Abstract
Malaria causes millions of deaths worldwide and is considered a huge burden to underdeveloped countries. The number of cases with resistance to all antimalarials is continuously increasing, making the identification of novel drugs a very urgent necessity. A potentially very interesting target for novel therapeutic intervention is the parasite mitochondrion. In this work, we studied in Plasmodium falciparum 3 genes coding for proteins homologues of the mammalian FIS1 (Mitochondrial Fission Protein 1) and DRP1 (Dynamin Related Protein 1) involved in mitochondrial fission. We studied the expression of P. falciparum genes that show ample sequence and structural homologies with the mammalian counterparts, namely FIS1, DYN1, and DYN2. The encoded proteins are characterized by a distinct pattern of expression throughout the erythrocytic cycle of P. falciparum, and their mRNAs are modulated by treating the parasite with the host hormone melatonin. We have previously reported that the knockout of the Plasmodium gene that codes for protein kinase 7 is essential for melatonin sensing. We here show that PfPk7 knockout results in major alterations of mitochondrial fission genes expression when compared to wild‐type parasites, and no change in fission proteins expression upon treatment with the host hormone. Finally, we have compared the morphological characteristics (using MitoTracker Red CMX Ros) and oxygen consumption properties of P. falciparum mitochondria in wild‐type parasites and PfPk7 Knockout strains. A novel GFP construct targeted to the mitochondrial matrix to wild‐type parasites was also developed to visualize P. falciparum mitochondria. We here show that, the functional characteristics of P. falciparum are profoundly altered in cells lacking protein kinase 7, suggesting that this enzyme plays a major role in the control of mitochondrial morphogenesis and maturation during the intra‐erythrocyte cell cycle progression.
Keywords: GFP, malaria parasites, melatonin, mitochondria, Plasmodium falciparum
1. INTRODUCTION
Malaria is one of the most lethal parasitic human diseases in the developing world, which caused more than 500 000 deaths in 2016 and 196 millions of new cases have been reported in the same year.1 Its etiological agents belong to the Plasmodium genus, and among these, Plasmodium falciparum is responsible for the most severe form of the disease. There are reported cases of parasite resistance to all available antimalarials and understanding the parasite biology and signaling events will help to identify new more effective drugs.2, 3
Mitochondria have uncommon evolutionary and functional characteristics in malaria parasites. An unconventional translation machinery and a reduction in the size of the mitochondrial genome, encoding just 3 proteins and the highly fragmented mitochondrial ribosomal RNA genes, appears to have originated at the point of divergence of dinoflagellates and apicomplexan parasites from ciliates.4 Mitochondria in plasmodia participate in numerous vital physiological processes, some of them unique for the parasites such as pyrimidine biosynthesis.5 Similarly to most other eukaryotes, mitochondria in P. falciparum and P. chabaudi also contribute to cellular Ca2+ homeostasis.6
Given the numerous and rapid cell divisions that the parasites undergo during the intra‐erythrocyte cycle, mitochondria should be able not only to synthesize and import rapidly a number of proteins from the cytosol, but also they must be able to divide and equally redistribute into the daughter cells at each division cycle.7 The dynamics of mitochondrial fission/fusion is extremely important for the survival of every eukaryote cell type, as through this process (i) new mitochondria are formed, (ii) organelle damage can be repaired, (iii) proteins, nucleic acids, and metabolites are rapidly exchanged among organelles.8, 9
Among the most important proteins controlling fission in mammalian mitochondria, 2 proteins appear particularly relevant, mitochondrial fission protein 1 (FIS1) and dynamin‐related protein 1 (DRP1). FIS1 is a small protein located in the mitochondrial outer membrane that is involved in the fragmentation of the mitochondrial network.10, 11 FIS1 plays a key role in the recruitment and association of the DRP1 to the mitochondrial surface. DRP1 is a GTPase that functions in several FIS1 controlled processes, like mitochondrial and peroxisomal division.12, 13
As Plasmodium mitochondria mediate various events related to drug action mechanisms and resistance,14, 15, 16, 17 the search for proteins involved in mitochondria functions that could be potential target of pharmacological intervention in malaria. That lead us to investigate the existence of plasmodial proteins analogous to those expressed in other eukaryotes and to investigate the changes in their transcripts at the different differentiation stages of the parasites. Van Dooren (2006) identified 3 Plasmodium genes (PF3D7_1325600, PF3D7_1037500 and PF3D7_1145400) that encode 3 Plasmodium proteins, that is FIS1, DYN1, and DYN2, that present strong sequence and structural homology with mammalian FIS1 and DRP1.7
In humans, melatonin has a number of functions that can be exercised in a receptor‐dependent or receptor‐independent manner, such as regulation of the circadian cycle, sleep, cancer inhibition and detoxification of free radicals.18, 19 Due to the wide range of processes in which it participates, this hormone has numerous applications in physiology/medicine and deserves more attention, as disturbances in melatonin levels are present in several neurodegenerative diseases. Temporally controlled administration of melatonin may be used as a form of treatment of some disorders.20, 21 Melatonin can act as an intracellular antioxidant, participating in aging processes and formation of free radicals, and its high concentration in the mitochondria of eukaryotic cells supports these roles.22, 23 In fact, mitochondrial concentrations of melatonin are unaffected by extra cellular concentrations, and there are reports that purple nonsulfur bacteria, which gave rise to the mitochondria of modern eukaryotes, are capable of producing this hormone.24
In P. falciparum, melatonin is able to modulate the plasmodial cell cycle through an intracellular Ca2+ controlled signaling pathway.25, 26, 27 The host hormone is also capable of increasing intracelular IP328 and cAMP levels within the parasite.27, 29 Blocking the IP3 receptor using the IP3R inhibitor 2‐APB was able to abolish the melatonin‐dependent Ca2+ increase.30 Additionally, melatonin can modulate the expression and ubiquitination of the transcription factor PfNF‐YB.31, 32 RNAseq experiments performed by our group showed that the host hormone can upregulate 38 genes in the asexual cycle of the parasite,33 whereas qRT‐PCR analysis revealed that melatonin is able to modulate genes involved in the ubiquitin proteasome system (UPS).34, 35 Taking together, these data reinforce that P. falciparum is able to sense and respond to changes in the external environment, including to the host hormone melatonin.
Plasmodium falciparum protein kinase 7 (PfPK7) is an orphan kinase (with no orthologues in mammalian cells). It has been previously shown that PfPK7 C‐terminal region has a sequence similar to mitogen‐activated protein kinase kinases (MAPKKs or MEK); meanwhile, the N‐terminal region has similarity with fungal protein kinase A.36 Disruption of pfpk7 gene resulted in slower parasite growth caused by a reduction in the number of merozoites.37 Interestingly, our group showed that the PfPK7 knockout strain (PfPK7−) was not able to respond to melatonin; as the ratio of asexual stages was not affected, there was no increase in cytosolic Ca2+ concentration upon melatonin addition and modulation of UPS genes did not occur.35 Despite these results, the target of this kinase was not yet identified and its role in P. falciparum is not fully understood.
