Abstract
Objective
To examine 6‐month outcomes for treatment of lateral nasal wall insufficiency with a bioabsorbable implant.
Study Design
Prospective, multicenter, nonrandomized, single‐blinded study.
Methods
One hundred and one patients with severe‐to‐extreme class of Nasal Obstruction Symptom Evaluation (NOSE) scores were enrolled at 14 U.S. clinics (September 2016–March 2017). Patients were treated with a bioabsorbable implant designed to support lateral wall, with or without concurrent septoplasty and/or turbinate reduction procedure(s). NOSE scores and visual analog scale (VAS) were measured at baseline and month 1, 3, and 6 postoperatively. The Lateral Wall Insufficiency (LWI) score was determined by independent physicians observing the lateral wall motion video.
Results
Forty‐three patients were treated with implant alone, whereas 58 had adjunctive procedures. Seventeen patients reported 19 adverse events, all of which resolved with no clinical sequelae. Patients showed significant reduction in NOSE scores at 1, 3, and 6 months postoperatively (79.5 ± 13.5 preoperatively, 34.6 ± 25.0 at 1 month, 32.0 ± 28.4 at 3 months, and 30.6 ± 25.8 at 6 months postoperatively; P < 0.01 for all). They also showed significant reduction in VAS scores postoperatively (71.9 ± 18.8 preoperatively, 32.7 ± 27.1 at 1 month, 30.1 ± 28.3 at 3 months, and 30.7 ± 29.6 at 6 months postoperatively; P < 0.01 for all). These results were similar in patients treated with the implant alone compared to those treated with the implant and adjunctive procedures. Consistent with patient‐reported outcomes, postoperative LWI scores were demonstrably lower (1.83 ± 0.10 and 1.30 ± 0.11 pre‐ and postoperatively; P < 0.01).
Conclusion
Stabilization of the lateral nasal wall with a bioabsorbable implant improves patients' nasal obstructive symptoms over 6 months.
Level of Evidence
2b. Laryngoscope, 2483–2489, 2018
Keywords: Nasal valve, nasal implant, lateral wall insufficiency, valve repair, nasal obstruction
INTRODUCTION
Nasal obstruction is one of the most often encountered clinical problems treated by otolaryngologists and facial plastic surgeons. Although the most common procedures used to address anatomic obstruction are septoplasty and turbinate reduction, reconstruction of the bony–cartilaginous vault with functional rhinoplasty has come to the fore as our understanding of the internal and external valves has evolved. The term functional rhinoplasty is used to describe an array of procedures performed to correct nasal obstruction due to variations of the bony–cartilaginous vault. The internal and external nasal valves were described in the clinical consensus statement from the American Academy of Otolaryngology–Head and Neck Surgery as the cross‐sectional area of the nasal cavity with the greatest overall resistance to airflow.1
Instability of the lateral nasal wall (i.e., lateral wall insufficiency [LWI]) is one of the anatomic contributors to nasal obstruction.2 The 2010 American Academy of Otolaryngology–Head and Neck Surgery clinical consensus statement also identifies multiple anatomical sources for nasal valve collapse (NVC), including those associated with LWI and the importance of surgical correction for its treatment.1 The efficacy of lateral various types of nasal wall repair in reducing symptoms of obstruction has been supported by a recent systematic review.3
Patient‐reported outcomes are an important component of measuring nasal obstruction and perhaps the most valid.4 The most studied and validated patient‐reported outcome measure for nasal obstruction is the Nasal Obstruction Symptom Evaluation (NOSE) score.5, 6 The NOSE score has also been used to develop a disease severity scale.7 A validated physician‐derived grading system has been developed for zone 1 LWI.8 Figure 1 illustrates the location of zone 1 and zone 2. With this system, using direct visualization of the lateral wall, the observer determines the amount of medialization at the level of the caudal margin of the lower lateral cartilage with normal (not forced) nasal inspiration. Grades 0 to 3 are assigned for 0, 1% to 33%, 34% to 66%, and 67% to 100% movement toward the nasal septum. This scale provides us with a valuable observer‐based objective assessment of LWI. Together, these physician‐ and patient‐derived measures of LWI and nasal obstruction, respectively, provide us with tools to measure the efficacy of various treatments.
Figure 1.
