Abstract
Purpose
To investigate the safety of gadoterate meglumine and identify the incidence of nephrogenic systemic fibrosis (NSF).
Materials and Methods
An international prospective observational study was conducted from November 2008 to June 2013. A total of 35,499 adults and children who were scheduled to undergo contrast‐enhanced MRI using gadoterate meglumine were analyzed (female, 53.1%; mean age: 49.5 years; range: 0‐98 years). At least 3‐month follow‐up was planned for patients with an estimated creatinine clearance or glomerular filtration rate <60 mL/min (/1.73 m2) to detect any suspicion or occurrence of NSF. Adverse events (AEs) were prospectively recorded. Demographic data, risk factors, indications for MRI examinations, characteristics of gadoterate meglumine administration, and efficacy were documented.
Results
MRI examinations were mainly for central nervous system (61%). The most frequent risk factor was renal insufficiency (14.7%). Seventy AEs were observed in 44 patients (0.12%). Among the 70 AEs, 38 in 32 patients (0.09% of all patients) were considered related to gadoterate meglumine and classified as adverse drug reaction (ADR).The most frequent ADRs were urticaria (9 patients, 0.03%), nausea (7 patients, 0.02%), and vomiting (4 patients, 0.01%). Within the pediatric population (1,629 patients), only one AE (vomiting) was observed. Nine adult patients (0.03%) experienced serious AEs. Moderate to severe renal insufficiency at inclusion was reported in 514 patients (1.5%). Among them, 476 (92.6%) were followed‐up. No patients were suspected of having NSF and no cases of NSF were observed.
Conclusion
Our study confirms the excellent safety profile of gadoterate meglumine in routine practice.
Level of Evidence: 1
J. Magn. Reson. Imaging 2017;45:988–997
Keywords: adverse event, contrast media, gadolinium‐based contrast agent, gadoteric acid, gadoterate meglumine, magnetic resonance imaging
MRI is now a pivotal examination for the assessment of a variety of pathological conditions involving many organs.1, 2, 3, 4, 5, 6 Although unenhanced MRI can be a diagnostic solving tool in some indications, contrast‐enhanced MRI is often needed to improve lesion detection and characterization and heighten confidence in diagnosis. In addition, in some specific areas MRI with intravenous (IV) administration of gadolinium‐based contrast agent (GBCA) remains the standard of reference for MRI.1, 2, 3, 4, 5, 6
Several reports have raised concerns regarding the use of GBCA because of the occurrence of nephrogenic systemic fibrosis (NSF) and acute, immediate adverse reactions after IV administration of GBCA.7, 8, 9, 10 However, the majority of these reports were retrospective so that the actual incidence of AEs along with that of NSF has not been fully addressed, or were based on single‐site or single‐country practices. Only a few large, prospective and multicenter studies were published recently,11, 12 and no multinational evaluation of the safety of gadoterate meglumine in routine practice is available yet.
Gadoterate meglumine is a macrocyclic paramagnetic GBCA with high thermodynamic stability.13 Gadoterate meglumine is commercially available in Europe since 1989 and its use has been approved in more than 80 countries for numerous indications.12, 14, 15, 16, 17 Gadoterate meglumine was approved in the United States in 2013 for imaging cerebral and spinal lesions and associated tissues with disrupted blood–brain barrier and/or abnormal vascularity in adults and children above 2 years.
The primary goal of this study was to prospectively investigate the safety of gadoterate meglumine in observational conditions. The study included countries that are less frequently represented in published studies with gadoterate meglumine or any other GBCA despite being emerging major users of GBCAs such as China and India, and thus could provide more recent and representative safety data. The secondary goal was to assess the overall incidence of NSF in patients with renal impairment who received IV administration of gadoterate meglumine for MRI.
Materials and Methods
Study Design
This prospective, observational, international study was designed to assess the safety and efficacy of gadoterate meglumine for MRI examinations in a large, unselected population with regard to demography, risk factors, and indications for MRI examination.
The study was performed from November 2008 to June 2013, inclusively in 118 centers in 10 countries: Argentina, Austria, China, France, Germany, India, Italy, Saudi Arabia, Spain, and United Kingdom. The centers were selected for their routine use of gadoterate meglumine for at least 100 MRI examinations per year. Local ethical committees approved the study protocol. Patients received clear information about the study before agreeing to participate and written informed consent was obtained when required. The study was registered on https://clinicaltrials.gov with identifier NCT01523873.
Patient Selection
Enrolled patients were consecutive adult patients (age ≥ 18 years) and children (age < 18 years) with or without renal impairment who were scheduled to undergo routine MRI with IV administration of gadoterate meglumine. A patient was not included in case of contra‐indication to MR examination or contra‐indication to gadoterate meglumine administration as defined in the local Summary of Product Characteristics.