Here we demonstrate that the host hormone melatonin increases the transcription of FIS1, DYN1, and DYN2 genes. To further confirm the specificity of melatonin action on fission gene expression, we also demonstrate that the P. falciparum kinase 7 (PfPK7)‐knockout strain is insensitive to the melatonin action35 and does not modify the expression levels of FIS1, DYN1, and DYN2 upon hormone treatment.
2. RESULTS
Given that the intra‐erythrocyte cycle of P. falciparum parasites involves a maturation process and several cell division events an equilibrated division and distribution of mitochondria among daughter cells is essential. We first verified the expression (by quantitative PCR) of the mRNA of the 3 Plasmodium genes potentially regulating the fission process, that is FIS1, DYN1, and DYN2. All 3 mRNAs were expressed, although their levels differed significantly depending on the cycle stage.
As far as FIS1 is concerned, a careful analysis of the aminoacid sequence of the encoded Plasmodium protein reveals that the parasite peptide is slightly smaller than the human homologue (17 kDa in humans and 16 kDa in P. falciparum), but it possesses the same structural domains at approximately equal positions (Figure 1). Previous studies have shown that the tetratricopeptide repeat domain (TPR) of FIS1 in mammals is responsible for recruiting a set of mitochondrial fission effector proteins such as DRP1 and MDV1.11 In Homo sapiens, phosphorylation of Ser 10 is responsible for the increased FIS1‐DRP1 interaction. Searching for that residue in the N‐terminal region of the Plasmodium protein revealed the presence of a serine at positions 3 and 23 and of a threonin at position 18, which may be phosphorylated and perform the same function.11 The presence of FIS1 conserved regions, including potential phosphorylation sites, in the Plasmodium protein suggests that the Plasmodium mitochondria fission mechanism may utilize the same principles and molecular machinery described in other eukaryotes.
Figure 1.
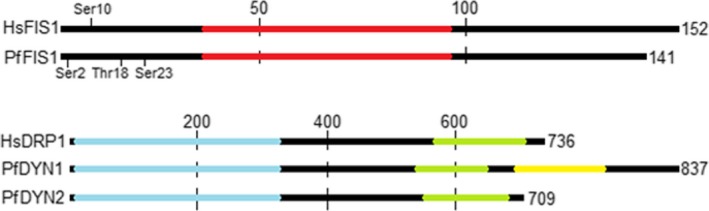
Protein alignment of FIS1 and DRP1 from Homo sapiens with FIS1 and DRP1 candidates from Plasmodium falciparum. Numbers shows length and positions, and residues that may regulate FIS1 by phosphorylation are evidenced. Regions in red shows TPR domain of FIS1, which are important for protein‐protein interactions. Regions in blue show GTP/co‐factors binding and dimerization domain of DRP1; Green regions represent GTPase activity motif; Yellow region is Forkhead domain for DNA interaction of DYN1
We next investigated 2 Plasmodium DRP1 candidates DYN1 (PF3D7_1145400) and DYN2 (PF3D7_1037500).7 DYN2 is a 81 kDa protein and possesses all typical structural domains of DRP1, including the GTP and Mg2+ binding domains, GTP hydrolysis catalytic sites, dimerization interaction sites and phosphorylation sites. DYN1 is a 96 kDa protein that also contains all typical domains of DRP1 positioned similarly in the polypeptide chain, like DYN2, and in addition a Forkhead structural domain. This latter domain is typical of transcription factors and other proteins that interact with nucleic acids (Figure 1).
The 3 genes selected had their expression validated by reverse transcription‐quantitative real‐time PCR (RT‐qPCR). Figure 2 shows the quantification of P. falciparum mRNA levels: FIS1, DYN1, and DYN2 are expressed throughout the intra‐erythrocytic parasite cycle in 3 different Plasmodium strains: wild‐type (3D7) parasites, the protein kinase 7 (PfPk7) knockout strain and the PK7 complement strain (knockout parasites that had the pk7 gene inserted in an episomal form). These latter 2 strains were used because knockout strains for kinase 7 (PfPK7) are incapable to respond to melatonin and to synchronize in response to the host hormone35, 37; accordingly, this plasmodia mutant represents a good control model to investigate the signaling pathways specifically controlled by melatonin.
Figure 2.
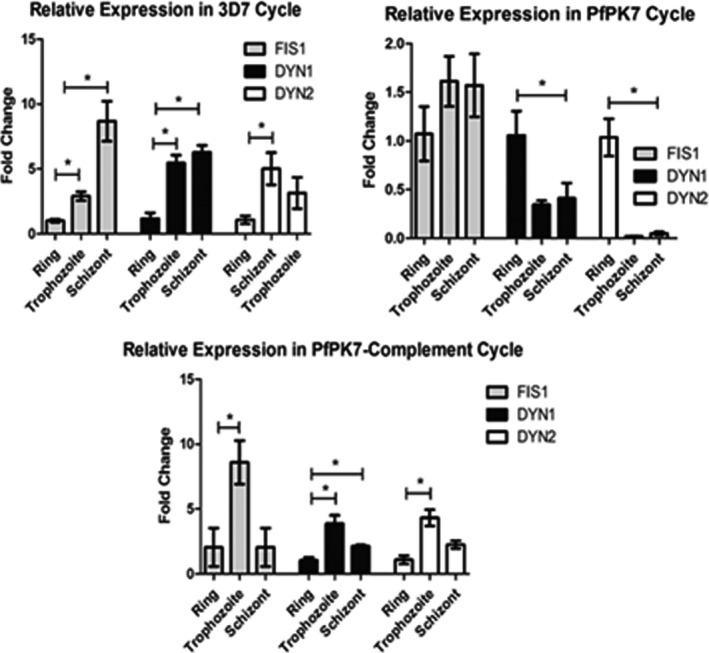
Relative expression of FIS1, DYN1, and DYN2 throughout 3D7, PfPk7 knockout, and PfPk7 complement strains. Parasites were synchronized to isolate ring, trophozoite, and schizont stages using sorbitol, and RNA samples were extracted, purified, and submitted to RT‐qPCR. Seryl‐tRNA Synthetase expression was quantified to normalize mRNA levels. The graphics reveal an increase in FIS1, DYN1, and DYN2 expression in mature forms of 3D7 parasites. In PfPk7 knockouts, DYN2 presented no change in expression throughout stages, while FIS1 had a small change only in trophozoite stage and DYN1 was expressed similarly to 3D7 strain. For PfPk7 complement strain, both FIS1 and DYN2 did not show increase in expression
As can be seen from Figure 2, in 3D7 (control) parasites, FIS1 mRNA is minimal at the ring stage and maximally expressed in the schizont stage. In the PK7 knockout parasites, the mRNA encoding FIS1 is already elevated at the ring stage and it increased only slightly in the more mature forms. In PK7 complement parasites, the mRNA encoding FIS1 is low at the ring and schizont stage and much more abundant in trophozoites. The expression pattern of both DYN 1 and DYN2 is also qualitatively and quantitatively different among the 3 strains analyzed. Noteworthy, the expression of DYN2 is almost undetectable in PK7 knockout plasmodia at the trophozoite and schizont stages.
