Abstract
Aim
Cerebral hypoxia has been associated with neurodevelopmental impairment. We studied whether reducing cerebral hypoxia in extremely preterm infants during the first 72 hours of life affected neurological outcomes at two years of corrected age.
Methods
In 2012‐2013, the phase II randomised Safeguarding the Brains of our smallest Children trial compared visible cerebral near‐infrared spectroscopy (NIRS) monitoring in an intervention group and blinded NIRS monitoring in a control group. Cerebral hypoxia was significantly reduced in the intervention group. We followed up 115 survivors from eight European centres at two years of corrected age, by conducting a medical examination and assessing their neurodevelopment with the Bayley Scales of Infant and Toddler Development, Second or Third Edition, and the parental Ages and Stages Questionnaire (ASQ).
Results
There were no differences between the intervention (n = 65) and control (n = 50) groups with regard to the mean mental developmental index (89.6 ± 19.5 versus 88.4 ± 14.7, p = 0.77), ASQ score (215 ± 58 versus 213 ± 58, p = 0.88) and the number of children with moderate‐to‐severe neurodevelopmental impairment (10 versus six, p = 0.58).
Conclusion
Cerebral NIRS monitoring was not associated with long‐term benefits or harm with regard to neurodevelopmental outcome at two years of corrected age.
Keywords: Ages and stages questionnaire, Bayley scales of infant and toddler development, Cerebral near‐infrared spectroscopy, Extremely preterm infants, Neurodevelopment
Abbreviations
- ASQ
Ages and stages questionnaire
- CP
Cerebral palsy
- GMFCS
Gross motor function classification system
- MDI
Mental developmental index
- NIRS
Near‐infrared spectroscopy
Key notes.
We studied whether reducing cerebral hypoxia in extremely preterm infants during the first 72 hours of life affected neurological outcomes at two years of corrected age.
The Safeguarding the Brains of our Smallest Children trial followed up 115 survivors from eight European centres by assessing their neurodevelopment.
Cerebral NIRS monitoring was not associated with long‐term benefits or harm with regard to neurodevelopment at two years of corrected age.
Introduction
Being born extremely preterm carries a 20% risk of death, and 25% of survivors have moderate‐to‐severe neurodevelopmental impairment, namely cerebral palsy (CP) or cognitive impairment 1. The respiratory and circulatory systems of extremely preterm infants are immature, and cerebral autoregulation is impaired, especially during the transition from intrauterine to extrauterine life 2, 3, 4. In addition, oxygen transport may be impaired by low haemoglobin levels 5 and this makes the developing premature brain susceptible to fluctuations in cerebral blood flow 6. In addition, haemodynamic instability may cause episodes of cerebral hypoxia and hyperoxia, which have been associated with intracranial haemorrhage, ischaemic lesions and later neurodevelopmental impairment 7. The phase II randomised Safeguarding the Brains of our smallest Children (SafeBoosC II) clinical trial demonstrated the possibility of reducing the burden of cerebral hypoxia and hyperoxia during the first 72 hours of life 8 and found that only hypoxia was reduced. The aim of this follow‐up study was to assess the neurodevelopmental outcomes of infants included in the SafeBoosC II trial when they reached two years of corrected age.
Methods
Patients
Between June 2012 and December 2013, 166 extremely preterm infants from eight European countries – Austria, Denmark, France, Ireland, Italy, Spain, the Netherlands and the United Kingdom – were included in the SafeBoosC II trial 8. The infants were randomised to either experimental or control groups before they were three hours of age, and both groups were monitored with continuous cerebral tissue oxygenation by near‐infrared spectroscopy (NIRS). In the intervention group, the cerebral oxygenation level was visible and the target range was set at 55% to 85%. The clinicians were provided with a dedicated treatment guideline listing possible interventions if the cerebral oxygenation was out of range 9. In the control group, the cerebral oxygenation levels were recorded, but the clinicians were blinded to the results and the infants were given standard treatment and care. Cerebral monitoring was conducted from three hours until 72 hours after birth. The burden of cerebral hypoxia and hyperoxia was reduced by 58% in the intervention group, with a 95% confidence interval (95% CI) of 35%–73% (p < 0.001) 8. The burden of cerebral hypoxia and hyperoxia was calculated as the time spent below or above the target limits, multiplied by the mean deviation from the lower (55%) or the upper limit (85%) during the first 72 hours of life, expressed in the percentage of hours. The burden was computed from un‐edited NIRS values and extrapolated to 72 hours, without the knowledge of any other outcomes of the trial. The protocol of the SafeBoosC II trial has been published 10 and is available in full at www.safeboosc.eu. The trial was registered at ClinicalTrial.gov (NCT01590316).
