Abstract
Pancreatic cancer is a highly lethal malignancy. Developments in recent years have broadened our therapeutic armamentarium. Novel drugs such as nab‐paclitaxel, liposomal irinotecan and chemotherapy regimens such as FOLFIRINOX have been successfully tested in clinical trials. Data on patients outside of clinical trials are scarce but necessary to assess and improve the standard of care. We present data on treatment and survival of 1,174 patients with locally advanced, inoperable, or metastatic pancreatic ductal adenocarcinoma. Between February 2014 and June 2017, patients were recruited by 104 sites at start of first‐line therapy into the ongoing, prospective clinical cohort study TPK (Tumour Registry Pancreatic Cancer). As first‐line therapy, 89% of patients received one of the three treatment regimens: gemcitabine monotherapy (23%), nab‐paclitaxel plus gemcitabine (42%), or FOLFIRINOX (24%). The corresponding subgroups differed: Patients receiving gemcitabine monotherapy were older and more comorbid (median age 78 years, 73% ECOG ≥ 1) than patients receiving nab‐paclitaxel plus gemcitabine (median age 71, 64% ECOG ≥ 1) or patients receiving FOLFIRINOX (median age 60, 52% ECOG ≥ 1). At least 40% of patients died before receiving second‐line treatment. First‐line progression‐free survival was 4.6 months (95% CI: 3.7–5.2) for gemcitabine, 5.6 months (95% CI: 5.0–6.2) for nab‐paclitaxel plus gemcitabine, and 6.3 months (95% CI: 5.5–6.9) for FOLFIRINOX. Our data represent the treatment reality in a German community setting. Although there are no stringent inclusion criteria for our cohort study, overall survival is comparable to that reported by randomised clinical trials.
Keywords: Pancreatic Neoplasms, Cohort Studies, Outpatients, Palliative Care
Short abstract
What's new?
More than four‐fifths of patients with pancreatic cancer present with locally advanced, inoperable (LAPC) or metastatic (MPC) disease at diagnosis. Beyond clinical trials, relatively little data is available on survival outcomes for these patients. Here, real‐world data, derived from an unselected cohort of 1,174 patients enrolled between 2014 and 2017 in a prospective study in Germany, show that the vast majority of first‐line therapies given to LAPC/MPC patients consisted of either gemcitabine monotherapy, nab‐paclitaxel plus gemcitabine, or FOLFIRINOX. About 40 percent of the patients received second‐line therapy. Overall cohort survival was comparable to that reported for randomized clinical trials.
Introduction
Pancreatic cancer is an aggressive malignancy with a very poor prognosis and high mortality. As it triggers no or only unspecific symptoms at an early stage, more than 80% of the tumours are locally advanced, inoperable (LAPC) or metastatic (MPC) at diagnosis. The median overall survival (OS) after diagnosis is less than 1 year, the relative 5‐year survival rate only 8%, both in Germany and the United States.1, 2 In the United States, pancreatic cancer has been projected to become the second leading cause of cancer‐related death by 2030.3 In Germany, incidence and mortality rates are almost equal: approximately 17,100 new cases and 16,600 deaths due to pancreatic cancer were registered in 2013.4
Since 1997, gemcitabine monotherapy has been the standard of care for patients with LAPC/MPC.5 Subsequently, several combination therapies have been studied, but only the combination of gemcitabine with erlotinib resulted in a minimal survival benefit of 2 weeks, yet also more side effects and higher costs.6 In 2011, the combination chemotherapy regimen FOLFIRINOX (leucovorin, fluorouracil, irinotecan, and oxaliplatin) was shown to provide a survival benefit compared to gemcitabine alone with a median OS of 11.1 vs. 6.8 months.7 However, FOLFIRINOX was also associated with increased toxicity, especially neutropenia, sensory neuropathy and diarrhoea, and is thus only suitable for patients with good performance status.7 In January 2014, the albumin‐based formulation of paclitaxel (nab‐paclitaxel) in combination with gemcitabine was approved in Germany for the first‐line treatment of pancreatic cancer and is currently recommended for first‐line and second‐line use (125 mg/m2 nab‐paclitaxel plus 1,000 mg/m2 gemcitabine, d 1,8,158). The combination therapy resulted in a markedly longer median OS compared to gemcitabine monotherapy (8.7 vs. 6.6 months) with increased, but still low rates of peripheral neuropathy and myelosuppression.9, 10 Due to differing toxicity profiles and only modest differences in OS, choosing the ideal regimen for the individual patient remains challenging.11 Moreover, the patients enrolled in clinical trials often differ from the general population in sociodemographic and medical characteristics (being younger and suffering from less comorbidities), hampering a generalisation of results. Data on treatment outside of clinical trials are needed to understand patient characteristics, treatment decision making, and effectiveness of treatments to assess and improve quality of care in daily routine.
In this article, we present data from the prospective clinical cohort study TPK (Tumour Registry Pancreatic Cancer), recruiting patients with LAPC/MPC treated by office‐based medical oncologists and clinics in Germany.
This comprehensive report presents real‐world data on 1,174 patients including type of therapy in first‐line and second‐line, best response, progression‐free survival (PFS), OS and disease‐specific survival (DSS).
