Abstract
Smoking exposure is associated with pregnancy complications, as are levels of folate, vitamin B12, and homocysteine. In nonpregnant adults, smoking exposure is associated negatively with folate and vitamin B12 levels and positively with homocysteine levels. A complete overview of the literature on this topic in pregnant women is lacking. To evaluate evidence of associations of maternal smoking exposure during pregnancy and levels of folate, homocysteine, and vitamin B12 in pregnancy and in cord blood, we searched MEDLINE, Embase, CINAHL, Cochrane, Scopus, Web of Science, and reference lists of relevant studies until August 2017. We selected studies in pregnant women describing the association of passive or active smoking and levels of folate, homocysteine, and/or vitamin B12. Data were extracted by two independent reviewers. We included 32 studies of 2,015 identified references with a total of 37,822 participants and more than 6,000 smokers. Twenty‐eight studies measured folate, 14 measured vitamin B12, and 13 measured homocysteine. Nineteen out of 28 studies assessing folate reported significantly lower levels in pregnant women exposed to smoking compared with those unexposed. Vitamin B12 levels were lower in smoking mothers in eight out of 14 studies. Homocysteine levels tended to be higher in mothers exposed to smoking. Smoking exposure during pregnancy is generally associated with lower folate and vitamin B12 levels and higher homocysteine levels. This may help raise further awareness about the consequences of smoking and the need to encourage stopping smoking in all, especially in pregnant women.
Keywords: folic acid, homocysteine, pregnancy, smoking, tobacco, vitamin B12
Key messages.
Smoking exposure during pregnancy still is a challenging problem for health care professionals and is associated with pregnancy complications, as are levels of folate, vitamin B12, and homocysteine.
A complete overview of literature on the associations of smoking exposure with folate, vitamin B12, and homocysteine in pregnant women is lacking.
We systematically reviewed literature on this topic and show smoking exposure during pregnancy is generally associated with lower folate/vitamin B12 levels and higher homocysteine levels.
This may help raise further awareness about the consequences of smoking and the need to encourage stopping smoking, especially in pregnant women.
1. INTRODUCTION
Maternal exposure to smoking in pregnancy is associated with a number of perinatal complications, including miscarriage, placental abruption, preterm birth, low birthweight, and congenital malformations (Hackshaw, Rodeck, & Boniface, 2011; Salihu & Wilson, 2007; U.S. Department of Health and Human Services, 2014). The mechanisms underlying these associations are not completely clear. Hypothesized mechanisms include changes in DNA methylation and vascular changes (Hackshaw et al., 2011; Joubert et al., 2016; Knopik, Francazio, & McGeary, 2012; Quinton, Cook, & Peek, 2008).
Adequate levels of folate during pregnancy are associated with a decreased risk of pregnancy complications such as neural tube defects, preterm delivery, low birthweight, and fetal growth restriction (Scholl & Johnson, 2000). Vitamin B12 is involved in the same metabolic pathway as folate and has also been associated with a decreased risk of neural tube defects (Ray & Blom, 2003). Vitamin B12 is a cofactor for methionine synthase, an enzyme that decreases levels of homocysteine by remethylating it into methionine, with 5‐methyltetrahydrofolate as a methyl donor (Ansari, Mahta, Mallack, & Luo, 2014). Thus, low levels of folate or vitamin B12 are associated with increased homocysteine concentrations, and high homocysteine levels during pregnancy increase the risk of birth defects and preterm birth (El‐Khairy, Vollset, Refsum, & Ueland, 2003; Ray & Blom, 2003; Scholl & Johnson, 2000).
In nonpregnant adults, exposure to smoking is negatively associated with folate and (Yamada et al., 2013) vitamin B12 levels and positively associated with homocysteine levels (Mannino, Mulinare, Ford, & Schwartz, 2003; O'Callaghan, Meleady, Fitzgerald, & Graham, 2002). A number of studies have examined this association in pregnant women, but a complete overview of published literature is currently not available (Bakker, Timmermans, Steegers, Hofman, & Jaddoe, 2011; Bergen et al., 2012; Nilsen et al., 2010; Relton, Pearce, & Parker, 2005; Yamada et al., 2013). Therefore, we aimed to systematically review the literature evaluating the associations of exposure to smoking during pregnancy with levels of folate, vitamin B12, and homocysteine in maternal and cord blood.
2. METHODS
We carried out a systematic review of studies that evaluated the associations of exposure to smoking during pregnancy with levels of folate, vitamin B12, and/or homocysteine in pregnant women and/or in cord blood. We systematically searched the databases MEDLINE (via Ovid), Embase (via http://embase.com), CINAHL (via EBSCOhost), Cochrane Central (via Wiley), Scopus, and Web of Science for papers until August 2017. Additional references were retrieved from Google Scholar and by checking reference lists of retrieved relevant articles. We used a detailed search strategy combining terms associated with pregnancy, folate/folic acid, vitamin B12, homocysteine, smoking, and blood levels (see Appendix S1 for full search strategy). The search was created by an experienced medical librarian (W. M. B.).
Two independent reviewers (varying pairs of the following authors: A. T., P. K. B., A. V., A. P., and J. F.) screened the titles and abstracts of all papers found in the search to decide if they met the selection criteria. We included cohort studies (prospective and retrospective), case–control studies, cross‐sectional studies, randomized controlled trials (RCTs) or randomized crossover studies. Studies among pregnant women at any time during pregnancy, reporting any type and amount of active or second‐hand smoking exposure, and reporting levels of folate, vitamin B12, homocysteine, or their metabolites in maternal serum/plasma or cord blood were included. We excluded letters, abstracts, or conference proceedings and studies into the effect of supplementation. Any disagreements were resolved through discussion or with the help of a third reviewer. Of the selected articles, we retrieved full texts, and these were assessed again by two independent reviewers. A third reviewer searched the reference lists of the included articles to detect any additional studies. In case of multiple publications on the same study population, the most recent paper or the paper describing the most complete dataset was included.
Data were extracted from the full text papers by two independent reviewers, using a predefined data collection. The data collection form contained information on study characteristics (e.g., authors, year of publication), design and methods (e.g., population characteristics, and inclusion and exclusion criteria), exposure (e.g., exposure definition and exposure assessment), outcome (e.g., type of outcome and outcome assessment), analysis and results (e.g., outcome measures and covariates), and conclusions. Data extraction of studies in any language other than English or Dutch was evaluated with the help of a person fluent in that particular language.
To evaluate the quality of the included studies, we used a predefined quality score (Leermakers et al., 2015). This quality score was based on existing scoring systems (Carter, Gray, Troughton, Khunti, & Davies, 2010; National Collaborating Centre for Methods and Tools, 2008). Studies received 0, 1, or 2 points on each of five items: (a) study design: 0 points for cross‐sectional studies, 1 point for longitudinal studies, and 2 points for intervention studies. (b) Study size: 0 points if the study population was smaller than 500, 1 point if it was between 500 and 2,000, and 2 points if it was larger than 2,000. (c) Exposure assessment: 0 points if the exposure assessment was inappropriate or not reported, 1 point if exposure assessment was of moderate quality (e.g., self‐report), and 2 points if exposure measurement was adequate (e.g., marker(s) of smoking in blood). (d) Outcome assessment: 0 points if no appropriate outcome measurement method was used or the outcome measurement method was not reported, 1 point if outcome measurement was of moderate quality (e.g., information from medical records), and 2 points if outcome measurement was adequate (e.g., measurement as part of a predefined study protocol using a standardized method. (e) Statistical adjustments for potential confounding: 0 points if the findings were not controlled for key confounders (pregnancy duration at the time of measurement, a measure of folic acid/vitamin B12 intake for studies into those respective outcomes), 1 point if controlled for at least these key confounders, and 2 points if controlled for at least two additional covariates out of maternal body mass index, alcohol use, socio‐economic status, maternal age, and parity. Studies could receive up to a maximum of 10 points, with 10 meaning the highest quality (Table S1).