Follow‐up at two years of corrected age
At a corrected age of two years, the children were invited to participate in the SafeBoosC II follow‐up study. Their neurodevelopment was assessed with the Bayley Scales of Infant and Toddler Development, Second Edition (Bayley‐II) or Third Edition (Bayley‐III), depending on what version the centre was using at the time of the study. In addition, their parents were asked to complete the Ages and Stages Questionnaire (ASQ) and the children also underwent a medical examination.
The Bayley‐II and Bayley‐III tests
At the corrected age of 23–25 months, the children underwent the Bayley‐II or Bayley‐III test. If the children were ill, tired or unable to be assessed on the day of the test for any other reason, the parents were offered a new consultation. We combined the results of the Bayley‐II and Bayley‐III tests by converting the Bayley‐III language and cognitive scores into a predicted mental developmental index (MDI) score according to a previously published algorithm 11. The psychologists conducting the tests were blinded to which group the infant was in.
The ASQ
The ASQ is a set of parental questionnaires covering the development of children aged from four months to five years. Each questionnaire contains five developmental domains, which each provides six questions covering communications, gross motor skills, fine motor skills, problem solving and personal–social skills. Previously, the agreement between ASQ and concurrent assessment by Bayley third edition has been good and ASQ may be a substitute for Bayley test in cases or at centres where the Bayley test is not possible 12. The ASQ was originally developed as a screening tool, and infants with normal development achieve near maximum scores, resulting in an overestimation of the mean. Therefore, the ASQ for slightly older children, aged 25 months and 16 days to 28 months and 15 days, was chosen to achieve normal distribution of the data 13. At the corrected age of 23–25 months, the ASQ was sent to the parents together with a prestamped envelope so that they could return questionnaire. The parents were not blinded to the intervention.
The medical examination
Basic growth measurements were collected, namely head circumference, height and weight, and vision and auditory functions were evaluated. If the children showed signs of cerebral palsy, the gross motor function was assessed using the Gross Motor Function Classification System (GMFCS). The doctor performing the medical examination was not blinded to the intervention.
Neurodevelopmental impairment was classified according to classification of health status at two years as a perinatal outcome, which was developed by the British Association of Perinatal Medicine 14. The child was diagnosed with severe neurodevelopmental disability if any of the following conditions were present: CP with a GMFCS score of 3–5; a cognitive function score below ‐3 standard deviations scores (MDI below 55); hearing impairment with no useful hearing even with aids; no meaningful words or signs; and blind or only able to perceive light or light‐reflecting objects. The child was diagnosed with moderate neurodevelopmental disability if any of the following conditions were present: CP with a GMFCS score of 1–2; a cognitive function score between ‐3 and ‐2 standard deviations scores (MDI 55–70); hearing impairment, but useful hearing with aids; fewer than five words or signs; and moderately impaired vision.
Statistical methods
The baseline characteristics and the two‐year outcomes were compared between the two intervention groups using unpaired t‐tests or the chi‐square test where appropriate. All p values are two‐tailed.
