Abstract
Purpose
To compare the anatomical outcomes of different extents of internal limiting membrane (ILM) peeling in idiopathic macular hole surgery.
Methods
Prospective, parallel‐group, randomized clinical trial. A total of 121 eyes of 121 patients with idiopathic macular hole underwent pars plana vitrectomy, and peeling of the ILM with a diameter of two disk diameters (DD) or 4DD based on randomization. The main outcome was the proportion of eyes with complete hole closure at 12 months. The second outcome was the hole closure grading stratified by macular hole closure index (MHCI) at each visit.
Results
At 12 months, there was no significant difference in anatomical outcomes with complete closure achieved in 52 (82.5%) of 63 eyes in the 2DD group and 53 (91.4%) of 58 eyes in the 4DD group (p = 0.15). For subjects with MHCI ≤0.5 (n = 24), complete closure rate was significantly lower in the 2DD group compared to the 4DD group (p = 0.012; 18.2% versus 75.9%, respectively). Average BCVA was lower in 2DD group than 4DD group (p = 0.014). By contrast, when MHCI was >0.5, the complete closure rate between the two groups showed no significant difference: 96.2% (50 patients) versus 95.6% (43 patients), respectively (p = 0.185).
Conclusion
In patients with idiopathic full‐thickness macular hole and MHCI ≤0.5, a larger ILM peel of 4DD tends to achieve better anatomical outcomes than a more limited 2DD peel.
Keywords: anatomical outcomes, different diameter of internal limiting membrane peel, functional outcomes, internal limiting membrane peeling, macular hole
Introduction
A surgical approach for the management of idiopathic macular hole (MH) was first reported by Kelly & Wendel (1991) in 1991 and has evolved over the last 25 years to feature not only a pars plana vitrectomy (PPV), but a combination of adjuvant techniques, including internal limiting membrane (ILM) peeling (Olsen et al. 1998), gas tamponade (Madreperla et al. 1994), and postoperative prone posturing. The use of ILM peeling was thought to facilitate closure of MHs by releasing tangential traction (Gass 1988; Tognetto et al. 2006; Bainbridge et al. 2008; Ho et al. 2018), and to prevent reopening of MHs by removing the ILM scaffold for retinal surface glial cell proliferation. Such a proliferation is thought to lead to epiretinal membrane formation after the surgery (Yooh et al. 1996; Gass 1999; Yamanishi et al. 2000; Cheng et al. 2002). On the other hand, it has been suggested that beneficial glial proliferation that could facilitate hole closure could be enhanced by the surgical trauma associated with ILM peeling (Funata et al. 1992; Gass 1995; Rosa et al. 1996).
A variety of studies have been performed evaluating the success of macular hole surgery. Various prognostic factors have been defined which may influence the success of MH surgery, including various preoperative optical coherence tomography (OCT) features, such as the macular hole index (Kusuhara et al. 2004), and tractional hole index (Ruiz‐Moreno et al. 2008). Most of these previous studies assessed success based on functional outcomes (Park et al. 1998; Haritoglou et al. 2007; Gupta et al. 2009; Chang et al. 2015; Hashimoto et al. 2015), which are potentially noisy and often require large sample sizes in order to assess the result reliably. When anatomical outcomes (Park et al. 1998; Ip et al. 2002; Wakely et al. 2012) were used they were often limited to a simple binary assessment of the presence or absence of anatomical hole closure. Such a simplistic assessment, however, may be insufficient variations in outcomes among patients in a surgical trial.
Another challenge in clinical trials involving surgical interventions is variation in surgical techniques or approaches between surgeons. Once such variation in MH surgery, is the extent of the ILM peel, for which there is no consensus. Some studies have reported limited peels measuring only a disk diameter (DD, roughly 1.8 mm) centred on the hole (Haritoglou et al. 2001; Lois et al. 2008), with others describing broad peels extending to the vascular arcades. Iezzi & Kapoor (2013), for example, illustrated that broad ILM peeling can facilitate MH closure even with shorter acting gas tamponade and no face‐down positioning. Nevertheless, the role of ILM peeling in MH surgery is still controversial. Deciding whether to peel the ILM or how large an extent to peel is not trivial as several studies have reported anatomic and functional deficits following ILM removal due to damage to the adjacent inner retina, including specific injury to the retinal nerve fiber layers (RNFL) and ganglion cell layers (Diaz et al. 2014).
Thus, careful and precise evaluation of impact of the extent of ILM peeling on the outcome of macular hole surgery would appear to be of importance. In the present study, we report the results of a prospective, randomized, comparative clinical trial that was conducted to investigate the relationship between the extent of ILM peeling and anatomical outcomes, adjusting for the macular hole closure index (MHCI; Liu et al. 2016) as a baseline anatomic prognostic factor.
Materials and Methods
Description of subjects
The present study was a prospective interventional, comparative and randomized clinical trial. A total of 128 eyes, from 128 patients who were diagnosed with idiopathic MH in Peking University People's Hospital Eye Centre between June 2015 and October 2015, were enrolled. The study adhered to the Declaration of Helsinki and was approved by the Peking University People's Hospital research ethics committees and the Peking University institutional review board.
Inclusion criteria for the MH study group included: (1) confirmed diagnosis of full‐thickness idiopathic MH using an indirect ophthalmoscope and spectral domain‐OCT (SD‐OCT; Optovue, Fremont, CA, US); and (2) less than or equals to 3 years duration (based on symptoms reported by the patient). The exclusion criteria included: (1) macular hole caused by high myopia (>6 diopters) or trauma; (2) macular hole secondary to other fundus disease; (3) presence of other causes of decreased vision (e.g. corneal scarring, age‐related macular degeneration, diabetic retinopathy, glaucoma if absolute visual field defects were present or uncontrolled by medicine); (4) retinal detachment due to macular hole; (5) history of previous PPV surgery; (6) patients whose MHCI was incalculable because of poor OCT image quality. Informed consent was obtained before the surgical intervention in all patients with confirmed eligibility after the risks and benefits were described in a comprehensive face‐to‐face discussion. For patients with bilateral MHs at presentation, only the eye selected for surgery first was enrolled in the study. The study adhered to the Consolidated Standards of Reporting Trials (CONSORT) statement and was registered in the clinicaltrials.gov database (NCT02930369).
Randomization and masking procedures
Participants were randomly allocated using a random number generator in a 1:1 ratio to the 2DD peel group (the region of ILM peeling extending in a circle 2 DD (approx. 3.6 mm) in size surrounding the macular hole) and the 4DD peel group (4DD (approx. 7.2 mm) extent of ILM peel). All patients, technicians, data managers, and examining physicians were masked to treatment allocation throughout the study. Only the surgeon was unmasked, but he/she did not participate in the postoperative study evaluations.