We next investigated whether the host hormone melatonin, that we showed can induce Ca2+ increases in the cytoplasm of plasmodia and cell cycle synchronization,25 modifies the transcript levels of the 3 genes, FIS1, DYN1 and DYN2. Infected RBC cells were thus incubated for 5 and 17 hours with 100 nmol L−1 melatonin, and the mRNA levels were monitored at the trophozoite stage (ie, 30 hours after infection). We compared the expression of these genes in the 3 P. falciparum lines (3D7, PfPK7−, and PfPK7 complement clones). Figure 3 shows that for 3D7 parasites there is a statistically significant change in mRNA expression upon melatonin treatment when compared to the control group for FIS1 (increase after 17 hours of melatonin treatment), DYN1 (increase after 5 and 17 hours) and DYN2 (a clear drop after 5 hours) (Figure 3A). For the parasite lines PfPK7− and PfPK7 complement as expected, there is no difference in mRNA expression of the 3 genes upon melatonin addition when compared to the untreated cells. To show that the parasites retain their morphology after melatonin treatment, we have included the microscopy images of 3D7, PfPK7−, and PfPK7 complement (Figure 3B).
Figure 3.
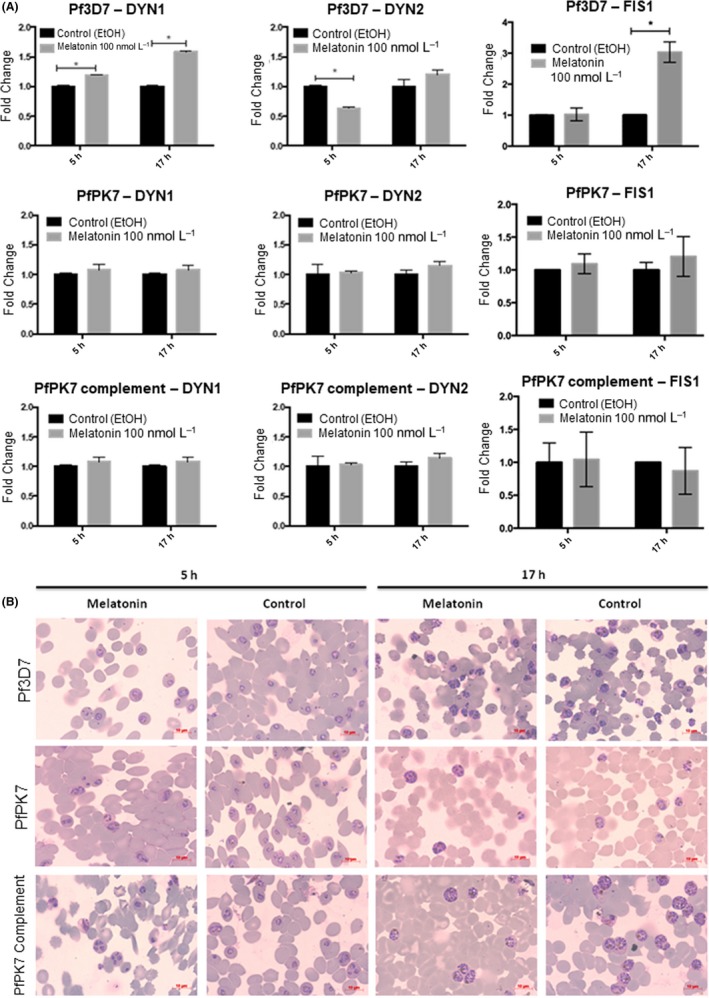
Relative expression of FIS1, DYN1, and DYN2 in 3D7, PfPk7−, and PfPk7 complement after melatonin treatment. Parasites at trophozoite stage (30 hours after invasion) were treated with 100 nmol L−1 of melatonin for 5 and 17 hours, and RNA samples were extracted, purified, and submitted to RT‐qPCR. Seryl‐tRNA Synthetase expression was quantified to normalize mRNA levels. The graphics reveal an increase in FIS1, DYN1, and DYN2 expression of 3D7 parasites. In PfPk7 knockouts and PfPk7 complement, we could not observe any change in expression of FIS1, DYN1, and DYN2 after treatment (A). Giemsa stained smears of infected red blood cells were made to make sure that the treatment did not interfered in parasites stage or morphology (B)
Given the differences in fission gene expression during cell cycle stages, one may expect alterations in mitochondrial morphology during progression from rings to trophozoites. In order to monitor continuously and in living parasites mitochondrial morphology, we transfected the P. falciparum wild type parasites with a novel construct, “mito‐emerald,” which encodes for a peptide containing the human cytochrome C oxidase signal peptide fused to the N‐terminal of emerald‐GFP fluorophore. This novel probe was expressed in an episomal way in the pDC plasmid. In Figure 4A, it is shown that a single Plasmodium mitochondrion/cell appears as a small fluorescent dot in the cytoplasm at the ring stage, but as an elongated tubular structure in trophozoites. In mature segmented schizont stages, the fluorescence appears distributed in numerous dots homogeneously distributed among individual merozoites. Figure 4A also shows the distribution of P. falciparum mitochondria, stained by the mitochondrial marker MitoTracker Red CMXRos (Mito‐tracker). The latter probe co‐localized nicely with the fluorescence of mito‐emerald throughout all the erythrocytic stages. Unlike “mito‐emerald” that can allow to monitor dynamically the shape of mitochondria in live cells, Mitotracker can be used only to verify the shape of the organelle in a precise moment, as prolonged incubation with this probe is toxic. Interestingly, in PfPK7 strain, the mitochondria shows an elongated form at both the ring and schizont stages; in addition, it is possible to observe that some of the new cells have a nucleus staining but lack mitochondrial fluorescence (Figure 4B). These morphological observations suggest that mitochondria during their intra‐erythrocyte cycle not only increase in number and equally divide during cell division, but also undergo major structural changes at the differentiation stage responsible for forming new cells capable of invading RBCs. Furthermore mitochondrial morphology in Pf3D7 wild‐type and PfPK7 knockout strains appears drastically abnormal, with altered separation of organelles during division.
Figure 4.