3. RESULTS
The search strategy identified 2,015 references. On the basis of title and abstract screening, 1,920 articles were excluded. Of the remaining 95 papers, 63 were excluded on the basis of the full text. The remaining 32 articles are included in this review, as shown in Supplemental S1 (Adaikalakoteswari et al., 2015; Ambroszkiewicz, Chelchowska, Lewandowski, Gajewska, & Laskowska‐Klita, 2007; Baker et al., 2009; Bakker et al., 2011; Bergen et al., 2012; Bodnar et al., 2010; Coker et al., 2011; Dayaldasani et al., 2014; Frery et al., 1992; D. Furness et al., 2013; D. L. Furness, Yasin, Dekker, Thompson, & Roberts, 2012; Gadowsky et al., 1995; Hay et al., 2010; Jauniaux, Johns, Gulbis, Spasic‐Boskovic, & Burton, 2007; Knight et al., 1994; Knudtson et al., 2004; Larroque et al., 1992; Matsuzaki et al., 2008; McDonald, Perkins, Jodouin, & Walker, 2002; Mito et al., 2007; Nilsen et al., 2010; Ozerol, Ozerol, Gokdeniz, Temel, & Akyol, 2004; Pagan, Hou, Goldenberg, Cliver, & Tamura, 2001; Prasodjo et al., 2014; Relton et al., 2005; Sram, Binkova, Lnenickova, Solansky, & Dejmek, 2005; Stark et al., 2005; Stark, Pawlosky, Sokol, Hannigan, & Salem Jr., 2007; Van Uitert et al., 2014; van Wersch, Janssens, & Zandvoort, 2002; Vandevijvere, Amsalkhir, Van Oyen, & Moreno‐Reyes, 2012; Yila et al., 2016).
The characteristics of the included articles are shown in Table 1. Eighteen studies were cohort studies, three were case–control studies, and 11 studies were cross‐sectional. The 32 studies included a total of 37,822 participants, of whom more than 6,000 smoked (data on the number of smokers not available for one study; Stark et al., 2005). Individual study samples ranged from 33 to 15,266 participants. All studies were done in Western countries (18 in Europe, nine in North America, three in Asia, and two in Australia). Quality scores of the included studies are shown in Table S1. The mean quality score was 4.9, with scores ranging from 3 to 9. There were no studies receiving the maximum amount of 2 points for study design, as there were no studies with an interventional study design. Seven out of 32 studies received the maximum of 2 points for appropriate exposure measurement, and outcome measurement was done adequately in 30 out of 32 studies.
Table 1.
Summary of the 32 studies included in this review that studied the association between exposure to smoking during pregnancy levels of folate, vitamin B12, and homocysteine
| First author (year) | Study setting | Outcome measuresa | Study design | Total N | Mean age (year) | Quality score |
|---|---|---|---|---|---|---|
| Adaikalakoteswari (2015) | United Kingdom | Folate, homocysteine, vitamin B12 | Cross‐sectional | 91 | 32.7 | 3 |
| Ambroszkiewicz (2007) | Poland | Folate, homocysteine | Cross‐sectional | 57 |
Median: s: 26 ns: 32 |
4 |
| Baker (2009) | United Kingdom | Folate, cobalamin | Prospective cohort | 306 | Range: 14–18 | 6 |
| Bakker (2011) | The Netherlands | Homocysteine | Prospective cohort | 6,294 | 29.9 | 8 |
| Bergen (2012) | The Netherlands | Folate, homocysteine, vitamin B12 | Prospective cohort | 5,805 | 29.8 | 6 |
| Bodnar (2010) | United States | Folate | Prospective cohort | 313 |
<20: 12% 22–29: 73% ≥30: 15% |
4 |
| Coker (2011) | Turkey | Folate, homocysteine | Prospective cohort | 58 |
s: 26.1 ns: 27.1 |
5 |
| Dayaldasani (2014) | Spain | Vitamin B12 | Prospective cohort | 204 | 30 | 5 |
| Frery (1992) | France | Vitamin B12 | Cross‐sectional | 188 | 29.2 | 4 |
| D. Furness (2013) | Australia | Folate, homocysteine, vitamin B12 | Prospective cohort | 137 | 33 | 4 |
| D. L. Furness (2012) | Australia | Folate | Retrospective case–control | 400 | 24.8 | 3 |
| Gadowsky (1995) | Canada | Folate, homocysteine, vitamin B12 | Cross‐sectional | 58 | 17.0 | 3 |
| Hay (2010) | Norway | Folate, homocysteine, cobalamin | Retrospective cohort | 340 | 29.9 | 6 |
| Jauniaux (2007) | United Kingdom | Folate | Cross‐sectional | 125 | nm | 4 |
| Knight (1994) | United States | Folate, vitamin B12 | Prospective cohort | 87 | Range: 16–35 | 5 |
| Knudtson (2004) | United States | Homocysteine | Case–control | 198 |
Cases: 25 Controls: 24 |
4 |
| Larroque (1992) | France | Folate | Prospective cohort | 245 |
≤22: 25% 23–29: 43% ≥30: 32% |
4 |
| Matsuzaki (2008) | Japan | Folate | Cross‐sectional | 537 | 30.5 | 4 |
| McDonald (2002) | Canada | Folate, homocysteine, vitamin B12 | Cross‐sectional | 80 |
s: 24.0 ns: 26.2 |
4 |
| Mito (2007) | Japan | Folate | Cross‐sectional | 70 | 29.9 | 4 |
| Nilsen (2010) | Norway | Folate | Prospective cohort | 2,934 | 29.8 | 8 |
| Ozerol (2004) | Turkey | Folate, homocysteine, vitamin B12 | Cross‐sectional | 33 | nm | 3 |
| Pagan (2001) | United States | Folate, homocysteine, vitamin B12 | Prospective cohort | 196 | 25.5 | 5 |
| Prasodjo (2014) | Canada/United States | Folate | Prospective cohort | 362 |
18–25: 23% 25–35: 60% ≥35: 17% |
7 |
| Relton (2005) | United Kingdom | Folate, vitamin B12 | Prospective cohort | 998 | 27.8 | 5 |
| Sram (2005) | Czech Republic | Folate | Case–control | 766 | nm | 5 |
| Stark (2005) | United States | 5‐MTHFA | Prospective cohort | 116 | 24.5 | 6 |
| Stark (2007) | United States | 5‐MTHFA | Prospective cohort | 58 | 24.8 | 6 |
| Van Uitert (2014) | The Netherlands | Folate | Prospective cohort | 77 | 32.7 | 4 |
| Van Wersch (2002) | The Netherlands | Folate, homocysteine, vitamin B12 | Cross‐sectional | 138 | nm | 3 |
| Vandevijvere (2012) | Belgium | Folate | Cross‐sectional | 1,285 | 28.5 ≤ 19: 1% | 6 |
| Yila (2016) | Japan | Folate | Prospective cohort | 15,266 |
<20: 1% 20–24: 12% 25–29: 31% 30–34: 37% ≥35: 19% |
9 |
Note. 5‐MTHFA: 5‐methyltetrahydrofolic acid; nm: not mentioned; ns: nonsmokers; s: smokers.
Nomenclature as in original paper.
Twenty‐eight studies measured folate or its metabolites, 14 reported on vitamin B12, and 13 measured homocysteine. Two studies included participants from the same study population, with one focusing on maternal blood levels and one on cord blood levels. Thus, we included data on maternal folate levels from the earlier paper and umbilical cord blood levels from the later paper (Stark et al., 2005; Stark et al., 2007).
3.1. Associations of maternal smoking exposure and folate levels
Table 2 shows the results of the 28 studies measuring levels of folate or its metabolites in a total of 30,938 participants (Adaikalakoteswari et al., 2015; Ambroszkiewicz et al., 2007; Baker et al., 2009; Bergen et al., 2012; Bodnar et al., 2010; Coker et al., 2011; D. Furness et al., 2013; D. L. Furness et al., 2012; Gadowsky et al., 1995; Hay et al., 2010; Jauniaux et al., 2007; Knight et al., 1994; Larroque et al., 1992; Matsuzaki et al., 2008; McDonald et al., 2002; Mito et al., 2007; Nilsen et al., 2010; Ozerol et al., 2004; Pagan et al., 2001; Prasodjo et al., 2014; Relton et al., 2005; Sram et al., 2005; Stark et al., 2005; Stark et al., 2007; Van Uitert et al., 2014; van Wersch et al., 2002; Vandevijvere et al., 2012; Yila et al., 2016). Twenty‐six studies measured folate levels in maternal blood (serum, plasma, or erythrocytes) and seven studies measured folate levels in umbilical cord blood. All studies in maternal blood showed negative associations of smoking exposure with folate levels, which were significant in 71% (n = 20) of the studies. The study with the highest quality score (9) showed odds ratios of 1.20 (95% CI [1.10–1.31]) and 1.91 (95% CI [1.70–2.14]) for passive and active smoking, respectively, for having a suboptimal folate status. Apart from this study, four other studies used cotinine (Jauniaux et al., 2007; Nilsen et al., 2010; Prasodjo et al., 2014) or thiocyanate (Pagan et al., 2001) levels to assess smoking exposure. Three of these studies showed significant negative associations of smoking with maternal folate levels (Jauniaux et al., 2007; Nilsen et al., 2010; Pagan et al., 2001). In the study measuring thiocyanate levels, only the difference at 30 weeks' gestational age was significant with a P value of <0.005 (Pagan et al., 2001). One study divided the participants with increased cotinine levels into passive and active smokers. No significant association of smoking exposure with folate levels was found in either group (Prasodjo et al., 2014).
Table 2.