Ethics
The SafeBoosC II trial was approved by each hospital's research ethics committee: the Hopital Femme Mere Enfants, Lyon, France; the Rigshospitalet, Copenhagen, Denmark; the La Paz University Hospital, Madrid, Spain; the Cork University Maternity Hospital, Cork, Ireland; the Wilhelmina Children's Hospital, Utrecht, the Netherlands; the Medical University of Graz, Graz, Austria; the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy, and the Rosie Hospital, Cambridge University Hospitals, the United Kingdom. Austria, Denmark, and France also required the approval of the authority responsible for medical devices. Written, informed parental consent was obtained before inclusion in the trial, and the parents gave their consent for the data collection and follow‐up before randomisation.
Results
Of the 166 extremely preterm infants included in the SafeBoosC II trial, 32 died before their expected date of delivery and three more died shortly afterwards. Of the remaining 131 children, the two‐year follow‐up was conducted in 115 children (Fig. 1). The 65 infants in the intervention group were predominantly male (54%), 24% were twins, and they had an average gestational age of 26.6 ± 1.1 weeks and average birthweight of 863 ± 208 grams. The 50 infants in the control group were predominantly female (62%), 22% were twins, and they had an average gestational age of 26.7 ± 1.2 weeks and an average birthweight of 901 ± 204 g (Table 1).
Figure 1.
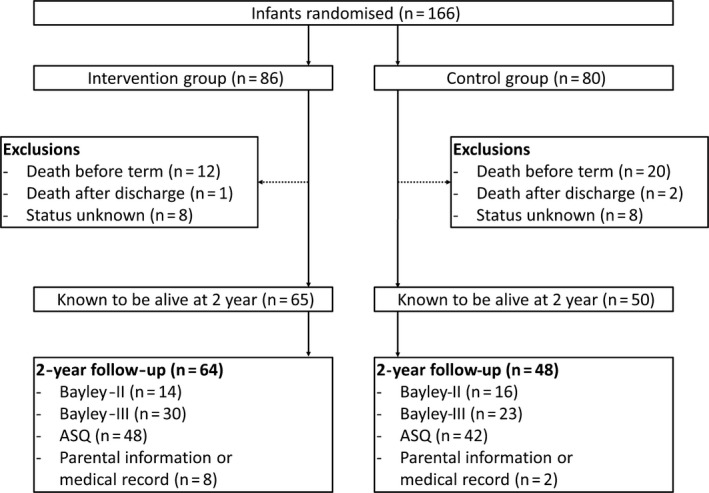
Trial participant flow in the SafeBoosC II clinical trial from randomisation within three hours of birth to follow‐up at two years of corrected age.
Table 1.
Baseline characteristics, burden of cerebral hypoxia and hyperoxia and neonatal complications in the intervention and control group
| Intervention (n = 65) | Control (n = 50) | p value | |
|---|---|---|---|
| Baseline characteristics | |||
| Gestational age (w) | 26.6 ± 1.1 | 26.7 ± 1.2 | |
| Birthweight (g) | 868 ± 208 | 901 ± 204 | |
| Male sex | 54 | 38 | |
| Twins | 24 | 22 | |
| Prenatal steroids | 72 | 81 | |
| Prolonged rupture of membranes | 26 | 40 | |
| Maternal clinical chorioamnionitis | 6 | 6 | |
| Apgar score <5 at five minutes | 18 | 16 | |
| Burden of cerebral hypoxia and hyperoxia | |||
| Burden of cerebral hypoxia (%h)* | 16 (4–63) | 50 (16–122) | <0.01 |
| Burden of cerebral hyperoxia (%h)* | 1 (0.2–10) | 0.7 (0.1–14) | 0.83 |
| Total burden of cerebral hypoxia and hyperoxia (%h)* | 32 (9–78) | 68 (38–143) | <0.01 |
| Neonatal complications | |||
| Severe brain injury on cUS** | 11 | 16 | 0.58 |
| Necrotising enterocolitis | 11 | 10 | >0.99 |
| Bronchopulmonary dysplasia | 57 | 48 | 0.35 |
| Retinopathy of prematurity | 18 | 10 | 0.29 |
Values are percentages unless stated otherwise.
*Median and (IQR) p values after log transformation.