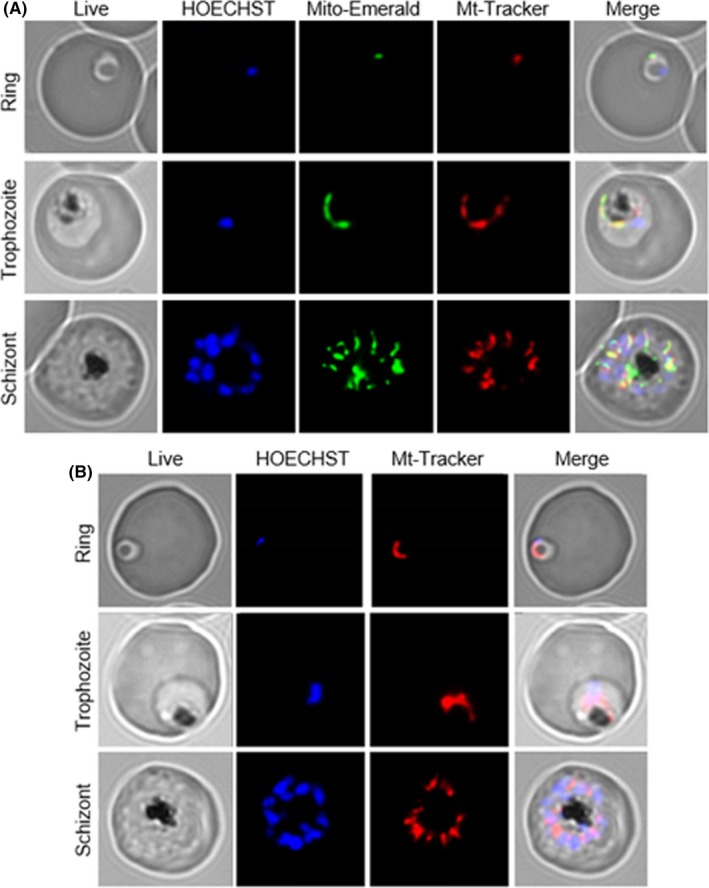
Mitochondria from Plasmodium falciparum seen by fluorescent microscopy using Mito‐Emerald construction. Images were taken from ring, trophozoites, and schizonts stages of Pf3D7 (A) The parasite nucleus was stained by HOECHST 33342 and mitochondria with MitoTracker Red CMX Ros, to demonstrate co‐localization with Mito‐Emerald. Mitochondria shape changes from small dots in ring stages to an elongated form in trophozoites and multiple mitochondria within segmented schizonts. MitoTracker Red CMX Ros was used for staining Pf PK7 mitochondria (B). In Pf PK7, mitochondria starts as an elongated form in ring, and then, it grows bigger until segmentation in schizogony (B)
We investigated whether the morphological alterations of mitochondria in the various cell cycle stages are accompanied by alterations in mitochondrial functions. In particular, we measured O2 consumption in Pf3D7 wild‐type and PfPK7 knockout strain in rings, trophozoites, and schizonts (Figure 5). Although no significant differences were observed in O2 consumption at the trophozoite and schizont stages, in the ring stage the oxygen consumption of PfPK7 strain was twofold higher than in the wild‐type strain, probably due to the larger size of its mitochondrion.
Figure 5.
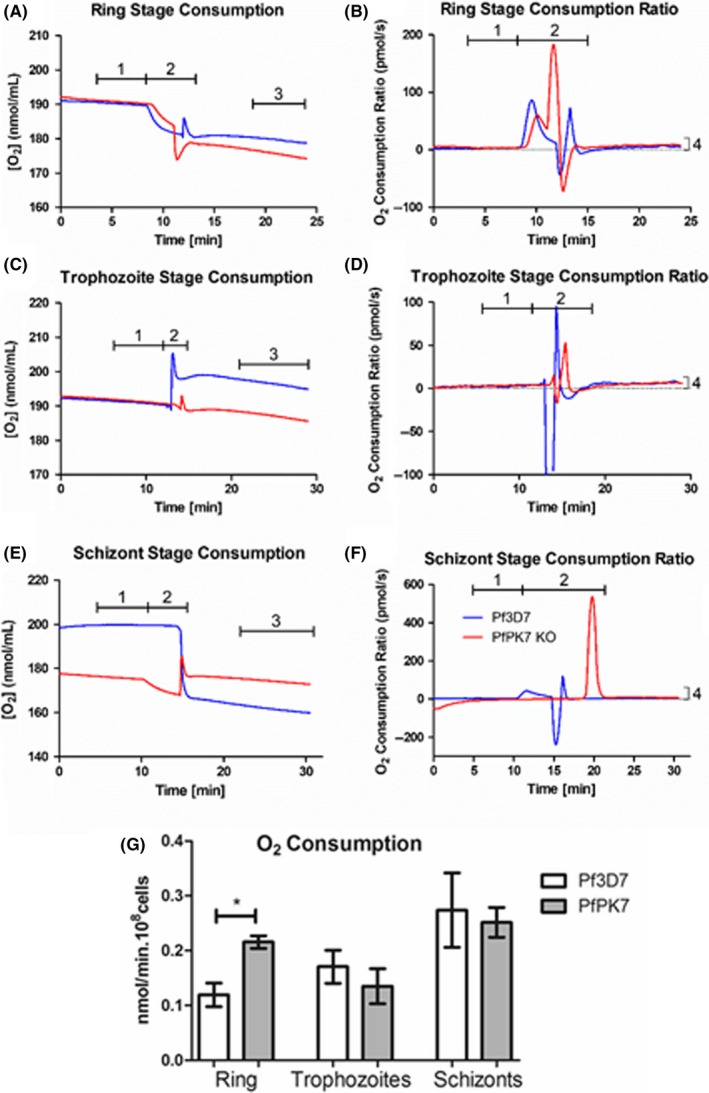
Oxygen consumption in Plasmodium falciparum Pf3D7 wild‐type and Pf PK7 strains at ring, trophozoites and schizonts. 108 synchronized parasites of each strain were incubated in O2K (Oroboros Instruments) at 37°C in complete RPMI media, oxygen concentration was monitored for 10‐30 minutes, and oxygen consumption rates were determined. Figures A‐F are typical recordings of O2K instrument, showing a time course of total oxygen concentration (A, C, and E), and its derivative function showing consumption ratio in pmol per second (B, D, and F). Total oxygen consumption is equal to consumption of parasites (3) minus consumption of RPMI media alone (1). Addition of cells to O2K instrument causes a disturbance in oxygen quantification (2). The consumption ratio of parasites is shown in (4), and mean corrected values of 3 independent experiments for each stage are in (G). Pf3D7 showed a consumption that increases along with the cycle, as expected. Pf PK7 has an unusual oxygen consumption: Rings of this strain have a twofold higher consumption when compared to wild‐type strain, and it is not observable a direct correlation between oxygen consumption and cycle development
We further investigated O2 consumption in 3D7 wild‐type parasites, to quantify O2 consumption related to ATP production and the maximum mitochondrial respiratory capacity due to uncoupling (Figure 6). At the ring stage addition of oligomycin results in no significant change in O2 consumption, suggesting that at this stage the ATP synthetic activity of mitochondria is negligible; uncoupling by carbonyl cyanide m‐chlorophenylhydrazone (CCCP) resulted in almost a doubling of O2 consumption. At the trophozoite and schizont stages, on the contrary, oligomycin drastically reduced O2 consumption, indicating that the organelle is fully involved in cellular ATP synthesis. Indeed complete uncoupling by CCCP resulted in a O2 consumption that was similar to that under resting conditions, as observed in many mammalian cell types under the same conditions.38
Figure 6.