Results of studies describing the association of maternal smoking and folate levels
| First author (year) | Definition of smokinga | Folate metaboliteb | Measured in | Timec of measurement | Statistical analysis | Measure of association | Results | SD/SE | 95% CI/IQR | P value | Adjustmentsd |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adaikalakoteswari (2015) | Any active smoking | Folate | Maternal serum | At birth | Student's t test | Difference in means |
sm: 10.1 μg/L nsm: 10.7 μg/L |
9.7–13.3e 10.6–13.9 |
ns | − | |
| Umbilical cord blood |
sm: 16.5 μg/L nsm: 16.9 μg/L |
15.8–17.7e 16.4–18.2 |
ns | − | |||||||
| Ambroszkiewicz (2007) | nm | Folate | Maternal serum | At birth | Student's t test | Difference in means |
sm: 12.80 ng/ml; nsm: 13.32 ng/ml; |
9.40f 3.19 |
ns | − | |
| Umbilical cord blood | Folic acid levels lower in smokers | ns | − | ||||||||
| Baker (2009) | nm | Folate | Maternal RBC | 30.3 ± 2.1 (mean ± SD) | Linear regression | Ratio of geometric means (nsm as reference) | 0.82g | 0.72–0.94e | 0.006 | − | |
| Maternal serum | 0.80g | 0.67–0.96e | 0.015 | − | |||||||
| Bergen (2012) | Any active smoking | Folate | Maternal serum | 13.2 (11.4–16.2), median (90% range) | Linear regression | Regression coefficient | −0.43 | −0.51 to −0.36e | <0.001 | − | |
| Bodnar (2010) | Any active smoking | Folate | Maternal serum | 9.4 (7.5–12.1), median (IQR) | Pearson chi‐square | Difference in number of smokers across folate tertiles |
Lowest folate tertile, sm: n = 67 (64%) Middle tertile, sm: n = 61 (58%) Upper tertile, sm: n = 47 (45%) |
<0.05 | − | ||
| Coker (2013) | ≥3 cigarettes/day for >3 years | Folic acid | Maternal serum, | At birth | Mann–Whitney U and Wilcoxon paired tests | Difference in means |
sm: 7.0 ng/ml nsm: 9.6 ng/ml |
4.2h 4.5 |
0.041 | − | |
| Umbilical cord blood |
sm: 15.4 ng/ml nsm: 16.9 ng/ml |
4.6h 4.5 |
0.207 | − | |||||||
| Furness (2013) | nm | Folate | Maternal RBC | 18–20 | t test | Difference in means (nmol/L) |
sm: 463 nsm: 687 |
354–571e 647–727 |
<0.001 | − | |
| Maternal serum |
sm: 22.6 nsm: 27.3 |
17.9–27.3e 25.5–29.0 |
0.035 | − | |||||||
| Furness (2012) | nm | Folate | Maternal RBC | 10–12 | ANOVA | Difference in means |
sm: 507.3 nmol/L nsm: 657.3 nmol/L |
<0.001 | − | ||
| Pearson correlation | Correlation coefficient | r = −0.256 | 0.002 | − | |||||||
| Gadowsky (1995) | ≥1 cigarette/day | Folate | Maternal plasma | 35.9 ± 0.2 | Pearson correlation | Correlation coefficient | nm | ns | − | ||
| Maternal RBC | r = −0.35 | <0.009 | − | ||||||||
| Hay (2010) | Any active smoking | Folate | Umbilical cord blood | At birth | Linear regression | Partial correlation coefficient | r = −0.17 | 0.052 | +++ | ||
| Jauniaux (2007) | Cotinine levels > 25 ng/ml | Folate | Maternal serum | Median: 9.2 | Least squares method and F test | Difference in medians |
sm: 7.5 nmol/L nsm: 14.3 nmol/L |
5.3–14.3i 11.1–20.0 |
<0.001 | − | |
| Knight (1994) | Any active smoking of marihuana | Folate | Maternal serum | 3rd trimester | Pearson correlation | Correlation coefficient | r = −0.25 | 0.02 | − | ||
| Larroque (1992) | ≥1 cigarette/day | Folate | Maternal serum | 33 (14–41) | Correlation | Correlation coefficient | r = −0.13 | 0.05 | − | ||
| Multiple linear regression | Beta | β = −0.02 | 0.39 | − | |||||||
| Maternal RBC | Correlation | Correlation coefficient | r = −0.12 | 0.06 | − | ||||||
| Multiple linear regression | Beta | β = −3.9 | 0.01 | − | |||||||
| Matsuzaki (2008) | Any active smoking | Folate | Maternal serum | 11–40 | Logistic regression | Odds ratio (of normal folic acid levels) | OR = 0.632 | 0.276–1.45e | ns | − | |
| McDonald (2002) | Any active smoking | Folate | Maternal serum | 1st and early 2nd trimesters | Unpaired t test | Difference in means |
sm: 22.7 nmol/L nsm: 29.4 nmol/L |
7.6h 8.9 |
0.001 | ||
| Maternal RBC |
sm: 766 nmol/L nsm: 900 nmol/L |
246h 317 |
0.038 | ||||||||
| Mito (2007) | Any active smoking | Folate | Maternal serum | 1st trimester | Chi‐square test | Percentage of smokers according to folate levels |
<9 ng/ml: 20.6 ≥9 ng/ml: 16.7 |
0.327 | − | ||
| Nilsen (2010) | Cotinine level ≥ 85 nmol/L | Folate | Maternal plasma | Median: 18 | Spearman correlation | Correlation coefficient | r = −0.12 | <0.001 | ++ | ||
| Ozerol (2004) | ≥2 cigarettes/day | Folate | Maternal serum | 16–22 | Mann–Whitney U test | Difference in means |
sm: 4.6 nmol/L nsm: 14.1 nmol/L |
0.4f 1.4 |
<0.001 | − | |
| Pagan (2001) | Thiocyanate blood levels in highest quartile | Folate | Maternal serum | 18 | Student's t test | Difference in means |
sm: 47 nmol/L nsm: 54 nmol/L |
31h 38 |
ns | − | |
| 30 |
sm: 38 nmol/L nsm: 54 nmol/L |
30h 39 |
<0.005 | − | |||||||
| Prasodjo (2014) | Active: Cotinine > 3 ng/ml | Maternal whole blood | 16 | Linear regression | Beta | −94 | −195 to 6e | 0.07 | ++ | ||
| Passive: >0 and 3 ng/ml | −26 | −84 to 32e | 0.38 | ++ | |||||||
| Relton (2005) | Any active smoking | Folate | Maternal RBC | 11.5 | Linear regression | Correlation coefficient | r = −1.38 | −1.92 to −0.86e | <0.001 | − | |
| Umbilical cord blood | At birth | r = 0.31 | −0.62 to 1.25e | 0.50 | − | ||||||
| Sram (2005) | Any (active or passive) | Folate | Maternal plasma | At birth | nm | Difference in means | Europeans: | Nm | − | ||
|
sm: 22.0 nmol/L nsm: 26.6 nmol/L |
15.9f 16.8 |
||||||||||
| Teplice Europeans: | |||||||||||
|
sm: 21.3 nmol/L nsm: 24.5 nmol/L |
16.3 16.8 |
||||||||||
| Prague Europeans: | |||||||||||
|
sm: 23.4 nmol/L nsm: 28.6 nmol/L |
14.8 16.3 |
||||||||||
| Umbilical cord blood | Europeans: | − | |||||||||
|
sm: 45.2 nmol/L nsm: 49.0 nmol/L |
15.7f 17.7 |
||||||||||
| Teplice Europeans: | |||||||||||
|
sm: 43.8 nmol/L nsm: 48.4 nmol/L |
15.4 17.4 |
||||||||||
| Prague Europeans: | |||||||||||
|
sm: 47.9 nmol/L nsm: 49.9 nmol/L |
15.7 17.5 |
||||||||||
| Stark (2005) | Any (active or passive) | 5‐MTHFA | Maternal plasma | 24 | Linear regression | Standardized beta | Maternal smoking: β = −0.12 | 0.23 | ++ | ||
| Paternal smoking: β = −0.26 | 0.019 | ||||||||||
| Pearson correlation | Regression coefficient | Maternal smoking: r = −0.03 |
ns |
++ |
|||||||
| Prepregnancy maternal smoking: r = −0.04 |
ns |
||||||||||
| Paternal smoking: r = −0.21 | 0.043 | ||||||||||
| Stark (2007) | Any active smoking | 5‐MTHFA | Umbilical cord blood | 24 | t test | Difference in means |
sm: 15.1 ng/ml nsm: 19.0 ng/ml |
7.6h 7.0 |
0.0498; adjusted: 0.034 | + | |
|
Pearson correlation |
Correlation coefficients | Number of cigarettes smoked at first prenatal visit: r = −0.31 | 0.019 | ||||||||
| Number of cigarettes smoked before pregnancy: r = −0.30 | 0.023 | ||||||||||
| Linear regression | Standardized beta | Maternal cigarettes smoked/day: β = −0.31 | 0.009 | ||||||||
| Van Uitert (2014) | Any active smoking | Folate | Maternal RBC | 7 (4–11) | Difference in means | sm: 1,257 nmol/L nsm: 1,627 nmol/L |
239h 475 |
<0.01 | − | ||
| Percentage of smokers per quartile |
Folate Q1: sm = 36.8%; Q2: sm = 20%; Q3: sm = 5.3%; Q4: sm = 5.3% |
0.024 | |||||||||
| Van Wersch (2002) | ≥20 cigarettes/day | Folate | Maternal serum | 0–10 | Mann–Whitney U test | Difference in medians |
sm: 8.2 nmol/L nsm: 12.2 nmol/L |
4.8–12.9i 8.8–48.0 |
ns | − | |
| 11–20 |
sm: 6.4 nmol/L nsm: 11.1 nmol/L |
3.0–10.1i 9.2–17.6 |
0.03 | − | |||||||
| 21–30 |
sm: 4.1 nmol/L nsm: 12.1 nmol/L |
2.0–12.2i 8.9–18.7 |
0.002 | − | |||||||
| 31–40 |
sm: 3.7 nmol/L nsm: 9.3 nmol/L |
1.6–6.9i 6.8–13.7 |
0.0002 | − | |||||||
| Vandevijvere (2012) | Any active smoking | Folate | Maternal RBC | 1st or 3rd trimester | Linear regression | Beta |
First trimester: β = 0.974j Third trimester: β = 0.098g |
0.313f 0.036f |
0.002 0.006 |
+++ | |
| Yila (2016) | Any (active/passive) | Folate | Maternal serum | 1st trimester | Logistic regression | Odds of low folate status |
psm: 1.20 sm: 1.91 |
1.10–1.31e 1.70–2.14 |
<0.001 | +++ |
Note. 5‐MTHFA: 5‐methyltetrahydrofolic acid; SE: standard error; CI: confidence interval; IQR: interquartile range; nm: not mentioned; ns: nonsignificant; sm: smoking women; nsm: nonsmoking women; psm: women exposed to passive smoking; RBC: red blood cell; ANOVA: analysis of variance.