Gender, age, body weight, renal function, and estimated creatinine clearance (eCC) or estimated glomerular filtration rate (eGFR) values, risk factors at inclusion, indications for MRI examination, and details regarding administration of gadoterate meglumine were recorded on a standardized data collection form for each individual patient.
Contrast Agent
Gadoterate meglumine or gadoteric acid (Dotarem®, Guerbet, Roissy‐Charles de Gaulle, France, or Magnescope®, Guerbet) is a macrocyclic and ionic gadolinium‐based molecule with a molecular weight of 753.86 g/mol on anhydrous basis and a concentration of 0.5 mmol/mL. This GBCA has a predominant renal elimination and a mean (±standard deviation [SD]) plasma elimination half‐life of 91 ± 14 min, similar to that of iodinated contrast agents (CAs).16
In each participating center, IV administration of gadoterate meglumine was performed according to local standard protocols, based on the recommended dose of 0.1 mmol/kg of body weight (i.e., 0.2 mL/kg); this dose was further referred as to the “standard dose.”
Safety Monitoring
All included patients who received gadoterate meglumine were followed up in each center and AEs were recorded with details regarding diagnosis, onset date, severity (mild, moderate, or severe) and outcome. The local principal investigator assessed the likelihood that an AE was related to gadoterate meglumine as follows: not related, related (doubtfully or possibly), or not assessable. AEs doubtfully or possibly related to gadoterate meglumine were defined as adverse drug reactions (ADRs). All AEs were classified according to the Medical Dictionary for Regulatory Activities (MedDRA) index terms.18 The data were recorded by the local investigators in 8/10 countries and on‐site monitoring was performed in 2/10 countries (China and India) by clinical research associates according to local regulations applicable to observational studies.
Patients with at least moderate renal impairment at inclusion (i.e., eCC < 60 mL/min or eGFR < 60 mL/min/1.73 m2 or kidney transplant) were entered into a specific safety assessment protocol. Local investigators sent a specific follow‐up questionnaire to the referring physician for completion and return after at least 3 months after IV administration of gadoterate meglumine to detect any symptoms or signs suggestive of NSF. More specifically, the referring physician was asked to examine the skin (presence of burning or itching, darkened patches, papules and subcutaneous nodules on extremities and trunk; painful skin swelling, hardening, and/or tightening), the eyes (yellow scleral plaques), the bones, the joints and muscles (presence of joint stiffness; limited range of motion in the arms, hands, legs, or feet; deep pain in the hip bone, or ribs; muscle weakness; calcifications of soft tissues), and to report presence of systemic fibrosis. This timeframe was determined based on the average time of occurrence of NSF as reported in the literature.15, 19, 20, 21
MRI and Assessment of Examination Efficacy
MRI examinations were performed with commercially available equipment according to standard protocols. Indications for MRI were classified as follows: (1) central nervous system (head/neck, brain, or spinal cord); (2) musculoskeletal system (bones/joints or soft tissues); (3) angiography (carotid arteries, renal arteries, aorto‐iliac arteries, lower limb arteries, or other arteries); (4) body (liver, kidney, pancreas, pelvis, lung, heart, or breast); or (5) others.
MRI efficacy was evaluated in terms of image quality which was graded using a five‐point scale from 0 (very poor) to 4 (very good) and diagnostic yield that was evaluated in a binary manner (diagnostic or not diagnostic).
Statistical Analysis
Statistical analysis was performed with SAS version 9.3 software (SAS® Institute Inc., Cary, NC). Descriptive statistics were calculated for all variables. Quantitative (continuous) data included means, standard deviations (SD), medians, and ranges. Qualitative (binary) data included raw numbers, frequencies, and 95% confidence intervals. Subgroup analysis was performed for several variables to identify predictive factors of AEs.
Qualitative variables were compared with the χ2 or Fisher exact test. Quantitative variables were first tested for normality in distribution. The Mann‐Whitney test was used to search for differences in variables when the variables were not normally distributed. Student t‐test was used when the continuous variables were normally distributed. All statistical tests were two‐tailed and significance was set at P < 0.05.
Results
Demographics and MRI Examination Characteristics
Completed patient questionnaires were received for 35,921 patients and 422 (1.2%) were excluded due to major deviations (compliance issues, n = 419; retrospective inclusion, n = 3); none of them corresponded to patients with AEs or suspicion of NSF. The remaining 35,499 patients (98.8%) were included in the “study” population used for description of demography and MRI indications; of them 35,474 patients (98.8% of received questionnaires) could be analyzed for safety (including risk factors, IV administration of gadoterate meglumine, AEs, ADRs, detection of NSF suspicion). Of these, 34,572 patients (96.2% of received questionnaires) had efficacy data available (efficacy population). Figure 1 shows the flow diagram of patients through the study.