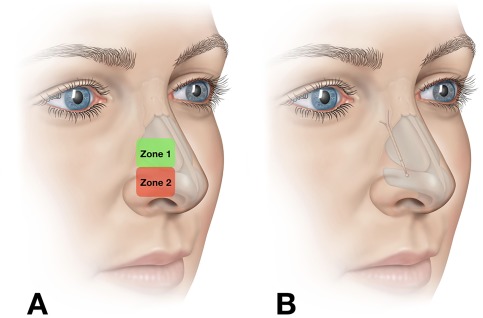
Zones of lateral wall insufficiency and implant placement. (A) Nasal valve collapse due to dynamic inward collapse of the nasal wall occurs in distinct zones.2 Zone 1 is more superior and roughly correlates to inward collapse at the level of the internal nasal valve. Zone 2 is more caudad and roughly corresponds to classically described external valve collapse. (B) Implant position in the nose.
In general, functional rhinoplasty procedures maybe divided along the two major types of anatomic obstruction treated: static and dynamic. In the case of the former, spreader grafts and extracorporeal septal reconstruction are two of the more common techniques used.9, 10, 11, 12 Dynamic collapse or LWI is postulated to occur as negative inspiratory forces overcome the strength of the lateral nasal wall, causing inward movement.13, 14 Thus, strategies for reduction in LWI can include optimizing the diameter of the nasal airway to reduce negative pressure generated and/or strengthening the lateral nasal wall. The most common methods for strengthening the lateral nasal wall include batten grafts (external or endonasal approach), bone‐anchored sutures, and lateral crural strut grafts (LCSG).1, 9, 15, 16
Recently, a first‐in‐human study has demonstrated the potential for a novel bioabsorbable implant to improve the nasal airway for 12 months.17 Ongoing follow‐up of the same cohort has demonstrated similar efficacy at 24 months postoperatively.18 The implant is placed via an endonasal approach into the nasal sidewall, parallel to the dorsum. The implant sits over the nasal bone and extends caudally to support the soft tissues of the area corresponding to upper and lower lateral cartilages. The implant may be placed either in the operating room in conjunction with septoplasty and/or turbinate reduction, or in the office under local anesthesia (typically as a stand‐alone procedure or in conjunction with turbinate reduction). Herein we examine the results of a study of patients undergoing the procedure either alone or in conjunction with septoplasty and/or turbinate reduction.
MATERIALS AND METHODS
Study Design
The data was collected from the first 101 consecutive patients enrolled in two prospective, multicenter, nonrandomized studies designed to obtain outcomes for an absorbable nasal implant comprised of a 70:30 blend of poly (L‐lactide) and poly (D‐lactide) (Spirox Inc., Redwood City, CA) in treating patients with severe‐to‐extreme severity class NOSE scores, with or without concurrent septoplasty and/or turbinate reduction procedure(s). All patients provided written informed consent, and approval was obtained from the institutional review board of each center. The studies are registered at clinicaltrials.gov (NCT02952313 and NCT02964312).
The baseline visit included a medical history review, evaluation of symptoms, modified Cottle maneuver, patient‐derived assessments, and endoscopic lateral wall motion video. The modified Cottle maneuver (intranasal stabilization of the lateral nasal wall) 1, 19 was performed by gently supporting the lateral wall cartilage on each side of the nose while the patient was asked to inspire in a normal fashion (Fig. 2). An LWI score8 was determined utilizing the lateral wall motion video. The endoscopic lateral wall motion videos were transferred to an independent reviewer to determine the LWI grade utilizing a standardized protocol in blinded fashion. The LWI grade was assigned without knowledge of the patient or the timepoint (baseline or 6 month) during video review.
Figure 2.
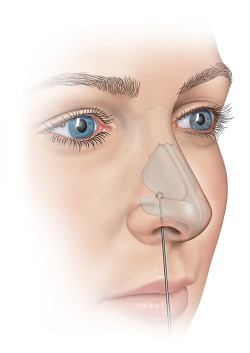
Modified Cottle manuever. This is performed by performed by gently supporting the lateral wall cartilage on each side of the nose while the patient is asked to inspire in a normal fashion.