Smoking refers to tobacco smoking unless mentioned otherwise.
Nomenclature in table as in original paper.
Weeks of gestation.
Adjustment level was categorized as follows: −, unadjusted; +, 4 covariates or less; ++, 5 to 8 covariates; +++, 9 or more covariates.
95% CI.
SE.
Log transformed.
SD.
IQR.
Root square transformed.
Three other studies described the association of maternal exposure to passive smoking with folate levels (Sram et al., 2005; Stark et al., 2005; Yila et al., 2016). Two of these reported a significantly negative association (Stark et al., 2005).
Seven studies measured folate levels in umbilical cord blood. One of these found a significant negative association (lower levels) of smoking with umbilical cord blood levels of folate (Stark et al., 2007). The other six studies did not report significant associations (Adaikalakoteswari et al., 2015; Ambroszkiewicz et al., 2007; Coker et al., 2011; Hay et al., 2010; Relton et al., 2005; Sram et al., 2005). One study described serum levels of folate during pregnancy measured at four different time points (0–10, 11–20, 21–30, and 31–40 weeks; van Wersch et al., 2002). At all time points, the association was negative, reaching statistical significance at the three latest time points.
One study did not report tobacco smoke exposure, but smoking of marihuana (Knight et al., 1994). This study found a significant negative correlation (r = −0.25, P value = 0.02) of smoking marihuana with maternal folate levels.
Figure 1a shows a harvest plot of the studies assessing the association of maternal smoking with folate levels. The harvest plots display the associations found and the corresponding quality scores of the studies. One study did not report information on significance and is therefore not presented in the harvest plot (Sram et al., 2005). If a study measured folate levels at different time points, the results of the latest time point were used for the harvest plot. If folate metabolites were measured in different components of blood (plasma/serum/red blood cells), the measurement most comparable with that of the other studies was presented in the harvest plot.
Figure 1.
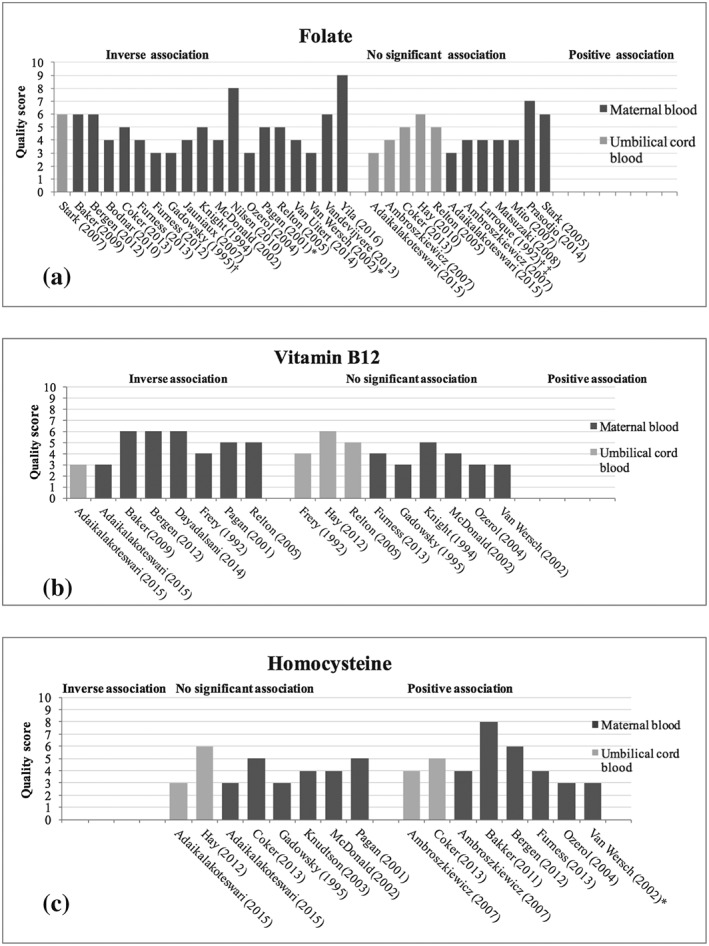
(a–c) Harvest plots of the evidence for associations of smoking with folate, vitamin B12, and homocysteine levels. One study did not report information on significance and is therefore not presented in the harvest plot (Sram et al., 2005). * indicates associations were determined at different time points in pregnancy. If there were discrepancies in the significance between the time points, the plot represents the significance of the measurement of the latest time point. † indicates levels of folate were measured in different blood components (e.g., maternal plasma, serum, or red blood cells). If there were discrepancies in the significance between the components, the plot represents the significance of the measurement most comparable with the measurements of the other studies (Table 2). The study of Gadowsky et al. measured levels of folate in maternal plasma and red blood cells; results of maternal RBC are shown. The study of Larroque et al. measure levels of folate in maternal serum and maternal RBC; results of maternal serum are shown. ‡ indicates significance was determined using different statistical tests. If there were discrepancies in the significance between tests, only the significance of the regression analysis is shown
3.2. Associations of maternal smoking exposure and vitamin B12 levels
Table 3 shows the results of the 13 studies measuring vitamin B12 levels in a total of 8,661 participants. The mean quality score was 4, and quality scores ranged from 3 to 6. Eleven studies reported lower levels of vitamin B12 in smokers, of which seven were significant (Adaikalakoteswari et al., 2015; Baker et al., 2009; Bergen et al., 2012; Dayaldasani et al., 2014; Frery et al., 1992; D. Furness et al., 2013; Gadowsky et al., 1995; Hay et al., 2010; Knight et al., 1994; McDonald et al., 2002; Ozerol et al., 2004; Pagan et al., 2001; Relton et al., 2005; van Wersch et al., 2002) The study assessing smoking using thiocyanate levels found significantly lower levels of vitamin B12 in smoking compared with nonsmoking mothers at two time points in pregnancy (Pagan et al., 2001). The study assessing the effect of marihuana smoking during pregnancy did not find a correlation with vitamin B12 levels (Knight et al., 1994). One of the four studies reporting measurements in umbilical cord blood showed a significant difference between the levels of vitamin B12 in smoking and nonsmoking pregnant women, with lower levels of vitamin B12 in women exposed to smoking (Adaikalakoteswari et al., 2015; Frery et al., 1992; Hay et al., 2010; Relton et al., 2005). The results of the associations of maternal smoking with levels of vitamin B12 are presented in Figure 1b.
Table 3.