Materials and Methods
Data source
The TPK is an ongoing, open, longitudinal, multicentre, observational, prospective cohort study, which started in 2014. Yearly descriptive statistical analyses were predefined regarding data on patient characteristics, choice of treatment, outcome, and course of disease. The TPK was reviewed by the responsible ethics committee and is registered as Tumour Registry Pancreatic Cancer at ClinicalTrials.gov (NCT02089269). The first patient was enrolled in February 2014. Eligible patients are ≥18 years of age with LAPC or MPC at the start of their palliative first‐line treatment. A maximum of 2 weeks’ time difference is allowed between start of first‐line therapy and signed informed consent. At the time of this analysis, 94 outpatient‐centres and 10 clinics for medical oncology located all over Germany were actively participating. Study sites are advised to recruit consecutively to minimise selection bias. At inclusion, patients’ sociodemographics, prognostic factors (stage, grading), ECOG performance status and tumour characteristics at diagnosis are collected. Comorbidities are assessed using the Charlson Comorbidity Index (CCI) according to Quan and colleagues, yet additional concomitant diseases are also documented.12, 13 Data on all previous treatments such as surgery, adjuvant chemotherapy and radiotherapy were collected. Details retrieved on systemic treatments include duration of each treatment, dose‐reductions (yes/no), and number of cycles of all agents applied per line of treatment. Systemic therapies are documented by specifying all agents separately rather than as predefined regimens to allow for documentation of individual combinations. The regimens FOLFOX and OFF differ only in dosage and administration intervals of the three agents leucovorin, fluorouracil, and oxaliplatin, and were often individually adjusted.14, 15 Therefore, all combinations thereof are labelled as FOLFOX/OFF.
Patients are treated according to physicians’ choice and visit their physician on their individual schedule. No specifications are imposed on the physicians’ assessment of treatment at any time. All patients are followed up for 2 years from enrolment (or until death, loss to follow‐up or withdrawal of consent). During the follow‐up period, data on all systemic antineoplastic treatments, outcome and course of the disease are collected. Outcome parameters assessed as per centre standard include absence or presence and location of distant metastasis or local recurrence, date(s) of progression(s) and date of death by any cause. The tumour response was documented as best (clinical) response by the physician and not at previously specified time points according to RECIST criteria. Patients’ data are transferred from medical records to a secure web‐based electronic case report form (eCRF) by designated site staff and are updated after each follow‐up visit, at any change in therapy or at least every 2 months. For quality assurance, data plausibility checks are performed and queries are generated automatically by the eCRF software. Manual checks on data completeness and plausibility are performed regularly to ensure the reliability of the data.
Cohort definition
A total of 1,219 patients had been recruited until data cut on June 30, 2017. Of these, 45 patients were excluded from this interim analysis as no start date of the palliative first‐line therapy had yet been documented, resulting in a cohort of 1,174 patients.
Statistical analysis
Overall survival is defined as the interval between start of first‐line therapy and the date of death from any cause. Patients alive or lost to follow‐up at data cut were censored at last contact according to the Kaplan–Meier method. Progression‐free survival was defined as the interval between start of first‐line therapy and the date of progression or death. Patients without such an event before start of second‐line therapy were censored at either the start of second‐line therapy or at last contact. Disease‐specific survival (DSS) was calculated from start of first‐line therapy until date of death due to pancreatic cancer. Patients alive, lost to follow‐up, or with other causes of death were censored at last contact or at date of death. The median observation time was calculated with a Kaplan–Meier estimate and was defined as time from start of first‐line treatment to death or end of study. Patients without such an event were censored at last contact. Documentation in the registry reflects clinical routine practice, thus missing data are expected. There was no imputation of missing data. The data analysis for this article was generated using SAS software, Version 9.4 of the SAS System for Windows. Copyright 2002–2012 SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA.
Results
Patient, tumour, and treatment characteristics
Table 1 presents demographic data and tumour characteristics of the 1,174 patients included into this analysis. Median age at start of therapy was 70 years; the age differed markedly between the chemotherapy regimens (60 years for FOLFIRINOX, 71 for nab‐paclitaxel plus gemcitabine and 78 years for gemcitabine monotherapy). Patients receiving one of these regimens differed in multiple demographic and tumour characteristics, due to the noninterventional design, and therefore these patient subgroups cannot be compared directly. There were slightly more male patients (54%). Only a third of the patients was in good general condition (ECOG = 0), and 83% of the patients had at least one concomitant disease, with a quarter of the patients having comorbidities considered for the CCI at start of treatment (28% CCI ≥ 1). Most of the patients with CCI ≥ 1 were also assigned a ECOG ≥ 1 (64%). Ninety percent of all patients had metastatic tumours at start of treatment, 54% of the tumours were located in the pancreas head. About 13% of the patients had received prior chemotherapy in curative intention and 20% had undergone surgery.
Table 1.
