Abstract
Lichen planopilaris (LPP) is an inflammatory cicatricial alopecia for which many different therapies are attempted with varying success. The Janus kinase (JAK) inhibitor, tofacitinib, has been shown to be effective in treating the noncicatricial alopecia, alopecia areata. As in alopecia areata, upregulation of interferon and JAK signaling may play a role in LPP. We retrospectively reviewed the cases of 10 patients with recalcitrant LPP who were treated with oral tofacitinib. Patients received oral tofacitinib 5 mg twice or three times daily for 2–19 months as either monotherapy or adjunctive therapy to other ongoing treatments including intralesional triamcinolone, hydroxychloroquine, and tacrolimus ointment. Eight patients had clinical improvement in LPP with tofacitinib as either monotherapy (4/10) or adjunctive therapy (4/10). LPP Activity Index (LPPAI) before and after treatment was measured in seven patients and was significantly different (6.22 before treatment, 3.08 after treatment; p value = .0014). Reduction in LPPAI ranged from 30 to 94%. One patient complained of 10 pound (4.5 kg) weight gain after 12 months on tofacitinib. No other adverse effects were reported. Treatment with oral tofacitinib either as monotherapy or adjunctive therapy can lead to measurable improvement in recalcitrant LPP.
Keywords: alopecia, frontal fibrosing alopecia, JAK inhibitors, lichen planopilaris, scarring alopecia, tofacitinib
1. INTRODUCTION
Lichen planopilaris (LPP) is a primary lymphocytic cicatricial alopecia of unclear etiology. Although several treatments are available, they are often unsuccessful. Treatment with Janus kinase (JAK) inhibitors has been shown to be effective for the noncicatricial inflammatory alopecia, alopecia areata (Kennedy et al., 2016; Liu, Craiglow, Dai, & King, 2017; Mackay‐Wiggan et al., 2016). We report a case series of 10 adult patients with LPP who were treated with the pan‐JAK inhibitor, tofacitinib.
2. METHODS
We reviewed the records of 10 adult patients who were treated with oral tofacitinib for LPP between October 2015 and May 2017. This study was approved by the Institutional Review Board of Columbia University. Clinical and demographic information for each patient was recorded, including age, sex, disease duration, biopsy results, prior treatments, tofacitinib dose, duration of tofacitinib treatment, and LPP Activity Index (LPPAI) (Chiang, Sah, Cho, Ochoa, & Price, 2010) when available. LPPAI is a numerical score that incorporates patient‐reported symptoms including pruritus, pain, and burning, as well as physical exam findings including erythema, perifollicular erythema, perifollicular scale, and evidence of disease activity via hair pull test positivity, and disease spreading (Chiang et al., 2010). Although this is not a validated scoring system, it facilitates statistical comparison. All patients were evaluated for complete blood count (CBC), comprehensive metabolic panel (CMP, including sodium, potassium, carbon dioxide, chloride, blood urea nitrogen, creatinine, glucose, albumin, total protein, and hepatic functional panel), and lipid panel prior to initiating treatment, at 4–8 weeks, and periodically thereafter.
3. RESULTS
Patient characteristics are detailed in Table 1. There were six females and four males and the average age at presentation was 55 years (range 33–68 years). The diagnosis of LPP was biopsy proven in five patients and was a clinical diagnosis in the remaining five patients. Patients 4 and 7 had scalp biopsies that were nondiagnostic, and they were determined clinically to be consistent with LPP. Prior attempted therapies included topical steroids (seven patients), intralesional triamcinolone (five patients), topical minoxidil (two patients), excimer laser (three patients), doxycycline (six patients), hydroxychloroquine (five patients), pioglitazone (four patients), finasteride (five patients), and mycophenolic acid (two patients). In all cases, there was inadequate control of disease; therefore, treatment with tofacitinib was pursued.
Table 1.