Surgical procedures
All patients received a standard 23‐ or 25‐gauge three‐port PPV carried out by one of four experienced surgeons. After indocyanine green (1 mg/ml solution, diluted in 5% glucose (Jaycock et al. 2005; Freeman et al. 1997)) staining for 60 seconds, the ILM was peeled off the retina with a peeling diameter of 2DD or 4DD. And the intra‐operative video or photo after the ILM peeling, including both papillary and ILM peeling area was saved for the masked researchers to evaluate the ILM peel range and record the actual range of ILM peeling after the surgery. Following a complete fluid‐air exchange, an air‐gas exchange was performed with a tamponade of 20% sulfur hexafluoride (SF6). Patients were instructed to maintain a facedown position until the gas was fully absorbed, which generally took 2 weeks. Patients with clear lenses would receive MH surgery only, otherwise would receive MH surgery combined with cataract surgery and intraocular lens implantation.
Baseline and follow‐up examination procedures
Participants were assessed at baseline prior to surgery and then had study visits at 3, 6 and 12 months after surgery. For each subject, baseline demographic data was recorded, which included the gender, age, duration of the MH, and lens status (phakic, pseudophakic or aphakic). Complete ophthalmic examinations were performed at baseline, including best‐corrected (following protocol refraction) visual acuity (BCVA), slit‐lamp biomicroscropy, intraocular pressure measurement, indirect ophthalmoscopy after pupil dilation and SD‐OCT. BCVA and OCT were repeated at each follow‐up study visit. Adverse events were specifically queried for a record at each follow‐up visit. The BCVA letter scores were measured with an Early Treatment Diabetic Retinopathy Study (ETDRS) chart at 4 m by certified vision examiners. All OCT scans were obtained by a single experienced OCT technician.
For subjects who developed a persistent macular hole after surgery, patients were advised to undergo repeat surgery with or without the broader ILM peeling. Subjects who developed visually significant cataract or posterior capsular opacification were treated with surgery or Nd:YAG laser treatment, respectively. BCVA was remeasured 1 week after the cataract surgery or laser treatment, and OCT scanning was repeated if the presurgical image quality was poor. Patients who developed retinal detachment postoperatively were treated in accordance with the treating physicians practices.
The preoperative and postoperative anatomic status of the MH was quantitatively assessed on the central OCT B‐scan, using the previously described macular hole closure index (MHCI; Liu et al. 2016). The MHCI (illustrated in Fig. 1) was calculated as (M + N)/BASE where: (1) M and N are the lengths of the detached photoreceptor arms, measured as a straight line from the broken end point of the external limiting membrane (ELM) to the junction of the detached photoreceptors with retinal pigment epithelium (RPE) band; and (2) BASE is the length of the RPE not in contact with the photoreceptors (i.e. the basal diameter of the macular hole). All lengths were measured using the built‐in caliper of the oct software (Optovue, Fremont, CA, USA).
Figure 1.

Measurement of macular hole closure index (MHCI). M and N represent the straight lengths of the detached photoreceptor arms. One end is located at the broken end point of the external limiting membrane (point E) and the other end is located at the junction of the detached photoreceptors and the retinal pigment epithelium (RPE) band (point D). The BASE is measured as the length of RPE band without attached photoreceptors.
Study outcome measures
The primary outcome measure was the proportion of eyes with complete closure within the intervention groups in the anatomy on OCT at the 12 months visit postoperatively. The secondary outcome measure was the anatomical outcomes and BCVA difference at the other scheduled visits, and the difference between the anatomical outcomes and BCVA in different subgroups when subjects were stratified by baseline MHCI. Postoperative anatomical outcome at each visit was recorded according to the OCT appearance. We classified the anatomical outcomes into three grades (Liu et al. 2016; Fig. 2): grade A, in which the macular hole was closed but with a bridge‐like shape due to persistent foveolar subretinal fluid; grade B, in which the macular hole was completely closed with a normal fovea morphology; grade C, in which the macular hole was poorly closed or not closed. The “not closed” macular hole with the edge detached was considered as a failure and was recommended to receive additional surgery (Fig. 2E).
Figure 2.

Macular hole closure grading based on OCT image. (A) Grade A postoperative outcome. Note that the full‐thickness defect in the macular hole is closed, but the foveal retina has a bridge‐like shape with persistence of foveal subretinal fluid. (B) Grade B postoperative outcome. The macular hole is closed with a normal‐appearing foveal morphology. (C) Grade C1 postoperative outcome. Note that the full‐thickness defect in the macular hole is closed, but the fovea is markedly abnormal and thinner with extensive or complete loss of ellipsoid zone and external limiting membrane with a V‐shape contour. (D) Grade C2 postoperative outcome. The macular hole remains open, but the neurosensory retina at the edge of the hole demonstrates relatively complete approximation with the underlying retinal pigment epithelium (RPE). (E) Grade C3 postoperative outcome. The macular hole remains open and the edges of the neurosensory retina also remain detached from the RPE. Such a configuration was deemed to represent a surgical failure and repeat surgery was advised.
Grading of OCT outcomes was performed by two independent, masked observers, and if a disagreement was present, a final adjudication/decision was made by a third masked observer. Since a Grade A closure was thought to be a transient phenomenon, and with time and resolution of fluid would transform to a Grade B closure, both Grade A and Grade B were thought to represent evidence of “complete closure” (CC), whereas all Grade C outcomes were thought to represent “poor closure” (PC).
The average diameter of ILM peeled on both vertical and horizontal direction smaller or larger within 0.5 DD based on the randomization result was considered as following the protocol (Fig. 3).
Figure 3.

The Intraoperative clinical photograph demonstrating the protocol of internal limiting membrane peel.
Statistical analysis
A standard power calculation was performed to calculate sample sizes based on a noninferiority assumption of an expected anatomic success of 92% after surgery, with a power of 80%, 1‐sided significance level of 2.5%, and a noninferiority margin of −15%. It was calculated that an estimated sample size of 52 eyes per surgical arm was required. However, to account for expected attrition and protocol noncompliance, the final estimate for enrollment consisted of 60 patients per group. The last observation carried forward method was used to impute missing values for the intention‐to‐treat population for analysis outcomes.
A noninferiority test based on the Wald method was applied to compare the OCT‐based anatomical outcomes at 12 months between the two groups. The main hypothesis tested in the present study was accepted if the lower limit of the 95% confidence interval (CI) was higher than the noninferiority margin set at −15%. And the chi‐square (χ 2) test or Fisher's exact test was added to test for superiority when noninferiority was not revealed.
A receiver operating characteristic curve (ROC) analysis was carried out in the 2DD group to evaluate the predictive ability of the MHCI and to find the cut‐off value for the prediction of complete and poor closure. The χ 2 test and Fisher's exact tests were also used in subgroup analysis divided by cut‐off value to compare the anatomical outcomes between 2DD and 4DD group. Paired samples t‐test was used to analyse the difference between baseline BCVA and BCVA at 12 months. The independent sampled t‐test was used to analyze the postoperative BCVA between groups. And Spearman's correlation analysis was calculated to assess correlation between the BCVA and anatomical grades. All analyses were conducted using spss software version 22.0 (SPSS Inc., Chicago, IL, USA). p values <0.05 indicated statistical significance.
