain Inventory.
All data are shown as mean ± SEM. For questionnaire references, see text.
*Results of independent sample t tests.
p values <0.05 were considered significant.
3.2. Pain thresholds and VAS ratings
Rectal sensory and pain thresholds, determined prior to scanning, did not differ between patients and controls (sensory threshold: 24.1 ± 2.0 mmHg in patients vs. 21.6 ± 2.0 mmHg in controls, p = 0.38; pain threshold: 36.5 ± 2.2 mmHg in patients vs. 34.0 ± 2.3 mmHg in controls, p = 0.44). VAS ratings of expected pain intensity (Figure 1a) and current arousal (Figure 1b) also revealed no group differences.
Figure 1.
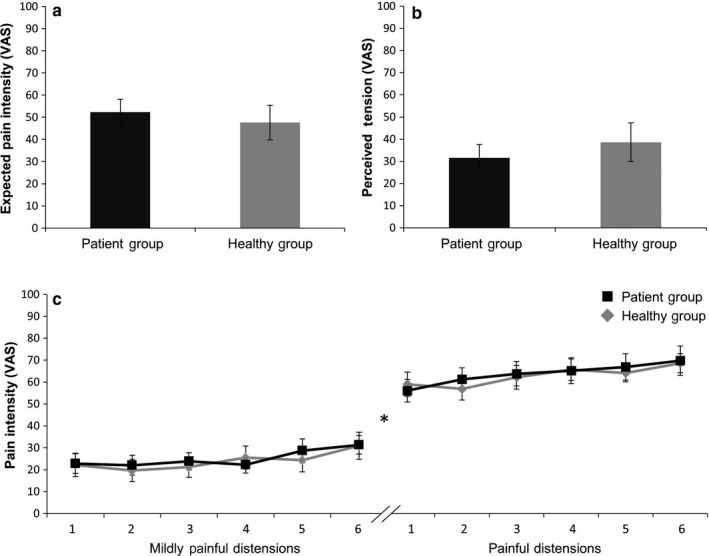
Visual analogue scale rating of expected pain intensity (a), current tension (b) and trial‐by‐trial distension intensity ratings of mildly painful distensions (c, left) and painful distensions (c, right) in patients and healthy controls. Data are shown as mean ± SEM. *Mildly painful distensions were significantly less intense compared to painful distensions in both groups (results of paired t tests), but group differences were found. For ANOVA results, see text
Trial‐by‐trial ratings of perceived distension intensity supported that as intended per individualized titration, mildly painful and painful distensions were clearly differentiated in both groups, with significantly lower VAS ratings of mildly painful distensions (25.2 ± 3.7 mm in patients; 24.0 ± 4.7 mm in controls) compared to painful distensions (64.6 ± 5.5 mm in patients; 62.8 ± 4.1 in controls; within‐group comparisons: both p < 0.001, for details, see Figure 1c). No group differences in trial‐by‐trial intensity ratings were observed for mildly painful or painful distensions (Figure 1c), as indicated by the absence of significant ANOVA group or interaction effects (for mildly painful distensions: F = 0.04, p = 0.85 group effect; F = 0.48, p = 0.74 interaction effect; for painful distensions: F = 0.07, p = 0.79 group effect; F = 0.67, p = 0.67 interaction effect). Accordingly, post hoc tests revealed no significant group differences for any trial (all p > 0.52). However, increasing ratings over repeated trials were observed in both groups, as indicated by trial/time effects that approached significance for mildly painful stimuli (F = 3.89, p = 0.07) and were significant for painful stimuli (F = 6.57, p < 0.001) distensions. Note that given a small age difference, all analyses were repeated with age as a covariate, but results remained unchanged (data not shown).
3.3. Prolactin, cortisol and state anxiety
Prolactin and cortisol were assessed together with state anxiety immediately prior to and after scanning. While ANOVA for cortisol revealed a significant effect of time point (F = 9.12, p = 0.005), but no effect of group (F = 1.71, p = 0.20) or interaction (F = 0.45, p = 0.52), no significant effects (not shown) were present for prolactin. Post hoc testing revealed no group differences before or after scanning for either neuroendocrine parameter (Table 2).
Table 2.
Neuroendocrine parameter and state anxiety before and after scanning
| Pre | Post | |||||
|---|---|---|---|---|---|---|
| Patients | Controls | p * | Patients | Controls | p * | |
| Prolactin (μg/L) | 10.6 ± 1.9 | 9.4 ± 1.6 | 0.63 | 11.3 ± 2.8 | 9.2 ± 1.5 | 0.50 |
| Cortisol (μg/L) | 154.7 ± 17.9 | 191.3 ± 18.9 | 0.17 | 142.5 ± 17.1 | 167.9 ± 18.7 | 0.33 |
| State anxiety (STAI‐S) | 33.5 ± 1.4 | 30.7 ± 1.2 | 0.12 | 33.4 ± 1.3 | 29.2 ± 1.2 | 0.02 |
All data are shown as mean ± SEM.
*Results of independent sample t tests for each time point; for ANOVA results, see text.
p values <0.05 were considered significant.
The ANOVA on state anxiety showed a significant effect of group (F = 5.14, p = 0.029), but no time point (F = 0.84, p = 0.37) or interaction (F = 0.64, p = 0.43) effects. Post hoc testing revealed no group differences before scanning, but significantly higher state anxiety after scanning in patients (p = 0.023, Table 2).
3.4. BOLD responses
The contrast of painful versus mildly painful stimulation in both groups revealed significant activations in the insular cortex, prefrontal, orbitofrontal and somatosensory cortices and cingulate cortex (p < 0.05 FWE‐corrected on peak level, minimum cluster size >10 voxel, Table 3, Figure 2).