During treatment, absorbable nasal implant(s) were delivered to patients under general or local anesthesia, with or without concurrent septoplasty and/or turbinate reduction procedure(s). The implant and delivery procedure have been previously described.17 Septoplasty and turbinate reduction were performed according to the physician's standard procedure. Follow‐up visits took place at months 1, 3, and 6 postprocedure for this report. Internal and external nasal exams as well as NOSE and visual analog scale (VAS) scores for nasal airway obstruction breathing assessment were performed at each visit, and lateral wall motion videos were obtained. Additional endpoints such as cosmetic analysis and patient tolerability were also collected and will be reported with the full data set. Physical examinations included an evaluation of nasal skin and nasal mucosa appearance and the presence of any implant extrusions, fractures, or migration. Primary efficacy endpoint evaluation included proportion of responders at 6 months postprocedure.
Enrollment
Enrollment occurred between September 2016 to March 2017 at 14 institutions across the United States. Eligible patients were adults seeking treatment for nasal airway obstruction due to nasal valve collapse (confirmed by positive modified Cottle maneuver). In addition, patients had NOSE scores ≥ 55 (severe, extreme) and had failed to benefit from appropriate maximal medical management (e.g., nasal steroid for at least 4 weeks; antihistamines; oral decongestants; nasal strips, stents, or cones). Patients were ineligible if they: 1) had septoplasty or turbinate reduction procedures within 6 months or rhinoplasty procedures within 12 months prior to the planned index procedure; or 2) were having a concurrent functional endoscopic sinus surgery; or 3) were planning to have other concurrent rhinoplasty procedure; or 4) were planning to have other rhinoplasty procedures or use external dilators within 12 months after the index procedure; or 5) were planning to have any surgical or nonsurgical treatment of their nasal valve other than the index procedure within 12 months of the study; or 6) had polyps or pathology other than septal deviation and/or turbinate hypertrophy and/or lateral wall insufficiency that would contribute to airway obstruction; or 7) had known or suspected allergy to polylactic acid or other absorbable materials.
Statistical Analysis
For purposes of analysis, we examined patients overall, implant alone (without adjunctive procedures), or implant + adjunctive procedures. Baseline characteristics were compared across subgroups using the t test for continuous variables and the chi‐square test or Fisher exact test for categorical variables. NOSE score results were converted to a 100‐point scale by multiplying the total score by 5. VAS scores were converted to a scale of 0 to 100. The analysis included the change in NOSE and VAS scores from baseline (preoperative) to 1, 3, and 6 months. Paired t tests were used to compare the mean baseline value to each of the follow‐up time points to determine whether there was evidence of significant reductions in NOSE scores.
A NOSE score severity classification system was developed by Lipan and Most.7 The analysis utilized this classification system to define a responder. Responders were defined as patients who have at least one NOSE class improvement or a NOSE score reduction of at least 20% from baseline. The response rate was calculated for 1, 3, and 6 months postprocedure.
An average LWI score was calculated at baseline and 6 months postprocedure. A mixed model for repeated measures with unstructured covariance matrices was used to account for repeated measures (nares) within patients. The least square means, standard errors, and P values were derived from these mixed models.
Statistical analyses were performed by an independent statistician using SAS version 9.4 (SAS Institute Inc., Cary, NC) and R version 3.2.3.
RESULTS
One hundred and one patients were examined for the analysis in this report. Of those, 43 patients were treated with implant alone (i.e., no adjunctive procedures), whereas 58 were concomitantly treated with septoplasty, turbinate reduction, or both. There were a total of 19 procedure‐ or implant‐related adverse events reported in 17 patients. These events included inflammation, foreign body sensation, skin irritation, hematoma, infection, and implant retrievals. The investigators confirmed the implant retrievals were not due to adverse physiologic tissue rejection. All events resolved with no clinical sequelae.
Demographic and relevant clinical history for the overall population, implant alone, and implant + adjunctive procedures are detailed in Table 1. Our analysis shows that subgroups based on concomitant procedures were generally similar. However, implant alone patients were more likely to have had prior septoplasty (46.5% vs. 12.1%, P < 0.01, for implant alone vs. implant + adjunctive procedures), turbinate reduction (51.2% vs. 13.8%, P < 0.01, for implant alone vs. implant + adjunctive) or prior ESS (32.6% vs. 15.5%, P = 0.04, for implant alone versus implant + adjunctive).
Table 1.