**Severe injury was defined as intraventricular haemorrhage grade III, posthaemorrhagic ventricular dilatation, parenchymal/periventricular haemorrhagic infarction, unilateral porencephalic cysts, cystic periventricular leucomalacia (bilateral), cerebellar haemorrhage, cerebral atrophy at term age or stroke 18.
Because we were unable to obtain any information about 16 of the 131 children, their status was recorded as unknown (Table 2). However, we do know that they had significantly fewer antenatal steroids and were more likely to have been born after prolonged rupture of the membranes than the 115 children who were followed up. There was also a tendency towards a lower mean gestational age (26.1 ± 1.1 weeks) in the children with unknown status at two years of age (Table 2). The flow of patients from inclusion to the two‐year follow‐up is presented in Figure 1.
Table 2.
Baseline characteristics, burden of cerebral hypoxia and hyperoxia and neonatal complications in the infants with and without follow‐up at two years
| Follow‐up (n = 115) | No follow‐up (n = 16) | p value | |
|---|---|---|---|
| Baseline characteristics | |||
| Gestational age (w) | 26.7 (1.1) | 26.1 (1.1) | 0.06 |
| Birthweight (g) | 882 (206) | 851 (172) | 0.56 |
| Male sex | 53 | 63 | 0.29 |
| Twins | 23 | 6 | 0.19 |
| Prenatal steroids | 76 | 44 | <0.05 |
| Prolonged rupture of membranes | 32 | 69 | <0.05 |
| Maternal clinical chorioamnionitis | 6 | 13 | 0.28 |
| Apgar score <5 at Five minutes | 17 | 7 | 0.46 |
| Burden of cerebral hypoxia and hyperoxia | |||
| Burden of cerebral hypoxia (% of hours)* | 27 (8–78) | 10 (4–86) | 0.29 |
| Burden of cerebral hyperoxia (% of hours)* | 1 (0.1–13) | 12 (3–32) | <0.05 |
| Total burden of cerebral hypoxia and hyperoxia (% of hours)* | 50 (15–102) | 42 (20–101) | 0.88 |
| Neonatal complications | |||
| Severe brain injury on cranial ultrasound** | 14 | 7 | 0.69 |
| Necrotising enterocolitis | 10 | 13 | 0.68 |
| Bronchopulmonary dysplasia | 53 | 36 | 0.26 |
| Retinopathy of prematurity | 15 | 13 | >0.99 |
Values are percentages unless stated otherwise.
*Median and (IQR) p values after log transformation.
**Severe injury was defined as intraventricular haemorrhage grade III, posthaemorrhagic ventricular dilatation, parenchymal/periventricular haemorrhagic infarction, unilateral porencephalic cysts, cystic periventricular leucomalacia (bilateral), cerebellar haemorrhage, cerebral atrophy at term age or stroke 18.
As expected in the infants followed up at two years of corrected age, there was a significantly lower burden of total cerebral hypoxia and hyperoxia in the intervention group (32% of hours) versus the control group (68% of hours, p < 0.01), whereas there were no differences in other patient characteristics (Table 1). The Bayley‐II and Bayley‐III and the ASQ findings did not differ between the groups (Table 3), and the number of infants with moderate‐to‐severe developmental delay was similar, at 10 (15%) in the intervention group and six (12%) in the control group (p = 0.58). The combined mean MDI did not differ between the groups, with a score of 89.l6 ± 19.5 for the intervention group and 88.4 ± 14.7 for the control group (p = 0.77).
Table 3.