Table 3.
BOLD responses to painful vs. mildly painful distensions in both groups
| Anatomical location | MNI coordinates | ||||||
|---|---|---|---|---|---|---|---|
| H | x | y | z | p a | z | Cluster size | |
| GFi, insula | R | 60 | 16 | −2 | 0.000 | 5.98 | 746 |
| GFm | L | −40 | 36 | 40 | 0.001 | 5.50 | 215 |
| GSupram, GTs | R | 66 | −38 | 30 | 0.001 | 5.46 | 370 |
| TPs, GFi | L | −52 | 12 | −10 | 0.003 | 5.29 | 22 |
| Insula, putamen | L | −40 | 2 | 2 | 0.004 | 5.23 | 172 |
| GFi | L | −48 | 6 | 24 | 0.004 | 5.21 | 42 |
| GPrC, GFm | L | −48 | 4 | 36 | 0.005 | 5.18 | 33 |
| Putamen, caudate | L | −16 | −2 | 12 | 0.006 | 5.13 | 80 |
| GSupram, GTs | L | −58 | −42 | 28 | 0.007 | 5.09 | 105 |
| Thalamus, caudate | R | 14 | −4 | 4 | 0.008 | 5.06 | 21 |
| LPi, GSupram | L | −58 | −38 | 42 | 0.009 | 5.04 | 64 |
| GFi, GFiorb | R | 50 | 46 | 0 | 0.010 | 5.02 | 18 |
| GPrC, GFm, GFi | R | 54 | 12 | 44 | 0.011 | 4.98 | 13 |
| Cerebellum (vermis) | L | −2 | −52 | −24 | 0.016 | 4.91 | 15 |
| GFsmed, Cingm | R | 4 | 22 | 44 | 0.017 | 4.90 | 48 |
| Caudate, putamen | R | 20 | 10 | 12 | 0.024 | 4.81 | 11 |
Cingm: middle cingulate gyrus; GFi: inferior frontal gyrus; GFiorb: inferior frontal orbital gyrus; GFm: middle frontal gyrus; GFm: middle frontal gyrus; GFsmed: superior medial frontal gyrus; GPrC: precentral gyrus; GSupram: supramarginal gyrus, superior temporal gyrus; H: hemisphere (R: right, L: left); LPi: inferior parietal lobule; MNI: Montréal Neurological Institute; TPs: superior temporal pole.
The first label gives the location of the peak voxel, and the following labels denote other areas that are part of the cluster. Anatomical labelling was accomplished with the automatic anatomical labelling (aal) toolbox (Tzourio‐Mazoyer et al., 2002).
FWE‐corrected p‐values at peak level; threshold: p < 0.05 FWE‐corrected, minimum cluster size >10.
Figure 2.
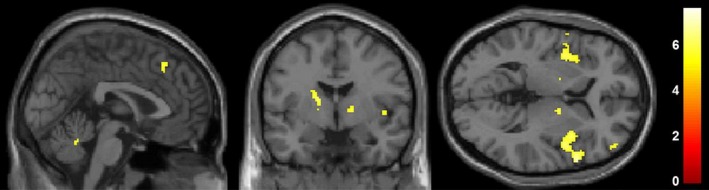
Second‐level analysis on neural activation specific to painful visceral stimuli in both groups. The contrast of painful versus mildly painful stimulation revealed pain‐specific activations in the insular cortex, prefrontal, orbitofrontal and somatosensory cortices and cingulate cortex (p < 0.05 FWE‐corrected, for details, see Table 3). No group differences were observed
No significant differences between the two groups were observed (patients > controls and controls > patients) in the whole‐brain analysis at a lower threshold of p < 0.001 uncorrected (minimum cluster size >50 voxel). We also analysed whether patients differed from controls with regard to BOLD responses to painful and mildly painful distensions against baseline, but found no significant differences in either direction (patients > controls and controls > patients). ROI analyses also revealed no group differences.
4. DISCUSSION
This is the first functional brain imaging study that implemented pressure‐controlled rectal distensions to address visceral sensitivity and to compare behavioural and neural responses to individually calibrated aversive visceral stimuli along with relevant cognitive and stress‐related measures in women with dysmenorrhoea and healthy women.