Characteristics, treatments, and outcomes of patients with lichen planopilaris treated with oral tofacitinib
| Patient # | Sex | Disease duration (years) | Type/biopsy proven | Prior treatmentsattempted | Daily tofacitinib dose (mg) | Treatment duration (months) | Concurrent therapies |
|---|---|---|---|---|---|---|---|
| 1 | M | 6 | Patchy | Topical steroids, intralesional triamcinolone, minoxidil, excimer laser, hydroxychloroquine, pioglitazone, prednisone | 15 | 10 | Intralesional triamcinolone |
| 2 | M | 15 | Patchy | Pioglitazone, mycophenolic acid | 10 | 17 | |
| 3 | M | 1 | Diffuse/yes | Topical steroids, intralesional triamcinolone, doxycycline, excimer laser, prednisone, hydroxychloroquine, pioglitazone, finasteride | 10 | 3 | |
| 4 | F | 2 | FFA | Topical steroids, intralesional triamcinolone, doxycycline, hydroxychloroquine | 10 | 9 | Intralesional triamcinolone |
| 5 | F | 1 | FFA/yes | Topical steroids, minoxidil, excimer laser, doxycycline, hydroxychloroquine, finasteride | 10 | 6 | Intralesional triamcinolone, hydroxychloroquine |
| 6 | F | 10 | Patchy | Topical steroids, minocycline, finasteride | 10 | 7 | Tacrolimus ointment, intralesional triamcinolone |
| 7 | F | 2 | Patchy | Intralesional triamcinolone | 15 | 19 | |
| 8 | M | 1 | Patchy/yes | Topical steroids, doxycycline, hydroxychloroquine, pioglitazone, finasteride, mycophenolic acid | 10 | 16 | Hydroxychloroquine |
| 9 | F | 1 | Patchy/yes | Intralesional triamcinolone, doxycycline, finasteride | 10 | 10 | |
| 10 | F | 2 | Diffuse/yes | Topical steroids, doxycycline | 10 | 2 |
| Patient # | LPPAI pre‐treatment | LPPAI post‐treatment | % changeLPPAI | Notes |
|---|---|---|---|---|
| 1 | 5.33 | 1.42 | −73 | |
| 2 | 5.58 | 1.75 | −68 | |
| 3 | 7.5 | 7.5 | 0 | No improvement |
| 4 | 6.67 | 4.58 | −32 | |
| 5 | 5.667 | 0.333 | −94 | |
| 6 | Not assessed | – | – | No improvement |
| 7 | Not assessed | – | – | Flared after stoppingtofacitinib |
| 8 | 6.67 | 2.08 | −69 | |
| 9 | 6 | 4.25 | −30 | |
| 10 | 6.33 | 2.75 | −5 |
Abbreviation: LPPAI = lichen planopilaris activity index.
Treatment with tofacitinib ranged from 2 to 19 months and was dosed 5 mg twice a day for eight patients. Patients 1 and 7 continued to have the progression of disease while on tofacitinib 5 mg twice a day for 2 and 4 months, respectively, and their doses were increased to 5 mg three times a day. Tofacitinib was used as monotherapy in five patients. Adjunctive therapies were used in five patients and included intralesional triamcinolone (two patients), hydroxychloroquine (one patient), intralesional triamcinolone and hydroxychloroquine (one patient), and intralesional triamcinolone and tacrolimus ointment (one patient).
In eight patients, disease severity was assessed using the LPPAI, which incorporates objective and subjective markers of disease into a numeric score. The pre‐treatment LPPAI and post‐treatment LPPAI scores were compared using a paired t‐test, which showed a significant improvement in LPPAI score (p value = .0014). LPPAI improvement ranged from 30 to 94%. The change in LPPAI was not significantly different between patients who were on tofacitinib as monotherapy and those who had combination therapy (p = .2). LPPAI was not calculated for Patient 7; however, she was noted to have regrowth after 6 months on tofacitinib 5 mg three times daily. Thus, there was clinical response in 8 of the 10 patients. Improvement in erythema, scaling, and hair density is shown for Patients 4 and 9 in Figure 1. Two patients did not improve on tofacitinib and included Patient 3 who was on 5 mg twice daily for 3 months and stopped due to his concern about a report of bladder cancer associated with tofacitinib and Patient 6 who has been on 5 mg twice daily for 7 months; her dose was unable to be increased due to the lack of insurance coverage. After 12 months on tofacitinib, Patient 7 noted a weight gain of 10 pounds prompting her to discontinue therapy. After discontinuation, she experienced new hair loss and was restarted on tofacitinib 5 mg twice daily with subsequent stabilization of hair loss. There were no significant changes in CBC, CMP, or lipid panel in any patients. No other adverse effects were reported.
Figure 1.