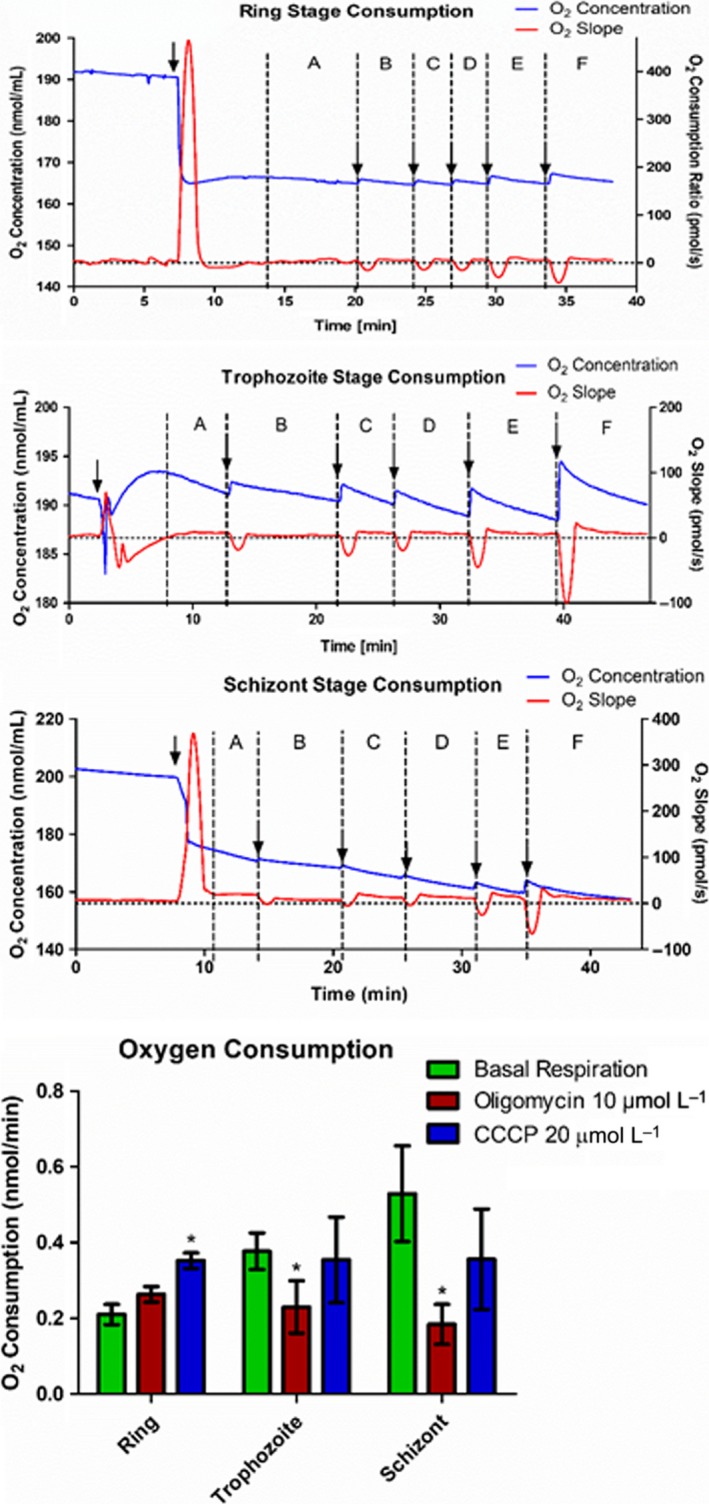
Oxygen consumption over time and after treatments with Oligomycin and carbonyl cyanide m‐chlorophenylhydrazone (CCCP) in 5 × 108 3D7 wild‐type parasites. Results of Oligomycin 10 μmol L−1 and CCCP 20 μmol L−1 were used for plotting bar graphs and analyzing the results because these were the lowest concentrations with highest consumption. Three independent experiments were performed for each stage. *Statistical significant differences by Student's t test. Results show that mitochondria operates in an uncoupled state in trophozoites and schizont stages, and ATP production is responsible for 50% or less O2 consumption in these stages. Ring stages do not use oxygen for ATP synthesis. Blue line shows total oxygen concentration inside the O2K chamber over time, and red line shows its derivative curve, oxygen consumption rate in pmol/s. Each arrow represents addition of cells (first), oligomycin (second), CCCP (third to sixth). The intervals used to calculate oxygen consumption in each timepoint are shown between dotted lines: basal respiration (A), with 10 μmol L−1 oligomycin (B), with 5, 10, 20, and 40 μmol L−1 CCCP (C, D, E, and F, respectively)
3. DISCUSSION
The nature and regulation of the proteins that control mitochondrial fission and fusion have been extensively investigated in yeast and mammalian cells, while little is known in plasmodia.4 In this contribution, we investigated in P. falciparum the mitochondrial fission candidate genes, FIS1, DYN1, and DYN2. The expression profile under melatonin treatment of these genes was analyzed throughout the cycle of wild‐type and PK7 knockout strains.
The initial stage of intra‐erythrocytic cycle, named ring, is a quiescent stage of low metabolic activity. Nevertheless, mRNAs were detected for all the 3 genes in both Pf3D7 and PfPK7 strains.
Trophozoite stage is characterized by high metabolic rate and extensive protein synthesis. At this point, in the control strain, Pf3D7, the expression of FIS1 genes, DYN1, and DYN2 increased about 5 times compared to the expression at the ring stage. On the contrary, in the PfPK7 strain no increase for FIS1 expression and a drastic decrease in expression of dynamins were observed. A significant increase in the mRNA of these proteins (though reduced compared to the controls) was observed when the PK7 gene was inserted in an episomal manner, indicating that somehow PK7 is engaged in events leading to early maturation of the mitochondrial division machinery.
During the final stage of the intra erythrocytic cycle, the expression of mitochondrial fission genes remains at high values relative to the ring stage in control strain, reaching a value 10 times higher for FIS1. In other cell types, it has been shown that overexpression of FIS1 is directly related to mitochondrial fission.39, 40 In the PfPK7 strain, the FIS1 expression remains at basal levels, while the expression of dynamins decreases. These data may contribute to explain the slow growth phenotype of PfPK7: The basal level of FIS1 expression and the low amount of dynamins are not sufficient to perform all successive mitochondrial divisions during schizogony.
We have then investigated the effect on the expression of the mRNA for the 3 genes of the treatment with the hormone melatonin (for 5 or 17 hours). Treatment of control P. falciparum‐infected cells at the trophozoite stage with 100 nmol L−1 melatonin induces the formation of mature forms of the parasite by mechanisms not yet fully elucidated.25, 35, 41 Our data indicate that after 17 hours of treatment with 100 nmol L−1 melatonin, the expression of FIS1 and DYN1 genes was significantly increased in the wild type strain, compared to the cells in the absence of the hormone. These results indicate that melatonin may also have a role in the maturation of mitochondria, a critical step for cell division of any organism. As described by Koyama et al, the PfPK7 strain is totally insensitive to melatonin‐induced maturation. As expected, no change in the expression of mitochondrial fission genes was observed in this strain upon treatment with melatonin.35
The mitochondrion appears to be a dynamic organelle that undergoes several morphological changes during the intra‐erythrocytic cycle. Recent studies have suggested that the mitochondrion appears as a single organelle for most of the asexual life cycle. Division apparently occurs very late in the schizogony with branched mitochondria remaining the predominant form in parasite. Van Dooren et al indicated that the mitochondrion lies in close proximity to the apicoplast during intra‐erythrocytic cycle and suggest a model for the mitochondrion‐apicoplast interaction: The 2 organelles are associated in ring and trophozoite stages.42
Plasmodia mitochondria, at the ring stage, appear not to be actively involved in cellular ATP synthesis, as revealed by the lack of oligomycin effect on O2 consumption, while at later stages, their O2 consumption in basal conditions is largely due to the ATP synthetizing activity (as revealed by the strong inhibition of Fo ATP synthase by oligomycin).