4.1. Visceral sensitivity and pain perception
Our results revealed comparable thresholds for first perception and pain induced by rectal distensions in PDM patients compared to healthy women. Trial‐by‐trial ratings of perceived distension intensity did not differ between groups neither for individually calibrated mildly painful nor for painful distensions that were repeatedly implemented during scanning. In fact, mean ratings for the groups were virtually identical, which is important to mention given limited statistical power and the possibility of Type II errors (see below). These negative results must be interpreted with due caution, but they nevertheless suggest unaltered visceral sensitivity and normal perceptual responses to individually calibrated aversive stimuli in this interoceptive visceral pain model in PDM. Hence, our findings do not support our hypotheses which were based on the only two existing studies which have addressed perceptual responses to interoceptive, visceral pain stimuli, namely distension of the uterine cervix (Arendt‐Nielsen et al., 2014) and rectum/colon (Brinkert et al., 2007) in PDM. In this published work, PDM patients had reduced distension volume thresholds in the sigmoid colon (Brinkert et al., 2007). In response to cervical distensions, PMD patients revealed a normal sensory threshold but a higher pain threshold to the first stimulus, which however decreased with repeated distensions (Arendt‐Nielsen et al., 2014). Further, pain reportedly increased during prolonged stimulation, indicating temporal summation (Arendt‐Nielsen et al., 2014). While our trial‐by‐trial pain ratings did not differ between PMD and controls, we also observed increases in perceived distension intensity for both mildly painful and painful distensions over repeated trials in both study groups. Results from studies implementing other types of mostly exteroceptive, somatic pain stimuli have been heterogeneous, ranging from unaltered (e.g. Aberger, Denney, & Hutchings, 1983; Amodei & Nelson‐Gray, 1989) over reduced (Giamberardino, Tana, & Costantini, 2014; Hapidou & De Catanzaro, 1988) to increased pain sensitivity (e.g. Bajaj et al., 2002; Iacovides, Avidon, & Baker, 2015b; Vincent et al., 2011) in PDM patients. This heterogeneity is likely due to differences between pain models and/or modalities, methodological differences in experimental procedures, small sample sizes in most studies, and differences between study samples in psychosocial patient characteristic (Iacovides et al., 2015a). Of note, even though our sample size is about the same size as other existing brain imaging studies in PDM patients, and we judge this sample to be large enough to produce stable effects, we must acknowledge that statistical power is limited and therefore small effects may not be detected. This is obviously particularly problematic when interpreting the lack of significant differences in terms of “negative findings.” However, in our opinion, the actual results do not indicate that lack of statistical power is a likely explanation for the negative findings that are reported herein. Instead, our findings support the possibility that hypersensitivity and central sensitization do not characterize all PDM patients, but may rather be organ‐ and/or pain‐modality specific in certain subgroups, possibly those with psychosocial impairment, as discussed in greater detail below. This calls for more systematic studies in larger samples and different subgroups, especially in patients with concurrent psychiatric comorbidity, as well as continued mechanistic work in animal models (Chen, Xie, Strong, Jiang, & Zhang, 2016).
4.2. Neural responses to mildly painful and painful visceral stimuli
Consistent with a large body of evidence on the central processing of aversive visceral stimuli (Mayer et al., 2015), herein rectal distension induced neural activation in multiple brain regions, with greater activation in response to painful than to mildly painful rectal distensions in the insula, cingulate and prefrontal regions, essentially validating our stimulation paradigm with distension stimuli of differing intensities, as previously accomplished in several studies from our group (Benson et al., 2015; Elsenbruch, Rosenberger, Bingel et al., 2010; Elsenbruch, Rosenberger, Enck et al., 2010; Icenhour et al., 2017; Rosenberger et al., 2009). However, we did not observe any group differences between PDM patients and healthy controls in any region, even at a relatively liberal statistical threshold. While again limitations in statistical power cannot be negated, this absence of group differences at the neural level is consistent with negative behavioural results, namely virtually identical perceived stimulus intensity, suggesting that our group of women with PDM revealed normal perceptual and neural responses to both mildly painful and painful visceral stimuli. In other words, in this first brain imaging study involving visceral stimuli in PDM, we could not find evidence suggestive of allodynia, hyperalgesia or central sensitization. The only other existing evoked pain fMRI study tested responses to thermal stimuli applied to the arm and abdomen in a sample of women with self‐reported menstrual pain. Patients demonstrated unaltered trial‐by‐trial pain ratings—consistent with our results—but altered neural responses when compared to healthy controls who demonstrated widespread deactivation in response to noxious stimulation that were not seen in patients (Vincent et al., 2011).
Deactivation in response to noxious visceral stimuli has rarely been reported (Mayer et al., 2015) and remains incompletely understood even in the broader somatic pain field (Kong et al., 2010). Importantly, differences in behavioural responses and neural processing of noxious visceral compared to somatic stimuli, including thermal cutaneous pain, are well‐documented in healthy participants (Aziz et al., 2000; Dunckley et al., 2005; Koenen et al., 2017; Strigo et al., 2003) and patients with IBS (Verne et al., 2003). Hence, it remains an important future research goal to study neural responses to different types of evoked pain stimuli, especially clinically relevant interoceptive stimuli, in PDM and more broadly in CPP in order to complement and extend a growing body of evidence documenting alterations in functional connectivity and brain structural measures (Liu et al., 2016; Tu et al., 2010, 2013) suggestive of central sensitization in recurrent or chronic interoceptive pain (Brawn et al., 2014; Giamberardino et al., 2014). It is indeed conceivable that as in IBS, alterations in the neural processing of visceral stimuli or in brain functional connectivity in PDM are shaped by the presence of visceral hypersensitivity (Icenhour et al., 2017; Larsson et al., 2012), which may not characterize all patients but only specific subgroups. This is underlined by comparatively large brain imaging studies in PDM patients revealing alterations of structure and functional connectivity (Liu et al., 2018) in the periaqueductal grey in association with a genetic polymorphism of the brain derived neurotrophic factor (BDNF), a known pain modulator in regards to adaptive neuroplasticity (Wei, Chao, Tu, Li et al., 2016; Wei, Chao, Tu, Lin et al., 2016).