Patient and tumour characteristics
| Characteristic | Gemcitabine | Nab‐paclitaxel + gemcitabine | FOLFIRINOX | All regimens | ||||
|---|---|---|---|---|---|---|---|---|
| (n = 272) | (n = 489) | (n = 284) | (n = 1,174) | |||||
| Median | Min‐Max | Median | Min‐Max | Median | Min‐Max | Median | Min‐Max | |
| Age at start of therapy, years | 78 | 45–94 | 71 | 40–87 | 60 | 39–79 | 70 | 39–94 |
| n | % | n | % | n | % | n | % | |
| Age ≥ 70 | 230 | 84.6% | 276 | 56.4% | 42 | 14.8% | 616 | 52.5% |
| Age ≥ 75 | 189 | 69.5% | 153 | 31.3% | 12 | 4.2% | 400 | 34.1% |
| Mean | StD | Mean | StD | Mean | StD | Mean | StD | |
| BMI at enrolment, kg/m2 | 24.3 | 4.4 | 24.7 | 4.2 | 24.7 | 4.4 | 24.6 | 4.3 |
| Sex | n | % | n | % | n | % | n | % |
| Female | 151 | 55.5% | 219 | 44.8% | 109 | 38.4% | 535 | 45.6% |
| Male | 121 | 44.5% | 270 | 55.2% | 175 | 61.6% | 639 | 54.4% |
| Patients with comorbidity1 , 2 | ||||||||
| Any comorbidity | 244 | 89.7% | 407 | 83.2% | 208 | 73.2% | 968 | 82.5% |
| CCI = 03 | 170 | 62.5% | 352 | 72.0% | 230 | 81.0% | 843 | 71.8% |
| CCI ≥ 13 | 102 | 37.5% | 137 | 28.0% | 54 | 19.0% | 331 | 28.2% |
| Hypertension | 170 | 62.5% | 266 | 54.4% | 116 | 40.8% | 625 | 53.2% |
| Diabetes mellitus | 98 | 36.0% | 147 | 30.1% | 66 | 23.2% | 358 | 30.5% |
| Coronary heart disease | 30 | 11.0% | 35 | 7.2% | 15 | 5.3% | 97 | 8.3% |
| Thyroid disorders | 21 | 7.7% | 47 | 9.6% | 26 | 9.2% | 103 | 8.8% |
| Chronic GI disorders | 25 | 9.2% | 44 | 9.0% | 22 | 7.7% | 100 | 8.5% |
| Performance status1 | ||||||||
| ECOG = 0 | 74 | 27.2% | 175 | 35.8% | 136 | 47.9% | 425 | 36.2% |
| ECOG = 1 | 145 | 53.3% | 263 | 53.8% | 136 | 47.9% | 623 | 53.1% |
| ECOG = 2 | 53 | 19.5% | 51 | 10.4% | 12 | 4.2% | 126 | 10.7% |
| Pancreatic tumour location | ||||||||
| Head | 159 | 58.5% | 256 | 52.4% | 149 | 52.5% | 635 | 54.1% |
| Body | 59 | 21.7% | 111 | 22.7% | 52 | 18.3% | 246 | 21.0% |
| Tail | 37 | 13.6% | 92 | 18.8% | 66 | 23.2% | 218 | 18.6% |
| Unknown | 18 | 6.6% | 30 | 6.1% | 17 | 6.0% | 75 | 6.4% |
| Metastases at start of therapy 4 | ||||||||
| Yes | 244 | 89.7% | 439 | 89.8% | 256 | 90.1% | 1,057 | 90.0% |
| Bilirubin | ||||||||
| ≤1.5 × ULN | 173 | 63.6% | 341 | 69.7% | 206 | 72.5% | 720 | 68.9% |
| 1.5–3 × ULN | 18 | 6.6% | 32 | 6.5% | 15 | 5.3% | 65 | 6.2% |
| >3 × ULN | 16 | 5.9% | 16 | 3.3% | 6 | 2.1% | 38 | 3.6% |
| Missing | 65 | 23.9% | 100 | 20.4% | 57 | 20.0% | 222 | 21.2% |
| Previous treatment5 | ||||||||
| Any prior treatment | 43 | 15.8% | 106 | 21.7% | 64 | 22.5% | 253 | 21.6% |
| Adjuvant chemotherapy | 7 | 2.6% | 68 | 14.0% | 47 | 16.5% | 154 | 13.2% |
| Radiotherapy | 1 | 0.4% | 8 | 1.6% | 6 | 2.1% | 16 | 1.4% |
| Surgery | 42 | 15.6% | 95 | 19.5% | 57 | 20.1% | 230 | 19.7% |
Abbreviations: BMI, body mass index; GI, gastrointestinal; Max, maximum; Min, minimum; StD, standard deviation; ULN: upper limit of normal.
At enrolment.
Comorbidity according to Charlson or additional concomitant diseases.
All metastases documented in the period of 8 weeks before until 4 weeks after start of first‐line treatment.
Multiple entries possible.
A total of 253 patients had received any prior treatment (adjuvant chemotherapy, surgery, and/or radiotherapy). 154 patients of this subgroup had received adjuvant chemotherapy, 126 of them (82%) gemcitabine monotherapy. In palliative first‐line treatment, 64 of the 253 pretreated patients (25%) received FOLFIRINOX, 106 (42%) received nab‐paclitaxel plus gemcitabine, and 43 (17%) gemcitabine monotherapy. The median time from diagnosis to start of palliative first‐line therapy (disease‐free interval) was 27 days for patients receiving FOLFIRINOX, 29 days for nab‐paclitaxel plus gemcitabine and 35 days for gemcitabine.
20% (236 out of 1,174 patients) of the cohort could be considered as “trial‐ineligible” patients, defined by the presence of at least one of the common exclusion criteria in clinical trials at start of first‐line therapy: ECOG ≥2, renal insufficiency, moderate or severe liver disease, chronic heart failure, or brain metastases. Of these, 28 patients (12%) received FOLFIRINOX in palliative first‐line treatment, 94 (40%) nab‐paclitaxel plus gemcitabine and 89 (38%) gemcitabine monotherapy.
Choice of chemotherapy regimens
From February 2014 until data cut at June 30, 2017, a total of 1,174 palliative first‐line treatments had been documented. The most frequently used first‐line regimens are shown in Figure 1 a. The three regimens gemcitabine alone (23%), nab‐paclitaxel plus gemcitabine (42%) and FOLFIRINOX (24%) accounted for 89% of all first‐line therapies. Patients receiving one of these three regimens differed in age, comorbidity and ECOG performance status. Patients receiving gemcitabine were the oldest, most comorbid and with poorest performance status, while patients receiving FOLFIRINOX were younger, less comorbid, and with better performance status than the patients receiving the combination nab‐paclitaxel plus gemcitabine. This indicates that these factors might affect treatment decision making. Of 391 second‐line treatments documented until data cut, the regimens nab‐paclitaxel plus gemcitabine accounted for 29%, FOLFOX/OFF for 24% and gemcitabine monotherapy for 12% of all second‐line therapies (Fig. 1 b).