Demographics and Key Characteristics for Each Group. Comparisons Between the Implant Alone and Implant + Adjunctive Procedures Group
| ALL | LATERA ALONE | IMPLANT + ADJUNCTIVE | ||
|---|---|---|---|---|
| (N = 101) | (N = 43) | (N = 58) | ||
| AGEa | N | 101 | 43 | 58 |
| Mean ± SD | 48.94 ± 13.79 | 53.42 ± 14.08 | 45.62 ± 12.70 | |
| BMI | N | 98 | 42 | 56 |
| Mean ± SD | 26.81 ± 4.36 | 26.12 ± 4.59 | 27.32 ± 4.15 | |
| GENDER | Female | 45 (44.6%) | 22 (51.2%) | 23 (39.7%) |
| Male | 56 (55.4%) | 21 (48.8%) | 35 (60.3%) | |
| ETHNICITY | Not Hispanic or Latino | 91 (91.9%) | 40 (95.2%) | 51 (89.5%) |
| Hispanic or Latino | 8 (8.1%) | 2 (4.8%) | 6 (10.5%) | |
| ALLERGIC RHINITIS | No | 60 (59.4%) | 28 (65.1%) | 32 (55.2%) |
| Yes | 41 (40.6%) | 15 (34.9%) | 26 (44.8%) | |
| SINUS DISEASE | No | 68 (67.3%) | 25 (58.1%) | 43 (74.1%) |
| Yes | 33 (32.7%) | 18 (41.9%) | 15 (25.9%) | |
| PRIOR SEPTOPLASTYa | No | 74 (73.3%) | 23 (53.5%) | 51 (87.9%) |
| Yes | 27 (26.7%) | 20 (46.5%) | 7 (12.1%) | |
| PRIOR TURBINATE REDUCTIONa | No | 71 (70.3%) | 21 (48.8%) | 50 (86.2%) |
| Yes | 30 (29.7%) | 22 (51.2%) | 8 (13.8%) | |
| PRIOR ESSb | No | 78 (77.2%) | 29 (67.4%) | 49 (84.5%) |
| Yes | 23 (22.8%) | 14 (32.6%) | 9 (15.5%) | |
| PRIOR RHINOPLASTY | No | 90 (89.1%) | 40 (93.0%) | 50 (86.2%) |
| Yes | 11 (10.9%) | 3 (7.0%) | 8 (13.8%) | |
P < 0.05.
P < 0.01.
SD = standard deviation.
Patients in all three groups showed short‐ and longer‐term improvement in nasal obstruction symptoms as measured by NOSE scores (NOSE) (Table 2). The baseline preoperative NOSE scores were similar overall and across subgroups. Overall, patients showed significant reduction in NOSE scores at 1, 3, and 6 months postoperatively (79.5 ± 13.5 preoperatively, 34.6 ± 25.0 at 1 month [P < 0.01], 32.0 ± 28.4 at 3 months [P < 0.01] and 30.6 ± 25.8 at 6 months postoperatively [P < 0.01]). The implant + adjunctive procedures group showed significant reduction in NOSE scores at 1, 3, and 6 months postoperatively (79.1 ± 13.6 preoperatively, 34.6 ± 26.0 at 1 month [P < 0.01], 27.9 ± 28.5 at 3 months [P < 0.01] and 24.0 ± 26.0 at 6 months postoperatively [P < 0.01]). The implant alone group showed significant reduction in NOSE scores at 1, 3, and 6 months postoperatively (80.0 ± 13.6 preoperatively, 34.6 ± 24.0 at 1 month [P < 0.01], 37.6 ± 27.7 at 3 months [P < 0.01), and 39.6 ± 23.0 at 6 months postoperatively [P < 0.01]).
Table 2.
Pre‐ and Postoperative NOSE scores for All Patients, Implant Alone, and Implant + Adjunctive
| N | NOSE Score (Mean ± SD) | |
|---|---|---|
| Implant Alone | ||
| Baseline | 43 | 80.0 ± 13.6 |
| 1 month | 42 | 34.6 ± 24.0a |
| 3 months | 41 | 37.6 ± 27.7a |
| 6 months | 37 | 39.6 ± 23.0a |
| Implant + Adjunctive | ||
| Baseline | 58 | 79.1 ± 13.6 |
| 1 month | 57 | 34.6 ± 26.0a |
| 3 months | 56 | 27.9 ± 28.5a |
| 6 months | 50 | 24.0 ± 26.0a |
| All Patients | ||
| Baseline | 101 | 79.5 ± 13.5 |
| 1 month | 99 | 34.6 ± 25.0a |
| 3 months | 97 | 32.0 ± 28.4a |
| 6 months | 87 | 30.6 ± 25.8a |
For details of each group composition, see Methods.
