Abstract
Aim
To describe the healthcare resource utilization (HRU), direct medical costs and clinical characteristics for Japanese patients with mild, moderate or severe systemic lupus erythematosus (SLE). The primary objectives were to describe HRU and examine the direct medical costs for Japanese patients with mild, moderate, or severe SLE over the 3‐year study period. Secondary objectives included recording patient demographics, clinical characteristics and frequency and cost of mild, moderate or severe flares. Exploratory objectives included a description of treatment patterns, and to explore which factors affect medical costs.
Methods
This retrospective, observational cohort study identified patients with SLE (diagnosed April 2010 to March 2012), from the Japan Medical Data Center claims database.
Result
The study cohort comprised 295 patients with mild (28, 9.5%), moderate (134, 45.4%), or severe (133, 45.1%) SLE. Outpatient visits, hospitalizations and emergency room stays were experienced by 295 (100%), 116 (39.3%) and 31 (10.5%) patients, respectively, over the 3‐year study period. Over the 3‐year period, the mean total direct medical cost was US$27 004, and cost increased with SLE severity: mild, $5549 moderate, $15 290; and severe, $43 322 (analysis of variance, P < 0.0001). During this period, the majority of patients (282, 95.6%) experienced at least one flare episode and the mean (standard deviation) frequency was 5.5 (3.3) flares. The mean total direct medical cost per flare increased with SLE severity.
Conclusion
This descriptive study provides information on the economic burden and clinical characteristics of Japanese patients with SLE based on claims data; high levels of HRU and direct medical costs were exhibited, particularly in patients with moderate or severe disease.
Keywords: Asian continental ancestry group, cost of illness, observational study, symptoms flare up, systemic lupus erythematosus
1. INTRODUCTION
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease characterized by periods of exacerbation (flare), and remission.1, 2, 3 Patients with SLE experience substantial impairment in their health‐related quality of life (HRQoL), both physically and mentally.4 Symptoms of SLE range from general malaise, arthralgia and fever, to more severe manifestations including fatigue, nephritis, cognitive impairment and cardiovascular diseases.2, 5, 6 Approximately 90% of patients with SLE are female and symptoms and diagnosis of SLE occur most often between the ages of 15 and 45 years. The global incidence and prevalence of SLE varies considerably, with the overall incidence ranging from 0.3 to 31.5 per 100 000 per year and the prevalence ranging from 3.2 to 517.5 per 100 000; in Japan the prevalence ranges from 3.7 to 37.7 per 100 000.7 Boers et al reported that Asian patients at a medical center in Australia presented with more severe disease than Caucasians; at baseline, south‐eastern Asian/Chinese patients had a median SLE Disease Activity Index (SLEDAI) of 13, compared with 8 (P = 0.002) among Caucasians.8
The treatment options for patients with SLE remain limited compared with those for other rheumatic diseases, such as rheumatoid arthritis, and many existing therapies are ineffective or poorly tolerated in some subsets of patients.9 In 2011, belimumab was the first drug to be approved in the USA and Europe for over 50 years as an add‐on treatment for SLE.10, 11, 12 In addition, belimumab has recently been approved in Japan.13 Given the ongoing need for more efficacious and cost‐effective therapies, it is important to understand the economic burden among Japanese patients with SLE.14
The disease burden of Japanese patients with SLE, in terms of costs and HRQoL, is not fully understood. The aim of this observational retrospective cohort study was to use medical and pharmacy administrative claims data to describe the healthcare resource utilization (HRU) and direct medical costs for Japanese patients with SLE.
2. METHODS
The primary objective of this study was to describe the HRU and direct medical costs of SLE for all patients with mild, moderate or severe SLE defined by a proxy disease severity algorithm, using insurance claims data. Other objectives were to describe clinical characteristics, and the frequency and cost of a mild, moderate or severe flares, a description of treatment patterns, and to explore which factors affect medical costs (cost predictors).
2.1. Study design
This observational retrospective study (GSK study HO‐15‐16208) was conducted using the Japan Medical Data Center Co. Ltd claims database (JMDC‐CDB).15 JMDC‐CDB is a nationwide database of fully anonymized records containing prescription, procedure and diagnostic information from over 90 healthcare insurance payers. JMDC‐CDB covers approximately 3% (approximately 4 million registrations) of the total Japanese population <75 years of age and consists of JMDC‐CDB employees and their family members. The study was conducted in accordance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects from The Ministry of Education, Culture, Sports, Science and Technology, The Ministry of Education, Technology and the Ministry of Health,16 privacy requirements and the guiding principles of the Declaration of Helsinki, and it was approved by the ethics committee of external healthcare providers (Kitamachi Clinic ethics committee, Tokyo).
Eligible patients (15 to 65 years of age) had: (a) an SLE‐related visit between April 2010 and March 2012 (SLE diagnosis code, International Classification of Diseases [ICD‐10] M32); (b) continuous inclusion eligibility for 6 months before and 3 years after the date of the first SLE‐related claim (index date); (b) a second diagnosis record of SLE determined during the 3‐year follow‐up period; and (d) records of SLE diagnosis and SLE‐related immunological blood tests in the latest 1‐year period available in the database. Immunological blood tests included complement levels, anti‐nuclear antibody (ANA), anti‐double‐stranded DNA (anti‐dsDNA) antibody, and anti‐phospholipid antibody. Duration of SLE treatment at the index date was determined by the oldest diagnosis record available. No specific exclusion criteria were applied.
2.2. Assessments
To assess the disease burden among Japanese patients with SLE by varying disease activity, the algorithm for determining SLE disease severity combined elements of disease activity with cumulative damage, based on SLEDAI, Systemic Lupus Erythematosus Activity Measure (SLAM), and British Isles Lupus Assessment Group (BILAG), with use of SLE medications and a consensus of expert clinical opinion to adapt to claims data.17, 18, 19 The algorithm for identifying SLE flares and categorizing severity is based on the Lupus Foundation of America (LFA) definition. The LFA categorizes flare severity as mild, moderate, or severe, encompassing the consensus of expert clinical opinion, and including outpatient visits, hospitalizations, and emergency room (ER) visits supported by a qualifying SLE diagnosis or SLE‐related condition.20
Detailed definitions of SLE severity and flare severity algorithms are shown in Tables S1 and S2. SLE severity was determined as the highest severity the patient experienced during the 3‐year follow‐up period.