An important aspect is the fact that the current sample of patients was comparatively healthy. Our patients revealed essentially normal quality‐of‐life scores and no anxiety or depression symptoms, despite chronic pain severe enough to interfere with everyday life activities. Psychological comorbidity (or lack thereof, as herein), illness burden, coping strategies, pain‐related cognitions and healthcare‐seeking behaviour are important factors in all conditions associated with chronic bodily symptoms and pain, especially in “medically unexplained” symptoms and diagnoses based solely on patient symptom reports in combination with exclusion of organic causes. Given that diagnostic criteria for dysmenorrhoea do not consider psychological or behavioural aspects (for a critical discussion, see e.g., Grandi et al., 2012), differences in patient samples regarding psychological factors likely contribute to contradictory findings on PDM in the literature, and conceivable explain the absence of group differences in behavioural and neural responses to visceral stimuli in the present study. Interestingly, Vincent et al. (2011) found no reduction in mental quality of life in PDM patients, similar to our cohort. In both studies, recruitment did not take place within a clinical setting where patients explicitly presented for clinical consultation due to dysmenorrhoea. Indeed, in women with endometriosis, recruitment strategy reportedly plays a role in quality of life outcomes (De Graaff et al., 2015). Psychiatric comorbidity, as well as psychological state factors including state anxiety and negative pain‐related cognitions, are important modulators of visceral pain processing (Boeckxstaens et al., 2016) and reportedly contribute to group differences between IBS and healthy controls (Elsenbruch, Rosenberger, Bingel et al., 2010; Elsenbruch, Rosenberger, Enck et al., 2010; Schmid et al., 2015). Except for slightly higher state anxiety after scanning, we observed no differences in our panel of psychological cognitive and emotional measures and stress markers of the HPA axis (here: cortisol and prolactin), indicating no or only subtle alterations in the stress system, which is consistent with the lack of psychosocial impairment in our sample and further supports the lack of group differences in pain‐specific measures in our study. Therefore, our findings may not generalize to dysmenorrhoea patients with affective disturbances, psychiatric comorbidities, maladaptive cognitions or those with enhanced stress responses and/or altered HPA axis functioning, all of which are intricately connected in shaping the response to evoked pain and actually any aversive or stressful stimulus. Studies examining the presence and role of anxiety and depression in PDM in adult women are very limited, but the role of mental health factors is increasingly recognized (for a recent review, see Bajalan, Moafi, MoradiBaglooei, & Alimoradi, 2018). Significantly higher rates of anxiety and depression have been found in adolescents with PDM (Balık, Ustüner, Kağıtcı, & Sahin, 2014; Beal et al., 2014; Gagua, Tkeshelashvili, Gagua, & McHedlishvili, 2013; Sahin, Kasap, Kirli, Yeniceri, & Topal, 2018) and recently in female students (Uçar, Timur Taşhan, Aksoy Derya, & Nacar, 2018) as well as in secondary dysmenorrhoea, especially endometriosis (Cavaggioni et al., 2014; Lorençatto, Petta, Navarro, Bahamondes, & Matos, 2006; Sepulcri & do Amaral, 2009). It is conceivable that menstrual disturbances increase the risk of poor psychosocial adjustment and disturbed illness behaviour, and vice versa that pre‐existing psychological impairment worsen coping with symptoms (Balık et al., 2014; Beal et al., 2014; Dorn et al., 2009) similar to the complex, bidirectional connections between disease‐specific symptoms (i.e. abdominal pain, bowel disturbance) and psychosocial impairments (e.g. anxiety, depression, low quality of life) that have been elegantly been demonstrated in functional gastrointestinal disorders (Koloski et al., 2012).
A major limitation is the definition of our patients as having primary rather than secondary dysmenorrhoea. Secondary dysmenorrhoea can only be excluded by laparoscopy, and although we excluded patients with a pre‐existing histological diagnosis of endometriosis, we cannot rule out the possibility that some of our patients may have had undetected endometriosis. Unless patient recruitment is restricted to women who have already undergone laparoscopy, this is a concern in all studies addressing PDM which may additionally contribute to conflicting results in the literature. Furthermore, it has been proposed that there might be no difference between primary and secondary dysmenorrhoea, assuming a progression of the symptoms of PDM to those of secondary dysmenorrhoea over time. CPP and/or dysmenorrhoea might therefore only be a predictor of future endometriosis (Chapron et al., 2011; Janssen, Rijkers, Hoppenbrouwers, Meuleman, & D'Hooghe, 2013; Steenberg, Tanbo, & Qvigstad, 2013). Clearly, future work is needed, ideally prospective studies comparing pain responses in patients with verified PDM, verified secondary dysmenorrhoea and women with self‐reported menstrual pain repeatedly across the menstrual cycle.
Our data were collected on days 1–5 of the menstrual cycle, despite the fact that maximum pain is encountered on days 1–2. Due to feasibility considerations, we extended this interval to 5 days, which may be one reason for the lack of differences between groups.
Given that dysmenorrhoea is still underdiagnosed and undertreated (Iacovides et al., 2015a), future studies are urgently needed to address the putative role of psychological trait and state factors in functional and structural measures of pain sensitization in PDM and to ascertain which vulnerability and resilience factors may explain why disturbed visceral pain processing may not unequivocally characterize all women with PDM.
CONFLICT OF INTEREST
Authors declare no conflict of interests.
AUTHOR CONTRIBUTIONS
ERG, SE, LW, JS and BB designed the study; BB, CS, RS, MV, DR, AI, JS and ERG involved in data acquisition; BB, CS, RS, MV, DR, AI, SE and ERG analysed the data; BB, ERG, CS and SE drafted the manuscript; and all authors critically revised the manuscript for important content and approved the final version.
ACKNOWLEDGEMENTS
The authors thank S. Grabmer, S. Kurz and J. Pfuner for their excellent technical and logistical support in conducting this project, Prof. B. Toth for critical reading of the manuscript and L.R. Koenen for help with statistical analyses.