P < 0.05
P < 0.01
NOSE = Nasal Obstruction Symptom Evaluation; SD = standard deviation.
Similarly, patients in all three groups showed short‐ and longer‐term improvement in nasal obstruction symptoms as measured by a VAS (Table 3). Overall, patients showed significant reduction in VAS scores at 1, 3, and 6 months postoperatively (71.9 ± 18.8 preoperatively, 32.7 ± 27.1 at 1 month [P < 0.01], 30.1 ± 28.3 at 3 months [P < 0.01], and 30.7 ± 29.6 at 6 months postoperatively [P < 0.01]). The implant + adjunctive procedures group showed significant reduction in VAS scores at 1, 3, and 6 months postoperatively (69.4 ± 19.5 preoperatively, 29.1 ± 24.8 at 1 month [P < 0.01], 26.2 ± 29.1 at 3 months [P < 0.01], and 24.5 ± 27.4 at 6 months postoperatively [P < 0.01]). The implant alone group showed significant reduction in VAS scores at 1, 3, and 6 months postoperatively (75.2 ± 17.6 preoperatively, 37.6 ± 29.5 at 1 month [P < 0.01], 35.5 ± 26.7 at 3 months [P < 0.01), and 39.0 ± 30.7 at 6 months postoperatively [P < 0.01]). The baseline preoperative VAS scores were similar overall and within subgroups.
Table 3.
Pre‐ and Postoperative VAS Scores for All Patients, Latera Alone, and Implant + Adjunctive Procedures
| VAS Score (Mean ± SD) | ||
|---|---|---|
| Implant Alone | ||
| Baseline | 43 | 75.2 ± 17.6 |
| 1 month | 42 | 37.6 ± 29.5a |
| 3 months | 41 | 35.5 ± 26.7a |
| 6 months | 36 | 39.0 ± 30.7a |
| Implant + Adjunctive Procedures | ||
| Baseline | 58 | 69.4 ± 19.5 |
| 1 month | 57 | 29.1 ± 24.8a |
| 3 months | 56 | 26.2 ± 29.1a |
| 6 months | 49 | 24.5 ± 27.4a |
| All Patients | ||
| Baseline | 101 | 71.9 ± 18.8 |
| 1 month | 99 | 32.7 ± 27.1a |
| 3 months | 97 | 30.1 ± 28.3a |
| 6 months | 85 | 30.7 ± 29.6a |
For details of each group composition, see Methods.
P < 0.05
P < 0.01.
SD = standard deviation; VAS = visual analog score.
Table 4.
Pre‐ and Postoperative LWI scores for All Patients, Implant Alone, and Implant + Adjunctive Procedures
| n | n | Baseline | 6 Month FU | Change | P | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pts. | Nares | LS Mean | SE | LS Mean | SE | LS Mean | SE | Value | |
| Implant alone | 33 | 61 | 2.03 | 0.16 | 1.51 | 0.17 | −0.53 | 0.17 | < 0.01 |
| Implant + adjunctive | 45 | 88 | 1.68 | 0.13 | 1.15 | 0.14 | −0.52 | 0.14 | < 0.01 |
| All patients | 78 | 149 | 1.83 | 0.10 | 1.30 | 0.11 | −0.53 | 0.11 | < 0.01 |
For details of each group composition, see Methods.
FU = follow‐up; LS = least square; LWI = lateral wall insufficiency; Pts. = patients; SE = standard error.
We sought to determine the percentage of patients who were considered responders to the treatment. A responder was determined as a patient with reduction in clinical severity by at least one category or a 20% reduction in NOSE score. As shown in Figure 3, when examining the entire group treated with implant, 86.9%, 87.6%, and 89.7% of patients were determined as responders at 1, 3, and 6 months postoperatively. A majority of patients treated with implant alone experienced a similar responder rate based on the NOSE score (90.5%, 87.8%, 89.2% at 1, 3, and 6 months, respectively). Patients treated with a combination of implant and adjunctive procedures also showed a similar pattern (84.2, 87.5, 90.0% at 1, 3, and 6 months, respectively).