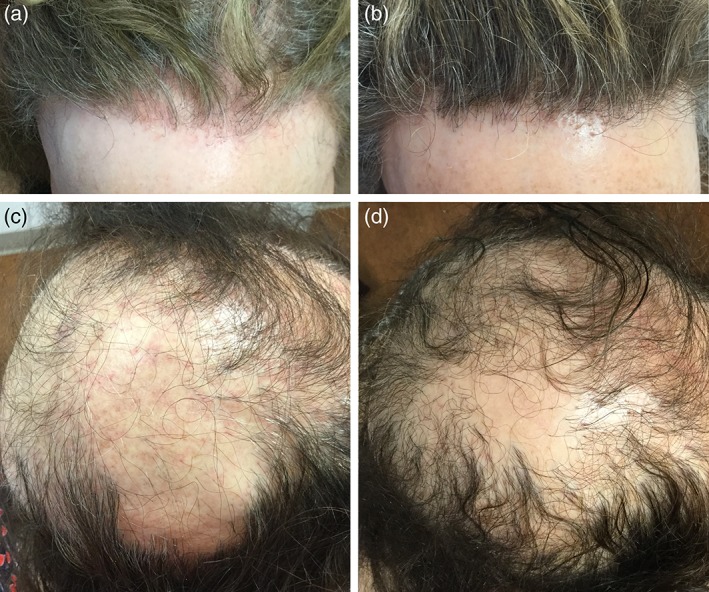
A case of frontal fibrosing alopecia before (a) and after (b) 9 months of tofacitinib 5 mg twice daily also treated with monthly intralesional triamcinolone, after having failed intralesional triamcinolone, topical steroids, doxycycline, and hydroxychloroquine. A case of severe lichen planopilaris before (c) and after (d) 10 months of tofacitinib 5 mg twice daily monotherapy after having failed intralesional triamcinolone, doxycycline, and finasteride
4. DISCUSSION
In this report, we demonstrate that treatment with the pan‐JAK inhibitor, tofacitinib, resulted in clinically a measurable improvement in LPP in 80% of patients, either as monotherapy or as adjunctive therapy. Tofacitinib was well tolerated by all patients. There were two patients who did not improve on the 10 mg daily dose and, then, did respond after the dose was increased to 15 mg; therefore, it is possible that the two patients who did not show any improvement require a higher dose. Although this study is limited by its retrospective nature, small sample size, and heterogeneous patient group, improvements were seen in patients using tofacitinib monotherapy and with other concomitant treatment suggesting that JAK inhibition with tofacitinib may be a promising strategy for treating LPP, which often requires a multipronged approach for therapy.
LPP is considered a follicular variant of lichen planus and presents with alopecic patches, perifollicular erythema, scale, pruritus, burning, or tenderness. The etiology of LPP is unclear, and hypotheses include an autoimmune mechanism, collapse of hair follicle bulge immune privilege and bulge stem cell destruction, decreased peroxisome proliferator‐activated receptor‐ϒ signaling, and others (Rongioletti & Christana, 2012). Numerous therapies have been used including topical, intralesional, and systemic corticosteroids, hydroxychloroquine, immunosuppressants such as cyclosporine and mycophenolate mofetil, doxycycline, excimer laser, minoxidil, griseofulvin, oral retinoids, and thalidomide; response rates vary, and relapse is common (Rácz et al., 2013).
Tofacitinib is a pan‐JAK inhibitor approved by the FDA for the treatment of moderate‐to‐severe rheumatoid arthritis. It has recently been shown in clinical trials to be effective for psoriasis (Mamolo, Harness, Tan, & Menter, 2014; Papp et al., 2012) and vitiligo (Craiglow & King, 2015). Our group and others have established the clinical utility of JAK inhibitors in alopecia areata, a T‐cell mediated autoimmune nonscarring alopecia characterized by increased interferon (IFN)‐γ signaling (Kennedy et al., 2016; Liu et al., 2017; Mackay‐Wiggan et al., 2016). In a mouse model of alopecia areata, JAK inhibition led to the normalization of IFN‐pathway gene expression, decreased T‐cell infiltration, and hair regrowth (Xing et al., 2014).
A recent study has demonstrated a similar key role of IFN signaling in LPP (Harries et al., 2013). Expression of IFN‐inducible chemokines is increased in the hair follicle bulge and may be important in recruiting cytotoxic CD8+ cells. There is also evidence of bulge epithelial cell immune privilege collapse in LPP, as demonstrated by increased expression of major histocompatibility complex (MHC) class I and II and decreased expression of transforming growth factor (TGF) β and cluster of differentiation (CD) 200. In skin organ culture, IFNγ was shown to induce hair follicle immune privilege collapse, raising the possibility of blocking IFN signaling and restoring immune privilege as a therapeutic approach in LPP (Harries et al., 2013). A recent study examining JAK expression in the skin of patients with various inflammatory skin conditions showed that JAK1 and JAK3 are significantly upregulated in dermal inflammatory cells of a subset of patients with lichen planus, including LPP (Medeiros et al., 2016). Therefore, JAK inhibition may reduce IFN‐mediated inflammation associated with LPP and prevent further hair follicle destruction; this mechanism may account for the improvement observed in our patients. The promising findings in our case series invite further clinical investigation of JAK inhibitors in LPP in the setting of randomized, placebo‐controlled clinical trials in the future.