Our results for wild‐type parasites are consistent with those described by.43, 44 Interestingly, in PfPK7 knockout strain, the maturation of mitochondrial metabolism during the cell cycle does not occur, as O2 consumption is already high at the ring stage. Noteworthy, mitochondria, at the ring stage, are small and rounded in Pf3D7 wild type, but are elongated in PfPK7 knockout.
In conclusion, our results provide conclusive evidence supporting the importance of mitochondrial fission genes in the appropriate distribution of mitochondria in P. falciparum during parasite division. The modulation of mitochondrial fission genes appears to require a functional protein kinase 7. In addition, we demonstrate that the host hormone melatonin modulates the transcription of the key genes involved in mitochondrial fission.
4. MATERIALS AND METHODS
4.1. Parasites
Plasmodium falciparum parasite lines (3D7, Pfpk7 −, and PfpK7 complement clones) are cultured in RPMI 1640 (Invitrogen) supplemented with 37.5 mmol L−1 HEPES, 7 mmol L−1 D‐glucose, 6 mmol L−1 NOH, 25/mL gentamicin sulfate, 2 mmol L−1 l‐glutamine, and 10% human serum) and maintained in human erythrocytes under a gas mixture of 5% O2, 5% CO2, and 90% N2.45 Culture is synchronized using 5% sorbitol.46 For Pfpk7 − and PfpK7 complement parasite, the same medium was supplemented with 2.5 μg/mL blasticidin.
4.2. Bioinformatic analysis
The complete human aminoacid sequence of FIS1 and DRP1 was retrieved in NCBI database. The gene sequences for P. falciparum 3D7 strain FIS1, DYN1, and DYN2 were found using a homology search based in human sequences, in Plasmodium database (PlasmoDB). Sequences alignments were performed at the European Bioinformatics Institute website (EMBL‐EBI) using the EMBOSS Needle tool and the Matrix EBLOSUM62. Structural domains were identified and analyzed by BLAST at the NCBI database.
4.3. Imaging Plasmodium falciparum mitochondria
Live parasites were observed by fluorescent microscopy using an AxioScope microscope (Zeiss) equipped with an AxioCam HRC digital camera (Zeiss) and analyzed with the AxioVision 4.8 software. To visualize the nucleus, parasitized erythrocytes were incubated with HOECHST 33342 with 10 μg/mL. To demonstrate co‐localization, parasites were incubated additionally with MitoTracker Red CMX Ros according to (Wrenger and Müller, 2004; Chan et al., 2013). Briefly, infected erythrocytes were incubated at 37°C with 25 nmol L−1 MitoTracker before pelleted at 1500 rpm for 5 minutes at room temperature. The supernatant was removed, and the parasitized cells were resuspended in prewarmed medium and immediately analyzed by fluorescent microscopy.
4.4. Melatonin treatment
The culture erythrocyte previously synchronized with parasites was transferred to a large flask and fresh medium added. Melatonin was dissolved in ethanol (40 mmol L−1) and immediately added to a final concentration of 100 nmol L−1 in RPMI and incubated for 535 and 17 hours25 at 37°C. The melatonin was added to synchronized parasites in culture at the trophozoite stage (30 hours after infection).
4.5. RNA extraction and real‐time RT‐PCR
Total RNA was extracted using Trizol reagent (Invitrogen) according to the manufacturer's instructions. RNA was quantified using the Nanodrop ND‐1000 UV/Vis spectrophotometer, and RNA integrity was checked by electrophoresis using an Agilent 2100 Bioanalyzer. Random‐primed reverse transcription (RT) was performed using 1.0 μg of total RNA according to the Super Script III kit protocol (Invitrogen). The relative transcriptional levels were determined through quantitative PCR (qPCR) with Sybr Green PCR Master Mix (Applied Biosystem) using the 7300 Real‐Time PCR System (Applied Biosystem). Real‐time data were normalized in relation to the level of expression of Seryl‐tRNA Synthetase. P‐value was determined for the biological triplicates with Student's t‐test, using one tail distribution and heteroscedastic variance.
4.6. Cloning of mt‐Emerald gene into Plasmodium falciparum expression vector pDC
The ORF encoding the mt‐Emerald gene was amplified from the original mammalian expression plasmid mEmerald‐Mito‐7 (Addgene, kindly supplied by Dr. Rosário Rizzuto) using the specific primers: 5′ CTCGAGATGTCCGTCCTGACGCCGC 3′ and 5′ CTCGAGTTACTTGTACAGCTCGTCCATGCCG 3′. The XhoI cleavage site was added in the 5′ end of both primers. Approximately 100 ng of plasmid was used in a PCR reaction in the following conditions: 94°C/2′, 35 cycles of 94°C/30″; 57°C/30″ and 68°C/120″ and a final step of 74°C/5′. The amplicons were purified from agarose gel using PureLinkTM Quick Gel Extraction Kit (Invitrogen) according with the manufacture's protocols. The purified amplicons were then cloned into bacterial propagation vector pJET (Fermentas) and used to transform Chemically Competent Escherichia coli DH5α strain. The transformant selection was made in LB agar plates at 37°C for 12 hours. Single colonies were grown in 5 mL of LB/ampicillin at 37°C for 12 hours at 180 rpm. Plasmid DNA was extracted with kit PureLink Quick Plasmid Miniprep (Invitrogen).
To test for the presence of mt‐Emerald gene, plasmid DNA was submitted to restriction analyses with the XhoI enzyme. The reaction was performed with approximately 10 U of enzyme at 37°C for 2 hours. The mt‐Emerald gene was then transferred to P. falciparum transfection plasmid pDC (kindly supplied by Dr. Gerhard Wunderlich). For this purpose, pJET/mt‐Emerald construct and pDC vector were submitted to a new restriction reaction with XhoI enzyme and the fragments, approximately 0.8 and 6 kb, corresponding to mt‐Emerald and pDC, respectively, were purified from the agarose gel as described above. The ligation reaction was carried out at 4°C overnight and was used to transform chemically competent E. coli using the heat shock method. From the 26 colonies obtained, 8 colonies were positive for mt‐Emerald gene. The correct insertion of the gene was confirmed by restriction analyses with NcoI, as digestion with this enzyme results in fragments with different sizes depending on the orientation of the insert. Colonies 2 and 5 showed the mt‐Emerald inserted in the correct orientation and the DNA extracted from these clones were used for transfection of P. falciparum.