Figure 1.
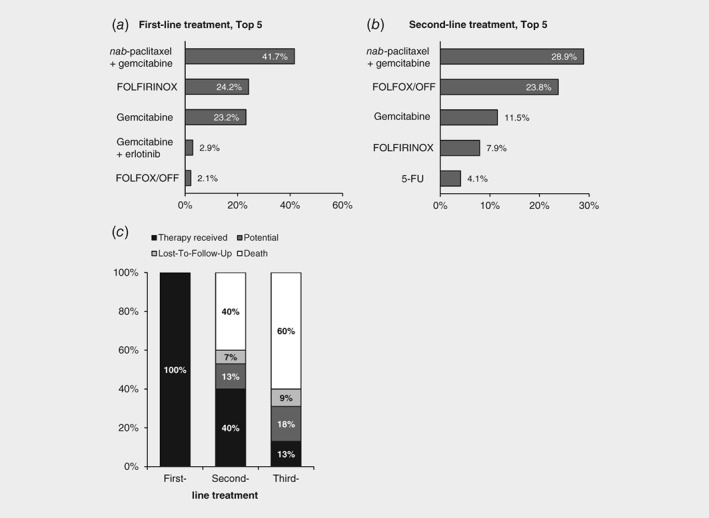
Treatment reality in LAPC/MPC. (a) Top 5 first‐line chemotherapy regimens sorted by frequency (n = 1,174), (b) Top 5 second‐line chemotherapy regimens sorted by frequency (n = 391). (c) All patients starting first‐line therapy until June 30, 2016 (n = 862) were included in this analysis. Shown is the proportion of patients receiving a palliative first‐line, second‐line, and third‐line treatment. Potential: further treatment possible (current line ongoing or therapy paused).
Dose‐reductions at start or during the course of first‐line treatment were documented in 280 patients (24%), corresponding to 34%, 21%, or 20% of the patients receiving FOLFIRINOX, nab‐paclitaxel plus gemcitabine or gemcitabine monotherapy, respectively. Toxicity was documented as reason for the dose‐reduction in 20%, 13%, or 11% of the respective treatment. Discontinuation of first‐line treatment due to toxicity was documented in 196 patients (17%) and occurred in 23%, 16%, or 11% of all patients receiving FOLFIRINOX, nab‐paclitaxel plus gemcitabine or gemcitabine monotherapy, respectively. The overall proportion of patients receiving a second‐line and third‐line therapy was estimated based on those patients recruited at least 1 year before data cut (until June 30, 2016; n = 862). This was done to allow for sufficient follow‐up and avoid underestimation of documented higher lines of treatment. In total, at least 40% (n = 346) of the patients received a second‐line, 13% (n = 111) a third‐line treatment, while 40% (n = 348) of the patients died prior to a second‐line and 60% (n = 514) of the patients prior to a third‐line therapy (Fig. 1 c). Patients marked as “potential” for another line of treatment (13%, n = 108 in second‐line and 18%, n = 158 in third‐line treatment) had either not yet completed the previous line of treatment or had finished the previous line but not yet started a new one. In total, 79 patients (9%) were lost to follow‐up.
Best response, progression‐free survival, overall survival, and disease‐specific survival
The outcome data are shown in Table 2, the survival curves in Figure 2. The disease control rate (CR/PR and SD) was 39% for the entire patient cohort. The median first‐line PFS of the cohort was 5.3 months (95% CI: 5.0–5.7, Fig. 2 a, Table 2) and the median OS was 9.2 months (95% CI: 8.5–10.0, Fig. 2 b, Table 2). The median observation time of all patients was 8.2 months (95% CI: 7.6–9.0).
Table 2.
Best response, PFS, OS, and DSS of the TPK cohort
| Gemcitabine | nab‐paclitaxel + gemcitabine (n = 489) | FOLFIRINOX | All regimens | |||||
|---|---|---|---|---|---|---|---|---|
| (n = 272) | (n = 284) | (n = 1,174) | ||||||
| Best response | n | % | n | % | n | % | n | % |
| CR/PR | 19 | 7.0% | 82 | 16.8% | 59 | 20.8% | 175 | 14.9% |
| SD | 66 | 24.3% | 118 | 24.1% | 67 | 23.6% | 286 | 24.4% |
| PD | 50 | 18.4% | 70 | 14.3% | 54 | 19.0% | 195 | 16.6% |
| Not yet evaluable1 | 137 | 50.4% | 219 | 44.8% | 104 | 36.6% | 518 | 44.1% |
| Number of cycles | n | Mean ± StD | n | Mean ± StD | n | Mean ± StD | n | Mean ± StD |
| 235 | 4.3 ± 3.9 | 415 | 4.3 ± 3.0 | 239 | 7.3 ± 7.3 | 1,002 | 5.0 ± 4.8 | |