Figure 3.
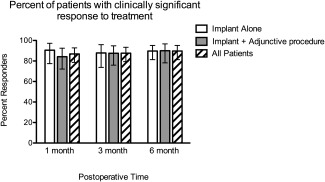
Percent of patients with clinically significant response to treatment. Patients were grouped as responders as noted in Methods. Shown are percent of patients who met criteria for clinical response at 1, 3, and 6 months posttreatment. Error bars indicate 95th percentile confidence intervals.
Disease severity category shifts show a decreasing in severity class from baseline to 6 months postoperatively for all patients and the two subgroups (Fig. 4). Based on the inclusion criteria, all study patients were categorized as severe or extreme with regard to nasal obstructive symptoms, preoperatively. Six months postoperatively, 22 patients improved their clinical symptoms by three categories, 35 patients improved by two categories, and 20 patients improved by one category. Resulting in a total of 77 patients (85.5%) improved by at least one clinical category. Similar trends were observed in the subgroup analysis, in which the implant alone group showed four patients improved by three categories, 15 patients improved by two categories, and 13 patients improved by one category. In the implant + adjunctive subgroup, there were a total of 18 patients who improved by three categories, 20 patients who improved by two categories, and seven patients who improved by one category.
Figure 4.
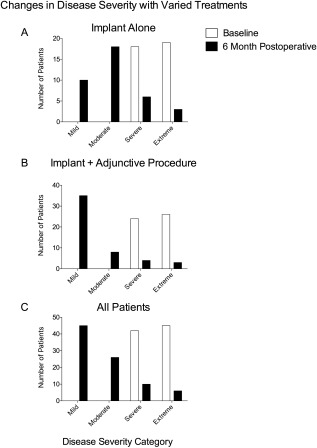
Changes in disease severity with varied treatments. Patients were grouped into disease severity classes at baseline and 6 months postoperatively, as noted in Methods. Shown severity classes pre‐ and postoperatively for (A) patients treated with implant alone, (B) patients treated with the implant and an adjunctive procedure (as described in Methods), or (C) all patients taken together.
In order to objectively quantify degree of lateral wall motion changes after treatment, LWI scores were generated by a blinded observer for each nares separately, as described in Methods. When examined as a group, postoperative LWI scores were demonstrably lower after lateral wall stabilization with the implant (1.83 ± 0.10 and 1.30 ± 0.11 pre‐ and postoperatively, respectively; P < 0.01). Patients who underwent both implant and intranasal adjunctive procedures demonstrated lower LWI scores (1.68 ± 0.13 and 1.15 ± 0.14 pre‐ and postoperatively, respectively; P < 0.01), as did patients who underwent lateral wall stabilization alone (2.03 ± 0.16 and 1.51 ± 0.17 pre‐ and postoperatively, respectively; P < 0.01).
DISCUSSION
Nasal valve collapse has many anatomical contributors, one of which is due to LWI. Strategies for addressing LWI include improving the strength of the lateral wall by focusing on either improving the cross‐sectional area of the nasal airway to reduce negative inspiratory forces or strengthening the lateral nasal wall to resist such forces, or both. One of the most common combinations of treatments in this regard is a septoplasty, turbinate reduction, and repair of nasal stenosis using some form of treatment for the lateral nasal wall, such as batten grafts, LCSG, or bone‐anchored sutures.1, 9, 16, 20, 21 In a recent case control study by the senior author, the most common strategies to treat zone 1 and zone 2 LWI were LCSG and rim grafts, respectively.15 Techniques currently in use are done via an external rhinoplasty approach or through an intranasal incision (in the case of batten grafts). Recently, a minimally invasive technique for stabilization of the lateral nasal wall was introduced utilizing an implant with an absorption profile of 18 months. The study showed benefit of the implant through 24 months postoperatively.17, 18 We sought to examine the effectiveness and safety of this new LWI repair either alone or in combination with adjunctive procedures conventionally used to widen the nasal airway (septoplasty and/or turbinate reduction).