Follow‐up at two years of corrected age using Bayley‐II, Bayley‐III and ASQ
| Intervention | Control | p value | |
|---|---|---|---|
| Bayley‐II (n) | 14 | 16 | |
| Age at Bayley‐II (months) | 25.0 (1.4) | 24.8 (1.1) | 0.65 |
| Mental Developmental Index | 96.9 (13.9) | 86.4 (13.8) | 0.05 |
| Psychomotor Developmental Index | 83.9 (14.3) | 87.4 (16.0) | 0.54 |
| Bayley‐III (n) | 30 | 23 | |
| Age at Bayley‐III (months) | 24.3 (3.6) | 25.2 (2.9) | 0.34 |
| Cognitive index | 95.8 (19.9) | 97.6 (10.2) | 0.69 |
| Language index | 94.4 (18.8) | 95.6 (13.4) | 0.81 |
| Motor index | 91.8 (15.6) | 99.1 (9.2) | 0.07 |
| Predicted Mental Developmental Index* | 85.6 (21.1) | 90.0 (15.4) | 0.43 |
| Ages and Stages Questionnaire (n) | 48 | 42 | |
| Age at ASQ (months) | 25.7 (3.3) | 25.6 (3.4) | 0.88 |
| Gross motor score | 39.7 (15.9) | 44.5 (14.6) | 0.14 |
| Fine motor score | 42.7 (14.9) | 44.3 (10.9) | 0.57 |
| Communication score | 46.1 (14.4) | 40.6 (15.7) | 0.08 |
| Personal social score | 43.4 (14.1) | 41.4 (13.2) | 0.48 |
| Problem solving score | 43.3 (12.3) | 42.6 (13.8) | 0.80 |
| Total ASQ score | 215.3 (58.0) | 213.4 (58.4) | 0.88 |
| Medical examination (n) | 45 | 37 | |
| Age (months) | 24.6 (3.1) | 24.8 (2.3) | 0.69 |
| Head circumference (centimetres) | 48.0 (1.8) | 48.1 (1.6) | 0.75 |
| Weight (kilograms) | 11.2 (1.7) | 11.0 (1.4) | 0.85 |
| Height (centimetres) | 85.4 (4.6) | 85.0 (4.8) | 0.67 |
| Cerebral palsy (number) | 3 | 2 | >0.99 |
| GMFCS for patients with CP** | 1, 2, 2 | 1, 4 | – |
| Hearing impairment (number) | 0 | 0 | – |
| Moderate vision impairment†(number) | 2 | 2 | >0.99 |
| Severe vision impairment††(number) | 0 | 0 | – |
| Combined outcome (n) | 45 | 38 | |
| Moderate neurodevelopmental impairment§(number) | 8 | 3 | 0.21 |
| Severe neurodevelopmental impairment§§ (number) | 2 | 3 | >0.99 |
*predicted MDI of the Bayley‐II as calculated from the cognitive and language indices.
**GMFCS, Gross Motor Function Classification System.
†vision impaired but better than severe vision impairment.
††blind or only able to perceive light or light‐reflecting objects.
§ and §§ see classification of neurodevelopmental impairment in Patients and Methods section.
The categorisations for the 16/115 (14%) of the children with moderate‐to‐severe developmental delay are presented in Table 4. The combined outcome of death or moderate‐to‐severe outcome at two years was not significant at 23 (26%) in the intervention group and 28 (35%) in the control group (p = 0.20).
Table 4.
Summary of the two‐year outcome in the 16 children diagnosed with moderate‐to‐severe developmental deficit
| Patient | Group* | Gestational age (weeks) | Sex | Cranial ultrasound classification** | Cranial ultrasound Findings | MRI Kidokoro score† | Bayleyscore | Vision impairment | GMFCS | Burden of cerebral hypoxia and hyperoxia (%h) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | C | 24 | m | S | Unilateral porencephalic cysts, cerebral atrophy | NA | 55 | No | 4 | 78.75 |
| 2 | C | 27 | f | S | IVH IV | NA | 57 | Moderate | 1 | 110.38 |
| 3 | I | 23 | m | M | IVH II, ventriculomegaly with ventricular index <p97 | 12 | 59 | No | 4.33 | |
| 4 | C | 27 | m | M | IVH II | 7 | 49 | No | 130.92 | |
| 5 | I | 25 | f | S | IVH IV | 16 | 62 | No | 2 | 8.94 |
| 6 | I | 26 | m | M | IVH II | NA | 61 | No | 8.93 | |
| 7 | I | 25 | m | S | IVH IV | NA | 68 | No | 79.16 | |
| 8 | C | 26 | m | N | Normal | NA | 56 | No | 70.64 | |
| 9 | I | 26 | m | N | Normal | 3 | 49 | No | 18.45 | |
| 10 | I | 25 | f | M | Inhomogeneous flaring after 7d, global thinning of corpus callosum | 6 | 50 | No | 173.87 | |
| 11 | I | 27 | m | M | Inhomogeneous flaring after 7d | NA | 80 | Moderate | 31.56 | |
| 12 | I | 26 | f | M | Ventriculomegaly with ventricular index <p97 | NA | 76 | Moderate | 80.77 | |
| 13 | I | 27 | f | S | Posthaemorrhagic ventricular dilatation | NA | 78 | 1 | 281.83 | |
| 14 | I | 26 | m | M | Inhomogeneous flaring after seven days | 6 | 100 | No | 2 | 28.31 |
| 15 | C | 25 | f | S | IVH IV | NA | 49 | NA | 83.9 | |
| 16 | C | 26 | f | N | Normal | NA | 108 | Moderate | 19.05 |
GMFCS = Gross Motor Function Classification System; MRI = magnetic resonance imaging.