Results
A total of 128 eyes from 128 consecutive patients with IMH were assessed initially, but five patients did not meet inclusion criteria and were not randomized. Among the 123 randomized subjects, two were lost to follow‐up. Thus, the final cohort for analysis, consistent of 121 eyes from 121 subjects (Fig. 4), with 63 randomized into the 2DD group and 58 into the 4DD group. The baseline demographic characteristics and clinical data of the 121 eyes and the two intervention groups are summarized in Table 1. No significant differences in any baseline variable were observed between the two groups. The patients with history over 12 months in 2DD and 4DD group were 6 and 3, respectively. And MHCI was significantly correlated with history (r = −0.34, p < 0.001, Pearson correlation) The earlier 30 patients received 23‐gauge PPV (13 versus 17 in 2DD and 4DD group, respectively) and the rest patients received 25‐gauge PPV. The mean vertical and horizontal ILM peeling diameter was 2.02 ± 0.20 DD and 2.05 ± 0.25 DD in 2DD group, 3.98 ± 0.13 DD and 3.97 ± 0.16 DD in 4DD group, respectively. One eye (0.83%) was pseudophakic at the time of surgery, and 120 (99.17%) were phakic. Nineteen patients (15.7%) underwent MH surgery only, and the distribution of phakic patients underwent MH surgery only between 2DD and 4DD groups (10 patients/15.8% versus eight patients/14.0% in two groups, respectively) showed no significance (p = 0.78, χ 2 test). The rest 102 patients (84.3%) underwent MH surgery combined with cataract surgery and intraocular lens implantation. Eight patients (6.61%) had bilateral MHs.
Figure 4.

Flowchart showing the progression of patients in the study.
Table 1.
Patient baseline characteristics
| Total | 2DD group | 4DD group | p Value | |
|---|---|---|---|---|
| No. eyes/patients | 121/121 | 63/63 | 58/58 | |
| Age (years) (Mean ± SD) (Range) | 64.59 ± 6.60 (45–78) | 65.52 ± 6.28 (50–77) | 63.57 ± 6.829 (45–78) | 0.104 |
| Gender (M/F) | 31/90 | 14/49 | 17/41 | 0.372 |
| Eye (R/L) | 53/68 | 29/34 | 24/34 | 0.606 |
| Symptom duration (months) (Mean ± SD) (Range) | 4.74 ± 7.06 (0.25–36) | 5.69 ± 8.29 (0.25–30) | 3.70 ± 5.29 (0.25–36) | 0.114 |
| MHCI (Mean ± SD) (Range) | 0.67 ± 0.19 (0.30–1.35) | 0.65 ± 0.21 (0.30–1.35) | 0.65 ± 0.17 (0.35–1.01) | 0.301 |
| MHD (μm) (Mean ± SD) (Range) | 484.50 ± 202.40 (127–1050) | 476.24 ± 210.28 (127–956) | 493.48 ± 195.04 (158–1050) | 0.642 |
| MHB (μm) (Mean ± SD) (Range) | 931.45 ± 281.51 (336–1870) | 932.63 ± 287.78 (336–1480) | 935.27 ± 278.68 (368–1870) | 0.962 |
| Preoperative BCVA (ETDRS letters) (Mean ± SD) (Range) | 41.88 ± 14.97 (15–74) | 41.51 ± 15.03 (15–70) | 42.28 ± 15.01 (15–74) | 0.779 |
| Preoperative spherical equivalent error (Mean ± SD) (Range) | 0.57 ± 1.32 (−3.25 to +1.50) | 0.49 ± 1.36 (−3.25 to +1.25) | 0.61 ± 1.29 (−2.00 to +1.50) | 0.669 |
BCVA = best‐corrected visual acuity; ETDRS = Early Treatment Diabetic Retinopathy Study visual acuity charts; MHCI = Macular Hole Closure Index; MHD = Macular Hole Minimum Diameter; MHB = Macular Hole Base Diameter.
Anatomic results
The primary outcome measurement in the two groups is shown in Table 2. A total of 105 patients (86.8%) attained CC, 16 patients (13.2%) attained PC.
Table 2.
Anatomical outcomes in two treatment groups at 12 months
| 2DD group | 4DD group | Difference between two groups (95% CI) | p Value | |
|---|---|---|---|---|
| Complete closure rate | 52 (82.5%) | 53 (91.4%) | 0.45 (0.15–1.37) | 0.185 |
| Poor closure rate | 11 (17.5%) | 5 (8.6%) |
CI = confidence interval; DD = disk diameter (size of the internal limiting membrane peel).
The mean difference in the CC rates between the 2DD and 4DD treatment groups was −8.8% (95% CI, −20.7% to 3.0%; p = 0.15, determined by use of the asymptotic Wald noninferiority test) at 12 months. Thus, the noninferiority hypothesis based on a margin set at −15% of 2DD diameters ILM peeling versus 4DD diameters ILM peeling for achieving CC of the MH was insufficiently powered to be demonstrated (Fig. 5). The Fisher's exact test was supplemented to test for superiority and demonstrated no significance between the 2DD group and 4DD group in CC rate at 12 months (82.5% versus 91.4%; p = 0.185; Table 2). The distribution of anatomical closure grades of two groups at 12 months was shown in Table 3.
Figure 5.
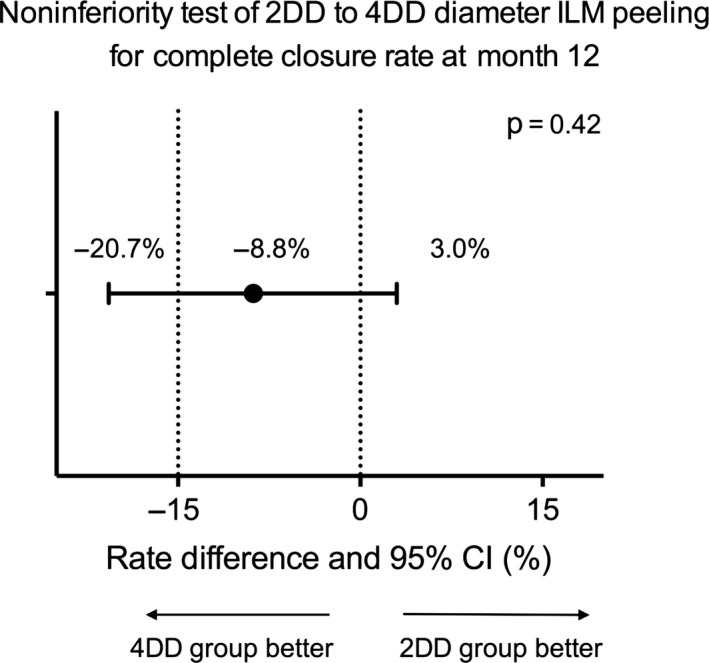
Noninferiority test of 2DD–4DD diameters ILM peeling for complete closure rate at month 12. CI = confidence interval; DD = disk diameter; ILM = internal limiting membrane.
Table 3.