Figure 1.
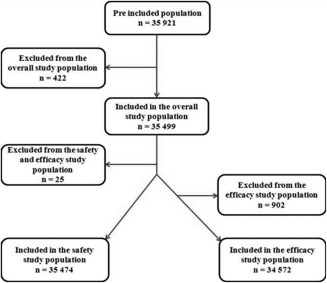
Flow chart diagram of the study.
The main recruiting countries were Germany (9988 patients; 28.1% patients), France (7692 patients; 21.7% patients), China (7064 patients; 19.9% patients), and India (6803 patients; 19.2% patients). Demographic data of patients according to participating country are given in Table 1. Among the 35,499 included patients, 46.9% were men and 53.1% were women. The patients had a mean age of 49.5 years ± 17.9 (SD) (median, 50 years; range: 0–98 years). The pediatric population included 1631 patients (4.6%). The patients had a mean body mass index (BMI) of 24.99 kg/m2 ± 5.33 (SD); 87.5% of patients had a BMI < 30 kg/m2 and 12.5% a BMI ≥ 30 kg/m2.
Table 1.
Demographic Data of Patients According to Participating Country
| Country | No. of centers | No. (%) of patients | Male/female ratio (%) | Age (years) median [range] | No. of pediatric patients (<18 years) |
|---|---|---|---|---|---|
| Austria | 8 | 703 (2.0%) | 40.3/59.7 | 55 [2 – 91] | 12 |
| France | 54 | 7692 (21.7%) | 44.9/55.1 | 53 [0 – 98] | 312 |
| Germany | 20 | 9988 (28.1%) | 45.8/54.2 | 52 [1 – 97] | 323 |
| Italy | 4 | 328 (0.9%) | 48.0/52.0 | 52 [5 – 86] | 12 |
| Spain | 3 | 1039 (2.9%) | 49.3/50.7 | 55 [0 – 91] | 22 |
| United Kingdom | 1 | 1467 (4.1%) | 20.9/79.1 | 52 [13 – 88] | 5 |
| India | 13 | 6803 (19.2%) | 53.8/46.2 | 40 [0 – 95] | 779 |
| China | 9 | 7064 (19.9%) | 49.5/50.5 | 54 [1 – 91] | 151 |
| Argentina | 2 | 43 (0.1%) | 41.9/58.1 | 57 [18 – 80] | 0 |
| Saudi Arabia | 4 | 372 (1.0%) | 47.0/53.0 | 40 [0 – 82] | 15 |
| Total | 118 | 35499 (100%) | 46.9/ 53.1 | 50 [0 – 98] | 1631 |
Indications for MRI examinations included central nervous system (61.0%), body (25.2%), musculoskeletal (14.3%), angiography (4.1%), or other organs (4.8%). A majority of patients (61.9%) had no risk factors. The most frequently reported risk factor was renal impairment (14.7%) (Table 2). A previous reaction to CAs, either with GBCAs or iodine‐based CAs, was reported in 407 patients (1.2%); they included previous reactions to GBCAs (42 patients), previous reactions to iodine‐based contrast agents (339), or not specified CAs (26 patients). Highest prevalences in risk factors were reported in France, Italy, and Spain, whereas China, India, and United Kingdom had the lowest prevalences of risk factors; with significant variations between countries (P < 0.001).
Table 2.
Pre‐existing Risk Factors and Adverse Drug Reactions in the Safety Populationa
| Pre‐existing risk factors | No. of patients with available data | n (%) | No. patients with ADR/ No. with risk factors | No. patients with ADR/ No. without risk factors | OR [95% CI] |
|---|---|---|---|---|---|
| At least one risk factor | 35,474 | 13,518 (38.1) | 19/13,518 | 13/21,956 | 2.376 [1.173‐4.812] |
| Any stage of renal impairment | 35,473 | 5,212 (14.7) | 6/5,212 | 26/30,261 | 1.341 [0.552‐3.259] |
| Allergies | 35,306 | 2,577 (7.3) | 6/2,577 | 25/32,729 | 3.053 [1.251‐7.449] |
| Previous reaction to CA | 35,288 | 407 (1.2) | 4/407 | 28/34,881 | 12.355 [4.314‐35.386] |
| Diabetes mellitus | 35,289 | 1,416 (4.0) | 1/1,416 | 31/33,873 | 0.772 [0.105‐5.656] |
| Bronchial asthma | 35,298 | 721 (2.0) | 2/721 | 30/34,577 | 3.204 [0.764‐13.430] |
| Heart insufficiency | 35,253 | 518 (1.5) | 1/518 | 31/34,735 | 2.165 [0.295‐15.892] |
| Cardiovascular disease other than heart insufficiency | 35,213 | 867 (2.5) | 2/867 | 30/34,346 | 2.645 [0.63111.085] |
Pre‐existing risk factors were analyzed in the safety population (N = 35,474).