Results of studies describing the association of maternal smoking and vitamin B12 levels
| First author (year) | Definition of smokinga | Measured in | Timeb of measurement | Statistical analysis | Measure of association | Results | SD/SE | 95% CI/IQR | P value | Adjustmentsc |
|---|---|---|---|---|---|---|---|---|---|---|
| Adaikalakoteswari (2015) | Any active smoking | Maternal serum | At birth | Student's t test | Difference in means |
sm: 189 ng/L nsm: 245 ng/L |
176–224d 227–357 |
<0.01 | − | |
| Umbilical cord blood |
sm: 252 ng/L nsm: 327 ng/L |
232–364d 305–502 |
<0.05 | − | ||||||
| Baker (2009) | nm | Maternal serum | 30.3 ± 2.1 | Simple and multiple regression | Ratio of geometric means (nsm as reference) | 0.77e | 0.68–0.88d | <0.001 | − | |
| Bergen (2012) | Any active smoking | Maternal serum | 13.2 (11.4–16.2), median (90% range) | Linear regression | Regression coefficient | −0.20 | −0.28 to –0.12d | <0.001 | − | |
| Dayaldasani (2014) | Any active smoking | Maternal serum | 30 | Student's t test | Difference in geometric means |
sm: 343.78 pmol/L nsm: 395.79 pmol/L |
0.035 | + | ||
| Stepwise multiple linear regression | Regression coefficient | −78.03 | −143.44 to –12.62d | 0.020 | − | |||||
| Frery (1992) | ≥1 cigarette/day | Maternal plasma | At birth | t test | Difference in geometric means |
sm: 189 pg/ml nsm: 245 pg/ml |
144–248d 226–267 |
<0.05 | − | |
| Umbilical cord blood | Difference in geometric means |
sm: 511 pg/ml nsm: 589 pg/ml |
378–690d 528–657 |
ns | − | |||||
| Furness (2013) | nm | Maternal serum | 18–20 | t test | Difference in means |
sm: 209 pmol/L nsm: 244 pmol/L |
144–273d 217–271 |
0.317 | − | |
| Gadowsky (1995) | ≥1 cigarette/day | Maternal plasma | 35.9 ± 0.2 | Pearson | Correlation coefficient | nm | ns | − | ||
| Hay (2010) | Any active smoking | Umbilical cord blood | At birth | Linear regression analysis | Correlation coefficient | nm | ns | +++ | ||
| Knight (1994) | Any active smoking of marihuana | Maternal serum | 1st, 2nd, and 3rd trimesters, birth | Pearson | Correlation coefficient | nm | ns | − | ||
| McDonald (2002) | Any active smoking | Maternal serum | 1st and early 2nd trimesters | Unpaired t test | Difference in means |
s: 195 pmol/L ns: 218 pmol/L |
87f 99 |
0.279 | − | |
| Ozerol (2004) | ≥2 cigarettes/day | Maternal serum | 16–22 | Mann–Whitney U test | Difference in means |
s: 236.4 nmol/L ns: 240 nmol/L |
15.4f 13.9 |
ns | − | |
| Pagan (2001) | Thiocyanate blood levels in highest quartile | Maternal serum | 18 | Student's t test | Difference in means |
s: 319 pmol/L ns: 379 pmol/L |
99f 139 |
<0.005 | − | |
| 30 |
s: 254 pmol/L ns: 309 pmol/L |
60f 102 |
0.0001 | − | ||||||
| Relton (2005) | Any active smoking | Maternal RBC | 11.5 and | Multiple linear regression | Correlation coefficient | r = −0.88 | −1.49 to –0.27d | 0.005 | − | |
| Umbilical cord blood | At birth | r = 0.23 | −0.50 to 0.96d | 0.54 | − | |||||
| Van Wersch (2002) | ≥20 cigarettes/day | Maternal plasma | 0–10 | Mann–Whitney U test | Difference in medians |
s: 0.27 nmol/L ns: 0.31 nmol/L |
0.21–0.31g 0.25–0.39 |
ns | − | |
| 11–20 |
s: 0.23 nmol/L ns: 0.24 nmol/L |
0.18–0.27g 0.21–0.25 |
ns | − | ||||||
| 21–30 |
s: 0.21 nmol/L ns: 0.21 nmol/L |
0.19–0.24g 0.19–0.26 |
ns | − | ||||||
| 31–40 |
s: 0.20 nmol/L ns: 0.20 nmol/L |
0.19–0.22g 0.18–0.23 |
ns | − |
Note. SD: standard deviation; SE: standard error; CI: confidence interval; IQR: interquartile range; nm: not mentioned; ns: nonsignificant; s: significant; sm: smoking women; nsm: nonsmoking women; RBC: red blood cell.
Smoking refers to tobacco smoking unless mentioned otherwise.
Weeks of gestation.
Adjustment level was categorized as follows: −, unadjusted; +, 4 covariates or less; ++, 5 to 8 covariates; +++, 9 or more covariates.
95% CI.
Log transformed.
SD.
IQR.
3.3. Associations of maternal smoking exposure and homocysteine levels
The results of the 13 studies reporting on homocysteine levels in a total of 13,485 participants are shown in Table 4. Quality scores ranged from 3 to 8 points, and three studies received a quality score of more than 5 points. In maternal blood, out of 12 studies, five studies showed significantly higher levels of homocysteine in pregnant women exposed to smoking (Adaikalakoteswari et al., 2015; Ambroszkiewicz et al., 2007; Bakker et al., 2011; Bergen et al., 2012; Coker et al., 2011; D. Furness et al., 2013; Gadowsky et al., 1995; Knudtson et al., 2004; McDonald et al., 2002; Ozerol et al., 2004; Pagan et al., 2001; van Wersch et al., 2002). The study measuring thiocyanate levels in blood as an objective measure of smoking exposure found higher levels of homocysteine in smokers as compared with nonsmokers, but this difference was nonsignificant (Pagan et al., 2001). The study with the highest quality score showed significantly higher homocysteine levels in smoking women compared with nonsmoking women with smoking being associated with a 0.05 unit increase in log‐transformed homocysteine levels (95% CI [0.03–0.06]; Bakker et al., 2011). Four studies used umbilical cord blood measurements (Adaikalakoteswari et al., 2015; Ambroszkiewicz et al., 2007; Coker et al., 2011; Hay et al., 2010), of which two showed a significant positive association of smoking with homocysteine levels (Ambroszkiewicz et al., 2007; Coker et al., 2011). Figure 1c shows harvest plots of the associations of maternal smoking and homocysteine levels.
Table 4.