In the current study, patients undergoing stand‐alone implant placement were more likely to have had prior septoplasty, turbinate reduction, or ESS. This may have been due to missed diagnosis of LWI or consequent development of LWI. The authors favor the former explanation. The group who underwent treatment with implant alone demonstrated significant reduction in nasal obstruction as measured by the NOSE scores. Interestingly, this is in congruence with a prior study that demonstrated the importance of initial diagnosis and treatment of NVC.22 Taken together, this would suggest patients are best served by recognition of NVC due to LWI and determination of its role in causing nasal obstruction. If it is determined that LWI significantly contributes to nasal obstructive symptoms, these patients should be considered for LWI treatment at the time of septoplasty and turbinate reduction.
Furthermore, the current study demonstrates the 6‐month efficacy of an implant for lateral wall stabilization for symptomatic LWI. This is demonstrated in two ways: First, there was a significant reduction in NOSE scores in patients who underwent the procedure alone or in combination with improvement of the intranasal airway. Secondly, a blinded physician‐derived score demonstrated the physical effect of the implant. A significant reduction in lateral wall medialization with inhalation was noted by core‐lab adjudicated rating of wall motion using a validated scale. These two independent measures, which both improved after treatment, provide evidence for the efficacy of the implant in improving the nasal airway in patients with LWI.
In this study, the majority of complications observed were related to implant retrievals. The implants were typically retrieved by the surgeon during a follow‐up visit in office following direct visualization of the implant exposed at the endonasal canula insertion point. Contributors to implants being exposed in this manner were hypothesized to be associated with insertion technique (e.g., proximal implant position close to entry point) and/or excessive postoperative patient manipulation of the nose. Other complications observed included localized inflammation and infection, which were subsequently resolved with pre‐and/or postprocedure antibiotics, one instance of pain, and one event in which a bump appeared on the external nasal skin. Complications discussed above were acute (within 3 month follow‐up) and resolved by the 6‐month follow‐up timepoint. Whereas intrinsic factors associated with poor outcome were not specifically tracked in this study, it may be that the most ideal candidate is a patient without excessively thick or thin skin, zone 2 collapse, or other medical issues that may affect healing.
The common nomenclature of NVC has been used previously and is described using specific and varied pathology to define the nature of the associated dynamic lateral wall motion. Lateral wall insufficiency is one of the described subsets. Importantly, treatment of LWI should be aimed at decreasing medial wall motion, not lateralization of the nasal wall. A modified Cottle maneuver that stabilizes but does not lateralize the nasal wall is the best method for determining if LWI is causative in nasal obstruction patients. Lateralization of the nasal wall is unlikely to occur with the implant described, lateral crural strut grafts, bone‐anchored sutures, or any of the other treatments currently in use without specific resection or repositioning of the lateral crura.
CONCLUSION
In sum, examination of patients with nasal obstruction should include evaluation for the presence of NVC due to LWI, utilizing the proper method of the modified Cottle technique, and consideration for treatment if LWI is determined to be a significant contributor to symptoms. Stabilization of the nasal wall with an implant improves nasal obstructive symptoms in patients with LWI, as shown by both a disease‐specific quality‐of‐life instrument and objective physical examination. Evidence for longer‐term improvement in a prior cohort of patients examined at 12 and 24 months postoperatively provides hope that these results may be maintained for 2 years or more.18 Limitations of the study include a single arm study design with short‐term follow‐up. A randomized placebo control study design should be considered in the future.