4.7. Transfection of Plasmodium falciparum with the pDC/mt‐Emerald constructs
Transfection was performed as Hasenkamp et al.47 A culture containing the 3D7 strain of P. falciparum synchronized ring stage with 10% parasitemia was collected in 50 mL Falcon tube and centrifuged for 5 minutes at 500g. The supernatant was discarded, and the pellet containing the infected erythrocytes was washed with 15 mL of buffer cytomix (120 mmol L−1 KCl, 0.15 mmol L−1 CaCl2, 2 mmol L−1 EGTA, 5 mmol L−1 MgCl2, 10 mmol L−1 K2HPO4/KH2PO4, 25 mmol L−1 HEPES). After washing, 150 μL of these erythrocytes was removed, resuspended in 150 μL of citomix buffer and placed on ice. Fifty micrograms of pDC vector containing the sequence of the mt‐Emerald was resuspended in 100 μL cytomix and added to erythrocytes infected with P. falciparum. Then, this material was transferred into a 0.2‐cm cuvette placed on ice. The electroporation conditions were as follows: 310 V, 960 μF, 200 Ω. The cells were then transferred to a 15‐mL Falcon tube with 10 mL of RPMI culture medium, centrifuged for 5 minutes and placed in flasks containing 20 mL of RPMI and placed in an incubator (5% CO2, 5% O2, and 90% N2). After 48 hours, 5 nmol L−1 WR99210 was added to the culture medium to select for transformants. The transfected parasites (Pfmt‐Emerald) started to appear approximately after 2 weeks, and the fluorescent parasites could be observed in a fluorescence microscope.
4.8. Oxygen consumption
Pf3D7 and PfPK7 strains were taken directly from the culture, and parasitemia was established by light microscopy on slides stained with GIEMSA. 2 × 108 parasites in complete RPMI medium were added directly to the O2K Oxygen Reading Chamber (Oroboros Instruments) under constant stirring at 37°C and final volume of 2 mL for the determination of oxygen consumption. The apparatus was calibrated with complete RPMI (zero consumption), and the analyses were performed in comparison with the consumption of empty erythrocytes. For oxymetry data, the oxygen consumption was established during a linearity interval of at least 10 minutes, and the values were plotted in GraphPad Prim 6 Software. The derivative of the generated function was used to infer the rate of consumption in nmol/min. The statistical test used was Tukey's t test for comparing 2 means. P values less than or equal to .05 were considered to indicate significant statistical differences.
Oligomycin was used to inhibit Fo ATP synthase in 3 different concentrations: 2.5, 5, and 10 μmol L−1. CCCP was used to uncouple respiratory chain and calculate the maximum oxygen consumption rate, in 4 different concentrations: 5, 10, 20, and 40 μmol L−1. For the experiments with oligomycin and CCCP, we used 5 × 108 parasites to increase resolution.
ACKNOWLEDGEMENTS
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) 2011/51295‐5, CNPQ PROCESS PVE 407287/2013‐2 to CG, 2012/12807‐3 and 2015/26722‐8 to KM and CW, respectively, and 2014/14347‐5 to PHSP.
Scarpelli PH, Tessarin‐Almeida G, Viçoso KL, et al. Melatonin activates FIS1, DYN1, and DYN2 Plasmodium falciparum related‐genes for mitochondria fission: Mitoemerald‐GFP as a tool to visualize mitochondria structure. J Pineal Res. 2019;66:e12484 10.1111/jpi.12484
REFERENCES
- 1. World Health Organization . World Malaria Report 2016; 2016.
- 2. Goodman CD, Siregar JE, Mollard V, et al. Parasites resistant to the antimalarial atovaquone fail to transmit by mosquitoes. Science. 2016;352:349‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mita T, Tanabe K. Evolution of Plasmodium falciparum drug resistance: implications for the development and containment of artemisinin resistance. Jpn J Infect Dis. 2012;65:465‐475. [DOI] [PubMed] [Google Scholar]
- 4. Vaidya AB, Mather MW. Mitochondrial evolution and functions in malaria parasites. Annu Rev Microbiol. 2009;63:249‐267. [DOI] [PubMed] [Google Scholar]
- 5. Painter HJ, Morrisey JM, Mather MW, Vaidya AB. Specific role of mitochondrial electron transport in blood‐stage Plasmodium falciparum . Nature. 2007;446:88‐91. [DOI] [PubMed] [Google Scholar]
- 6. Gazarini ML, Garcia CRS. The malaria parasite mitochondrion senses cytosolic Ca2 + fluctuations. Biochem Biophys Res Commun. 2004;321:138‐144. [DOI] [PubMed] [Google Scholar]
- 7. Van Dooren GG, Stimmler LM, McFadden GI. Metabolic maps and functions of the Plasmodium mitochondrion. FEMS Microbiol Rev. 2006;30:596‐630. [DOI] [PubMed] [Google Scholar]
- 8. Westermann B. Bioenergetic role of mitochondrial fusion and fission. Biochem Biophys Acta. 2012;1817:1833‐1838. [DOI] [PubMed] [Google Scholar]
- 9. Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062‐1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iwasawa R, Mahul‐Mellier A‐L, Datler C, Pazarentzos E, Grimm S. Fis1 and Bap31 bridge the mitochondria‐ER interface to establish a platform for apoptosis induction. EMBO J. 2011;30:556‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wells RC, Picton LK, Williams SCP, Tan FJ, Hill RB. Direct binding of the dynamin‐like GTPase, Dnm1, to mitochondrial dynamics protein Fis1 is negatively regulated by the Fis1 N‐terminal arm. J Biol Chem. 2007;282:33769‐33775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang CR, Blackstone C. Dynamic regulation of mitochondrial fission through modification of the dynamin‐related protein Drp1. Ann N Y Acad Sci. 2010;1201:34‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cereghetti GM, Stangherlin A, Martins de Brito O, et al. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci USA. 2008;105:15803‐15808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Torrentino‐Madamet M, Desplans J, Travaillé C, James Y, Parzy D. Microaerophilic respiratory metabolism of Plasmodium falciparum mitochondrion as a drug target. Curr Mol Med. 2010;10:29‐46. [DOI] [PubMed] [Google Scholar]
- 15. Peatey CL, Chavchich M, Chen N, et al. Mitochondrial membrane potential in a small subset of artemisinin‐induced dormant Plasmodium falciparum parasites in vitro. J Infect Dis. 2015;212:426‐434. [DOI] [PubMed] [Google Scholar]
- 16. Wang J, Huang L, Li J, et al. Artemisinin directly targets malarial mitochondria through its specific mitochondrial activation. PLoS ONE. 2010;5:e9582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Srivastava IK, Rottenberg H, Vaidya AB. Atovaquone, a broad spectrum antiparasitic drug, collapses mitochondrial membrane potential in a malarial parasite. J Biol Chem. 1997;272:3961‐3966. [DOI] [PubMed] [Google Scholar]