| Progression‐free survival | n | % | n | % | n | % | n | % |
| Events | 178 | 65.4% | 317 | 64.8% | 154 | 54.2% | 740 | 63.0% |
| Median PFS | Months | 95% CI | Months | 95% CI | Months | 95% CI | Months | 95% CI |
| 4.6 | 3.7–5.2 | 5.6 | 5.0–6.2 | 6.3 | 5.5–6.9 | 5.3 | 5.0–5.7 | |
| Survival rate | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI |
| 6 months | 33.1% | 26.7–39.6 | 46.6% | 41.5–51.6 | 52.7% | 45.8–59.2 | 43.6% | 40.3–46.8 |
| 12 months | 16.1% | 10.9–22.2 | 17.6% | 13.4–22.2 | 24.3% | 17.6–31.7 | 18.4% | 15.6–21.5 |
| Overall survival | n | % | n | % | n | % | n | % |
| Events | 151 | 55.5% | 296 | 60.5% | 147 | 51.8% | 670 | 57.1% |
| Median OS | Months | 95% CI | Months | 95% CI | Months | 95% CI | Months | 95% CI |
| 6.8 | 6.1–9.0 | 9.1 | 8.2–10.1 | 11.3 | 10.5–12.5 | 9.2 | 8.5–10.0 | |
| Survival rate | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI |
| 6 months | 57.6% | 50.6–64.0 | 65.1% | 60.1–69.6 | 80.0% | 74.2–84.7 | 66.2% | 63.1–69.1 |
| 12 months | 28.4% | 21.6–35.6 | 36.6% | 31.4–41.8 | 43.6% | 36.2–50.8 | 35.8% | 32.4–39.3 |
| Disease‐specific survival | n | % | n | % | n | % | n | % |
| Events | 128 | 47.1% | 231 | 47.2% | 124 | 43.7% | 548 | 46.7% |
| Median DSS | Months | 95% CI | Months | 95% CI | Months | 95% CI | Months | 95% CI |
| 9.0 | 6.6–10.4 | 10.6 | 9.3–11.6 | 11.9 | 11.2–13.4 | 10.7 | 9.8–11.3 | |
| Age at start of therapy | Median | Min‐Max | Median | Min‐Max | Median | Min‐Max | Median | Min‐Max |
| Years | 78 | 45–94 | 71 | 40–87 | 60 | 39–79 | 70 | 39–94 |
| Performance status2 | n | % | n | % | n | % | n | % |
| ECOG ≥ 1 | 198 | 72.8% | 314 | 64.2% | 148 | 52.1% | 749 | 63.8% |
Abbreviations: CR, complete response; DSS, disease‐specific survival; OS, overall survival; PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease; StD, standard deviation.
Due to the high number of ongoing treatments and assessment of response as per local site standard (noninterventional design without independent review), a high percentage of responses is not yet evaluable.
At enrolment.
Figure 2.
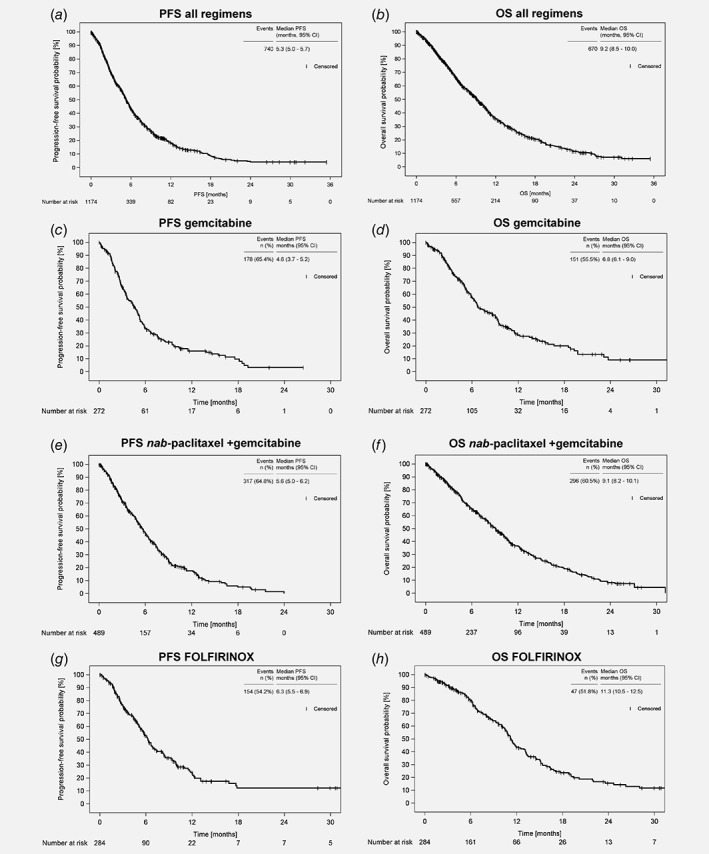
Progression‐free survival (PFS) and overall survival (OS) since start of first‐line therapy of patients with LAPC/MPC. (a) PFS and (b) OS of the whole TPK cohort (n = 1,174), (c) PFS and (d) OS of the patients receiving gemcitabine monotherapy (n = 272), (e) PFS, and (f) OS of the patients receiving nab‐paclitaxel plus gemcitabine (n = 489), (g) PFS and (h) OS of the patients receiving FOLFIRINOX (n = 284). Abbreviations: CI, confidence interval; OS, overall survival; PFS, progression‐free survival.
Outcome data for patients receiving one of the three main treatment regimens are shown in Figure 2 c–g, as well as in Table 2. Of note, the patients receiving these regimens differed considerably in important sociodemographic and medical parameters (see Table 1). The proportion of patients with compromised performance status (ECOG ≥ 1) was 73% for gemcitabine alone, 64% for nab‐paclitaxel plus gemcitabine, and 52% for FOLFIRINOX; furthermore the median age at start of therapy differed markedly, ranging from 78 years (gemcitabine alone), 71 years (nab‐paclitaxel plus gemcitabine) to 60 years (FOLFIRINOX).