All data, including demographics, were collected individually by disease, medication, procedure names or codes such as ICD‐10, Anatomical Therapeutic Chemical or procedure codes, in the 3‐year follow‐up period, and at baseline (6‐month period prior to index date). HRU, direct medical costs, comorbidities and treatments were assessed. A continuous prescription treatment period was defined by intervals of 6 months. HRU outcomes (hospital clinic services [inpatient admission, ER admission, and outpatient visit], prescribed class of medications, laboratory services, operations and procedures) were collected by SLE‐severity status. The claims of interest to analyze were separated two ways, by “all‐cause” or “SLE‐related.” Direct medical cost was computed based on the medical fee points (1 point = 10 Japanese yen [JPY]) or drug prices. Costs in JPY were converted to US dollars (1 JPY = 0.01 US$).
2.3. Analysis
All analyses were conducted using SAS software version 9.3 (SAS Institute Inc., Cary, NC, USA). Statistical significance of the results were reported by 95% confidence intervals. Descriptive statistics included means (standard deviation [SD]), median and frequency for continuous and categorical data, respectively. Appropriate tests for comparisons across the disease severity cohorts (Fisher's exact test or analysis of variance [ANOVA]) were used based on value types and the distribution of the measure.
Systemic lupus erythematosus‐related costs and related damage are hard to ascertain using claims data; hence this analysis is based on all‐cause costs. Cost predictors associated with all‐cause direct medical costs were identified using multivariate regression. Since direct medical cost (dependent variable) showed the normal distribution as a value of logarithm, a logarithm‐transformed linear regression model was applied to estimate the incremental direct medical costs associated with SLE. Independent variables in the model included gender, age, SLE‐treated period, principal insured person or family, facility types, baseline corticosteroid dose, baseline comorbidity, organ damage, medications, number of flares, and inpatient admission. The forward, stepwise and backward selection methods were used to determine the best fitting model to predict direct medical costs. In addition, in order to assess the adjusted direct medical costs, SLE severity was included, with a severity category of mild applied as the reference.
3. RESULTS
3.1. Patient population and demographics
Among the 4 685 857 patients identified in the JMDC‐CDB (March 2016), 2368 patients had at least one confirmed M32 SLE ICD‐10 code between April 2010 and March 2012; 295 met the inclusion criteria for this study. Of the 295 eligible patients, 256 (86.8%) were female; the mean (SD) age at the time of inclusion was 41.6 (10.3) years. The mean (SD) treatment duration was 6.5 (6.0) years. The numbers of patients with lupus nephritis and neuropsychiatric (NP) lupus were 110 (41.2%) and 12 (4.5%), respectively. The numbers of patients with mild, moderate, or severe SLE were 28 (9.5%), 134 (45.4%), and 133 (45.1%), respectively (Table 1).
Table 1.
Baseline patient characteristics and demographics
| Patient demographics | Total (N = 295) | Mild (n = 28) | Moderate (n = 134) | Severe (n = 133) |
|---|---|---|---|---|
| Female, n (%) | 256 (86.8) | 25 (89.3) | 112 (83.6) | 119 (89.5) |
| Age at index date, y, mean (SD) | 41.6 (10.3) | 43.8 (9.1) | 40.7 (11.0) | 42.0 (9.8) |
| Principal insurer, full‐time worker, n (%) | 107 (36.3) | 12 (42.9) | 50 (37.3) | 45 (33.8) |
| Treatment period at index date, y, mean (SD) | 6.5 (6.0) | 4.9 (4.7) | 6.6 (6.1) | 6.9 (6.1) |
| NP lupus | 12 (4.5) | 0 | 4 (3.0) | 8 (6.0) |
| Lupus nephritis | 110 (41.2) | 0 | 48 (35.8) | 62 (46.6) |
NP, neuropsychiatric; SD, standard deviation.
3.2. HRU: all‐cause
All patients in the study cohort experienced outpatient visits (295, 100%) (Table 2), and 39.3% (116 patients) and 10.5% (31 patients) experienced hospitalization and ER stays, respectively. Over the 3‐year period there were 64.9 outpatient visits, 1.8 ER stays and 2.3 hospital stays (mean duration was 19.9 days per hospitalization). The proportion of patients experiencing irregular outpatient visits (out of office hours, late night, holiday, or emergency treatment) was 51.9% and the mean frequency per patient of the study cohort was 3.0 times over the study period. The majority of HRU (295/295 [100%] outpatient visits, 100/116 [86.2%] inpatient stays, and 31/31 [100%] emergency stays) were directly attributed to SLE.
Table 2.