Anatomical outcomes grades of two groups at 12 months
| 2DD group | 4DD group | |
|---|---|---|
| Grade B | 52 (82.54%) | 53 (91.38%) |
| Grade C1 | 4 (6.35%) | 3 (5.17%) |
| Grade C2 | 3 (4.76%) | 0 (0.0%) |
| Grade C3 | 4 (6.35%) | 2 (3.45%) |
Furthermore, we found that anatomical closure grades at follow‐up were significantly correlated with MHCI in the 2DD group (Spearman's rank correlation coefficient = −0.674, p < 0.05). Accordingly, to exclude the effect of baseline macular hole configuration on anatomical outcomes, we stratified patients by a MHCI cut‐off value, which was calculated by ROC curve within the 2DD group. First, an ROC analysis was performed to test the predictive ability of MHCI (Fig. 6). The area under the ROC curve (AUC) for the MHCI as a predictor of a CC prognosis was 0.928 (95% CI 0.839–1.00, p < 0.001). This analysis provided a MHCI cut‐off value of 0.505. The associated sensitivity and specificity were 96.2% and 81.8%. Finally, we chose 0.5 as the cut‐off value to separate CC and PC outcomes for clinical application.
Figure 6.

Receiver operating characteristic (ROC) curve for macular hole closure index (MHCI). When we analysed these grade A and grade B closure status combined together, we found the area under ROC curve was 0.928 (p < 0.05) compared with grade C closure, obtaining the MHCI cut‐off value as 0.505.
Second, the comparison analysis between the 2DD and 4DD arms were performed on the subgroups of patients with MHCI values above and below the 0.5 cutpoint (Table 4). By 12 months, within the subgroup of MHCI ≤0.5, two patients (18.2%) receiving a 2DD ILM peel achieved CC outcome, compared to 10 patients (75.9%) receiving a 4DD peel. In contrast, within the subgroup of MHCI >0.5, CC rate was 96.2% (50 patients) in the 2DD group versus 95.6% (43 patients) in the 4DD group. Overall, when MHCI was ≤0.5, the anatomic outcomes contributed to significant differences between the two treatment groups (p = 0.08, 0.036, 0.012 for Months 3, 6 and 12, respectively, Fisher's exact test). When MHCI was >0.5, the anatomic outcomes no longer differed significantly between the two treatments at any visit (p = 1.00, 1.00, 1.00 for Months 3, 6 and 12, respectively, Fisher's exact test; Table 4).
Table 4.
Anatomical outcome differences between groups at each visit following stratification by MHCI
| MHCI ≤ 0.5 | MHCI > 0.5 | |||||
|---|---|---|---|---|---|---|
| 2DD group | 4DD group | p Value | 2DD group | 4DD group | p Value | |
| 3 months N = 115 | ||||||
| CC n (%) | 1 (9.1%) | 8 (72.7%) | 0.008a | 48 (96%) | 41 (95.3%) | 1.00a |
| PC n (%) | 10 (90.9%) | 3 (27.3%) | 2 (4%) | 2 (4.7%) | ||
| 6 months N = 120 | ||||||
| CC n (%) | 2 (20%) | 9 (69.2%) | 0.036a | 50 (96.2%) | 43 (95.6%) | 1.00a |
| PC n (%) | 8 (80%) | 4 (30.8%) | 2 (3.8%) | 2 (4.4%) | ||
| 12 months N = 121 | ||||||
| CC n (%) | 2 (18.2%) | 10 (76.9%) | 0.012a | 50 (96.2%) | 43 (95.6%) | 1.00a |
| PC n (%) | 9 (81.8%) | 3 (23.1%) | 2 (3.8%) | 2 (4.4%) | ||
CC = complete closure; includes anatomical outcomes grade A and grade B; MHCI = macular hole closure index; PC = poor closure; included anatomical outcomes grade C1, grade C2 and grade C3.
2DD Group: patients underwent macular hole surgery with 2 DD (disk diameters) of internal limiting membrane (ILM) peeling.
4DD Group: patients underwent macular hole surgery with 4DD of ILM peeling.
Fisher Exact test.
Visual acuity outcomes
Best corrected visual acuity of ETDRS letters improved significantly from a mean ± SD value of 41.88 ± 14.97 (range 15–74) before surgery to 60.63 ± 12.30 (range 20–82) at 3 months (t = −13.16, p < 0.001, paired t‐test), 64.35 ± 12.85 (range 15–83) at 6 months (t = −11.41, p < 0.001, paired t‐test) and 67.60 ± 13.34 (range 20–85) at 12 months (t = −15.41, p < 0.001, paired t‐test; Table 5). The mean BCVA letters between the 2DD group and 4DD group showed no difference at 12 months: 66.57 and 68.53, respectively, (t = −0.763, p = 0.447, independent t‐test). The greatest improvement in both groups occurred between baseline and Month 3 (Fig. 7). BCVA at 12 months also showed a significant correlation with anatomical outcomes (r = −0.369, p < 0.05, Spearman's correlation), with better vision associated with complete closure type. The mean BCVA of each closure grade at 12 months was showed in Table 6. In the subgroup with MHCI ≤0.5, BCVA of 12 months showed a significant difference between the 2DD and 4DD groups (t = −2.759, p = 0.014, independent sampled t‐test). In the MHCI >0.5 subgroup, BCVA of 12 months showed no evidence of a significant difference between the two treatment groups (t = 0.208, p = 0.836, independent sampled t‐test).
Table 5.
BCVA in each visit
| MHCI ≤ 0.5 | MCHI > 0.5 | ||||||
|---|---|---|---|---|---|---|---|
| Total | 2DD group | 4DD group | p Value (95% CI) | 2DD group | 4DD group | p Value (95% CI) | |
| Baseline BCVA | 41.88 ± 14.97 (15–74) | 34.36 ± 14.69 (19–59) | 34.54 ± 19.61 (15–67) | 0.981 (−15.08 to 14.731) | 43.02 ± 14.80 (15–70) | 44.51 ± 12.82 (15–74) | 0.60 (−7.12 to 4.14) |
| BCVA at 3 months | 60.63 ± 12.30 (20–82) | 45.11 ± 14.87 (20–64) | 57.70 ± 10.86 (35–71) | 0.049 (−25.10 to −0.79) | 63.52 ± 10.82 (33–82) | 61.74 ± 11.15 (35–76) | 0.465 (−3.05 to 6.62) |
| BCVA at 6 months | 64.35 ± 12.85 (15–83) | 53.50 ± 3.42 (50–58) | 57.43 ± 19.36 (15–75) | 0.703 (−26.52 to 18.66) | 68.57 ± 8.40 (48–83) | 63.47 ± 14.03 (20–81) | 0.101 (−1.03 to 11.24) |
| BCVA at 12 months | 67.60 ± 13.34 (20–85) | 46.57 ± 19.82 (20–70) | 66.10 ± 10.35 (50–81) | 0.014 (−34.52 to −4.52) | 69.90 ± 12.38 (25–85) | 69.37 ± 10.49 (45–85) | 0.836 (−4.60 to 5.67) |
BCVA = best corrected visual acuity; MHCI = macular hole closure index.