*C = control group; I = intervention group.
**The categorisation of brain injury on cranial ultrasound (cUS) has previously been described in detail 18: S = severe brain injury on cUS, M = moderate brain image on cUS, N = normal cUS.
†The Kidokoro score 22.
Discussion
At two years of corrected age, 88% of the surviving infants from the SafeBoosC II trial were followed up. We found no difference between the MDI and ASQ results or the number of children with moderate‐to severe developmental delays between the intervention and the control groups. Moreover, there was no difference between the groups when the composite adverse outcomes of death or moderate‐to‐severe neurodevelopmental outcome were assessed.
SafeBoosC II was a randomised clinical trial that investigated the possibility of reducing the burden of cerebral hypoxia and hyperoxia during the first 72 hours of life in extremely preterm infants, and the burden of cerebral hypoxia was the primary outcome. This outcome enabled us to calculate the sample size needed for a trial of this nature 10, and we concluded that a trial powered to show a potential difference between neurodevelopmental outcomes would require approximately 10 times the number of infants in the SafeBoosC II trial. Therefore, it is not surprising that we found no difference in the neurodevelopmental outcomes between the intervention and control groups.
In the SafeBoosC II trial, the eight participating centres were allowed to conduct the two‐year assessment using either Bayley‐II or Bayley‐III. This approach was taken as the centres used different editions for the routine follow‐up of preterm infants and we did not think it was reasonable for the children to undergo two similar tests. Bayley‐III is known to yield higher scores than Bayley‐II, particularly in infants who perform in the low range 15, but this was not expected to bias the comparisons between the groups. Therefore, we converted the cognitive and language indexes from Bayley‐III into a predicted MDI score 11. We found no significant differences between the intervention groups in either of the Bayley tests or in the combined MDI score. Almost a quarter of the infants in the follow‐up were twins, but only six pairs of twins participated in one of the Bayley tests and only seven pairs of twins took part in the ASQ test. As the statistical effects were small and all the neurodevelopmental effects were insignificant, we choose not to correct the data for any twin correlation.
Observational studies have found that cerebral hypoxia during the transition from intrauterine to extrauterine life has been associated with an increased risk of intracranial haemorrhage, ischaemic lesions and later developmental impairment 7, 16. The benefits of cerebral NIRS monitoring need to be proven in randomised clinical trials, before the method is routinely applied to extremely premature infants 17. The SafeBoosC II trial was the first randomised clinical trial to investigate the possibility of reducing the burden of cerebral hypoxia and hyperoxia in extremely preterm infants. During the trial, fewer infants died and fewer infants had severe brain injuries on cranial ultrasound in the intervention group than in the control group, but these results were not statistically significant 8, 18. Moreover, the biomarkers of brain injury used in the SafeBoosC II trial did not differ between the intervention groups. These were amplitude‐integrated electroencephalography at 64 hours of age and S100 beta, neuroketal and brain fatty acid‐binding protein at six and 64 hours of age 19. However, post hoc analysis showed clear associations between the burden of cerebral hypoxia, severe brain injuries on cranial ultrasound, reduced electrical activity on amplitude‐integrated electroencephalography and death 20.