The disease control rate was 30% with gemcitabine monotherapy, 41% with nab‐paclitaxel plus gemcitabine, and 44% with FOLFIRINOX. The median PFS ranged from 4.6 months (95% CI: 3.7–5.2) for gemcitabine monotherapy and 5.6 months (95% CI: 5.0–6.2) for nab‐paclitaxel plus gemcitabine to 6.3 months (95% CI: 5.5–6.9) for FOLFIRINOX (Fig. 2 c,e,g). The 6 months PFS rates ranged from 33% (gemcitabine monotherapy) and 47% (nab‐paclitaxel plus gemcitabine) to 53% (FOLFIRINOX). The 6 month OS rates were 58%, 65%, and 80% in the patients receiving gemcitabine alone, nab‐paclitaxel plus gemcitabine or FOLFIRINOX, respectively. The median OS was 6.8 months (95% CI: 6.1–9.0) for the patients treated with gemcitabine alone, 9.1 months (95% CI: 8.2–10.1) for those treated with nab‐paclitaxel plus gemcitabine and 11.3 months (95% CI: 10.5–12.5) for the patients receiving FOLFIRINOX (Fig. 2 d,f,h).
At the time of this analysis, pancreatic cancer was documented as cause of death for 47% of the patients receiving gemcitabine monotherapy or nab‐paclitaxel plus gemcitabine and for 44% of the patients receiving FOLFIRINOX (Table 2). Median DSS was 9.0 months (95% CI: 6.6–10.4) for the patients treated with gemcitabine monotherapy, 10.6 months (95% CI: 9.3–11.6) for the patients treated with nab‐paclitaxel plus gemcitabine and 11.9 months (95% CI: 11.2–13.4) for the patients treated with FOLFIRINOX (Fig. 3 b–d).
Figure 3.
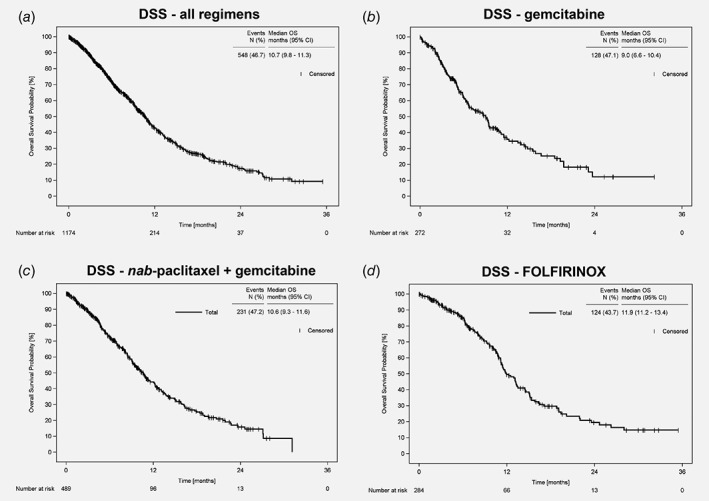
Disease‐specific survival (DSS) since start of first‐line therapy of patients with LAPC/MPC. DSS of (a) the whole TPK cohort (n = 1,174), (b) of the patients receiving gemcitabine monotherapy (n = 272), (c) of the patients receiving nab‐paclitaxel plus gemcitabine (n = 489), and (d) of the patients receiving FOLFIRINOX (n = 284). Abbreviations: CI, confidence interval; DSS, disease‐specific survival.
To assess the robustness of the outcome data, a sensitivity analysis was performed, including all patients starting first‐line therapy until June 30, 2016 (n = 862), allowing at least 1 year follow‐up for each patient. The results indicate that the outcome data can be considered robust: first‐line PFS and OS were almost identical: PFS 5.3 months, 95% CI: 5.0–5.8, 73% events, OS 9.4 months, 95% CI: 8.5–10.1, 69% events. Regarding the three main treatment regimens, PFS and OS were highly similar: gemcitabine monotherapy, PFS 4.7 months (95% CI: 3.6–5.3, 75% events), OS 6.7 months (95% CI: 5.9–9.0, 67% events); nab‐paclitaxel plus gemcitabine, PFS 5.8 months (95% CI: 5.2–6.4, 76% events), OS 9.4 months (95% CI: 8.4–10.4, 73% events); FOLFIRINOX, PFS 6.3 months (95% CI: 5.6–7.5, 62% events) and OS 11.3 months (95% CI: 10.6–13.0, 66% events).
Discussion
Pancreatic cancer with its high mortality rates is a major medical and economic burden. Although there are many studies and pivotal trials aimed at finding new treatment options,5, 7, 9, 16 there are—to our knowledge—no recent real‐world data on the systemic treatment of a population‐based cohort with LAPC/MPC.17 This prospective cohort study evaluated data from 1,174 patients with LAPC/MPC. We show that the three main treatment regimens gemcitabine monotherapy, nab‐paclitaxel plus gemcitabine and FOLFIRINOX account for 89% of all first‐line therapies in German routine practice. As expected, the corresponding patient subgroups differ considerably regarding age, performance status and comorbidities. Although the median age in our population is considerably higher than in the pivotal trials, we could demonstrate that the outcome of the real‐world population is similar to the outcome of patients enrolled in randomised clinical trials with stringent inclusion criteria.
Our study was designed to examine the treatment and outcome of patients receiving systemic therapy, results may not be generalised to the small group of patients not receiving any systemic treatment. The noninterventional design of our study (no randomisation) precludes causal conclusions on differences of effectiveness of the different treatment strategies. In the TPK registry, there are no specifications as to the timing, frequency or criteria of tumour assessment, and thus, PFS data should be considered as the best clinical approximation and might not be identical to the PFS determined in clinical trials. Strengths of this project are the prospective data collection and the participation of oncologists all over Germany recruiting into a large study cohort.