All‐cause healthcare resource utilization in Japanese patients with SLE over the study period
| Total (N = 295) | Mild (n = 28) | Moderate (n = 134) | Severe (n = 133) | P value | |
|---|---|---|---|---|---|
| Inpatient | |||||
| ≥1 inpatient stay, n (%) | 116 (39.3) | 3 (10.7) | 34 (25.4) | 79 (59.4) | <0.001 |
| Number, mean (SD) | 2.3 (2.6) | 1.3 (0.6) | 1.5 (0.8) | 2.7 (3.0) | 0.054 |
| Duration, days, per event, mean (SD) | 19.9 (32.4) | 3.5 (2.4) | 13.0 (19.2) | 21.9 (34.9) | 0.126 |
| ≥1 ER stays, n (%) | 31 (10.5) | 0 | 4 (3.0) | 27 (20.3) | <0.001 |
| Number, mean (SD) | 1.8 (1.4) | ‐ | 1.3 (0.5) | 1.9 (1.5) | 0.442 |
| Outpatient | |||||
| ≥1 outpatient visit, n (%) | 295 (100.0) | 28 (100.0) | 134 (100.0) | 133 (100.0) | NA |
| Number, mean (SD) | 64.9 (65.3) | 40.4 (23.4) | 53.4 (32.8) | 81.6 (88.1) | <0.001 |
| ≥1 outpatient visit, irregular, n (%) | 153 (51.9) | 14 (50.0) | 64 (47.8) | 75 (56.4) | 0.353 |
| Number, mean (SD) | 3.0 (4.3) | 3.9 (8.4) | 2.7 (4.3) | 3.0 (3.1) | 0.639 |
| Laboratory services | |||||
| ANA, n (%) | 214 (72.5) | 21 (75.0) | 94 (70.1) | 99 (74.4) | 0.722 |
| Antibody for double‐stranded DNA | |||||
| n (%) | 250 (84.7) | 21 (75.0) | 111 (82.8) | 118 (88.7) | 0.129 |
| Complement, n (%) | 275 (93.2) | 25 (89.3) | 126 (94.0) | 124 (93.2) | 0.547 |
| Anti‐phospholipid antibody, n (%) | 126 (42.7) | 6 (21.4) | 46 (34.3) | 74 (55.6) | <0.001 |
| Proteinuria, n (%) | 59 (20.0) | 0 | 19 (14.2) | 40 (30.1) | <0.001 |
| Imaging | |||||
| Imaging, n (%) | 275 (93.2) | 21 (75.0) | 124 (92.5) | 130 (97.7) | <0.001 |
| X‐ray, n (%) | 258 (87.5) | 19 (67.9) | 116 (86.6) | 123 (92.5) | 0.003 |
| Head CT, PET, SPECT and MRI, n (%) | 61 (20.7) | 2 (7.1) | 20 (14.9) | 39 (29.3) | 0.003 |
| Body CT, PET and MRI, n (%) | 172 (58.3) | 9 (32.1) | 65 (48.5) | 98 (73.7) | <0.001 |
| Bone mineral density, n (%) | 102 (34.6) | 1 (3.6) | 46 (34.3) | 55 (41.4) | <0.001 |
| Electrocardiogram/echocardiography/cardiac ultrasonic imaging, n (%) | 158 (53.6) | 10 (35.7) | 58 (43.3) | 90 (67.7) | <0.001 |
| Operation/procedure | |||||
| Dialysis, n (%) | 9 (3.1) | 0 | 0 | 9 (6.8) | 0.003 |
| Apheresis, n (%) | 6 (2.0) | 0 | 0 | 6 (4.5) | 0.032 |
| Medications | |||||
| Corticosteroids (oral and IV) | 255 (86.4) | 16 (57.1) | 118 (88.1) | 121 (91.0) | <0.001 |
| Immunosuppressants, oral, n (%) | 118 (40.0) | 0 | 52 (38.8) | 66 (49.6) | <0.001 |
| NSAIDs, n (%) | 256 (86.8) | 18 (64.3) | 120 (89.6) | 118 (88.7) | 0.003 |
| IVIg, n (%) | 8 (2.7) | 0 (0.0) | 1 (0.7) | 7 (5.3) | 0.069 |
| Steroid ointment, n (%) | 168 (56.9) | 14 (50.0) | 72 (53.7) | 82 (61.7) | 0.323 |
| Other biologics, n (%) | 9 (3.1) | 0 | 3 (2.2) | 6 (4.5) | 0.451 |
| Osteoporosis therapeutic medication, n (%) | 201 (68.1) | 7 (25.0) | 93 (69.4) | 101 (75.9) | <0.001 |
ANA, anti‐nuclear antibody; CT, computed tomography; ER, emergency room; IVIg, intravenous immunoglobulin; MRI, magnetic resonance imaging; NSAIDs, non‐steroidal anti‐inflammatory drugs; PET, positron emission tomography; SD, standard deviation; SLE, systemic lupus erythematosus; SPECT, single‐photon emission computed tomography.
Laboratory blood test services were utilized for all patients during their visits. Immunological tests were recorded for ANAs (72.5%), anti‐dsDNA (84.7%), complement (93.2%), anti‐phospholipid antibodies (42.7%), and other disease‐related auto‐antibodies (69.2%). The mean number of blood tests for anti‐dsDNA, complement, and serum creatinine correlated with SLE severity (Fisher's exact test, P < 0.01). Proteinuria was examined in 59 (20.0%) patients with moderate and severe SLE. Imaging services (X‐ray, computed tomography, positron emission tomography, single‐photon emission computed tomography, magnetic resonance imaging, and bone mineral density) and cardiovascular examinations (echocardiography, electrocardiogram [ECG] and cardiac ultrasonic imaging) were provided more frequently for patients with higher severity of SLE. In severe cases, 9 (6.8%), 7 (5.3%) and 6 (4.5%) patients were treated with dialysis (mean frequency, 289.9), intravenous immunoglobulin (IVIg) and apheresis, respectively. Medication classes for patients were non‐steroidal anti‐inflammatory drugs (86.8%), corticosteroid (oral and IV) (86.4%), osteoporosis therapeutic medications (68.1%), oral immunosuppressants (40.0%), other biologicals (3.1%) and IVIg (2.7%).
3.3. Direct medical cost
The mean total all‐cause direct medical cost per patient over the 3‐year study period was $29 135 (2 913 509 JPY) and the corresponding costs increased with SLE severity: mild ($7184), moderate ($16 862), and severe ($46 122) (ANOVA, P < 0.001) (Figure 1A). The mean costs for each type of claim were: inpatient, $7995; outpatient, $12 981; pharmacy, $8159 (Figure 1A). Outpatient claims formed the largest proportion of overall costs (mild, 62.4%; moderate, 49.5%; and severe, 42.1%; P < 0.001). The proportion of inpatient claims ranged from 9.5% in patients with mild disease to 34.0% in those with severe disease (P < 0.001). Pharmacy claims comprised 28.0% of the direct medical cost. The breakdown distributions (mean [SD]) of direct medical cost in inpatient claims were management (55.6% [28.7]), medication (17.3% [24.9]), operation services (10.1% [19.2]), laboratory services (9.4% [12.8]), and imaging and pathology services (4.5% [9.5]). The corresponding distributions (mean [SD]) in outpatient claims were management (10.3% [6.8]), medication (52.4% [23.1]), operation services (2.8% [11.3]), laboratory services (29.0% [17.6]), and imaging and pathology services (4.0% [5.4]).
Figure 1.