When the extremely preterm infants reached term age, the mortality in the SafeBoosC II trial was 19%. We found that five children in the trial had severe developmental delay, according to the British Association of Perinatal Medicine definition 14, and further 11 children were diagnosed with moderate developmental delay. These numbers correspond to 6% (5/83) with severe developmental delay and 19% (16/83) with moderate‐to‐severe developmental delay. These numbers were slightly lower than expected for extremely preterm infants. We were unable to obtain follow‐up data on 16 of the 131 children. In observational studies, infants lost to follow‐up often have lower socioeconomic status and tend to have more developmental delays than infants attending follow‐up programmes 21. If all of these 16 infants lost to follow‐up were diagnosed with moderate‐to‐severe developmental delay, the percentage affected would have been 28%. In our trial, the number of infants lost to follow‐up was similar in the intervention and control groups. The burden of cerebral hyperoxia was higher for the infants lost to follow‐up than the children attending the follow‐up programme, and we suggest this was caused by multiple comparisons, as the burden of hyperoxia was negligible in the SafeBoosC II trial 8.
Conclusions
In conclusion, cerebral oxygenation monitoring was not associated with adverse long‐term outcomes in extremely preterm infants. Successfully reducing cerebral hypoxia in the SafeBoosC II trial intervention group was not reflected in the MDI or the ASQ at two years of corrected age, and the numbers of infants with moderate‐to‐severe neurodevelopmental impairment were similar in the two intervention groups. The SafeBoosC II trial was a feasibility trial that was powered to prove only the possibility of reducing the burden of cerebral hypoxia and hyperoxia. Larger randomised clinical trials are needed to document clinically relevant benefits, such as reductions in mortality, severe brain injuries and/or neurodevelopmental deficits at follow‐up, before monitoring of cerebral oxygenation is routinely introduced to the care of extremely preterm infants.
Funding
This work was supported by an unconditional and unrestricted grant from the Danish Council for Strategic Research (number 0603‐00482B). The funder had no role in any aspect the trial.
Conflict of interest
The authors do not have any conflict of interests to declare.
References
- 1. Volpe JJ. Brain injury in the premature infant. Neuropathology, clinical aspects, pathogenesis, and prevention. Clin Perinatol 1997; 24: 567–87. [PubMed] [Google Scholar]
- 2. Soul JS, Hammer PE, Tsuji M, Saul JP, Bassan H, Limperopoulos C, et al. Fluctuating pressure‐passivity is common in the cerebral circulation of sick premature infants. Pediatr Res 2007; 61: 467–73. [DOI] [PubMed] [Google Scholar]
- 3. Boylan GB, Young K, Panerai RB, Rennie JM, Evans DH. Dynamic cerebral autoregulation in sick newborn infants. Pediatr Res 2000; 48: 12–7. [DOI] [PubMed] [Google Scholar]
- 4. Riera J, Cabañas F, Serrano JJ, Madero R, Pellicer A. New developments in cerebral blood flow autoregulation analysis in preterm infants: a mechanistic approach. Pediatr Res 2016; 79: 460–5. [DOI] [PubMed] [Google Scholar]
- 5. Andersen CC, Karayil SM, Hodyl NA, Stark MJ. Early red cell transfusion favourably alters cerebral oxygen extraction in very preterm newborns. Arch Dis Child Fetal Neonatal Ed 2015; 100: F433–5. [DOI] [PubMed] [Google Scholar]