Results of studies describing the association of maternal smoking and homocysteine levels
| First author (year) | Definition of smokinga | Measured in | Timeb of measurement | Statistical analysis | Measure of association | Results | SD/SE | 95% CI/IQR | P value | Adjustmentsc |
|---|---|---|---|---|---|---|---|---|---|---|
| Adaikalakoteswari (2015) | Any active smoking | Maternal serum | At birth | Student's t test | Difference in means | sm: 6.33 μmol/L | 5.90–7.45d | ns | − | |
| nsm: 6.15 μmol/L | 5.61–8.08 | |||||||||
| Umbilical cord blood |
sm: 6.21 μmol/L nsm: 5.42 μmol/L |
5.80–7.68d | ns | − | ||||||
| 5.06–6.58 | ||||||||||
| Ambroszkiewicz (2007) | nm | Maternal serum | At birth | Student's t test | Difference in means | sm: 5.95 μmol/L | 2.50e | <0.05 | − | |
| nsm: 4.60 μmol/L | 0.9 | |||||||||
| Umbilical cord blood | sm: 6.43 μmol/L | 2.21e | <0.001 | |||||||
| nsm: 4.70 μmol/L | 0.89 | |||||||||
| Bakker (2011) | nm | Maternal plasma | Median: 14.4 | Linear regression | Regression coefficient | β = 0.05f | 0.03–0.06d | <0.01 | +++ | |
| Bergen (2012) | Any active smoking | Maternal serum | 13.2 (11.4–16.2), median (90% range) | Linear regression | Regression coefficient | 0.35 | 0.28–0.42d | <0.001 | − | |
| Coker (2013) | ≥3 cigarettes/day for >3 years | Maternal serum | At birth | Mann–Whitney U and Wilcoxon paired tests | Difference in means |
sm: 6.7 μmol/L nsm: 5.9 μmol/L |
2.5g 2.5 |
0.237 | − | |
| Umbilical cord blood | sm: 8.2 μmol/L | 2.5g | 0.006 | − | ||||||
| nsm: 6.4 μmol/L | 2.0 | |||||||||
| Furness (2013) | nm | Maternal plasma | 18–20 | t test | Difference in means |
sm: 6.0 μmol/L nsm: 4.4 μmol/L |
5.0–6.9d 4.1–4.6 |
<0.001 | − | |
| Gadowsky (1995) | ≥1 cigarette/day | Maternal plasma | 35.9 ± 0.2 | Pearson correlation | Correlation coefficient | nm | ns | − | ||
| Hay (2010) | Any active smoking | Umbilical cord blood | At birth | Linear regression analysis | Correlation coefficient | nm | ns | +++ | ||
| Knudtson (2003) | Any active smoking | Maternal serum | 24–32 | Pearson correlation | Correlation coefficient | r = −0.08 | 0.57 | − | ||
| McDonald (2002) | Any active smoking | Maternal serum | 1st and early 2nd trimesters | Unpaired t test | Difference in means | sm: 5.4 μmol/L | 1.3g | 0.613 | − | |
| nsm: 5.2 μmol/L | 1.8 | |||||||||
| Ozerol (2004) | ≥2 cigarettes/day | Maternal serum | 16–22 | Mann–Whitney U test | Difference in means | sm: 13.1 μmol/L | 1.1e | <0.001 | − | |
| nsm: 6.9 μmol/L | 0.7 | |||||||||
| Pagan (2001) | Thiocyanate blood levels in highest quartile | Maternal serum | 18 | Student's t test | Difference in means | sm: 5.2 μmol/L | 2.4g | ns | − | |
| nsm: 5.0 μmol/L | 1.6 | |||||||||
| 30 | sm: 5.7 μmol/L | 3.4g | ns | |||||||
| nsm: 4.9 μmol/L | 1.6 | |||||||||
| Van Wersch (2002) | ≥20 cigarettes/day | Maternal plasma | 0–10 | Mann–Whitney U test | Difference in medians |
sm: 6.4 μmol/L nsm: 7.2 μmol/L |
5.6–11.4h 6.5–7.8 |
ns | − | |
| 11–20 | sm: 6.1 μmol/L | 5.4–8.0h | ns | |||||||
| nsm: 5.9 μmol/L | 5.1–6.3 | |||||||||
| 21–30 | sm: 6.4 μmol/L | 5.7–7.9h | 0.02 | |||||||
| nsm: 5.1 μmol/L | 4.1–6.2 | |||||||||
| 31–40 | sm: 7.5 μmol/L | 6.5–9.1h | 0.04 | |||||||
| nsm: 6.5 μmol/L | 4.8–7.6 |
Note. SE: standard error; SD: standard deviation; CI: confidence interval; IQR: interquartile range; nm: not mentioned; ns: nonsignificant; sm: smoking women; nsm: nonsmoking women.
Tobacco unless mentioned otherwise.
Weeks of gestation.
Adjustment level was categorized as follows: −, unadjusted; +, 4 covariates or less; ++, 5 to 8 covariates; +++, 9 or more covariates.
95% CI.
SE.
Log transformed.
SD.
IQR.
4. DISCUSSION
4.1. Main findings
Our systematic review shows that levels of both folate and vitamin B12 were lower in blood samples of pregnant women exposed to smoking than in blood samples of pregnant women who were not exposed to smoking. The same association was generally seen in cord blood, but the number of studies measuring cord blood levels was lower (eight out of 32 studies), and there were fewer significant associations. Homocysteine levels tended to be higher in maternal and cord blood of pregnant women exposed to smoking.
4.2. Interpretation of results
Low folate and vitamin B12 levels and high homocysteine levels during pregnancy are associated with poor perinatal outcomes (Mannino et al., 2003; Nelen, Blom, Steegers, den Heijer, & Eskes, 2000; O'Callaghan et al., 2002; Ray & Blom, 2003; Scholl & Johnson, 2000). Folate and vitamin B12 are important regulators of homocysteine levels. Decreased levels of folate and vitamin B12 cause an increase in homocysteine, because both micronutrients are cofactors in the methylation of homocysteine to methionine (Refsum, Ueland, Nygard, & Vollset, 1998).
A number of mechanisms may underlie the negative associations of smoking exposure with folate and vitamin B12 levels and the positive associations with homocysteine levels. Firstly, several components of cigarette smoke, such as organic nitrites, nitrous oxide, cyanates, and isocyanates, can increase oxidative stress and interact with folate and vitamin B12, causing these micronutrients to become inactive (Northrop‐Clewes & Thurnham, 2007). This leads to lower levels of folate and vitamin B12 and higher levels of homocysteine.
Secondly, nicotine use can lead to a change in basal metabolic rate by increasing serum levels of catecholamines (Walker et al., 1999). This leads to higher nutritional demands in smokers, which are already increased during pregnancy (Picciano, 2003).
A third pathway that can cause lower micronutrient levels in smokers is a difference in diet between smokers and nonsmokers (Dallongeville, Marecaux, Fruchart, & Amouyel, 1998). It is not completely clear what causes the difference in dietary preferences of smokers as compared with nonsmokers. Hypotheses include a reduction of monoamine oxidase in smokers, leading to changes in mood and appetite, a direct influence of nicotine on taste receptors, and a generally unhealthier lifestyle (Dallongeville et al., 1998; Leroy et al., 2009). Differences in dietary habits may have larger effects in pregnancy, when the nutritional demands are different (King, 2000; Picciano, 2003). Previous work has shown that the association of maternal smoking with low birthweight may be modified by periconceptional folic acid supplementation, underlining the interactive effects of these exposures on fetal growth (Bakker et al., 2011).
4.3. Strengths and limitations
The quality of the studies included in this review varied widely, as reflected in the quality scores. In some studies, a low score was mainly given because of a small number of participants, but there also was room for improvement in assessing exposure status with more objective measures. Not all studies adjusted for key confounders such as dietary intake, supplementation, maternal age, and duration of gestation at the time of measurement of the outcome. Due to the large diversity in study designs and reporting of measures of association, it was not possible to include a meta‐analysis of the individual study results.
As smoking during pregnancy is generally considered a socially undesired habit, pregnant women may report a lower amount of cigarettes smoked or not having smoked at all, which can lead to misclassification of the exposure. Therefore, we cannot comment on a possible dose–effect association of smoking with nutrient levels. The majority of studies assessed smoking exposure by questionnaire, and only five studies used cotinine or thiocyanate blood levels to objectively determine smoking exposure (Jauniaux et al., 2007; Nilsen et al., 2010; Pagan et al., 2001; Prasodjo et al., 2014; Yila et al., 2016). The results of these studies were in line with those measuring smoking exposure by questionnaire, with lower levels of folate and vitamin B12 and higher levels of homocysteine. If anything, the misclassification that can occur by using questionnaires would bias results towards the null, as it is unlikely that misclassification would occur in the opposite direction (i.e., overreporting of smoking habits in pregnant women).
In theory, the association of smoking exposure with micronutrient levels would ideally be assessed using an RCT, but this would raise important ethical issues. All studies in this review are cohorts or case–control studies, which are the best options for this specific research question.
The total number of participants in all studies combined was 37,822, which is substantial, but there was a large range in study sizes, with the smallest study consisting of 33 participants (Ozerol et al., 2004) and the largest of 15,266 participants (Yila et al., 2016).
5. CONCLUSION
In general, smoking was associated with lower levels of folate and vitamin B12 and higher levels of homocysteine in maternal blood, with the strongest evidence for folate. This may help raise further awareness about the consequences of smoking and the need to encourage stopping smoking in all, especially in pregnant women. Also, they may highlight a potential need for more intense and targeted advice about supplementation of mainly folic acid to pregnant women, depending on smoking status.
CONFLICTS OF INTEREST
OHF works in ErasmusAGE, a centre for aging research across the life course funded by Nestlé Nutrition (Nestec Ltd.) and Metagenics Inc. Nestlé Nutrition (Nestec Ltd.) and Metagenics Inc. had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
CONTRIBUTIONS
AT and JFF were involved in the conception and design of the study. AT, PKB, AV, AP, WMB, OHF, and JFF acquired the data. AT, OHF, and JFF analysed and interpreted the data. AT and JFF drafted the article. AT, PKB, AV, AP, WMB, OHF, and JFF revised the article critically for important intellectual content. AT, PKB, AV, AP, WMB, OHF, and JFF agreed to be accountable for all aspects of the work.