OR = odds ratio; CI = confidence interval.
There were more adult than pediatric patients at risk for each individual risk factor (P < 0.001). Risk factor frequency varied according to gender. Risk factors were significantly more frequent (P < 0.001) in patients with BMI ≥ 30 kg/m2 except for previous reaction to CAs. Premedication was given in 1.9% of patients.
The mean total volume of injected gadoterate meglumine was 14.01 mL ± 4.98 (SD) (median, 15.00 mL; range: 0.5–53.0 mL). The mean dose of gadoterate meglumine was 0.21 mL/kg ± 0.07 (SD) (median, 0.20 mL/kg; range: 0.02–2.1 mL/kg). In the pediatric population, the mean total volume was 7.53 mL ± 4.50 (SD) (range: 0.5–36.0 mL) and the mean dose was 0.24 mL/kg ± 0.14 (SD) (range: 0.0–2.1 mL/kg). In the adult population, the mean total volume was 14.32 mL ± 4.78 (SD) (range: 0.80–53.00 mL) and the mean dose was 0.21 mL/kg ± 0.07 (SD) (range: 0.02–1.0 mL/kg). The median dose was the same (0.20 mL/kg) in the adult and pediatric population. Regarding BMI, the mean dose was greater in nonobese patients (BMI < 30 kg/m2) than in obese patients (0.21 mL/kg ± 0.07 [SD]) versus 0.17 mL/kg ± 0.07 [SD], respectively), with a median dose of 0.20 mL/kg and 0.17 mL/kg, respectively.
Adverse Events and Safety
Seventy AEs were observed in 44 patients (0.12%) during MRI examination or during the follow‐up after IV administration of gadoterate meglumine (Table 3). On a per‐country basis, AEs were more frequent in Italy (6/328 patients [1.83%]; 6 AEs), Spain (5/1037 patients [0.48%]; 7 AEs), and France (13/7683 patients [0.17%], 21 AEs) followed by patients from United Kingdom (2/1465 patients [0.14%], 2 AEs), Germany (10/9980 patients [0.10%]; 24 AEs), India (5/6803 patients [0.07%]; 7 AEs), and China (3/7064 patients [0.04%]; 3 AEs). No AEs were reported in the three other countries. There were 18 men and 26 women, with a mean age of 49.6 years ± 19.(SD) years (range: 2–90 years). No significant differences in gender (P = 0.428), age distribution (P = 0.754), and BMI class (<30 versus ≥ 30 kg/m2; P = 0.253) were found between the patients who experienced AEs and those who did not. The frequencies of AEs per MedDRA preferred term and System Organ Class are presented in Table 3.
Table 3.
Frequency of Adverse Events per Preferred Term and System Organ Classa
| System organ class Preferred term | Safety population (n = 35,474) | |||
|---|---|---|---|---|
| AEs | ADRs | |||
| No. (%) of patients with at least one AE | No. of AEs | No. (%) of patients with at least one ADR | No. of ADRs | |
| Any AE | 44 (0.12%) | 70 | 32 (0.09%) | 38 |
| Skin and subcutaneous tissue disorders | 18 (0.05%) | 19 | 16 (0.05%) | 17 |
| Urticaria | 9 (0.03%) | 9 | 9 (0.03%) | 9 |
| Angioedema | 2 (0.006%) | 2 | 2 (0.006%) | 2 |
| Pruritus | 2 (0.006%) | 2 | 2 (0.006%) | 2 |
| Rash | 2 (0.006%) | 2 | ‐ | ‐ |
| Rash macular | 1 (0.003%) | 1 | 1 (0.003%) | 1 |
| Dermatitis allergic | 1 (0.003%) | 1 | 1 (0.003%) | 1 |
| Erythema | 1 (0.003%) | 1 | 1 (0.003%) | 1 |
| Swelling face | 1 (0.003%) | 1 | 1 (0.003%) | 1 |