2DD Group: patients underwent macular hole surgery with 2 DD (disk diameters) of internal limiting membrane (ILM) peeling.
4DD Group: patients underwent macular hole surgery with 4DD of ILM peeling.
Figure 7.

Improvement in best‐corrected visual acuity (BCVA) Letters From Baseline at Each Visit. (A) The improvement of all participants in BCVA letters during all visits. (B) The improvement of two intervention arms in BCVA letters at each visit separately.
Table 6.
The mean BCVA of each grade at 12 months
| N | BCVA | |
|---|---|---|
| Grade B | 105 | 68.08 ± 12.16 |
| Grade C1 | 7 | 57.86 ± 8.88 |
| Grade C2 | 3 | 47.33 ± 23.86 |
| Grade C3 | 6 | 49.33 ± 9.35 |
BCVA = best corrected visual acuity.
Adverse events
Over 12 months follow‐up, eight patients (7.8%) had post capsular opacification (PCO) among the 102 patients who underwent combined phacovitrectomy. Seven of these patients with PCO received a capsulotomy with Nd: YAG laser. Seven patients (38.9%) among the 18 phakic patients underwent MH surgery only, eventually proceeded to cataract surgery after a mean period of 13.71 months. Three patients (2.5%) developed postoperative retinal detachment which occurred at 1.5, 9 and 15 months after initial macular hole surgery. All three maintained complete closure of the MH through the last follow‐up, and all received retinal reattachment surgery.
Five patients had a persistent macular hole after the primary surgery. Two patients were assigned to the 4DD group: one had a history of MH for 36 months with a baseline MHCI value of 0.52, and the other patient had an MHCI of 0.35. These two patients received a second vitrectomy, with additional peeling of the ILM beyond 4DD, and attained successful closure of the hole. The remaining three patients were assigned to the 2DD group: two patients had a long history of MH for 24 months, including one with a small MHCI value of 0.35. These two patients underwent additional surgery with broader ILM peeling to 4DD diameters around the hole and attained complete closure. The final 2DD patient had a history of MH for 2 weeks and a MHCI value of 0.56, and underwent additional surgery, without extending the ILM peeling range. The patient attained a poor MH closure outcome where the neurosensory retina attached to the RPE, but the gap between the edges remained. No holes that were closed, reopened in the present study.
Discussion
In this randomized clinical trial of patients with idiopathic macular holes undergoing MH surgery, we observed that patients with a baseline MHCI ≤0.5, had better anatomic and visual outcomes with extended (4DD) ILM peeling compared to limited (2DD) ILM peeling. The ILM may act as a scaffold for cellular proliferation – glial cells may migrate onto the surface of the ILM and contribute to the tangential contractile force, which thought to be important in the pathogenesis of MH (Gass 1988; Tognetto et al. 2006; Bainbridge et al. 2008). Therefore, the procedure of ILM peeling is thought to release this tangential traction force and to increase retinal compliance, allowing the retina to move more freely to facilitate MH repair. Benefits of ILM peeling have been suggested by many researchers (Eckardt et al. 1997; Brooks 2000; Spiteri Cornish et al. 2013; Spiteri et al. 2014). Furthermore, a few of studies have suggested that broader and more complete ILM peeling could facilitate MH closure (Lois et al. 2008). Hejsek et al. (2014) performed enlargement of the ILM peeling region in a second surgery for those patients who initially failed MH, and obtained 100% success eventually. However, some concerns (Abdelkader & Lois 2008) have been raised about potential deleterious side‐effects of ILM peeling regarding the role of ILM in retinal function and the procedure of ILM peeling itself, particularly since the ILM represents the basal lamina of Müller cells which have a critical function in the retina. Tadayoni et al. (2001) first described the occurrence of dissociated optic nerve fiber layer after idiopathic epiretinal membrane removal, and subsequently described after ILM removal by Ito et al. (2005). Alkabes et al. (2011) observed that inner retinal defects frequently occurred after the ILM was peeled, and it consisted of numerous concentric macular dark spots in the same orientiation as the optic nerve fibers. Similar investigations have suggested that ILM peeling might reduce retinal sensitivity, and notably increase the incidence of microscotomas (Tadayoni et al. 2012). Furthermore, removal of the ILM might selectively delay the recovery of the focal macular electroretinograms b‐wave, implying some dysfunction or physiological alteration to the Müller cells of the macular region (Terasaki et al. 2001). Spaide (2012) and Amouyal et al. (2014) confirmed the presence of similar inner retinal defects resembling pits or dimples coursing along the nerve fiber layer using SD‐OCT, and showed further enlargement of the dimples in the postoperative period. Steel et al. (2017) observed that ILM peel size correlate with the shortening of the fovea to disc distance, the appearance of dissociated optic nerve fiber layer and postoperative visual acuity. Considering these observations, it would seem prudent to limit the ILM peel size necessary to achieve the maximal visual outcome. The studies mentioned above which concerned the retinal function defection caused by ILM peeling included the cases that achieved anatomical success after the surgery. Thus, achieving complete closure of MH should be a precondition when considering the detriment of ILM peeling. And the present study observed that in 4DD group, which was without limiting the surface peeled to the bare minimum, achieved more encouraging anatomical outcomes in subgroup of subjects with MHCI ≤0.5. This would appear to highlight that baseline anatomy may need to be considered when planning MH surgery.
However, several recently published studies have shown controversial results with regards to baseline anatomical characteristics. Goker et al. (2016) retrospectively analyzed 41 eyes with Gass stage 3–4 MH, which was divided into two groups based on anatomic success or failure. The multiple regression analysis showed an association between anatomical outcomes and two factors: basal MH diameter and peeled ILM range. However, limited by the retrospective intrinsic, small sample and the fact that the peeled ILM borders were only assessed using SD‐OCT, the validity of the study was weakened. In contrast, a study conducted by Modi et al. (2016) concluded that increasing the size of ILM peeling did not increase the final closure rate of MHs, irrespective of its size, duration, or staging. Even though Modi study was a prospective trial, some factors may have contributed to the apparent different result compared to our study. First, their sample size of 30 patients may have left the study underpowered to detect a difference. Second, 3‐mm‐diameter and 5‐mm‐diameter ILM peels were used for the two treatment groups (approximating sizes of two and three disc diameters). This magnitude of difference in diameters may have been too small to lead a difference in closure rates. Finally, the Modi study analysed their subgroups based on MH stage and MH size, rather than MHCI. MHCI, of course, proved to be an important factor in our study. Another study conducted by Bae et al. (2016) indicated that larger extent of ILM peeling (3 DD diameter) is beneficial to alleviate metamorphosia and asymmetric elongation of foveal tissue than smaller extent group (1.5 DD diameter). The complete closure rate of the two groups was 97.0%. However, the same anatomical outcomes of two groups might be due to the different gas selection strategy based on the size and duration of MH. For MH smaller than 400 μm with symptom duration shorter than 3 months, 25% SF6 was used. Otherwise 14% perfluoropropane gas was used. Longer duration of perfluoropropane gas sustained in vitreous cavity might improve the complete closure rate of macular hole larger than 400 μm, which leading to the comparable results of anatomical outcomes between the different ILM peeling extent groups.