Böttcher B, Gizewski ER, Siedentopf C, et al. Behavioural and neural responses to aversive visceral stimuli in women with primary dysmenorrhoea. Eur J Pain. 2019;23:272–284. 10.1002/ejp.1302
Funding information
This research did not receive any specific grant funding from funding agencies in the public, commercial or not‐for‐profit sectors. Resources were kindly provided by the Department of Neuroradiology, the Department of Gynecological Endocrinology and Reproductive Medicine, both Medical University Innsbruck, Austria, and the Institute of Medical Psychology & Behavioral Immunobiology, University Hospital Essen, University of Duisburg‐Essen, Germany.
REFERENCES
- Aberger, E. W. , Denney, D. R. , & Hutchings, D. F. (1983). Pain sensitivity and coping strategies among dysmenorrheic women: Much ado about nothing. Behavior Research and Therapy, 21, 119–127. 10.1016/0005-7967(83)90156-0 [DOI] [PubMed] [Google Scholar]
- Amodei, N. , & Nelson‐Gray, R. O. (1989). Reactions of dysmenorrheic and nondysmenorrheic women to experimentally induced pain throughout the menstrual cycle. Journal of Behavioral Medicine, 12, 373–385. 10.1007/BF00844930 [DOI] [PubMed] [Google Scholar]
- Arendt‐Nielsen, L. , Madsen, H. , Jarrell, J. , Gregersen, H. , & Drewes, A. M. (2014). Pain evoked by distension of the uterine cervix in women with dysmenorrhea: Evidence for central sensitization. Acta Obstetricia et Gynecologica Scandinavica, 93, 741–748. 10.1111/aogs.12403 [DOI] [PubMed] [Google Scholar]
- Aziz, Q. , Thompson, D. G. , Ng, V. W. , Hamdy, S. , Sarkar, S. , Brammer, M. J. , … Williams, S. C. (2000). Cortical processing of human somatic and visceral sensation. Journal of Neuroscience, 20, 2657–2663. 10.1523/JNEUROSCI.20-07-02657.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj, P. , Bajaj, P. , Madsen, H. , & Arendt‐Nielsen, L. (2002). A comparison of modality‐specific somatosensory changes during menstruation in dysmenorrheic and nondysmenorrheic women. Clinical Journal of Pain, 18, 180–190. 10.1097/00002508-200205000-00007 [DOI] [PubMed] [Google Scholar]
- Bajalan, Z. , Moafi, F. , MoradiBaglooei, M. , & Alimoradi, Z. (2018). Mental health and primary dysmenorrhea: A systematic review. Journal of Psychosomatic Obstetrics and Gynecology, 71, 1–10. 10.1080/0167482X.2018.1470619 [DOI] [PubMed] [Google Scholar]
- Balık, G. , Ustüner, I. , Kağıtcı, M. , & Sahin, F. K. (2014). Is there a relationship between mood disorders and dysmenorrhea? Journal of Pediatric and Adolescent Gynecology, 27, 371–374. 10.1016/j.jpag.2014.01.108 [DOI] [PubMed] [Google Scholar]
- Baranowski, A. P. , Lee, J. , Price, C. , & Hughes, J. (2014). Pelvic pain: A pathway for care developed for both men and women by the British Pain Society. British Journal of Anaesthesia, 112, 452–459. 10.1093/bja/aet421 [DOI] [PubMed] [Google Scholar]
- Beal, S. J. , Dorn, L. D. , Sucharew, H. J. , Sontag‐Padilla, L. , Pabst, S. , & Hillman, J. (2014). Characterizing the longitudinal relations between depressive and menstrual symptoms in adolescent girls. Psychosomatic Medicine, 76, 547–554. 10.1097/PSY.0000000000000099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson, S. , Rebernik, L. , Wegner, A. , Kleine‐Borgmann, J. , Engler, H. , Schlamann, M. , … Elsenbruch, S. (2015). Neural circuitry mediating inflammation‐induced central pain amplification in human experimental endotoxemia. Brain, Behavior, and Immunity, 48, 222–231. 10.1016/j.bbi.2015.03.017 [DOI] [PubMed] [Google Scholar]
- Boeckxstaens, G. , Camilleri, M. , Sifrim, D. , Houghton, L. A. , Elsenbruch, S. , Lindberg, G. , … Parkman, H. P. (2016). Fundamentals of neurogastroenterology: Physiology/motility – sensation. Gastroenterology, 150, 1292–1304. e1292. 10.1053/j.gastro.2016.02.030 [DOI] [PubMed] [Google Scholar]
- Böttcher, B. , Laterza, R. M. , Wildt, L. , Seufert, R. J. , Buhling, K. J. , Singer, C. F. , … Smith, R. P. (2014). A first‐in‐human study of PDC31 (prostaglandin F2α receptor inhibitor) in primary dysmenorrhea. Human Reproduction, 29, 2465–2473. 10.1093/humrep/deu205 [DOI] [PubMed] [Google Scholar]
- Brawn, J. , Morotti, M. , Zondervan, K. T. , Becker, C. M. , & Vincent, K. (2014). Central changes associated with chronic pelvic pain and endometriosis. Human Reproduction Update, 20, 737–747. 10.1093/humupd/dmu025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkert, W. , Dimcevski, G. , Arendt‐Nielsen, L. , Drewes, A. M. , & Wilder‐Smith, O. H. G. (2007). Dysmenorrhoea is associated with hypersensitivity in the sigmoid colon and rectum. Pain, 132(Suppl 1), S46–S51. 10.1016/j.pain.2006.12.011 [DOI] [PubMed] [Google Scholar]