The three main systemic treatment options gemcitabine monotherapy, nab‐paclitaxel plus gemcitabine or FOLFIRINOX are administered to three distinctly different patient subgroups and effectiveness can therefore not be compared directly.11, 18 Interestingly, the outcome of our cohort is comparable to that of the pivotal studies: Conroy and colleagues enrolled fit patients (ECOG score 0 or 1) younger than 76 years and reported a median OS of 11.1 months for FOLFIRINOX (median age 61 years) vs. 6.8 months for gemcitabine (median age 61 years),7 while we observed a median OS of 11.3 months for FOLFIRINOX (median age 60 years) and 6.8 months for gemcitabine (median age 78 years). Of note, it has been discussed that only approximately 10% of the population fulfil the criteria for receiving the FOLFIRINOX regimen,7, 19 a rate which cannot be directly verified in our cohort which excluded patients not being fit enough to receive any systemic treatment. Fitness is an important predictive factor for survival, which could be shown within the MPACT trial: a prespecified subgroup of fit patients (Karnofsky performance status = 100) had a median OS of 12.6 months with nab‐paclitaxel plus gemcitabine vs. 10.9 months with gemcitabine monotherapy.20 In total, the MPACT trial reported a median OS of 6.6 months for gemcitabine (median age 63 years) vs. 8.7 months for nab‐paclitaxel plus gemcitabine (median age 62 years).9, 10 The latter survival time is comparable to the TPK‐cohort, which has a median OS of 9.1 months for nab‐paclitaxel plus gemcitabine. This is even more interesting, as the median age of the patients receiving nab‐paclitaxel plus gemcitabine in our cohort was 71 years compared to 62 years in the MPACT trial.9, 10 A retrospective, population‐based analysis in a US community setting showed a median database persistence (used as proxy for OS) of 8.6 months for nab‐paclitaxel plus gemcitabine and a similar effectiveness as FOLFIRINOX, determined by time to deterioration and database persistence.21
Looking at other cohort studies in Europe, which were mainly performed before the era of nab‐paclitaxel and FOLFIRINOX, the median survival ranged from 2 to 8 months as reviewed by Carrato and colleagues.17 The median OS of the TPK‐cohort was 9.2 months, which certainly reflects the effectiveness of these new treatment options. The subgroup analysis of the Western European cohort of the MPACT trial on 38 patients treated with nab‐paclitaxel plus gemcitabine reported a median PFS of 5.3 months (TPK: 5.6 months) and a median OS of 10.7 months (TPK: 9.1 months).22 The Canadian subgroup analysis of the MPACT trial on 33 patients receiving nab‐paclitaxel plus gemcitabine reported a median OS of 11.9 months.23 In both analyses, the patients were considerably younger than the patients within the TPK with a median age of 60–61 vs. 70 years.22, 23 A retrospective analysis including 41 patients with metastatic pancreatic ductal adenocarcinoma treated with at least 1 cycle of nab‐paclitaxel plus gemcitabine in Italy reported a median PFS of 6.7 months and a median OS of 10 months.24
A single institution retrospective chart review of young and fit patients (n = 50, median age 55, WHO performance status 0/1) with 82% receiving FOLFIRINOX reported a median OS of 14.8 months for patients with locally advanced and 9.0 months for metastatic pancreatic cancer, with dose modifications in 90% of the patients and 52% grade 3–4 toxicity.25
Outcome data from published clinical trials always reflect the unique patient population and methodology of the specific study, but not necessarily differences in the effectiveness of the respective chemotherapy regimens received. In the absence of a randomised clinical trial comparing FOLFIRINOX vs. nab‐paclitaxel plus gemcitabine, it is tempting to speculate what the outcome of both regimens might be in a patient subgroup of similar age, comorbidity, and performance status. An exploratory comparative analysis is planned once sufficient numbers of patients with FOLFIRINOX treatment have been recruited.
In the TPK‐cohort, median DSS was slightly higher than median OS for patients receiving gemcitabine monotherapy or nab‐paclitaxel plus gemcitabine, implicating that not only the pancreatic cancer, but also other factors (e.g. age, comorbidities) influence the survival of these patients. Indeed, 70% of the patients treated with gemcitabine were aged ≥75 years, compared to 31% of the patients receiving nab‐paclitaxel plus gemcitabine and 4% receiving FOLFIRINOX.
The increase in treatment options paved the way for second‐line treatments. We showed that at least 40% of the patients received a second‐line therapy, while a similar percentage died during or after first‐line treatment. This is in range of the published data reporting 38%–50% second‐line treatments.7, 9 Subsequent to nab‐paclitaxel plus gemcitabine, most patients received a regimen containing 5‐fluorouracil, leucovorin, and oxaliplatin with or without irinotecan (FOLFOX, OFF or FOLFIRINOX). After first‐line FOLFIRINOX, the most frequently administered second‐line treatment was nab‐paclitaxel plus gemcitabine. It will be very interesting to follow up on the second‐line treatments as new therapy options, such as nanoliposomal irinotecan, become available.
Conclusion
The real‐world data from the population‐based TPK cohort study demonstrates an overall survival comparable to the data retrieved from pivotal trials, although the median age and comorbidities were higher. The three main treatment regimens gemcitabine monotherapy, nab‐paclitaxel plus gemcitabine or FOLFIRINOX are given to three distinct patient populations. Prognosis for patients with LAPC/MPC remains unfavourable despite improvements in treatment options in recent years. This is particularly obvious for older, frail, and comorbid patients who cannot receive combination therapies. Prospective cohort studies provide an important measure to assess treatment reality, implementation of novel treatments and guidelines, and to further improve quality of care.
Acknowledgments
The authors thank all patients, physicians, and study teams participating in the TPK. We thank Stefanie Kopfmann (iOMEDICO) for support and comments during design and set‐up of the project and Dr. Karin Potthoff (iOMEDICO) for critical comments on the manuscript. The authors thank Dr. Stephanie Dille (iOMEDICO) for preparation of the manuscript. The TPK and its satellite projects are designed, managed, and analysed by iOMEDICO and have received continuous financial support from Baxter Deutschland GmbH and Celgene GmbH. None of the funding companies had any role in study design, data collection and analysis, interpretation of results, or decision to publish.