CONFLICTS OF INTEREST
Columbia University has filed patents on Dr. Christiano's research on the use of JAK inhibitors in the treatment of hair loss disorders, which have been licensed to Aclaris Therapeutics, Inc. Dr. Yang, Dr. Sallee, Dr. Bordone, and Ms. Khanna have no conflicts of interest.
Yang CC, Khanna T, Sallee B, Christiano AM, Bordone LA. Tofacitinib for the treatment of lichen planopilaris: A case series. Dermatologic Therapy. 2018;31:e12656. 10.1111/dth.12656
REFERENCES
- Chiang, C. , Sah, D. , Cho, B. K. , Ochoa, B. E. , & Price, V. H. (2010). Hydroxychloroquine and lichen planopilaris: Efficacy and introduction of lichen Planopilaris Activity Index scoring system. Journal of the American Academy of Dermatology, 62(3), 387–392. [DOI] [PubMed] [Google Scholar]
- Craiglow, B. G. , & King, B. A. (2015). Tofacitinib citrate for the treatment of vitiligo. JAMA Dermatology, 151(10), 1110–1112. [DOI] [PubMed] [Google Scholar]
- Kennedy, Crispin, M. K. , Ko, J. M. , Craiglow, B. G. , Li, S. , Shankar, G. , Urban, J. R. , … King, B. A. (2016). Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight, 1(15), e89776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries, M. J. , Meyer, K. , Chaudhry, I. , Kloepper, J. E. , Poblet, E. , Griffiths, C. E. , & Paus, R. (2013). Lichen planopilaris is characterized by immune privilege collapse of the hair follicles epithelial stem cell niche. The Journal of Pathology, 231(2), 236–247. [DOI] [PubMed] [Google Scholar]
- Liu, L. Y. , Craiglow, B. G. , Dai, F. , & King, B. A. (2017). Tofacitinib for the treatment of severe alopecia areata and variants: A retrospective cohort study of 90 patients. Journal of the American Academy of Dermatology, 76(1), 22–28. [DOI] [PubMed] [Google Scholar]
- Mackay‐Wiggan, J. , Jabbari, A. , Nguyen, N. , Cerise, J. E. , Clark, C. , Ulerio, G. , … Clynes, R. (2016). Oral ruxolitinib induces hair regrowth in patients with moderate‐to‐severe alopecia areata. JCI Insight, 1(15), e89790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamolo, C. , Harness, J. , Tan, H. , & Menter, A. (2015). Tofacitinib (CP‐690,550), an oral Janus kinase inhibitor, improves patient‐reported outcomes in a phase 2b, randomized, double‐blind, placebo‐controlled study in patients with moderate‐to‐severe psoriasis. Journal of the European Academy of Dermatology and Venereology, 28(2), 192–203. [DOI] [PubMed] [Google Scholar]
- Medeiros, A. K. , Speeckaert, R. , Desmet, E. , Gele, M. V. , Schepper, S. D. , & Lambert, J. (2016). JAK3 as an emerging target for topical treatment of inflammatory skin diseases. PLoS One, 11(10), e0164080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp, K. , Menter, A. , Strober, B. , Langley, R. , Buonanno, M. , Wolk, R. , … Harness, J. (2012). Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: A phase 2b randomized placebo‐controlled dose‐ranging study. British Journal of Dermatology, 167(3), 668–677. [DOI] [PubMed] [Google Scholar]
- Rácz, E. , Gho, C. , Moorman, P. , Hegt, V. N. , & Neumann, H. (2013). Treatment of frontal fibrosing alopecia and lichen planopilaris: A systematic review. Journal of the European Academy of Dermatology and Venereology, 27(12), 1461–1470. [DOI] [PubMed] [Google Scholar]
- Rongioletti, F. , & Christana, K. (2012). Cicatricial (scarring) alopecias. American Journal of Clinical Dermatology, 13(4), 247–260. [DOI] [PubMed] [Google Scholar]
- Xing, L. , Dai, Z. , Jabbari, A. , Cerise, J. E. , Higgins, C. A. , Gong, W. , … Clynes, R. (2014). Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nature Medicine, 20(9), 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]