- Cavaggioni, G. , Lia, C. , Resta, S. , Antonielli, T. , Benedetti Panici, P. , Megiorni, F. , & Porpora, M. G. (2014). Are mood and anxiety disorders and alexithymia associated with endometriosis? A preliminary study. BioMed Research International, 2014, 786830–786835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapron, C. , Souza, C. , Borghese, B. , Lafay‐Pillet, M.‐C. , Santulli, P. , Bijaoui, G. , … de Ziegler, D. (2011). Oral contraceptives and endometriosis: The past use of oral contraceptives for treating severe primary dysmenorrhea is associated with endometriosis, especially deep infiltrating endometriosis. Human Reproduction, 26, 2028–2035. 10.1093/humrep/der156 [DOI] [PubMed] [Google Scholar]
- Chen, S. , Xie, W. , Strong, J. A. , Jiang, J. , & Zhang, J.‐M. (2016). Sciatic endometriosis induces mechanical hypersensitivity, segmental nerve damage, and robust local inflammation in rats. European Journal of Pain, 20, 1044–1057. 10.1002/ejp.827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco, A. S. (1999). Primary dysmenorrhea. American Family Physician, 60, 489–496. [PubMed] [Google Scholar]
- Collins, S. L. , Moore, R. A. , & McQuay, H. J. (1997). The visual analogue pain intensity scale: What is moderate pain in millimetres? Pain, 72, 95–97. 10.1016/S0304-3959(97)00005-5 [DOI] [PubMed] [Google Scholar]
- De Graaff, A. A. , Dirksen, C. D. , Simoens, S. , De Bie, B. , Hummelshoj, L. , D'Hooghe, T. M. , & Dunselman, G. A. J. (2015). Quality of life outcomes in women with endometriosis are highly influenced by recruitment strategies. Human Reproduction, 30, 1331–1341. 10.1093/humrep/dev084 [DOI] [PubMed] [Google Scholar]
- Dorn, L. D. , Negriff, S. , Huang, B. , Pabst, S. , Hillman, J. , Braverman, P. , & Susman, E. J. (2009). Menstrual symptoms in adolescent girls: Association with smoking, depressive symptoms, and anxiety. Journal of Adolescent Health, 44, 237–243. 10.1016/j.jadohealth.2008.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunckley, P. , Wise, R. G. , Aziz, Q. , Painter, D. , Brooks, J. , Tracey, I. , & Chang, L. (2005). Cortical processing of visceral and somatic stimulation: Differentiating pain intensity from unpleasantness. Neuroscience, 133, 533–542. 10.1016/j.neuroscience.2005.02.041 [DOI] [PubMed] [Google Scholar]
- Elsenbruch, S. , & Enck, P. (2015). Placebo effects and their determinants in gastrointestinal disorders. Nature Reviews Gastroenterology & Hepatology, 12, 472–485. 10.1038/nrgastro.2015.117 [DOI] [PubMed] [Google Scholar]
- Elsenbruch, S. , & Enck, P. (2017). The stress concept in gastroenterology: From Selye to today. F1000 Research, 6, 2149 10.12688/f1000research [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsenbruch, S. , & Labrenz, F. (2018). Nocebo effects and experimental models in visceral pain In Colloca L. (Ed.), Neurobiology of the placebo effect part I (pp. 285–306). New York, NY: Elsevier; 10.1016/bs.irn.2018.01.010 [DOI] [PubMed] [Google Scholar]
- Elsenbruch, S. , Rosenberger, C. , Bingel, U. , Forsting, M. , Schedlowski, M. , & Gizewski, E. R. (2010). Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology, 139, 1310–1319. 10.1053/j.gastro.2010.06.054 [DOI] [PubMed] [Google Scholar]
- Elsenbruch, S. , Rosenberger, C. , Enck, P. , Forsting, M. , Schedlowski, M. , & Gizewski, E. R. (2010). Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: An fMRI study. Gut, 59, 489–495. 10.1136/gut.2008.175000 [DOI] [PubMed] [Google Scholar]
- Elsenbruch, S. , Schmid, J. , Kullmann, J. S. , Kattoor, J. , Theysohn, N. , Forsting, M. , & Kotsis, V. (2014). Visceral sensitivity correlates with decreased regional gray matter volume in healthy volunteers: A voxel‐based morphometry study. Pain, 155, 244–249. 10.1016/j.pain.2013.09.027 [DOI] [PubMed] [Google Scholar]
- Flor, H. , Rudy, T. E. , Birbaumer, N. , Streit, B. , & Schugens, M. M. (1990). The applicability of the West Haven‐Yale multidimensional pain inventory in German‐speaking countries. Data on the reliability and validity of the MPI‐D. Schmerz, 4, 82–87. 10.1007/BF02527839 [DOI] [PubMed] [Google Scholar]
- Gagua, T. , Tkeshelashvili, B. , Gagua, D. , & McHedlishvili, N. (2013). Assessment of anxiety and depression in adolescents with primary dysmenorrhea: A case‐control study. Journal of Pediatric and Adolescent Gynecology, 26, 350–354. 10.1016/j.jpag.2013.06.018 [DOI] [PubMed] [Google Scholar]
- Giamberardino, M. A. , Berkley, K. J. , Iezzi, S. , de Bigontina, P. , & Vecchiet, L. (1997). Pain threshold variations in somatic wall tissues as a function of menstrual cycle, segmental site and tissue depth in non‐dysmenorrheic women, dysmenorrheic women and men. Pain, 71, 187–197. 10.1016/S0304-3959(97)03362-9 [DOI] [PubMed] [Google Scholar]