Conflict of Interest: SHB, AA, TW, BKS, RSS, DH, and MJ declare no conflict of interest concerning the topic of this publication. NM has received honoraria by Celgene for talks and attendance of conferences. HL received sponsoring for attendance of conferences and honoraria for NIS by Celgene.
References
- 1. Robert Koch Institut , ed. Krebs in Deutschland 2011/2012 [Internet]. 10 Berlin: Robert Koch‐Institut, 2015 [cited 2016]. 74‐77. Available from: http://www.gekid.de/Doc/krebs_in_deutschland_2015.pdf [Google Scholar]
- 2. Noone A, Howlader N, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis D, Chen H, Feuer E, et al., eds. SEER Cancer Statistics Review, 1975–2015. Based on November 2017 SEER data submission, posted to the SEER web site, April 2018, https://seer.cancer.gov/csr/1975_2015/. Bethesda, MD: National Cancer Institute, 2017. [Google Scholar]
- 3. Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–21. [DOI] [PubMed] [Google Scholar]
- 4. Robert‐Koch‐Institut Zentrum für Krebsregisterdaten im RK‐I , ed. Bericht zum Krebsgeschehen in Deutschland 2016. Ther Ber: 2016. [Google Scholar]
- 5. Burris HA, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first‐line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol Off J Am Soc Clin Oncol 1997;15:2403–13. [DOI] [PubMed] [Google Scholar]
- 6. Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol Off J Am Soc Clin Oncol 2007;25:1960–6. [DOI] [PubMed] [Google Scholar]
- 7. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–25. [DOI] [PubMed] [Google Scholar]
- 8. Oettle H, Bauernhofer T, Borner M, et al. Onkopedia Leitlinie Pankreaskarzinom ICD‐10 C25, Herausgeber DGHO Deutsche Gesellschaft für Hämatologie und Medizinische Onkologie, Berlin: DGHO, 2018. [Google Scholar]
- 9. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab‐paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goldstein D, El‐Maraghi RH, Hammel P, et al. nab‐Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long‐term survival from a phase III trial. J Natl Cancer Inst 2015;107 pii: dju413. 10.1093/jnci/dju413. Print 2015 Feb. [DOI] [PubMed] [Google Scholar]
- 11. Thota R, Pauff JM, Berlin JD. Treatment of metastatic pancreatic adenocarcinoma: a review. Oncol Williston Park 2014;28:70–4. [PubMed] [Google Scholar]
- 12. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 13. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011;173:676–82. [DOI] [PubMed] [Google Scholar]
- 14. Pelzer U, Kubica K, Stieler J, et al. A randomized trial in patients with gemcitabine refractory pancreatic cancer. Final results of the CONKO 003 study. J Clin Oncol Off J Am Soc Clin Oncol 2008;26:4508. [Google Scholar]
- 15. Zaanan A, Trouilloud I, Markoutsaki T, et al. FOLFOX as second‐line chemotherapy in patients with pretreated metastatic pancreatic cancer from the FIRGEM study. BMC Cancer 2014;14:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spadi R, Brusa F, Ponzetti A, et al. Current therapeutic strategies for advanced pancreatic cancer: a review for clinicians. World J Clin Oncol 2016;7:27–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carrato A, Falcone A, Ducreux M, et al. A systematic review of the burden of pancreatic cancer in Europe: real‐world impact on survival, quality of life and costs. J Gastrointest Cancer 2015;46:201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Al‐Batran S‐E, Geissler M, Seufferlein T, et al. Nab‐paclitaxel for metastatic pancreatic cancer: clinical outcomes and potential mechanisms of action. Oncol Res Treat 2014;37:128–34. [DOI] [PubMed] [Google Scholar]
- 19. Bjerregaard JK, Mortensen MB, Schønnemann KR, et al. Characteristics, therapy and outcome in an unselected and prospectively registered cohort of pancreatic cancer patients. Eur J Cancer Oxf Engl 2013;49:98–105. [DOI] [PubMed] [Google Scholar]
- 20. Tabernero J, Chiorean EG, Infante JR, et al. Prognostic factors of survival in a randomized phase III trial (MPACT) of weekly nab‐Paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Oncologist 2015;20:143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Braiteh F, Patel MB, Parisi M, et al. Comparative effectiveness and resource utilization of nab‐paclitaxel plus gemcitabine vs FOLFIRINOX or gemcitabine for the first‐line treatment of metastatic pancreatic adenocarcinoma in a US community setting. Cancer Manag Res 2017;9:141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tabernero J, Kunzmann V, Scheithauer W, et al. nab‐Paclitaxel plus gemcitabine for metastatic pancreatic cancer: a subgroup analysis of the Western European cohort of the MPACT trial. OncoTargets Ther 2017;10:591–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tehfe M, Dowden S, Kennecke H, et al. nab‐Paclitaxel plus gemcitabine versus gemcitabine in patients with metastatic pancreatic adenocarcinoma: Canadian subgroup analysis of the phase 3 MPACT trial. Adv Ther 2016;33:747–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Vita F, Ventriglia J, Febbraro A, Laterza MM, Fabozzi A, Savastano B, Petrillo A, Diana A, Giordano G, Troiani T, Conzo G, Galizia G, et al. NAB‐paclitaxel and gemcitabine in metastatic pancreatic ductal adenocarcinoma (PDAC): from clinical trials to clinical practice. BMC Cancer [Internet] 2016. [cited 2017 Sep 26];16:709 Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5010686/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rombouts SJ, Mungroop TH, Heilmann MN, et al. FOLFIRINOX in locally advanced and metastatic pancreatic cancer: a single centre cohort study. J Cancer 2016;7:1861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