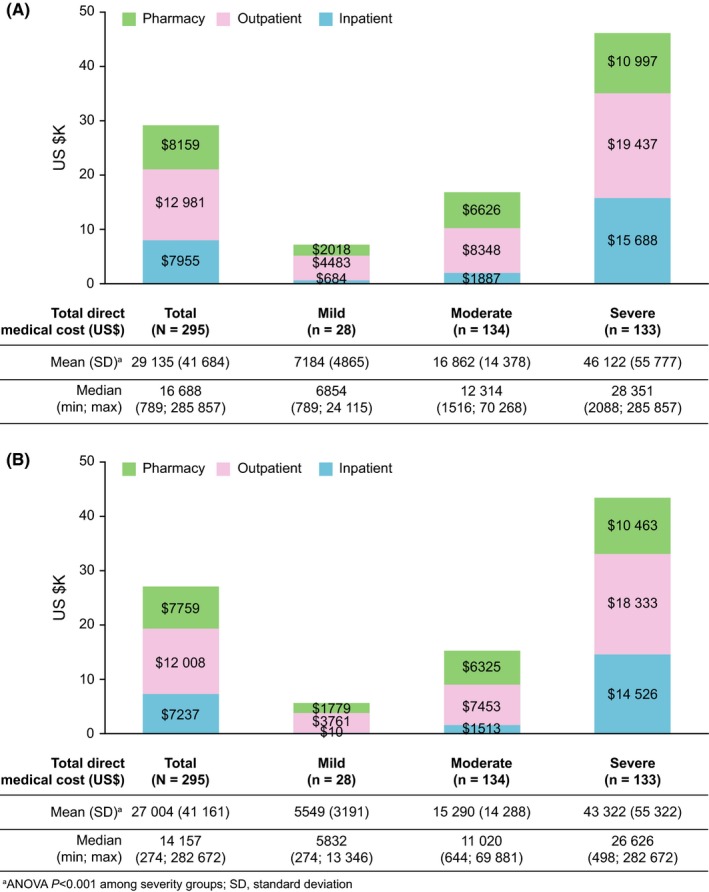
(A) All‐cause mean direct and systemic lupus erythematosus (SLE)‐related and (B) medical costs over the 3‐year follow‐up period. SLE, systemic lupus erythematosus
As most all‐cause claims were related to SLE treatment, the direct medical cost limited to SLE‐related claims was lower than that for all‐cause claims. However, higher SLE‐related costs were associated with a higher level of SLE disease burden: $27 004 (total cohort); $5549 (mild); $15 290 (moderate); $43 322 (severe) (ANOVA, P < 0.001) (Figure 1B). The mean SLE‐related costs for the total cohort for each type of claim were: $7237 (inpatient); $12 008 (outpatient); and $7759 (pharmacy). In this study, age (P = 0.941), gender (P = 0.707), and disease duration (P = 0.064) were not associated with direct medical costs in univariate regression analyses. Disease duration (P = 0.342) or numbers of flare episodes (P = 0.438) were not associated with direct medical costs in multivariate regression analyses.
3.4. Clinical manifestations and comorbidities
The most frequently reported clinical manifestations and comorbidities (not used for SLE or flare severity definitions) were other infections (93.9%) and inflammation (94.2%) (Table 3). Infections included herpes infections (19.3%) such as herpes zoster and herpes simplex. Hematological, endocrine, cardiovascular, psychiatric or cutaneous symptoms were present in many patients with SLE. Solid organ/hematological malignancies occurred in 10.2% of patients (Table 3). Major SLE‐related conditions used to define SLE severity were arthritis/arthralgia (50.8%), dry eye/tear film insufficiency (46.4%), kidney disease: nephritis (45.1%), kidney disease: nephrotic syndrome (23.1%), arterial/venous thrombosis (21.0%), mononeuropathy/polyneuropathy (20.7%), vasculitis excluding aortitis (16.9%), and stroke/transient ischemic attack (4.6%) (Table S3).
Table 3.
Prevalence of clinical manifestations and comorbidities by SLE disease severity (at index and during the study)a
| Clinical manifestation, n (%) | Total (N = 295) | Mild (n = 28) | Moderate (n = 134) | Severe (n = 133) | P value |
|---|---|---|---|---|---|
| Infection | 277 (93.9) | 24 (85.7) | 126 (94.0) | 127 (95.5) | 0.150 |
| Respiratory infection | 254 (86.1) | 24 (85.7) | 115 (85.8) | 115 (86.5) | 1.000 |
| Herpes infection | 57 (19.3) | 3 (10.7) | 23 (17.2) | 31 (23.3) | 0.262 |
| Inflammation | 278 (94.2) | 24 (85.7) | 126 (94.0) | 128 (96.2) | 0.088 |
| Malignancy: solid organ and hematological | 30 (10.2) | 2 (7.1) | 13 (9.7) | 15 (11.3) | 0.847 |
| Solid organ malignancy | 19 (6.4) | 2 (7.1) | 6 (4.5) | 11 (8.3) | 0.441 |
| Hematological malignancy | 12 (4.1) | 0 | 7 (5.2) | 5 (3.8) | 0.629 |
| Blood disease | 205 (69.5) | 17 (60.7) | 89 (66.4) | 99 (74.4) | 0.198 |
| Anemia | 153 (51.9) | 8 (28.6) | 65 (48.5) | 80 (60.2) | 0.005 |
| Purpura, bleed and coagulation dysfunction | 115 (39.0) | 9 (32.1) | 44 (32.8) | 62 (46.6) | 0.057 |
| Other immune and blood dysfunction/deficiency | 64 (21.7) | 3 (10.7) | 27 (20.1) | 34 (25.6) | 0.199 |
| Endocrine disorder | 245 (83.1) | 18 (64.3) | 110 (82.1) | 117 (88.0) | 0.012 |
| Thyroid dysfunction | 84 (28.5) | 6 (21.4) | 33 (24.6) | 45 (33.8) | 0.180 |
| Diabetes mellitus | 116 (39.3) | 7 (25.0) | 52 (38.8) | 57 (42.9) | 0.213 |
| Hyperlipidemia | 156 (52.9) | 8 (28.6) | 66 (49.3) | 82 (61.7) | 0.003 |
| Hypertension and related cardiovascular diseases | 218 (73.9) | 17 (60.7) | 88 (65.7) | 113 (85.0) | <0.001 |
| Nervous and psychological symptoms | 124 (42.0) | 9 (32.1) | 44 (32.8) | 71 (53.4) | 0.002 |
| Systemic manifestation: headache, pain, fatigue, and anorexia | 106 (35.9) | 8 (28.6) | 44 (32.8) | 54 (40.6) | 0.305 |
| Headache | 70 (23.7) | 5 (17.9) | 33 (24.6) | 32 (24.1) | 0.827 |
| Fever | 24 (8.1) | 1 (3.6) | 5 (3.7) | 18 (13.5) | 0.011 |
| Other pain | 23 (7.8) | 1 (3.6) | 7 (5.2) | 15 (11.3) | 0.148 |
| Fatigue | 3 (1.0) | 0 (0.0) | 1 (0.7) | 2 (1.5) | 0.720 |
| Food concern | 13 (4.4) | 1 (3.6) | 5 (3.7) | 7 (5.3) | 0.837 |
| Pulmonary heart disease | 10 (3.4) | 1 (3.6) | 4 (3.0) | 5 (3.8) | 0.901 |
| Cutaneous symptoms: dermatitis, eczema, cornification, xeroderma, and cutaneous ulcer | 219 (74.2) | 19 (67.9) | 98 (73.1) | 102 (76.7) | 0.547 |
| Other connective tissue disease | 81 (27.5) | 5 (17.9) | 37 (27.6) | 39 (29.3) | 0.491 |
| Other renal and urological dysfunctions | 98 (33.2) | 6 (21.4) | 37 (27.6) | 55 (41.4) | 0.025 |
| Steroidal and bone symptoms | 234 (79.3) | 11 (39.3) | 107 (79.9) | 116 (87.2) | <0.001 |
| Female genital disease | 106 (35.9) | 9 (32.1) | 46 (34.3) | 51 (38.3) | 0.766 |
SLE, systemic lupus erythematosus.