| Gastrointestinal disorders | 12 (0.03%) | 13 | 10 (0.03%) | 11 |
| Nausea | 7 (0.02%) | 7 | 7 (0.02%) | 7 |
| Vomiting | 4 (0.01%) | 4 | 4 (0.01%) | 4 |
| Crohn disease | 1 (0.003%) | 1 | ‐ | ‐ |
| Intra‐abdominal hemorrhage | 1 (0.003%) | 1 | ‐ | ‐ |
| General disorders and administration site conditions | 11 (25.0%) | 15 | 5 (0.01%) | 7 |
| Adverse event | 2 (0.006%) | 2 | ‐ | ‐ |
| Death | 2 (0.006%) | 2 | 1 (0.003%) | 1 |
| Multi‐organ failure | 2 (0.006%) | 2 | ‐ | ‐ |
| Pyrexia | 2 (0.006%) | 2 | 1 (0.003%) | 1 |
| Chills | 1 (0.003%) | 1 | 1 (0.003%) | 1 |
| Extravasation | 1 (0.003%) | 1 | ‐ | ‐ |
| Feeling hot | 1 (0.003%) | 1 | 1 (0.003%) | 1 |
| General physical health deterioration | 1 (0.003%) | 1 | ‐ | ‐ |
| Injection site erythema | 1 (0.003%) | 1 | 1 (0.003%) | 1 |
| Injection site extravasation | 1 (0.003%) | 1 | 1 (0.003%) | 1 |
| Injection site warmth | 1 (0.003%) | 1 | 1 (0.003%) | 1 |
| Respiratory, thoracic and mediastinal disorders | 3 (0.008%) | 6 | ‐ | ‐ |
| Bronchial obstruction | 1 (0.003%) | 1 | ‐ | ‐ |
| Dyspnea | 1 (0.003%) | 1 | ‐ | ‐ |
| Lung disorder | 1 (0.003%) | 1 | ‐ | ‐ |
| Pleurisy | 1 (0.003%) | 1 | ‐ | ‐ |
| Pneumothorax | 1 (0.003%) | 1 | ‐ | ‐ |
| Respiratory distress | 1 (0.003%) | 1 | ‐ | ‐ |
| Nervous system disorders | 3 (0.008%) | 3 | 1 (0.003%) | 1 |
| Clonus | 1 (0.003%) | 1 | ‐ | ‐ |
| Migraine | 1 (0.003%) | 1 | 1 (0.003%) | 1 |
| Presyncope | 1 (0.003%) | 1 | ‐ | ‐ |
| Renal and urinary disorders | 3 (0.008%) | 3 | 1 (0.003%) | 1 |
| Renal failure | 2 (0.006%) | 2 | 1 (0.003%) | 1 |
| Acute renal failure | 1 (0.003%) | 1 | ‐ | ‐ |
| Cardiac disorders | 2 (0.006%) | 4 | ‐ | ‐ |
| Cardiac failure | 1 (0.003%) | 1 | ‐ | ‐ |
| Cardio‐respiratory arrest | 1 (0.003%) | 1 | ‐ | ‐ |
| Cardiovascular insufficiency | 1 (0.003%) | 1 | ‐ | ‐ |
| Mitral valve incompetence | 1 (0.003%) | 1 | ‐ | ‐ |
| Infections and infestations | 2 (0.006%) | 2 | ‐ | ‐ |
| Candida sepsis | 1 (0.003%) | 1 | ‐ | ‐ |
| Sepsis | 1 (0.003%) | 1 | ‐ | ‐ |
| Blood and lymphatic system disorders | 1 (0.003%) | 1 | ‐ | ‐ |
| Hemorrhagic diathesis | 1 (0.003%) | 1 | ‐ | ‐ |
| Immune system disorders | 1 (0.003%) | 1 | 1 (0.003%) | 1 |
| Hypersensitivity | 1 (0.003%) | 1 | 1 (0.003%) | 1 |
| Metabolism and nutrition disorders | 1 (0.003%) | 1 | ‐ | ‐ |
| Acidosis | 1 (0.003%) | 1 | ‐ | ‐ |
| Vascular disorders | 1 (0.003%) | 2 | ‐ | ‐ |
| Angiopathy | 1 (0.003%) | 1 | ‐ | ‐ |
| Shock | 1 (0.003%) | 1 | ‐ | ‐ |
All reported adverse events were coded in “Preferred term” according to current MedDRA version. One patient could experience more than one ADR. AE = adverse event; ADR = adverse drug reaction.
None of the patients who experienced AEs had received premedication before IV administration of gadoterate meglumine. Most AEs were mild (47.6%) or moderate (19.0%) in intensity, and most AEs resolved within the reporting timeframe (66.7%). A total of 21 AEs (33.3%) were considered severe and one was considered related to gadoterate meglumine (urticaria in a 39‐year‐old woman who recovered after treatment).