We selected MHCI as the preferred index to predict anatomical prognosis based on our previous work (Liu et al. 2016). Because the preoperative configuration is another key variable that may influence MH closure rates, many studies have been performed to investigate the predictors of anatomical or visual outcomes based on baseline OCT features. Among those predictors, MH minimum diameter was popularized as a predictor of MH surgical outcomes. Ip et al. (2002), for example, observed that a MH diameter smaller than 400 μm was associated with a higher success rate following surgery. However, our previous work (Liu et al. 2016) demonstrated that MHCI showed a greater predictive power than other parameters, including MH minimum diameter, MH height,MH base diameter, the macular hole index, the diameter hole index and the tractional hole index. Thus, we chose MHCI as the indicator of prognosis in the present study, and selected the MHCI cut‐point based on the ROC curve. Within the 2DD peeling group in our present study, MHCI once again proved to be an important predictor. Our MHCI is similar to another prognostic index named hole formed factor (HFF), which was defined by Desai et al. (1999). However, limited by the resolution of time‐domain OCT (TD‐OCT), it was difficult to consistently identify microstructures such as the ELM. Thus the start point of the arm length for HFF calculation was at the axial position of minimum diameter of the MH diameter – which may or may not precisely correspond to the length of detached photoreceptors. In our study, we measured the detached photoreceptor from the broken end point of the ELM, which was reported as an important factor for survival of photoreceptor cells (Wakabayashi et al. 2009, 2010). Since the use of SD‐OCT with much higher resolution compared with TD‐OCT in the present study, we believe that the MHCI can offer more accurate information than the HFF.
Another advantage of our study, compared to previous reports is that we graded the anatomical outcomes in a more granular fashion with regards to the postoperative morphology. Using our grading system, the primary complete closure (defined as closure with normal foveal morphology) rate for our study was 86.8%. Postoperative anatomic success rates in previous studies vary between 86% and 100% in the literature (Tornambe et al. 1997; Park et al. 1999; Tadayoni et al. 2006; Kapoor et al. 2012), but the morphology of the closed holes was generally not subdivided in detail. If we do not subdivide the closed hole morphology, the primary closure rate in our study was 95%.
The postoperative BCVA improved significantly at each visit point. And BCVA at the 12 months visit was correlated with anatomy, patients with complete MH closure could achieve better BCVA than those with poor closure configurations. This further highlights that achieving complete MH closure (and not simply closure) should be the primary goal of MH surgery. What's more, the patients received 23‐ or 25‐gauge PPV carried out by four different surgeons might be a bias for the outcomes. The earlier 30 patients enrolled received 23‐gauge PPV and the rest of patients received 25‐gauge PPV, which might influence the outcome. However, Kusuhara et al. (2008) proved that both 23‐ and 25‐gauge PPV were safe and effective in MH surgery. And the surgically‐induced astigmatism was corrected by testing BCVA at each visit to eliminate the difference between the patients in the present study. And to reduce the bias induced by different surgeons, the surgeons reached agreement on the surgical protocol to ensure the MH surgery procedure was standardized.
In analysing the reasons why five patients failed to achieve closure with primary surgery, a longer duration, smaller MHCI, and a smaller ILM peeling range were thought to be important, but the small number of cases makes it difficult to draw any definitive conclusions. Although the history between the two groups was comparable, the patients with history more than 12 months differed between the two groups, which might affect the results. Of note, when the ILM peeling range was extended in the second operation, complete closure was obtained in these cases which would seem to in accordance with previous reports from Eckardt et al. (1997) and Iezzi & Kapoor (2013).
The present study has several limitations. First, the calculation of sample was based on the simple anatomic success rate rather than the complete closure rate since such data were absent in present literature. However, the primary outcome in the present study was a complete closure rate, which was lower than the success rate, leading to an underestimation of the sample size. And the smaller sample size might weaken the validity of the conclusion. Second, the small sample of 24 patients in the subgroup of MHCI ≤0.5 may have led to a lack of statistical difference between the 2DD and 4DD groups with regards to anatomical outcomes when the Fisher's exact test was applied. And the sample of patients with MHCI ≤0.5 was too small to draw an established conclusion. To address this issue, we can stratify the patients into different subgroups divided by MHCI cut‐off value preoperatively and then assign patients randomly in subsequent studies. Furthermore,the present study is a single centre clinical trial. Thus, a multi‐centred, prospective, randomized and controlled clinical trial with large sample should be conducted to replicate our results and to demonstrate more established conclusion. In addition, most literatures excluded patients with history of >12 months, however, the present study included nine eyes with history more than 12 months. Since the history is correlated with the MHCI, in order to enroll patients with smaller MHCI, the present study defined the inclusion criteria of history within 36 months, which might be a confounding factor for the results. Finally, MHCI had to be manually calculated by trained OCT graders in our study. To make MHCI a more clinical practical parameter, it will need to be developed as an automatic tool built into OCT instruments.
In conclusion, our study demonstrates that more extensive ILM peel in those MHs with MHCI ≤0.5 can achieve better anatomic and visual outcome in MH surgery. And for those MH with MHCI >0.5, ILM peeling area with 2DD diameters is sufficient to achieve satisfactory anatomical and visual outcomes. This highlights the potential benefit of careful assessment of preoperative OCT anatomy in order to individualize and optimize surgical planning for MH surgery.
This work was supported by the Beijing Municipal Science and Technology Commission (Capital Characteristic Clinic Applied Research Project, Z161100000516037) and National Natural Science Foundation of China Grant (81770943, 81470651). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
The authors thank Xiulan Xu, Yujing Bai for their technologic support in the trial conduction.
Research design: Mingwei Zhao, Chongya Dong. Acquisition, analysis and interpretation of data: All authors. Wrote or contributed to writing the manuscript: Yuou Yao, Jinfeng Qu. Reviewed the manuscript: Mingwei Zhao. Critical revised the manuscript: Srinivas R. Sadda. Statistical analysis: Yuou Yao, Chongya Dong. Grant obtained: Mingwei Zhao. Administrative, technical, or material support: All authors. Study supervision: Mingwei Zhao, Xiaoxin Li, Jianhong Liang, Hong Yin. Mingwei Zhao had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Jinfeng Qu contributed equally to this work and should be considered as the co‐first author. Srinivas R. Sadda contributed equally to this work and should be viewed as corresponding author.