Patients have been defined based on diagnostic code only.
3.5. Flare incidence
The majority of patients (282, 95.6%) experienced at least one flare episode and 105 (35.6%) experienced severe flare; the frequency of overall, moderate and severe flares increased with SLE severity (Table 4). The mean (SD) frequency was 5.5 (3.3) times over the 3‐year study period, and also increased with SLE severity (ANOVA P < 0.001). Mean direct medical cost per flare episode was $1576 (median $541) per study cohort patient, and increased with SLE severity.
Table 4.
Frequency of flares by SLE severity and costs per flare over the 3‐year study period
| Total (N = 295) | Mild (n = 28) | Moderate (n = 134) | Severe (n = 133) | P valuea | |
|---|---|---|---|---|---|
| ≥1 flare, n (%) | 282 (95.6) | 20 (71.4) | 131 (97.8) | 131 (98.5) | <0.001 |
| Mild flare, n (%) | 203 (68.8) | 17 (60.7) | 97 (72.4) | 89 (66.9) | 0.394 |
| Moderate flare, n (%) | 246 (83.4) | 10 (35.7) | 118 (88.1) | 118 (88.7) | <0.001 |
| Severe flare, n (%) | 105 (35.6) | 1 (3.6) | 26 (19.4) | 78 (58.6) | <0.001 |
| Frequency of flares/patient, mean (SD) | 5.5 (3.3) | 2.9 (3.0) | 5.0 (2.8) | 6.5 (3.5) | <0.001 |
| Direct medical cost/flare, US$, mean (SD) | 1576 (3159) | 315 (238) | 739 (1134) | 2,344 (4048) | <0.001 |
SD, standard deviation; SLE, systemic lupus erythematosus.
P value represents comparison between the three severity groups.
3.6. Treatment patterns
Eleven (8.3%) patients were treated with pulses of steroid and 7 (5.3%) with cyclophosphamide, considered for emergency use for patients with SLE in Japan (mean frequency over study period: steroid pulse, 1.3; cyclophosphamide pulse, 6.4). There were 40 patients who did not receive corticosteroid treatment over the study period; 24 (60.0%) of these patients received loxoprofen sodium hydrate, 18 (45.0%) received acetaminophen, and 17 (42.5%) received rebamipide, a frequently prescribed medication for patients with SLE in Japan who have previously experienced steroid‐induced ulcers.
More patients (44, 14.9%) initiated corticosteroid treatment over the study period than terminated (19, 6.4%). The specified treatments that were initiated included: 19 (6.4%) azathioprine; 11 (3.7%) cyclosporine; 4 (1.4%) methotrexate; 2 (0.7%) mycophenolate mofetil; 24 (8.1%) tacrolimus; 8 (2.7%) IVIg; 9 (3.1%) cyclophosphamide; 1 (0.3%) rituximab; and 17 (5.8%) mizoribine. The specified treatments that were terminated included: 14 (4.7%) azathioprine; 6 (2.0%) cyclosporine; 3 (1.0%) methotrexate; 1 (0.3%) mycophenolate mofetil; 8 (2.7%) tacrolimus; 10 (3.4%) cyclophosphamide; 1 (0.3%) rituximab; 7 (2.4) IVIg; and 7 (2.4%) mizoribine. It should be noted that there are currently no treatment guidelines for patients with SLE in Japan; the above treatment patterns highlight realistic clinical practice in Japan, which has previously been otherwise unknown.
3.7. Cost predictors
The direct medical costs, adjusted for corticosteroid dose, were $5538 for patients with mild SLE severity (reference), $11 621 for moderate (110% increase), and $24 942 for severe (350% increase). In multivariate analyses, patients with skin and renal organ manifestations had 84.5% ($5538) and 78.3% ($5129) greater costs, respectively, than those without (P < 0.001) (Table 5). Other predictors (P < 0.05) of higher medical costs are shown in Table 5.
Table 5.