Supporting information
Appendix S1. Full search strategy
Figure S1. Literature flow diagram
Table S1. Quality score
Tuenter A, Bautista Nino PK, Vitezova A, et al. Folate, vitamin B12, and homocysteine in smoking‐exposed pregnant women: A systematic review. Matern Child Nutr. 2019;15:e12675 10.1111/mcn.12675
REFERENCES
- Adaikalakoteswari, A. , Vatish, M. , Lawson, A. , Wood, C. , Sivakumar, K. , McTernan, P. G. , … Saravanan, P. (2015). Low maternal vitamin B12 status is associated with lower cord blood HDL cholesterol in white Caucasians living in the UK. Nutrients, 7(4), 2401–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambroszkiewicz, J. , Chelchowska, M. , Lewandowski, L. , Gajewska, J. , & Laskowska‐Klita, T. (2007). Serum folate and homocysteine concentrations in women smoking during pregnancy and in umbilical cord blood of newborns. Przegla̧d Lekarski, 64(10), 674–678. [PubMed] [Google Scholar]
- Ansari, R. , Mahta, A. , Mallack, E. , & Luo, J. J. (2014). Hyperhomocysteinemia and neurologic disorders: A review. Journal of Clinical Neurology, 10(4), 281–288. 10.3988/jcn.2014.10.4.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, P. N. , Wheeler, S. J. , Sanders, T. A. , Thomas, J. E. , Hutchinson, C. J. , Clarke, K. , … Poston, L. (2009). A prospective study of micronutrient status in adolescent pregnancy. The American Journal of Clinical Nutrition, 89(4), 1114–1124. [DOI] [PubMed] [Google Scholar]
- Bakker, R. , Timmermans, S. , Steegers, E. A. , Hofman, A. , & Jaddoe, V. W. (2011). Folic acid supplements modify the adverse effects of maternal smoking on fetal growth and neonatal complications. The Journal of Nutrition, 141(12), 2172–2179. [DOI] [PubMed] [Google Scholar]
- Bergen, N. E. , Jaddoe, V. W. , Timmermans, S. , Hofman, A. , Lindemans, J. , Russcher, H. , … Steegers, E. A. (2012). Homocysteine and folate concentrations in early pregnancy and the risk of adverse pregnancy outcomes: The Generation R Study. BJOG, 119(6), 739–751. [DOI] [PubMed] [Google Scholar]
- Bodnar, L. M. , Himes, K. P. , Venkataramanan, R. , Chen, J. Y. , Evans, R. W. , Meyer, J. L. , & Simhan, H. N. (2010). Maternal serum folate species in early pregnancy and risk of preterm birth. The American Journal of Clinical Nutrition, 92(4), 864–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, P. , Gray, L. J. , Troughton, J. , Khunti, K. , & Davies, M. J. (2010). Fruit and vegetable intake and incidence of type 2 diabetes mellitus: Systematic review and meta‐analysis. BMJ, 18(341). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker, I. , Colak, A. , Gunaslan Hasturk, A. , Yildiz, O. , Turkon, H. , & Halicioglu, O. (2011). Maternal and cord blood homocysteine and folic acid levels in smoking and nonsmoking pregnant women. Gynecologic and Obstetric Investigation, 71(4), 245–249. [DOI] [PubMed] [Google Scholar]
- Dallongeville, J. , Marecaux, N. , Fruchart, J. C. , & Amouyel, P. (1998). Cigarette smoking is associated with unhealthy patterns of nutrient intake: A meta‐analysis. The Journal of Nutrition, 128(9), 1450–1457. [DOI] [PubMed] [Google Scholar]
- Dayaldasani, A. , Ruiz‐Escalera, J. , Rodriguez‐Espinosa, M. , Rueda, I. , Perez‐Valero, V. , & Yahyaoui, R. (2014). Serum vitamin B12 levels during the first trimester of pregnancy correlate with newborn screening markers of vitamin B12 deficiency. International Journal for Vitamin and Nutrition Research, 84(1–2), 92–97. [DOI] [PubMed] [Google Scholar]
- El‐Khairy, L. , Vollset, S. E. , Refsum, H. , & Ueland, P. M. (2003). Plasma total cysteine, pregnancy complications, and adverse pregnancy outcomes: The Hordaland Homocysteine Study. The American Journal of Clinical Nutrition, 77(2), 467–472. [DOI] [PubMed] [Google Scholar]
- Frery, N. , Huel, G. , Leroy, M. , Moreau, T. , Savard, R. , Blot, P. , & Lellouch, J. (1992). Vitamin B12 among parturients and their newborns and its relationship with birthweight. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 45(3), 155–163. [DOI] [PubMed] [Google Scholar]
- Furness, D. , Fenech, M. , Dekker, G. , Khong, T. Y. , Roberts, C. , & Hague, W. (2013). Folate, vitamin B12, vitamin B6 and homocysteine: Impact on pregnancy outcome. Maternal & Child Nutrition, 9(2), 155–166. 10.1111/j.1740-8709.2011.00364.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness, D. L. , Yasin, N. , Dekker, G. A. , Thompson, S. D. , & Roberts, C. T. (2012). Maternal red blood cell folate concentration at 10–12 weeks gestation and pregnancy outcome. The Journal of Maternal‐Fetal & Neonatal Medicine, 25(8), 1423–1427. 10.3109/14767058.2011.636463 [DOI] [PubMed] [Google Scholar]
- Gadowsky, S. L. , Gale, K. , Wolfe, S. A. , Jory, J. , Gibson, R. , & O'Connor, D. L. (1995). Biochemical folate, B12, and iron status of a group of pregnant adolescents accessed through the public health system in southern Ontario. The Journal of Adolescent Health, 16(6), 465–474. 10.1016/1054-139x(94)00001-u [DOI] [PubMed] [Google Scholar]
- Hackshaw, A. , Rodeck, C. , & Boniface, S. (2011). Maternal smoking in pregnancy and birth defects: A systematic review based on 173 687 malformed cases and 11.7 million controls. Human Reproduction Update, 17(5), 589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay, G. , Clausen, T. , Whitelaw, A. , Trygg, K. , Johnston, C. , Henriksen, T. , & Refsum, H. (2010). Maternal folate and cobalamin status predicts vitamin status in newborns and 6‐month‐old infants. The Journal of Nutrition, 140(3), 557–564. [DOI] [PubMed] [Google Scholar]
- Jauniaux, E. , Johns, J. , Gulbis, B. , Spasic‐Boskovic, O. , & Burton, G. J. (2007). Transfer of folic acid inside the first‐trimester gestational sac and the effect of maternal smoking. American Journal of Obstetrics and Gynecology, 197(1), e1–e6. [DOI] [PubMed] [Google Scholar]
- Joubert, B. R. , Felix, J. F. , Yousefi, P. , Bakulski, K. M. , Just, A. C. , Breton, C. , … London, S. J. (2016). DNA methylation in newborns and maternal smoking in pregnancy: Genome‐wide consortium meta‐analysis. American Journal of Human Genetics, 98(4), 680–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, J. C. (2000). Physiology of pregnancy and nutrient metabolism. The American Journal of Clinical Nutrition, 71(5 Suppl), 1218S–1225S. [DOI] [PubMed] [Google Scholar]
- Knight, E. M. , Spurlock, B. G. , Edwards, C. H. , Johnson, A. A. , Oyemade, U. J. , Cole, O. J. , … Laryea, H. (1994). Biochemical profile of African American women during three trimesters of pregnancy and at delivery. The Journal of Nutrition, 124(6 Suppl), 943S–953S. [DOI] [PubMed] [Google Scholar]
- Knopik, V. S. , Maccani, M. A. , Francazio, S. , & McGeary, J. E. (2012). The epigenetics of maternal cigarette smoking during pregnancy and effects on child development. Development and Psychopathology, 24(4), 1377–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudtson, E. J. , Smith, K. , Mercer, B. M. , Miodovnik, M. , Thurnau, G. R. , Goldenberg, R. L. , … Das, A. (2004). Serum homocysteine levels after preterm premature rupture of the membranes. American Journal of Obstetrics and Gynecology, 191(2), 537–541. 10.1016/j.ajog.2003.12.005 [DOI] [PubMed] [Google Scholar]
- Larroque, B. , Kaminski, M. , Lelong, N. , d'Herbomez, M. , Dehaene, P. , Querleu, D. , & Crepin, G. (1992). Folate status during pregnancy: Relationship with alcohol consumption, other maternal risk factors and pregnancy outcome. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 43(1), 19–27. [DOI] [PubMed] [Google Scholar]
- Leermakers, E. T. , Moreira, E. M. , Kiefte‐de Jong, J. C. , Darweesh, S. K. , Visser, T. , Voortman, T. , … Franco, O. H. (2015). Effects of choline on health across the life course: A systematic review. Nutrition Reviews, 73(8), 500–522. [DOI] [PubMed] [Google Scholar]
- Leroy, C. , Bragulat, V. , Berlin, I. , Gregoire, M. C. , Bottlaender, M. , Roumenov, D. , … Trichard, C. (2009). Cerebral monoamine oxidase A inhibition in tobacco smokers confirmed with PET and [11C]befloxatone. Journal of Clinical Psychopharmacology, 29(1), 86–88. [DOI] [PubMed] [Google Scholar]
- Mannino, D. M. , Mulinare, J. , Ford, E. S. , & Schwartz, J. (2003). Tobacco smoke exposure and decreased serum and red blood cell folate levels: Data from the Third National Health and Nutrition Examination Survey. Nicotine & Tobacco Research, 5(3), 357–362. [DOI] [PubMed] [Google Scholar]
- Matsuzaki, M. , Haruna, M. , Ota, E. , Sasaki, S. , Nagai, Y. , & Murashima, S. (2008). Dietary folate intake, use of folate supplements, lifestyle factors, and serum folate levels among pregnant women in Tokyo, Japan. The Journal of Obstetrics and Gynaecology Research, 34(6), 971–979. 10.1111/j.1447-0756.2008.00821.x [DOI] [PubMed] [Google Scholar]
- McDonald, S. D. , Perkins, S. L. , Jodouin, C. A. , & Walker, M. C. (2002). Folate levels in pregnant women who smoke: An important gene/environment interaction. American Journal of Obstetrics and Gynecology, 187(3), 620–625. [DOI] [PubMed] [Google Scholar]
- Mito, N. , Takimoto, H. , Umegaki, K. , Ishiwaki, A. , Kusama, K. , Fukuoka, H. , … Yoshiike, N. (2007). Folate intakes and folate biomarker profiles of pregnant Japanese women in the first trimester. European Journal of Clinical Nutrition, 61(1), 83–90. 10.1038/sj.ejcn.1602497 [DOI] [PubMed] [Google Scholar]
- National Collaborating Centre for Methods and Tools . (2008, Updated 13 April, 2010). Quality assessment tool for quantitative studies. Retrieved from http://www.nccmt.ca/registry/view/eng/14.html.