- Giamberardino, M. A. , Tana, C. , & Costantini, R. (2014). Pain thresholds in women with chronic pelvic pain. Current Opinion in Obstetrics and Gynecology, 26, 253–259. 10.1097/GCO.0000000000000083 [DOI] [PubMed] [Google Scholar]
- Grandi, G. , Ferrari, S. , Xholli, A. , Cannoletta, M. , Palma, F. , Romani, C. , … Cagnacci, A. (2012). Prevalence of menstrual pain in young women: What is dysmenorrhea? Journal of Pain Research, 5, 169–174. 10.2147/JPR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapidou, E. G. , & De Catanzaro, D. (1988). Sensitivity to cold pressor pain in dysmenorrheic and non‐dysmenorrheic women as a function of menstrual cycle phase. Pain, 34, 277–283. 10.1016/0304-3959(88)90123-6 [DOI] [PubMed] [Google Scholar]
- Herrmann‐Lingen, C. , Buss, U. , & Snaith, R. L. (2011). Hospital anxiety and depression scale: HADS‐D; deutsche version. Göttingen, Germany: Hogrefe. [Google Scholar]
- Iacovides, S. , Avidon, I. , & Baker, F. C. (2015a). What we know about primary dysmenorrhea today: A critical review. Human Reproduction Update, 21, 762–778. 10.1093/humupd/dmv039 [DOI] [PubMed] [Google Scholar]
- Iacovides, S. , Avidon, I. , & Baker, F. C. (2015b). Women with dysmenorrhoea are hypersensitive to experimentally induced forearm ischaemia during painful menstruation and during the pain‐free follicular phase. European Journal of Pain, 19, 797–804. 10.1002/ejp.604 [DOI] [PubMed] [Google Scholar]
- Icenhour, A. , Witt, S. T. , Elsenbruch, S. , Lowén, M. , Engström, M. , Tillisch, K. , … Walter, S. (2017). Brain functional connectivity is associated with visceral sensitivity in women with Irritable Bowel Syndrome. NeuroImage Clinical, 15, 449–457. 10.1016/j.nicl.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen, E. B. , Rijkers, A. C. M. , Hoppenbrouwers, K. , Meuleman, C. , & D'Hooghe, T. M. (2013). Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: A systematic review. Human Reproduction Update, 19, 570–582. 10.1093/humupd/dmt016 [DOI] [PubMed] [Google Scholar]
- Koenen, L. R. , Icenhour, A. , Forkmann, K. , Pasler, A. , Theysohn, N. , Forsting, M. , … Elsenbruch, S. (2017). Greater fear of visceral pain contributes to differences between visceral and somatic pain in healthy women. Pain, 158, 1599–1608. 10.1097/j.pain.0000000000000924 [DOI] [PubMed] [Google Scholar]
- Koloski, N.A. , Jones, M. , Kalantar, J. , Weltman, M. , Zaguirre, J. , Talley, N.J. (2012). The brain‐gut pathway in functional gastrointestinal disorders is bidirectional: a 12‐year prospective population‐based study. Gut 61, 1284‐90. [DOI] [PubMed] [Google Scholar]
- Kong, J. , Loggia, M. L. , Zyloney, C. , Tu, P. , Laviolette, P. , & Gollub, R. L. (2010). Exploring the brain in pain: Activations, deactivations and their relation. Pain, 148, 257–267. 10.1016/j.pain.2009.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson, M. B. O. , Tillisch, K. , Craig, A. D. , Engström, M. , Labus, J. , Naliboff, B. , … Walter, S. A. (2012). Brain responses to visceral stimuli reflect visceral sensitivity thresholds in patients with irritable bowel syndrome. Gastroenterology, 142(463–472), e463 10.1053/j.gastro.2011.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux, L. , Schaffner, P. , & Glanzmann, P. (1981). Das state‐trait angst angstinventar (STAI).
- Leyendecker, G. , Bilgicyildirim, A. , Inacker, M. , Stalf, T. , Huppert, P. , Mall, G. , … Wildt, L. (2015). Adenomyosis and endometriosis. Re‐visiting their association and further insights into the mechanisms of auto‐traumatisation. An MRI study. Archives of Gynecology and Obstetrics, 291, 917–932. 10.1007/s00404-014-3437-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyendecker, G. , Wildt, L. , & Mall, G. (2009). The pathophysiology of endometriosis and adenomyosis: Tissue injury and repair. Archives of Gynecology and Obstetrics, 280, 529–538. 10.1007/s00404-009-1191-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, P. , Liu, Y. , Wang, G. , Li, R. , Wei, Y. , Fan, Y. , … Qin, W. (2018). Changes of functional connectivity of the anterior cingulate cortex in women with primary dysmenorrhea. Brain Imaging and Behavior, 12, 710–717. 10.1007/s11682-017-9730-y [DOI] [PubMed] [Google Scholar]
- Liu, P. , Yang, J. , Wang, G. , Liu, Y. , Liu, X. , Jin, L. , … Calhoun, V. D. (2016). Altered regional cortical thickness and subcortical volume in women with primary dysmenorrhoea. European Journal of Pain, 20, 512–520. 10.1002/ejp.753 [DOI] [PubMed] [Google Scholar]
- Lorençatto, C. , Petta, C. A. , Navarro, M. J. , Bahamondes, L. , & Matos, A. (2006). Depression in women with endometriosis with and without chronic pelvic pain. Acta Obstetricia et Gynecologica Scandinavica, 85, 88–92. 10.1080/00016340500456118 [DOI] [PubMed] [Google Scholar]