Multivariate model of direct medical cost over the 3‐year period
| Variable | Coefficient | Error | P value | Increments (%) | Increasing amount (US$) |
|---|---|---|---|---|---|
| Intercept | 5.816 | 0.0539 | <0.001 | ‐ | 6550 (ref) |
| Number of flares experienced | 0.005 | 0.0067 | 0.438 | 1.202 | 79 |
| Baseline maximum daily dose of steroid (mg) | 0.003 | 0.0011 | 0.013 | 0.624 | 41 |
| SLE treated year (continuous variable) | 0.004 | 0.0037 | 0.342 | 0.818 | 54 |
| Organ damage | |||||
| Ocular (either eye) | 0.018 | 0.0534 | 0.732 | 4.300 | 282 |
| Renal: proteinuria, end‐stage renal disease | 0.251 | 0.0514 | <0.001 | 78.304 | 5129 |
| Pulmonary | 0.151 | 0.0516 | 0.004 | 41.612 | 2725 |
| Peripheral vascular | 0.184 | 0.0713 | 0.010 | 52.862 | 3462 |
| Musculoskeletal | 0.111 | 0.0535 | 0.039 | 29.068 | 1904 |
| Skin: scarring chronic alopecia, extensive scarring or panniculum other than scalp and pulp space, skin ulceration | 0.266 | 0.0935 | 0.005 | 84.548 | 5538 |
| Gonadal failure | 0.094 | 0.0711 | 0.187 | 24.214 | 1586 |
| Diabetes | 0.173 | 0.0863 | 0.046 | 48.922 | 3204 |
| Malignancy: malignant tumor (excluding dysplasia) | 0.200 | 0.0708 | 0.005 | 58.588 | 3837 |
| Comorbidities | |||||
| Arthritis/arthralgia | 0.045 | 0.0455 | 0.324 | 10.887 | 713 |
| Anemia | 0.144 | 0.0458 | 0.002 | 39.470 | 2585 |
| Hypertension and related cardiovascular diseases | 0.0325 | 0.0466 | 0.486 | 7.776 | 509 |
| Nervous and psychological symptom | 0.096 | 0.0510 | 0.062 | 24.661 | 1615 |
SLE, systemic lupus erythematosus.
4. DISCUSSION
This study reports high levels of HRU and SLE‐related costs in Japanese patients with SLE, particularly among patients with moderate and severe disease severity. A recent report states that over 60 000 Japanese patients with SLE receive subsidies from the Japanese government‐funded intractable disease program, with an estimated prevalence of 46 per 100 000.21 In the present study, a population of Japanese patients with SLE was identified from the JMDC‐CDB claims database. Among this population, the majority had moderate (45%) or severe (45%) disease; 10% were identified with mild disease. In comparison, a study of patients with SLE in the USA identified fewer patients with severe disease (22%) and more patients with mild disease (26%); the percentage with moderate disease was similar (52%).20 These differences may have been caused by the stringent inclusion criteria in the present study. However, the gender ratio and age distribution, with the exception of the elderly population, were similar to those described in previous studies.22, 23, 24
The proportion of patients experiencing systemic comorbid conditions increased in correlation with increased SLE disease severity. In particular, incidence rates of hypertension and related cardiovascular disease, hyperlipidemia and anemia were significantly higher. An increase was also observed for malignancies and herpes, although this was not significant. These findings are consistent with other studies and these conditions are linked with an increased risk of mortality and deterioration of QoL.25, 26, 27
It is challenging to ascertain which HRU and costs are attributable directly to SLE rather than other clinical manifestations and comorbidities. However, SLE‐related costs in this study showed a similar trend and values to all‐cause costs. The majority of outpatient/inpatient visits or ER stays were directly attributed to SLE‐related systemic symptoms and damages in this study cohort. Unadjusted all‐cause direct medical costs (i.e before multivariate model regression adjustment) were high (mean: $29 135 over 3 years) and increased with SLE severity (P < 0.001). After multivariate regression model adjustment, patients with moderate disease had 110% higher costs (P < 0.001), and patients with severe disease 350% higher costs (P < 0.001) compared with patients with mild disease. A separate report assessed direct medical costs of Japanese patients with SLE using a hospital‐based claims database.28 This report included patients with SLE who received a subsidy from the intractable disease program and those with confirmed diagnoses. The mean direct medical cost per year was reported as $7361 (median $3885),28 which is lower than the $29 135 over 3 years reported in this study. Total direct costs per patient per year in the USA and Canada were reported as $10 000 to $33 000.7 The corresponding costs of European countries ranged from $4800 to $10 000.7 These differences may be attributed to variations in background and treatment of eligible patients, differing disease severity profiles, study designs, healthcare systems and relative prices.
In this study, the proportions of costs from health insurance claims increased significantly with disease severity. Pharmacy claims constituted over a quarter of the direct medical costs. The average cost of medical services was more than six times higher in patients with severe disease compared with those with mild disease, and for patients with moderate disease it was approximately twice as costly compared with mild disease.
A variety of cost predictors for patients with SLE have been reported in other studies and include younger patients, high disease activity at onset/over the disease course, flare, greater disease damage, disease severity, active glomerulonephritis and NP involvement.29, 30, 31, 32, 33, 34 In this study, age, gender or disease duration were not associated with the direct medical costs by both univariate and multivariate regression models. The majority of patients (282, 95.6%) experienced at least one flare episode and the number of patients experiencing moderate and severe flares increased with SLE severity. The mean (SD) number of flares per patient was 5.5 (3.3) times over the 3‐year study period, which increased with SLE severity (ANOVA, P < 0.001). The proportion of patients experiencing a severe flare during the study (105 patients, 35.6%) was consistent with the proportion of patients (31.9%) reported in a long‐term follow‐up, monocentric SLE cohort.24 The mean costs per flare increased with SLE severity, consistent with results of a study that applied chart reviews and patient‐reported questionnaires in patients with SLE in Hong Kong.31 Reduction of flare could facilitate important benefits for economical and clinical burden. Costs were significantly elevated by 84.5%, 78.3% and 39.5%, in patients with skin, renal involvement, and anemia, respectively; these patients may have required additional treatment such as immunosuppressant therapies, laboratory tests or inpatient stays. In particular, patients with NP lupus/lupus nephritis showed considerably higher levels of economic burden (Tables S4 and S5).35, 36, 37
The treatments administered to Japanese patients with SLE were similar to those in other countries except for anti‐malarial therapies, which only appeared on the Japanese market after the end of the study assessment period.38 However, the majority of the population received typical therapies for moderate or severe SLE patients (such as cyclophosphamide pulse or mycophenolate) despite the limited therapeutic guidelines for SLE in Japan to date. Corticosteroid treatment was the main standard of care therapy received. However, as long‐term steroid use is associated with adverse events, reduction in treatment with steroids should be considered.39 Anti‐malarial drugs, emerging biologicals9 or combinations of immunosuppressive agents may also prevent episodes of flare and therefore could be applied as an alternative method to improve the long‐term prognosis of SLE and related damage.