This study was funded by Entellus, Inc. Pablo Stolovitzky, MD; Douglas M. Sidle, MD; Randall A. Ow, MD; Nathan E. Nachlas, MD; and Sam P. Most, MD, were paid consultants of Entellus, Inc. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1. Rhee JS, Weaver EM, Park SS, et al. Clinical consensus statement: diagnosis and management of nasal valve compromise. Otolaryngol Head Neck Surg 2010;143:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Most SP. Trends in functional rhinoplasty. Arch Facial Plast Surg 2008;10:410–413. [DOI] [PubMed] [Google Scholar]
- 3. Kandathil CK, Spataro EA, Laimi K, Moubayed SP, Most SP, Saltychev M. Repair of the lateral nasal wall in nasal airway obstruction: a meta‐analysis. JAMA Facial Plast Surg. 2018. doi: 10.1001/jamafacial.2018.0036. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lam DJ, James KT, Weaver EM. Comparison of anatomic, physiological, and subjective measures of the nasal airway. Am J Rhinol 2006;20:463–470. [DOI] [PubMed] [Google Scholar]
- 5. Stewart MG, Witsell DL, Smith TL, Weaver EM, Yueh B, Hannley MT. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngol Head Neck Surg 2004;130:157–163. [DOI] [PubMed] [Google Scholar]
- 6. Stewart MG, Smith TL, Weaver EM, et al. Outcomes after nasal septoplasty: results from the Nasal Obstruction Septoplasty Effectiveness (NOSE) study. Otolaryngol Head Neck Surg 2004;130:283–290. [DOI] [PubMed] [Google Scholar]
- 7. Lipan MJ, Most SP. Development of a severity classification system for subjective nasal obstruction. JAMA Facial Plast Surg 2013;15:358–361. [DOI] [PubMed] [Google Scholar]
- 8. Tsao GJ, Fijalkowski N, Most SP. Validation of a grading system for lateral nasal wall insufficiency. Allergy Rhinol (Providence) 2013;4:e66–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goode RL. Surgery of the incompetent nasal valve. Laryngoscope 1985;95:546–555. [DOI] [PubMed] [Google Scholar]
- 10. Most SP. Anterior septal reconstruction: outcomes after a modified extracorporeal septoplasty technique. Arch Facial Plast Surg 2006;8:202–207. [DOI] [PubMed] [Google Scholar]
- 11. Sheen JH. Spreader graft: a method of reconstructing the roof of the middle nasal vault following rhinoplasty. Plast Reconstr Surg 1984;73:230–239. [PubMed] [Google Scholar]
- 12. Surowitz J, Lee MK, Most SP. Anterior septal reconstruction for treatment of severe caudal septal deviation: clinical severity and outcomes. Otolaryngol Head Neck Surg 2015;153:27–33. [DOI] [PubMed] [Google Scholar]
- 13. Keeler J, Most SP. Measuring nasal obstruction. Facial Plast Surg Clin North Am 2016;24:315–322. [DOI] [PubMed] [Google Scholar]
- 14. Most SP. Functional rhinoplasty In: Kennedy DW, Hwang PH, eds. Rhinology: Diseases of the Nose, Sinuses, and Skull Base. New York, NY: Thieme Publishers; 2012: 457. [Google Scholar]
- 15. Vaezeafshar R, Moubayed SP, Most SP. Repair of lateral wall insufficiency. JAMA Facial Plast Surg 2018;20:111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roofe SB, Most SP. Placement of a lateral nasal suspension suture via an external rhinoplasty approach. Arch Facial Plast Surg 2007;9:214–216. [DOI] [PubMed] [Google Scholar]
- 17. San Nicolo M, Stelter K, Sadick H, Bas M, Berghaus A. Absorbable implant to treat nasal valve collapse. Facial Plast Surg 2017;33:233–240. [DOI] [PubMed] [Google Scholar]
- 18. San Nicolo M, Stelter K, Sadick H, Bas M, Berghaus A. A 2‐year follow‐up study of an absorbable implant to treat nasal valve collapse. Presented at the American Academy of Facial Plastic and Reconstructive Surgery Meeting, Oct 26, 2017, Phoenix, AZ.
- 19. Fung E, Hong P, Moore C, Taylor SM. The effectiveness of modified cottle maneuver in predicting outcomes in functional rhinoplasty. Plast Surg Int 2014;2014:618313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lindsay RW. Disease‐specific quality of life outcomes in functional rhinoplasty. Laryngoscope 2012;122:1480–1488. [DOI] [PubMed] [Google Scholar]
- 21. Paniello RC. Nasal valve suspension . An effective treatment for nasal valve collapse. Arch Otolaryngol Head Neck Surg 1996;122:1342–1346. [DOI] [PubMed] [Google Scholar]
- 22. Chambers KJ, Horstkotte KA, Shanley K, Lindsay RW. Evaluation of improvement in nasal obstruction following nasal valve correction in patients with a history of failed septoplasty. JAMA Facial Plast Surg 2015;17:347–350. [DOI] [PubMed] [Google Scholar]