- Mayer, E. A. , Gupta, A. , Kilpatrick, L. A. , & Hong, J.‐Y. (2015). Imaging brain mechanisms in chronic visceral pain. Pain, 156(Suppl 1), S50–S63. 10.1097/j.pain.0000000000000106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfeld, M. , Kirchberger, I. , & Bullinger, M. (2011). SF‐36 Fragebogen zum gesundheitszustand. Göttingen, Germany: Hogrefe. [Google Scholar]
- Olafsdottir, L. B. , Gudjonsson, H. , Jonsdottir, H. H. , Björnsson, E. , & Thjodleifsson, B. (2012). Natural history of irritable bowel syndrome in women and dysmenorrhea: A 10‐year follow‐up study. Gastroenterology Research and Practice, 2012, 534204–534207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger, C. , Elsenbruch, S. , Scholle, A. , de Greiff, A. , Schedlowski, M. , Forsting, M. , & Gizewski, E. R. (2009). Effects of psychological stress on the cerebral processing of visceral stimuli in healthy women. Neurogastroenterology and Motility, 21, e740–e745. 10.1111/j.1365-2982.2009.01295.x [DOI] [PubMed] [Google Scholar]
- Sahin, N. , Kasap, B. , Kirli, U. , Yeniceri, N. , & Topal, Y. (2018). Assessment of anxiety‐depression levels and perceptions of quality of life in adolescents with dysmenorrhea. Reproductive Health, 15, 2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid, J. , Langhorst, J. , Gaß, F. , Theysohn, N. , Benson, S. , Engler, H. , … Elsenbruch, S. (2015). Placebo analgesia in patients with functional and organic abdominal pain: A fMRI study in IBS, UC and healthy volunteers. Gut, 64, 418–427. 10.1136/gutjnl-2013-306648 [DOI] [PubMed] [Google Scholar]
- Sepulcri, R. P. , & do Amaral, V. F. (2009). Depressive symptoms, anxiety, and quality of life in women with pelvic endometriosis. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 142, 53–56. 10.1016/j.ejogrb.2008.09.003 [DOI] [PubMed] [Google Scholar]
- Steenberg, C. K. , Tanbo, T. G. , & Qvigstad, E. (2013). Endometriosis in adolescence: Predictive markers and management. Acta Obstetricia et Gynecologica Scandinavica, 92, 491–495. 10.1111/aogs.12121 [DOI] [PubMed] [Google Scholar]
- Strigo, I. A. , Duncan, G. H. , Boivin, M. , & Bushnell, M. C. (2003). Differentiation of visceral and cutaneous pain in the human brain. Journal of Neurophysiology, 89, 3294–3303. 10.1152/jn.01048.2002 [DOI] [PubMed] [Google Scholar]
- Tu, C.‐H. , Niddam, D. M. , Chao, H.‐T. , Chen, L.‐F. , Chen, Y.‐S. , Wu, Y.‐T. , … Hsieh, J.‐C. (2010). Brain morphological changes associated with cyclic menstrual pain. Pain, 150, 462–468. 10.1016/j.pain.2010.05.026 [DOI] [PubMed] [Google Scholar]
- Tu, C.‐H. , Niddam, D. M. , Yeh, T.‐C. , Lirng, J.‐F. , Cheng, C.‐M. , Chou, C.‐C. , … Hsieh, J.‐C. (2013). Menstrual pain is associated with rapid structural alterations in the brain. Pain, 154, 1718–1724. 10.1016/j.pain.2013.05.022 [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer, N. , Landeau, B. , Papathanassiou, D. , Crivello, F. , Etard, O. , Delcroix, N. , … Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage, 15, 273–289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Uçar, T. , Timur Taşhan, S. , Aksoy Derya, Y. , & Nacar, G. (2018). An analysis of dysmenorrhoea and depressive symptoms in university students: A case‐control study. International Journal of Nursing Practice, 16, e12678. [DOI] [PubMed] [Google Scholar]
- Verne, G. N. , Himes, N. C. , Robinson, M. E. , Gopinath, K. S. , Briggs, R. W. , Crosson, B. , & Price, D. D. (2003). Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain, 103, 99–110. 10.1016/S0304-3959(02)00416-5 [DOI] [PubMed] [Google Scholar]
- Vincent, K. , Warnaby, C. , Stagg, C. J. , Moore, J. , Kennedy, S. , & Tracey, I. (2011). Dysmenorrhoea is associated with central changes in otherwise healthy women. Pain, 152, 1966–1975. 10.1016/j.pain.2011.03.029 [DOI] [PubMed] [Google Scholar]
- Wei, S.‐Y. , Chao, H.‐T. , Tu, C.‐H. , Li, W.‐C. , Low, I. , Chuang, C.‐Y. , … Hsieh, J.‐C. (2016). Changes in functional connectivity of pain modulatory systems in women with primary dysmenorrhea. Pain, 157, 92–102. 10.1097/j.pain.0000000000000340 [DOI] [PubMed] [Google Scholar]
- Wei, S.‐Y. , Chao, H.‐T. , Tu, C.‐H. , Lin, M.‐W. , Li, W.‐C. , Low, I. , … Hsieh, J.‐C. (2016). The BDNF Val66Met polymorphism is associated with the functional connectivity dynamics of pain modulatory systems in primary dysmenorrhea. Scientific Reports, 6, 23639 10.1038/srep23639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zondervan, K. T. , Yudkin, P. L. , Vessey, M. P. , Jenkinson, C. P. , Dawes, M. G. , Barlow, D. H. , & Kennedy, S. H. (2001). The community prevalence of chronic pelvic pain in women and associated illness behaviour. British Journal of General Practice, 51, 541–547. [PMC free article] [PubMed] [Google Scholar]