References
- Abdelkader E & Lois N (2008): Internal limiting membrane peeling in vitreo‐retinal surgery. Surv Ophthalmol 53: 368–396. [DOI] [PubMed] [Google Scholar]
- Alkabes M, Salinas C, Vitale L, Burés‐Jelstrup A, Nucci P & Mateo C (2011): En face optical coherence tomography of inner retinal defects after internal limiting membrane peeling for idiopathic macular hole. Invest Ophthalmol Vis Sci 52: 8349–8355. [DOI] [PubMed] [Google Scholar]
- Amouyal F, Shah SU, Pan CK, Schwartz SD & Hubschman JP (2014): Morphologic features and evolution of inner retinal dimples on optical coherence tomography after internal limiting membrane peeling. Retina 34: 2096–2102. [DOI] [PubMed] [Google Scholar]
- Bae K, Kang SW, Kim JH, Kim SJ, Kim JM & Yoon JM (2016): Extent of internal limiting membrane peeling and its impact on macular hole surgery outcomes: a randomized trial. Am J Ophthalmol 169: 179–188. [DOI] [PubMed] [Google Scholar]
- Bainbridge J, Herbert E & Gregor Z (2008): Macular holes: vitreoretinal relationships and surgical approaches. Eye 22: 1301–1309. [DOI] [PubMed] [Google Scholar]
- Brooks HL (2000): Macular hole surgery with and without internal limiting membrane peeling. Ophthalmology 107: 1939–1948. [DOI] [PubMed] [Google Scholar]
- Chang YC, Lin WN, Chen KJ, Wu HJ, Lee CL, Chen CH, Wu KY & Wu WC (2015): Correlation between the dynamic postoperative visual outcome and the restoration of foveal microstructures after macular hole surgery. Am J Ophthalmol 160: 100–106. e101. [DOI] [PubMed] [Google Scholar]
- Cheng L, Azen SP, El‐Bradey MH, Toyoguchi M, Chaidhawangul S, Rivero ME, Scholz BM & Freeman WR (2002): Effects of preoperative and postoperative epiretinal membranes on macular hole closure and visual restoration. Ophthalmology 109: 1514–1520. [DOI] [PubMed] [Google Scholar]
- Desai VN, Hee MR & Puliafito CA (1999): Optical coherence tomography of macular holes In: Mad‐reperla SA. & MzCuen BW. (eds). Macular hole: pathogenesis, diagnosis and treatment. Oxford: Butterworth‐Heinemann; 37–47. [Google Scholar]
- Diaz RI, Randolph JC, Sigler EJ & Calzada JI (2014): Intraoperative grasp site correlation with morphologic changes in retinal nerve fiber layer after internal limiting membrane peeling. Ophthalmic Surg Lasers Imaging Retina 45: 45–49. [DOI] [PubMed] [Google Scholar]
- Eckardt C, Eckardt U, Groos S, Luciano L & Reale E (1997): [Removal of the internal limiting membrane in macular holes. Clinical and morphological findings]. Ophthalmologe 94: 545–551. [DOI] [PubMed] [Google Scholar]
- Freeman WR, Azen SP, Kim JW, Elhaig W, Mishell DR 3rd & Bailey I (1997): Vitrectomy for the treatment of full‐thickness stage 3 or 4 macular holes. Results of a multicentered randomized clinical trial. The Vitrectomy for Treatment of Macular Hole Study Group. Arch Ophthalmol 115: 11–21. [DOI] [PubMed] [Google Scholar]
- Funata M, Wendel R, La Cruz Z & Green W (1992): Clinicopathologic study of bilateral macular holes treated with pars plana vitrectomy and gas tamponade. Retina 12: 289–298. [DOI] [PubMed] [Google Scholar]
- Gass JD (1988): Idiopathic senile macular hole. Its early stages and pathogenesis. Arch Ophthalmol 106: 629–639. [DOI] [PubMed] [Google Scholar]
- Gass JD (1995): Reappraisal of biomicroscopic classification of stages of development of a macular hole. Am J Ophthalmol 119: 752–759. [DOI] [PubMed] [Google Scholar]
- Gass JDM (1999): Müller cell cone, an overlooked part of the anatomy of the fovea centralis: hypotheses concerning its role in the pathogenesis of macular hole and foveomacular retinoschisis. Arch Ophthalmol 117: 821–823. [DOI] [PubMed] [Google Scholar]
- Goker YS, Koc M, Yuksel K, Yazici AT, Demir A, Gunes H & Ozpinar Y (2016): Relationship between peeled internal limiting membrane area and anatomic outcomes following macular hole surgery: a quantitative analysis. J Ophthalmol 2016: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta B, Laidlaw DAH, Williamson TH, Shah SP, Wong R & Wren S (2009): Predicting visual success in macular hole surgery. Br J Ophthalmol 93: 1488–1491. [DOI] [PubMed] [Google Scholar]
- Haritoglou C, Gass CA, Schaumberger M, Ehrt O, Gandorfer A & Kampik A (2001): Macular changes after peeling of the internal limiting membrane in macular hole surgery. Am J Ophthalmol 132: 363–368. [DOI] [PubMed] [Google Scholar]
- Haritoglou C, Neubauer AS, Reiniger IW, Priglinger SG, Gass CA & Kampik A (2007): Long‐term functional outcome of macular hole surgery correlated to optical coherence tomography measurements. Clin Exp Ophthalmol 35: 208–213. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Saito W, Fujiya A et al. (2015): Changes in inner and outer retinal layer thicknesses after vitrectomy for idiopathic macular hole: implications for visual prognosis. PLoS ONE 10: e0135925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejsek L, Dusova J, Stepanov A & Rozsival P (2014): Re‐operation of idiopathic macular hole after failed initial surgery. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 158: 596–599. [DOI] [PubMed] [Google Scholar]
- Ho TC, Ho A & Chen MS (2018): Vitrectomy with a modified temporal inverted limiting membrane flap to reconstruct the foveolar architecture for macular hole retinal detachment in highly myopic eyes. Acta Ophthalmol 96: e46–e53. [DOI] [PubMed] [Google Scholar]
- Iezzi R & Kapoor KG (2013): No face‐down positioning and broad internal limiting membrane peeling in the surgical repair of idiopathic macular holes. Ophthalmology 120: 1998–2003. [DOI] [PubMed] [Google Scholar]
- Ip MS, Baker BJ, Duker JS, Reichel E, Baumal CR, Gangnon R & Puliafito CA (2002): Anatomical outcomes of surgery for idiopathic macular hole as determined by optical coherence tomography. Arch Ophthalmol 120: 29–35. [DOI] [PubMed] [Google Scholar]
- Ito Y, Terasaki H, Takahashi A, Yamakoshi T, Kondo M & Nakamura M (2005): Dissociated optic nerve fiber layer appearance after internal limiting membrane peeling for idiopathic macular holes. Ophthalmology 112: 1415–1420. [DOI] [PubMed] [Google Scholar]
- Jaycock PD, Bunce C, Xing W, Thomas D, Poon W, Gazzard G, Williamson TH & Laidlaw DA (2005): Outcomes of macular hole surgery: implications for surgical management and clinical governance. Eye (Lond) 19: 879–884. [DOI] [PubMed] [Google Scholar]