There are some limitations to the data and study design. First, using administrative claims data to ascertain disease severity can be challenging. The algorithm of SLE disease and flare severity using claims databases has not been previously validated, but has been assessed using a US‐managed care health plan database20 and HRU and direct medical costs were shown to be correlated with SLE severity. Second, patients were defined based on diagnostic code only; this did not capture cases of misdiagnosis and in addition, the presence of diagnosis or examination procedure codes on a medical claim does not confirm presence of the disease of interest, as these may not be correctly coded for a prescription, or may be included as rule‐out criteria. Other indicators of this limitation include the lower frequency of neuropsychiatric lupus (4.5%) compared with other lupus cohorts, and the low frequency of protein urea testing (20.0%) given 41% were coded as having lupus. Third, the database population is limited to the insurers of JMDC‐contracted healthcare societies. Therefore, the socio‐demographic characteristics are not directly representative of the general Japanese population, in particular those patients over 60 years of age. In addition, the principle insurer may not have been able to continue working, which may have resulted in cessation of insurance and therefore, these patients were not captured in this study. Finally, not all information is readily available in claims data and this could affect study outcomes.
In summary, this descriptive study demonstrated that Japanese patients with SLE showed high levels of HRU and associated costs, particularly in patients with moderate or severe disease activity.
CONFLICT OF INTERESTS
Y.T. has received consulting fees, speaking fees, and/or honoraria from Daiichi‐Sankyo, Astellas, Pfizer, Mitsubishi‐Tanabe, Bristol‐Myers Squibb, Chugai, YL Biologics, Eli Lilly, Sanofi, Janssen, and UCB and has received research grants from Mitsubishi‐Tanabe, Takeda, Bristol‐Myers Squibb, Chugai, Astellas, AbbVie, MSD, Daiichi‐Sankyo, Pfizer, Kyowa‐Kirin, Eisai, and Ono. A.M., A.K. and T.M. are employees of GSK group of companies and A.K. holds shares in the company. C.I. is an employee of Japan Medical Data Center.
AUTHOR CONTRIBUTIONS
Y.T., T.M., A.M. and A.K. contributed to the study conception or study design and data analysis or interpretation. C.I. contributed to data acquisition and data analysis or interpretation.
Supporting information
ACKNOWLEDGEMENTS
This study was funded by GSK. Medical writing support was provided by Jennie McLean, PhD, Fishawack Indicia Ltd, UK, and Nicole Cash, PhD, and was funded by GSK. Mr Gen Terashima, Ms Miho Fukuhara, and Ms Shiori Tsuchiya of JMDC Co. Ltd. assisted with study design and analysis and conducted data management.
Tanaka Y, Mizukami A, Kobayashi A, Ito C, Matsuki T. Disease severity and economic burden in Japanese patients with systemic lupus erythematosus: A retrospective, observational study. Int J Rheum Dis. 2018;21:1609–1618. 10.1111/1756-185X.13363
REFERENCES
- 1. Zhu TY, Tam LS, Lee VW, Lee KK, Li EK. Relationship between flare and health‐related quality of life in patients with systemic lupus erythematosus. J Rheumatol. 2010;37(3):568‐573. [DOI] [PubMed] [Google Scholar]
- 2. Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358(9):929‐939. [DOI] [PubMed] [Google Scholar]
- 3. Petri M. Disease activity assessment in SLE: do we have the right instruments? Ann Rheum Dis. 2007;66(suppl 3):iii61‐iii64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zheng Y, Ye DQ, Pan HF, et al. Influence of social support on health‐related quality of life in patients with systemic lupus erythematosus. Clin Rheumatol. 2009;28(3):265‐269. [DOI] [PubMed] [Google Scholar]
- 5. Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365(22):2110‐2121. [DOI] [PubMed] [Google Scholar]
- 6. Bruce IN, Mak VC, Hallett DC, Gladman DD, Urowitz MB. Factors associated with fatigue in patients with systemic lupus erythematosus. Ann Rheum Dis. 1999;58(6):379‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol. 2016;12(10):605‐620. [DOI] [PubMed] [Google Scholar]
- 8. Boers A, Li Q, Wong M, Miller M, Littlejohn G. Differences in SLE disease activity between patients of Caucasian and South‐East Asian/Chinese background in an Australian hospital. Int J Rheum Dis. 2006;9(1):43‐48. [Google Scholar]
- 9. Leone A, Sciascia S, Kamal A, Khamashta M. Biologicals for the treatment of systemic lupus erythematosus: current status and emerging therapies. Expert Rev Clin Immunol. 2015;11(1):109‐116. [DOI] [PubMed] [Google Scholar]
- 10. GlaxoSmithKline . Benlysta. http://wwwbenlystacom/about/indexhtml. Accessed March 2017.
- 11. Furie R, Petri M, Zamani O, et al. A phase III, randomized, placebo‐controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63(12):3918‐3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Navarra SV, Guzmán RM, Gallacher AE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo‐controlled, phase 3 trial. Lancet. 2011;377(9767):721‐731. [DOI] [PubMed] [Google Scholar]
- 13. GSK . GSK receives approval for Benlysta in Japan for the treatment of systemic lupus erythematosus 2017. https://us.gsk.com/en-us/media/press-releases/2017/gsk-receives-approval-for-benlysta-in-japan-for-the-treatment-of-systemic-lupus-erythematosus/. Accessed 27 September 2017.
- 14. NIfHaCE (NICE) . Belimumab for treating active autoantibody‐positive systemic lupus erythematosus 2016. https://www.nice.org.uk/guidance/ta397/resources/belimumab-for-treating-active-autoantibodypositive-systemic-lupus-erythematosus-pdf-82602915211717. Accessed 12 December 2017.
- 15. JMDC . Japan Medical Data Center (JMDC) Claims Database. https://www.jmdc.co.jp/en/about/database.html. Accessed March 2017.
- 16. The Ministry of Education C, Sports, Science and, Technology and the Ministry of Health LaWetE, Research GfE . Ethical Guidelines for Medical and Health Research Involving Human Subjects (Provisional Translation as of March 2015). http://www.mhlw.go.jp/file/06-Seisakujouhou-10600000-Daijinkanboukouseikagakuka/0000080278.pdf. Accessed March 2017.