- 6. Wong FY, Silas R, Hew S, Samarasinghe T, Walker AM. Cerebral oxygenation is highly sensitive to blood pressure variability in sick preterm infants. PLoS ONE 2012; 7: e43165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 2009; 8: 110–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hyttel‐Sørensen S, Pellicer A, Alderliesten T, Austin T, Van Bel F, Benders M, et al. Cerebral near infrared spectroscopy oximetry in extremely preterm infants: phase II randomised clinical trial. BMJ 2015; 350: g7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pellicer A, Greisen G, Benders M, Claris O, Dempsey E, Fumagalli M, et al. The safeboosc phase II randomised clinical trial: a treatment guideline for targeted near‐infrared‐derived cerebral tissue oxygenation versus standard treatment in extremely preterm infants. Neonatology 2013; 104: 171–8. [DOI] [PubMed] [Google Scholar]
- 10. Hyttel‐Sørensen S, Austin T, Van Bel F, Benders M, Claris O, Dempsey E, et al. A phase II randomized clinical trial on cerebral near‐infrared spectroscopy plus a treatment guideline versus treatment as usual for extremely preterm infants during the first three days of life (SafeBoosC): study protocol for a randomized controlled trial. Trials 2013; 14: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moore T, Johnson S, Haider S, Hennessy E, Marlow N. Relationship between test scores using the second and third editions of the Bayley Scales in extremely preterm children. J Pediatr 2012; 160: 553–8. [DOI] [PubMed] [Google Scholar]
- 12. Gollenberg AL, Lynch CD, Jackson LW, McGuinness BM, Msall ME. Concurrent validity of the parent‐completed ages and stages questionnaires, 2nd Ed. with the Bayley scales of infant development ii in a low‐risk sample. Child Care Health Dev 2010; 36: 485–90. [DOI] [PubMed] [Google Scholar]
- 13. Plomgaard AM, Hansen BM, Greisen G. Measuring developmental deficit in children born at gestational age less than 26 weeks using a parent‐completed developmental questionnaire. Acta Paediatr 2006; 95: 1488–94. [DOI] [PubMed] [Google Scholar]
- 14. Marlow N, Abbott J, Field D, Johnson S, Huertas A, Jones H et al. Report of a BAPM/RCPCH Working Group: classification of health status at 2 years as a perinatal outcome. British Association of Perinatal Medicine, 2008.
- 15. Jary S, Whitelaw A, Walløe L, Thoresen M. Comparison of Bayley‐2 and Bayley‐3 scores at 18 months in term infants following neonatal encephalopathy and therapeutic hypothermia. Dev Med Child Neurol 2013; 55: 1053–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alderliesten T, Lemmers PMA, Van Haastert IC, de Vries LS, Bonestroo HJC, Baerts W, et al. Hypotension in preterm neonates: low blood pressure alone does not affect neurodevelopmental outcome. J Pediatr 2014; 164: 986–91. [DOI] [PubMed] [Google Scholar]
- 17. Hyttel‐Sørensen S, Greisen G, Als‐Nielsen B, Gluud C. Cerebral near‐infrared spectroscopy monitoring for prevention of brain injury in very preterm infants. Cochrane Database Syst Rev 2017; 2: CD011506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Plomgaard AM, Hagmann C, Alderliesten T, Austin T, Van Bel F, Claris O, et al. Brain injury in the international multicentre randomised SafeBoosC phase II feasibility trial: cranial ultrasound and magnetic resonance imaging assessments. Pediatr Res 2016; 79: 466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Plomgaard AM, van Oeveren W, Petersen TH, Alderliesten T, Austin T, Van Bel F, et al. The SafeBoosC II randomised trial: treatment guided by near‐infrared spectroscopy reduces cerebral hypoxia without changing early biomarkers of brain injury. Pediatr Res 2016; 79: 528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Plomgaard AM, Alderliesten T, Austin T, Van Bel F, Benders M, Claris O, et al. Early biomarkers of brain injury and cerebral hypo‐ and hyperoxia in the SafeBoosC II trial. PLoS ONE 2017; 12: e0173440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Howe LD, Tilling K, Galobardes B, Lawlor DA. Loss to follow‐up in cohort studies: bias in estimates of socioeconomic inequalities socioeconomic inequalities. Epidemiology 2013; 24: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kidokoro H, Neil JJ, Inder TE. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am J Neuroradiol 2013; 34: 2208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