- Kapoor KG, Khan AN, Tieu BC & Khurshid GS (2012): Revisiting autologous platelets as an adjuvant in macular hole repair: chronic macular holes without prone positioning. Ophthalmic Surg Lasers Imaging 43: 291–295. [DOI] [PubMed] [Google Scholar]
- Kelly NE & Wendel RT (1991): Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol 109: 654–659. [DOI] [PubMed] [Google Scholar]
- Kusuhara S, Escaño MFT, Fujii S et al. (2004): Prediction of postoperative visual outcome based on hole configuration by optical coherence tomography in eyes with idiopathic macular holes. Am J Ophthalmol 138: 709–716. [DOI] [PubMed] [Google Scholar]
- Kusuhara S, Ooto S, Kimura D et al. (2008): Outcomes of 23‐ and 25‐gauge transconjunctival sutureless vitrectomies for idiopathic macular holes. Br J Ophthalmol 92: 1261–1264. [DOI] [PubMed] [Google Scholar]
- Liu P, Sun Y, Dong C et al. (2016): A new method to predict anatomical outcome after idiopathic macular hole surgery. Graefes Arch Clin Exp Ophthalmol 254: 683–688. [DOI] [PubMed] [Google Scholar]
- Lois N, Burr J, Norrie J, Vale L, Cook J & McDonald A (2008): Clinical and cost‐effectiveness of internal limiting membrane peeling for patients with idiopathic full thickness macular hole. Protocol for a Randomised Controlled Trial: FILMS (Full‐thickness macular hole and Internal Limiting Membrane peeling Study). Trials 9: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madreperla SA, Geiger GL, Funata M, de la Cruz Z & Green WR (1994): Clinicopathologic correlation of a macular hole treated by cortical vitreous peeling and gas tamponade. Ophthalmology 101: 682–686. [DOI] [PubMed] [Google Scholar]
- Modi A, Giridhar A & Gopalakrishnan M (2016): Comparative analysis of outcomes with variable diameter internal limiting membrane peeling in surgery for idiopathic macular hole repair. Retina 37: 265–273. [DOI] [PubMed] [Google Scholar]
- Olsen TW, Sternberg P Jr, Capone A Jr, Martin DF, Lim JI, Grossniklaus HE & Aaberg TM Sr (1998): Macular hole surgery using thrombin‐activated fibrinogen and selective removal of the internal limiting membrane. Retina 18: 322–329. [DOI] [PubMed] [Google Scholar]
- Park DW, Lee JH & Min WK (1998): The use of internal limiting membrane maculorrhexis in treatment of idiopathic macular holes. Korean J Ophthalmol 12: 92–97. [DOI] [PubMed] [Google Scholar]
- Park DW, Sipperley J, Sneed S, Dugel P & Jacobsen J (1999): Macular hole surgery with internal‐limiting membrane peeling and intravitreous air. Ophthalmology 106: 1392–1398. [DOI] [PubMed] [Google Scholar]
- Rosa RH, Glaser BM, Cruz ZDL & Green WR (1996): Clinicopathologic correlation of an untreated macular hole and a macular hole treated by vitrectomy, transforming growth factor‐β2, and gas tamponade. Am J Ophthalmol 122: 853–863. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Moreno JM, Staicu C, Piñero DP, Montero J, Lugo F & Amat P (2008): Optical coherence tomography predictive factors for macular hole surgery outcome. Br J Ophthalmol 92: 640–644. [DOI] [PubMed] [Google Scholar]
- Spaide RF (2012): “Dissociated optic nerve fiber layer appearance” after internal limiting membrane removal is inner retinal dimpling. Retina 32: 1719–1726. [DOI] [PubMed] [Google Scholar]
- Spiteri Cornish K, Lois N, Scott N et al. (2013): Vitrectomy with internal limiting membrane (ILM) peeling versus vitrectomy with no peeling for idiopathic full‐thickness macular hole (FTMH). Cochrane Database Syst Rev 6(6): CD009306. [DOI] [PubMed] [Google Scholar]
- Spiteri CK, Lois N, Scott NW et al. (2014): Vitrectomy with internal limiting membrane peeling versus no peeling for idiopathic full‐thickness macular hole. Ophthalmology 121: 649–655. [DOI] [PubMed] [Google Scholar]
- Steel DHW, Chen Y, Latimer J et al. (2017): Does internal limiting membrane peeling size matter? J Vitreoretin Dis 1: 27–33. [Google Scholar]
- Tadayoni R, Paques M, Massin P, Mouki‐Benani S, Mikol J & Gaudric A (2001): Dissociated optic nerve fiber layer appearance of the fundus after idiopathic epiretinal membrane removal. Ophthalmology 108: 2279–2283. [DOI] [PubMed] [Google Scholar]
- Tadayoni R, Gaudric A, Haouchine B & Massin P (2006): Relationship between macular hole size and the potential benefit of internal limiting membrane peeling. Br J Ophthalmol 90: 1239–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadayoni R, Svorenova I, Erginay A, Gaudric A & Massin P (2012): Decreased retinal sensitivity after internal limiting membrane peeling for macular hole surgery. Br J Ophthalmol 96: 1513–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki H, Miyake Y, Nomura R, Piao CH, Hori K, Niwa T & Kondo M (2001): Focal macular ERGs in eyes after removal of macular ILM during macular hole surgery. Invest Ophthalmol Vis Sci 42: 229–234. [PubMed] [Google Scholar]
- Tognetto D, Grandin R, Sanguinetti G, Minutola D, Di NM, Di MR & Ravalico G (2006): Internal limiting membrane removal during macular hole surgery: results of a multicenter retrospective study. Ophthalmology 113: 1401–1410. [DOI] [PubMed] [Google Scholar]
- Tornambe PE, Poliner LS & Grote K (1997): Macular hole surgery without face‐down positioning. A pilot study. Retina 17: 179–185. [DOI] [PubMed] [Google Scholar]
- Wakabayashi T, Oshima YH, Murakami Y, Sakaguchi H, Kusaka S & Tano Y (2009): Foveal microstructure and visual acuity after retinal detachment repair: imaging analysis by Fourier‐domain optical coherence tomography. Ophthalmology 116: 519–528. [DOI] [PubMed] [Google Scholar]
- Wakabayashi T, Fujiwara M, Sakaguchi H, Kusaka S & Oshima Y (2010): Foveal microstructure and visual acuity in surgically closed macular holes: spectral‐domain optical coherence tomographic analysis. Ophthalmology 117: 1815–1824. [DOI] [PubMed] [Google Scholar]
- Wakely L, Rahman R & Stephenson J (2012): A comparison of several methods of macular hole measurement using optical coherence tomography, and their value in predicting anatomical and visual outcomes. Br J Ophthalmol 96: 1003–1007. [DOI] [PubMed] [Google Scholar]
- Yamanishi S, Oshima Y, Emi K & Motokura M (2000): Optical cross‐sectional evaluation of successfully repaired idiopathic macular holes by retinal thickness analyzer. Retina 20: 450–458. [DOI] [PubMed] [Google Scholar]
- Yooh H, Brooks H, Capone A, L'hernault N & Grossniklaus H (1996): Ultrastructural features of tissue removed during idiopathic macular hole surgery. Am J Ophthalmol 122: 67–75. [DOI] [PubMed] [Google Scholar]