Among the 70 AEs, 38 (54.3%) in 32 patients (0.09% of all patients) were considered doubtfully or possibly related to gadoterate meglumine and classified as ADRs. The most frequent ADRs were urticaria (9 patients, 0.03%), nausea (7 patients, 0.02%), and vomiting (4 patients, 0.01%) (Table 3). One ADR was reported in the pediatric population (0.06%) consisting of vomiting of mild intensity in a 2‐year‐old girl. There was no statistically significant difference between patients with and without ADRs according to gender, age, BMI, mean volume, or mean dose of gadoterate meglumine injected (Table 4). Overall, 19 patients with at least one pre‐existing risk factor experienced at least one ADR. Differences were found between patients who experienced ADR and those who did not (Tables 2 and 4). The presence of at least one of the listed risk factors was more frequently observed in patients with ADR than in patients without ADR (59.4% versus 38.1%; P = 0.017).
Table 4.
Characteristics of Patients with and without ADRa
| Patients without ADR (n = 35442) | Patients with at least one ADR (n = 32) | P‐Value (Test) | |
|---|---|---|---|
| Gender | 0.288 (χ2) | ||
| Missing | 41 | 0 | |
| Male | 16594 (46.9%) | 12 (37.5%) | |
| Female | 18807 (53.1%) | 20 (62.5%) | |
| Age (years) | 0.108 (Wilcoxon) | ||
| Missing | 142 | 0 | |
| Mean ± SD | 49.5 ± 17.9 | 45.6 ± 17.7 | |
| Median | 50.0 | 43.0 | |
| Range | 0.0 ‐ 98.0 | 2.0 ‐90.0 | |
| BMI (kg/m2) | 0.422 (Fisher exact) | ||
| Missing | 922 | 0 | |
| < 30 | 30199 (87.5%) | 30 (93.8%) | |
| ≥ 30 | 4321 (12.5%) | 2 (6.3%) | |
| Premedication | 1.000 (Fisher exact) | ||
| Missing | 571 | 0 | |
| No | 34225 (98.1%) | 32 (100.0%) | |
| Yes | 646 (1.9%) | 0 (0.0%) | |
| At least one pre‐existing risk factor | 0.017 (Fisher exact) | ||
| No | 21943 (61.9%) | 13 (40.6%) | |
| Yes | 13499 (38.1%) | 19 (59.4%) | |
| Total volume (mL) | 0.802 (Wilcoxon) | ||
| Missing | 25 | 0 | |
| Mean ± SD | 14.01 ± 4.98 | 14.38 ± 7.87 | |
| Median | 15.00 | 15.00 | |
| Range | 0.5 ‐53.0 | 3.0 ‐ 53.0 | |
| Dose (mL/kg) | 0.895 (Wilcoxon) | ||
| Missing | 167 | 0 | |
| Mean ± SD | 0.21 ± 0.07 | 0.22 ± 0.11 | |
| Median | 0.20 | 0.20 | |
| Range | 0.0 ‐ 2.1 | 0.1 ‐ 0.8 | |
| Dose in class (mL/kg) | < 0.001 (χ2) | ||
| Missing | 167 | 0 | |
| < 0.18 | 9333 (26.5%) | 6 (18.8%) | |
| 0.18 ‐ 0.66 | 25893 (73.4%) | 25 (78.1%) | |
| > 0.66 | 49 (0.1%) | 1 (3.1%) |
Data were extracted from the safety population (n = 35,474 patients).
Medical history of allergies and previous reactions to CAs were two specific risk factors that were more frequently observed in patients with ADRs than in with patients without ADRs (19.4% vs. 7.3%, P = 0.023; 12.5% versus 1.1%, P < 0.001; respectively). Six patients with pre‐existing allergies experienced one nonserious ADR each consisting of urticaria (3 patients), rash macular (1 patient), warmness (1 patient), and hypersensitivity (1 patient).
Four patients with previous reaction to CAs (one to GBCA and three to iodine‐based CAs) experienced five nonserious ADRs consisting of urticaria (2 patients), rash (1 patient), and nausea and vomiting (both in same patient, on the same day).
Nine adult patients (0.03%) experienced serious AEs. Three serious AEs were considered possibly related to gadoterate meglumine. They consisted of renal failure in one patient, and angioedema with erythema occurring on the same day in one patient; both patients recovered. One death was considered as doubtfully related to gadoterate meglumine in a 84‐year‐old man with a complex medical history including severe impaired renal function and class IV heart failure according to the New York Heart Association, proteinuria, and chronic arterial occlusive disease. This death occurred 9 days after IV administration of 53 mL of gadoterate meglumine given in three automatic injections for MR angiography of the aorto‐iliac arteries, after a sequence of five other AEs that included candida sepsis, acute renal failure, cardiovascular insufficiency, acidosis, and mitral valve incompetence.