- 18. Reiter RJ, Tan DX, Galano A. Melatonin: exceeding expectations. Physiology. 2014;29:325‐333. [DOI] [PubMed] [Google Scholar]
- 19. Reiter RJ. Melatonin: clinical relevance. Best Pract Res Clin Endocrinol Metab. 2003;17:273‐285. [DOI] [PubMed] [Google Scholar]
- 20. Hardeland R, Madrid JA, Tan DX, Reiter RJ. Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling. J Pineal Res. 2012;52:139‐166. [DOI] [PubMed] [Google Scholar]
- 21. Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi‐Perumal SR. Melatonin – a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93:350‐384. [DOI] [PubMed] [Google Scholar]
- 22. Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre‐Jimenez M, Qin L. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res. 2016;61:253‐278. [DOI] [PubMed] [Google Scholar]
- 23. Hardeland R. Melatonin and the theories of aging: a critical appraisal of melatonin's role in antiaging mechanisms. J Pineal Res. 2013;55:325‐356. [DOI] [PubMed] [Google Scholar]
- 24. Manchester LC, Coto‐Montes A, Boga JA, et al. Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J Pineal Res. 2015;59:403‐419. [DOI] [PubMed] [Google Scholar]
- 25. Hotta CT, Gazarini ML, Beraldo FH, et al. Calcium‐dependent modulation by melatonin of the circadian rhythm in malarial parasites. Nat Cell Biol. 2000;2:466‐468. [DOI] [PubMed] [Google Scholar]
- 26. Alves E, Bartlett PJ, Garcia CRS, Thomas AP. Melatonin and IP3‐induced Ca2 + release from intracellular stores in the malaria parasite Plasmodium falciparum within infected red blood cells. J Biol Chem. 2011;286:5905‐5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beraldo FH, Almeida FM, da Silva AM, Garcia CRS. Cyclic AMP and calcium interplay as second messengers in melatonin‐dependent regulation of Plasmodium falciparum cell cycle. J Cell Biol. 2005;170:551‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alves E, Bartlett PJ, Garcia CRS, Thomas AP. Melatonin and IP3‐induced Ca2 + release from intracellular stores in the malaria parasite Plasmodium falciparum within infected red blood cells. J Biol Chem. 2011;286:5905‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Furuyama W, Enomoto M, Mossaad E, Kawai S, Mikoshiba K, Kawazu S. An interplay between 2 signaling pathways: melatonin‐cAMP and IP3–Ca2 + signaling pathways control intraerythrocytic development of the malaria parasite Plasmodium falciparum . Biochem Biophys Res Commun. 2014;446:125‐131. [DOI] [PubMed] [Google Scholar]
- 30. Beraldo FH, Mikoshiba K, Garcia CRS. Human malarial parasite, Plasmodium falciparum, displays capacitative calcium entry: 2‐aminoethyl diphenylborinate blocks the signal transduction pathway of melatonin action on the P. falciparum cell cycle. J Pineal Res. 2007;43:360‐364. [DOI] [PubMed] [Google Scholar]
- 31. Lima WR, Moraes M, Alves E, Azevedo MF, Passos DO, Garcia CRS. The PfNF‐YB transcription factor is a downstream target of melatonin and cAMP signalling in the human malaria parasite Plasmodium falciparum . J Pineal Res. 2013;54:145‐153. [DOI] [PubMed] [Google Scholar]
- 32. Lima WR, Holder AA, Garcia CRS. Melatonin signaling and its modulation of PfNF‐YB transcription factor expression in Plasmodium falciparum . Int J Mol Sci. 2013;14:13704‐13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lima WR, Tessarin‐Almeida G, Rozanski A, et al. Signaling transcript profile of the asexual intraerythrocytic development cycle of Plasmodium falciparum induced by melatonin and cAMP. Genes Cancer. 2016;7:323‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koyama FC, Azevedo MF, Budu A, Chakrabarti D, Garcia CRS. Melatonin‐induced temporal up‐regulation of gene expression related to ubiquitin/proteasome system (UPS) in the human malaria parasite Plasmodium falciparum . Int J Mol Sci. 2014;15:22320‐22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koyama F, Ribeiro R, Garcia J, Mauro F, Chakrabarti D, Garcia C. Ubiquitin Proteassome System and the atypical kinase PfPK7 are involved in melatonin signaling in Plasmodium falciparum . J Pineal Res. 2013;53:147‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dorin D, Semblat JP, Poullet P, et al. PfPK7, an atypical MEK‐related protein kinase, reflects the absence of classical three‐component MAPK pathways in the human malaria parasite Plasmodium falciparum . Mol Microbiol. 2005;55:184‐196. [DOI] [PubMed] [Google Scholar]
- 37. Dorin‐Semblat D, Sicard A, Doerig C, Ranford‐Cartwright L, Doerig C. Disruption of the PfPK7 gene impairs schizogony and sporogony in the human malaria parasite Plasmodium falciparum . Eukaryot Cell. 2008;7:279‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pancrazi L, Di Benedetto G, Colombaioni L, et al. Foxg1 localizes to mitochondria and coordinates cell differentiation and bioenergetics. Proc Natl Acad Sci. 2015;112:13910‐13915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gomes LC, Scorrano L. High levels of Fis1, a pro‐fission mitochondrial protein, trigger autophagy. Biochim Biophys Acta. 2008;1777:860‐866. [DOI] [PubMed] [Google Scholar]
- 40. Losón OC, Song Z, Chen H, Chan DC. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell. 2013;24:659‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Beraldo FH, Garcia CRS. Products of tryptophan catabolism induce Ca2 + release and modulate the cell cycle of Plasmodium falciparum malaria parasites. J Pineal Res. 2005;39:224‐230. [DOI] [PubMed] [Google Scholar]
- 42. Van Dooren GG, Marti M, Tonkin CJ, Stimmler LM, Cowman AF, McFadden GI. Development of the endoplasmic reticulum, mitochondrion and apicoplast during the asexual life cycle of Plasmodium falciparum . Mol Microbiol. 2005;57:405‐419.15978074 [Google Scholar]
- 43. Murphy AD, Lang‐unnasch N. Alternative oxidase inhibitors potentiate the activity of atovaquone against Plasmodium falciparum alternative oxidase inhibitors potentiate the activity of atovaquone against Plasmodium falciparum . Antimicrob Agents Chemother. 1999;43:651‐654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Murphy AD, Doeller JE, Hearn B, Lang‐Unnasch N. Plasmodium falciparum: cyanide‐resistant oxygen consumption. Exp Parasitol. 1997;87:112‐120. [DOI] [PubMed] [Google Scholar]
- 45. Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673‐675. [DOI] [PubMed] [Google Scholar]
- 46. Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418‐420. [PubMed] [Google Scholar]
- 47. Hasenkamp S, Russell KT, Horrocks P. Comparison of the absolute and relative efficiencies of electroporation‐based transfection protocols for Plasmodium falciparum . Malar J. 2012;11:210. [DOI] [PMC free article] [PubMed] [Google Scholar]