- 17. Bombardier C, Gladman DD, Urowitz MB, et al. Derivation of the SLEDAI. A disease activity index for lupus patients. Arthritis Rheum. 1992;35(6):630‐640. [DOI] [PubMed] [Google Scholar]
- 18. Liang MH, Socher SA, Larson MG, Schur PH. Reliability and validity of six systems for the clinical assessment of disease activity in systemic lupus erythematosus. Arthritis Rheum. 1989;32(9):1107‐1118. [DOI] [PubMed] [Google Scholar]
- 19. Isenberg DA, Rahman A, Allen E, et al. BILAG 2004. Development and initial validation of an updated version of the British Isles Lupus Assessment Group's disease activity index for patients with systemic lupus erythematosus. Rheumatology. 2005;44(7):902‐906. [DOI] [PubMed] [Google Scholar]
- 20. Garris C, Jhingran P, Bass D, Engel‐Nitz NM, Riedel A, Dennis G. Healthcare utilization and cost of systemic lupus erythematosus in a US managed care health plan. J Med Econ. 2013;16(5):667‐677. [DOI] [PubMed] [Google Scholar]
- 21. MHLW . Ministry of Health, Labour and Welfare (MHLW), Intractable Disease program. http://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/nanbyou/index.html. Accessed March 2017.
- 22. Terao C, Yamada R, Mimori T, Yamamoto K, Sumida T. A nationwide study of SLE in Japanese identified subgroups of patients with clear signs patterns and associations between signs and age or sex. Lupus. 2014;23(13):1435‐1442. [DOI] [PubMed] [Google Scholar]
- 23. Ohta A, Nagai M, Nishina M, Tomimitsu H, Kohsaka H. Age at onset and gender distribution of systemic lupus erythematosus, polymyositis/dermatomyositis, and systemic sclerosis in Japan. Mod Rheumatol. 2013;23(4):759‐764. [DOI] [PubMed] [Google Scholar]
- 24. Minowa K, Amano H, Ando S, et al. Disease flare patterns and predictors of systemic lupus erythematosus in a monocentric cohort of 423 Japanese patients during a long‐term follow‐up: the JUDE study. Mod Rheumatol. 2017;27(1):72‐76. [DOI] [PubMed] [Google Scholar]
- 25. Cao L, Tong H, Xu G, et al. Systemic lupus erythematous and malignancy risk: a meta‐analysis. PLoS ONE. 2015;10(4):e0122964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jakes RW, Bae S‐C, Louthrenoo W, Mok C‐C, Navarra SV, Kwon N. Systematic review of the epidemiology of systemic lupus erythematosus in the Asia‐Pacific region: prevalence, incidence, clinical features, and mortality. Arthritis Care Res. 2012;64(2):159‐168. [DOI] [PubMed] [Google Scholar]
- 27. Yurkovich M, Vostretsova K, Chen W, Aviña‐Zubieta JA. Overall and cause‐specific mortality in patients with systemic lupus erythematosus: a meta‐analysis of observational studies. Arthritis Care Res. 2014;66(4):608‐616. [DOI] [PubMed] [Google Scholar]
- 28. Kawahara K. Analysis of medical cost required for SCD and SLE (in Japanese). Nanbyo Zaitaku Care. 2013;19(3):21‐23. [Google Scholar]
- 29. Lau CS, Mak A. The socioeconomic burden of SLE. Nat Rev Rheumatol. 2009;5(7):400‐404. [DOI] [PubMed] [Google Scholar]
- 30. Zhu TY, Tam L‐S, Lee VWY, Lee KK, Li EK. Systemic lupus erythematosus with neuropsychiatric manifestation incurs high disease costs: a cost‐of‐illness study in Hong Kong. Rheumatology. 2009;48(5):564‐568. [DOI] [PubMed] [Google Scholar]
- 31. Zhu TY, Tam L‐S, Lee VWY, Lee KKC, Li EK. The impact of flare on disease costs of patients with systemic lupus erythematosus. Arthritis Care Res. 2009;61(9):1159‐1167. [DOI] [PubMed] [Google Scholar]
- 32. Clarke AE, Panopalis P, Petri M, et al. SLE patients with renal damage incur higher health care costs. Rheumatology. 2008;47(3):329‐333. [DOI] [PubMed] [Google Scholar]
- 33. Wilson ECF, Jayne DRW, Dellow E, Fordham RJ. The cost‐effectiveness of mycophenolate mofetil as firstline therapy in active lupus nephritis. Rheumatology. 2007;46(7):1096‐1101. [DOI] [PubMed] [Google Scholar]
- 34. Sutcliffe N, Clarke AE, Taylor R, Frost C, Isenberg DA. Total costs and predictors of costs in patients with systemic lupus erythematosus. Rheumatology. 2001;40(1):37‐47. [DOI] [PubMed] [Google Scholar]
- 35. Tanaka E, Hoshi D, Igarashi A, et al. Analysis of direct medical and nonmedical costs for care of rheumatoid arthritis patients using the large cohort database, IORRA. Mod Rheumatol. 2013;23(4):742‐751. [DOI] [PubMed] [Google Scholar]
- 36. Higashiyama A, Okamura T, Watanabe M, et al. Effect of chronic kidney disease on individual and population medical expenditures in the Japanese population. Hypertens Res. 2009;32(6):450. [DOI] [PubMed] [Google Scholar]
- 37. Igarashi A, Kuwabara H, Fahrbach K, Schenkel B. Cost‐efficacy comparison of biological therapies for patients with moderate to severe psoriasis in Japan. J Dermatolog Treat. 2013;24(5):351‐355. [DOI] [PubMed] [Google Scholar]
- 38. Touma Z, Urowitz MB, Gladman DD. Systemic lupus erythematosus: an update on current pharmacotherapy and future directions. Expert Opin Biol Ther. 2013;13(5):723‐737. [DOI] [PubMed] [Google Scholar]
- 39. Tanaka Y, Takeuchi T, Miyasaka N, et al. Efficacy and safety of rituximab in Japanese patients with systemic lupus erythematosus and lupus nephritis who are refractory to conventional therapy. Mod Rheumatol. 2016;26(1):80‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