NSF Suspicion in Patients With Impaired Renal Function
A total of 514 patients with impaired renal function were included in the safety population. In these patients, impaired renal function was moderate in 417/514 (81.1%), severe in 58/514 (11.3%), and end‐stage or dialysis in 7/514 patients (1.4%), with eCC or eGFR between 30 and 60, between 15 and 30, and <15 mL/min/1.73 m2, respectively. Follow‐up could be retrieved for 476/514 patients (92.6%). None of them developed NSF or had suspicion of NSF after a mean follow‐up of 148 days (up to 996 days). The follow‐up duration was ≥ 3 months for 316/476 patients (66.4%). Patients with impaired renal function who were excluded from the safety population were also checked for NSF suspicion but none was identified.
Image Quality
Among the 34,572 patients analyzed for MRI efficacy, image quality was good or very good for 34,009 patients (98.8%) (Table 5). Very good quality MR images were more frequent in patients with BMI<30 kg/m2 compared with patients with BMI≥30 kg/m2 (70.7% versus 65.6%, respectively; P < 0.001). MR images of good to very good quality were reported for 99.1%, 98.7%, 99.4%, 95.2%, and 97.5% of patients for CNS, body, musculoskeletal, angiography, and other indications, respectively.
Table 5.
Image Quality and Diagnostic Efficacy According to the Total Dose of Gadoterate Meglumine Administereda
| Dose (mL/kg) | All N = 34,572* | |||||
|---|---|---|---|---|---|---|
| < 0.18 | 0.18 to 0.22 | >0.22 to 0.66 | >0.66 | P‐Value (test) | ||
| N = 9,271 | N = 15,317 | N = 9,769 | N = 50 | |||
| MR image quality rating | < 0.001 (χ2) | |||||
| Missing | 20 | 85 | 58 | 0 | 164 | |
| Very poor | 1 (<0.1%) | 3 (<0.1%) | 2 (<0.1%) | 0 (0.0%) | 6 (<0.1%) | |
| Poor | 5 (0.1%) | 31 (0.2%) | 16 (0.2%) | 0 (0.0%) | 52 (0.2%) | |
| Fair | 58 (0.6%) | 196 (1.3%) | 85 (0.9%) | 1 (2.0%) | 341 (1.0%) | |
| Good | 3,402 (36.8%) | 4,201 (27.6%) | 2,524 (26.0%) | 25 (50.0%) | 10,268 (29.8%) | |
| Very good | 5,785 (62.5%) | 10,801 (70.9%) | 7,084 (72.9%) | 24 (48.0%) | 23,741 (69.0%) | |
| Diagnostic efficacy | 0.101 (χ2) | |||||
| Missing | 33 | 201 | 72 | 0 | 308 | |
| No | 104 (1.1%) | 160 (1.1%) | 78 (0.8%) | 0 (0.0%) | 343 (1.0%) | |
| Yes | 9,134 (98.9%) | 14,956 (98.9%) | 9,619 (99.2%) | 50 (100.0%) | 33,921 (99.0%) | |
Data were extracted from the efficacy population (n = 34,572 patients). The total includes 165 patients with unknown received dose of gadoterate meglumine.
Analysis of MR image quality showed that fewer patients in the ≥0.66 mL/kg dose class had very good image quality (48.0% versus 62.5–72.9% in the lower dose classes; P < 0.001) (Table 5). Good to very good images were reported for 98.0% of patients in the ≥0.66 mL/kg dose class versus 98.5–99.3% for patients in the lower dose classes (P < 0.001).
A diagnosis was established for 33,921 patients yielding an efficacy rate of 99.0%. No differences in diagnostic efficacy were observed according to age class, gender, BMI, degree of renal impairment, condition of administration, or occurrence of AEs.
Discussion
Our study involving 35,499 patients of all ages is to date the largest prospective study on the safety of gadoterate meglumine that was designed to include patients from a multinational environment. This study confirms the excellent safety profile of gadoterate meglumine. More specifically, the results of this study showed that gadoterate meglumine is a well‐tolerated GBCA, with a relatively low incidence of AEs (0.12%). In addition, no cases of NSF were reported, supporting that gadoterate meglumine is associated with a negligible risk of NSF. Moreover, gadoterate meglumine showed high degrees of efficacy considering the high proportion of MRI examinations that allowed reaching a diagnosis and/or that yielded high quality images.