- Nelen, W. L. , Blom, H. J. , Steegers, E. A. , den Heijer, M. , & Eskes, T. K. (2000). Hyperhomocysteinemia and recurrent early pregnancy loss: A meta‐analysis. Fertility and Sterility, 74(6), 1196–1199. [DOI] [PubMed] [Google Scholar]
- Nilsen, R. M. , Vollset, S. E. , Monsen, A. L. , Ulvik, A. , Haugen, M. , Meltzer, H. M. , … Ueland, P. M. (2010). Infant birth size is not associated with maternal intake and status of folate during the second trimester in Norwegian pregnant women. The Journal of Nutrition, 140(3), 572–579. [DOI] [PubMed] [Google Scholar]
- Northrop‐Clewes, C. A. , & Thurnham, D. I. (2007). Monitoring micronutrients in cigarette smokers. Clinica Chimica Acta, 377(1–2), 14–38. [DOI] [PubMed] [Google Scholar]
- O'Callaghan, P. , Meleady, R. , Fitzgerald, T. , & Graham, I. (2002). Smoking and plasma homocysteine. European Heart Journal, 23(20), 1580–1586. [DOI] [PubMed] [Google Scholar]
- Ozerol, E. , Ozerol, I. , Gokdeniz, R. , Temel, I. , & Akyol, O. (2004). Effect of smoking on serum concentrations of total homocysteine, folate, vitamin B12, and nitric oxide in pregnancy: A preliminary study. Fetal Diagnosis and Therapy, 19(2), 145–148. [DOI] [PubMed] [Google Scholar]
- Pagan, K. , Hou, J. , Goldenberg, R. L. , Cliver, S. P. , & Tamura, T. (2001). Effect of smoking on serum concentrations of total homocysteine and B vitamins in mid‐pregnancy. Clinica Chimica Acta, 306(1–2), 103–109. [DOI] [PubMed] [Google Scholar]
- Picciano, M. F. (2003). Pregnancy and lactation: physiological adjustments, nutritional requirements and the role of dietary supplements. The Journal of Nutrition, 133(6), 1997S–2002S. [DOI] [PubMed] [Google Scholar]
- Prasodjo, A. , Pfeiffer, C. M. , Fazili, Z. , Xu, Y. , Liddy, S. , Yolton, K. , n Braun, J. M. (2014). Serum cotinine and whole blood folate concentrations in pregnancy. Annals of Epidemiology, 24(7), 498‐503.e491. doi: 10.1016/j.annepidem.2014.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinton, A. E. , Cook, C. M. , & Peek, M. J. (2008). The relationship between cigarette smoking, endothelial function and intrauterine growth restriction in human pregnancy. BJOG, 115(6), 780–784. [DOI] [PubMed] [Google Scholar]
- Ray, J. G. , & Blom, H. J. (2003). Vitamin B12 insufficiency and the risk of fetal neural tube defects. QJM, 96(4), 289–295. [DOI] [PubMed] [Google Scholar]
- Refsum, H. , Ueland, P. M. , Nygard, O. , & Vollset, S. E. (1998). Homocysteine and cardiovascular disease. Annual Review of Medicine, 49, 31–62. [DOI] [PubMed] [Google Scholar]
- Relton, C. L. , Pearce, M. S. , & Parker, L. (2005). The influence of erythrocyte folate and serum vitamin B12 status on birth weight. The British Journal of Nutrition, 93(5), 593–599. [DOI] [PubMed] [Google Scholar]
- Salihu, H. M. , & Wilson, R. E. (2007). Epidemiology of prenatal smoking and perinatal outcomes. Early Human Development, 83(11), 713–720. [DOI] [PubMed] [Google Scholar]
- Scholl, T. O. , & Johnson, W. G. (2000). Folic acid: Influence on the outcome of pregnancy. The American Journal of Clinical Nutrition, 71(5 Suppl), 1295S–1303S. [DOI] [PubMed] [Google Scholar]
- Sram, R. J. , Binkova, B. , Lnenickova, Z. , Solansky, I. , & Dejmek, J. (2005). The impact of plasma folate levels of mothers and newborns on intrauterine growth retardation and birth weight. Mutation Research, 591(1–2), 302–310. 10.1016/j.mrfmmm.2005.04.015 [DOI] [PubMed] [Google Scholar]
- Stark, K. D. , Pawlosky, R. J. , Beblo, S. , Murthy, M. , Flanagan, V. P. , Janisse, J. , … Salem, N. Jr. (2005). Status of plasma folate after folic acid fortification of the food supply in pregnant African American women and the influences of diet, smoking, and alcohol consumption. The American Journal of Clinical Nutrition, 81(3), 669–677. [DOI] [PubMed] [Google Scholar]
- Stark, K. D. , Pawlosky, R. J. , Sokol, R. J. , Hannigan, J. H. , & Salem, N. Jr. (2007). Maternal smoking is associated with decreased 5‐methyltetrahydrofolate in cord plasma. The American Journal of Clinical Nutrition, 85(3), 796–802. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (2014). The health consequences of smoking: 50 years of progress In A report of the surgeon general. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 10.1111/dar.12309 [DOI] [Google Scholar]
- Van Uitert, E. M. , Van Ginkel, S. , Willemsen, S. P. , Lindemans, J. , Koning, A. H. J. , Eilers, P. H. C. , … Steegers‐Theunissen, R. P. M. (2014). An optimal periconception maternal folate status for embryonic size: The Rotterdam Predict study. BJOG, 121(7), 821–829. 10.1111/1471-0528.12592 [DOI] [PubMed] [Google Scholar]
- van Wersch, J. W. , Janssens, Y. , & Zandvoort, J. A. (2002). Folic acid, Vitamin B(12), and homocysteine in smoking and non‐smoking pregnant women. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 103(1), 18–21. [DOI] [PubMed] [Google Scholar]
- Vandevijvere, S. , Amsalkhir, S. , Van Oyen, H. , & Moreno‐Reyes, R. (2012). Determinants of folate status in pregnant women: Results from a national cross‐sectional survey in Belgium. European Journal of Clinical Nutrition, 66(10), 1172–1177. [DOI] [PubMed] [Google Scholar]
- Walker, J. F. , Collins, L. C. , Rowell, P. P. , Goldsmith, L. J. , Moffatt, R. J. , & Stamford, B. A. (1999). The effect of smoking on energy expenditure and plasma catecholamine and nicotine levels during light physical activity. Nicotine & Tobacco Research, 1(4), 365–370. [DOI] [PubMed] [Google Scholar]
- Yamada, T. , Morikawa, M. , Yamada, T. , Kishi, R. , Sengoku, K. , Endo, T. , … Minakami, H. (2013). First‐trimester serum folate levels and subsequent risk of abortion and preterm birth among Japanese women with singleton pregnancies. Archives of Gynecology and Obstetrics, 287(1), 9–14. [DOI] [PubMed] [Google Scholar]
- Yila, T. A. , Araki, A. , Sasaki, S. , Miyashita, C. , Itoh, K. , Ikeno, T. , … Kishi, R. (2016). Predictors of folate status among pregnant Japanese women: The Hokkaido Study on Environment and Children's Health, 2002–2012. The British Journal of Nutrition, 115(12), 2227–2235. 10.1017/s0007114516001628 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Full search strategy
Figure S1. Literature flow diagram
Table S1. Quality score