Our study demonstrates that gadoterate meglumine is responsible for AEs that are predominantly mild or moderate in intensity (66.6%) and transient (66.7%). Among the 32 patients with gadoterate meglumine‐related AEs, the most common ADRs were urticaria, nausea, and vomiting. In addition, no cases of NSF were observed in the subgroup of patients with renal insufficiency, including 65 patients with an eGRF < 60 mL/min/1.73 m2.
Prior reports showed that NSF was observed in renally‐compromised patients who had GBCA‐enhanced MRI examinations.19, 20, 22, 23, 24 Such category of patients consisted of 514 patients in our study. Although no fully and definitely elucidated cases of NSF secondary to IV administration of gadoterate meglumine have been observed in our study or reported in the literature so far,25 we recommend caution for renally impaired patients who are scheduled to undergo IV administration of gadoterate meglumine.26 In this regard, screening for NSF risk should not be disregarded or omitted.23
Our results in terms of AEs favorably compare with those of Ishiguchi and Takahashi.11 In their prospective study involving 3444 patients from 127 Japanese institutions, 40 AEs were reported in 32 patients, yielding an overall incidence of AEs of 1.16% on a per‐AE basis and of 0.929% on a per‐patient basis.11 Ishiguchi and Takahashi reported AEs of mild intensity in 36/40 AEs (90%) and moderate in 4/40 AEs (10%). The most frequent AEs were gastrointestinal, skin, and subcutaneous tissue disorders.11 Herborn et al reported minor AEs in 94/24,308 patients (0.4%), including nausea, vomiting, feeling of warmness, and taste alteration.14 In the multicenter study by Maurer et al involving 84,621 patients from 129 German institutions, 421 AEs were reported in 285 patients yielding an AE rate of 0.34%.12 Oudkerk et al observed minor AEs in 0.97% of patients in their study involving 1038 patients.17
In the pediatric population, we observed only one ADR (0.06%), consistent with prior results.27 Balassy et al have analyzed the results of seven clinical trials and six postmarketing studies performed with gadoterate meglumine.27 They found among a total of 3,810 pediatric patients, 20 AEs in 10 patients; 7 of them were considered as ADRs to gadoterate meglumine, for an overall incidence of 0.262% for AEs and 0.184% for ADRs on a per‐patient basis.
In our study, only nine patients experienced serious AEs. In the study by Ishiguchi and Takahashi, no patients experienced serious AEs, probably because of the limited population size.11 In the study by Maurer et al, only eight patients (<0.01%) had serious AEs with a favorable outcome.12 Herborn et al have reported the occurrence of anaphylactic shock in only 1/24,308 patients (0.004%) after IV gadoterate meglumine with a favorable outcome.14
We found an overall diagnostic efficacy rate of 99% for MRI examinations using gadoterate meglumine, consistent with prior studies (99.5–99.7%).11, 12, 14 In addition, image quality was good or excellent in 98.8% of patients, consistent with the results of Herborn et al14 and Maurer et al.12 Regarding image quality and given dose, we observed that best image quality was reported for patients who received recommended doses of gadoterate meglumine (0.18–0.66 mL/kg) and not for those who received greater doses. This result is of importance, because GBCAs are often used with greater doses than those that are recommended.28 Our results suggest that high doses do not improve image quality.
In our study, we addressed the issue of AEs due to gadoterate meglumine and we also aimed to determine if individual patient variables may predict the occurrence of AE. Ishiguchi and Takahashi found in a retrospective study that several variables including patient general condition, liver disease, renal disorder, concomitant treatment with a variety of drugs and high liquid dose of gadoterate meglumine were significantly associated with AEs.11 In our prospective study, the dose of gadoterate meglumine did not affect the incidence of ADRs. No specific demographic characteristics were associated with a higher risk of ADR to gadoterate meglumine.
Our study has several limitations. First, many patients with renal insufficiency did not receive gadoterate meglumine because at many participating centers local policies recommend performance of unenhanced MRI examinations in patients with renal impairment. However, a prerequisite of our study was to not alter local practices to best reflect the actual rate of AEs and ADRs. Second, because of the low incidence of NSF, it may be argued that our study population might have been too small to detect cases of NSF. Third, studies have reported that the time interval between GBCA administration and the onset of NSF ranges between <10 days and up to 68 months.19, 20, 21, 22, 23, 24, 29, 30 It may be thus argued that the follow‐up duration in our study may be not long enough to definitely exclude any case of NSF that appeared late. However, the majority of reported cases of NSF have developed less than 3 months after GBCA administration.24, 29 Finally, in our study, we did not compare gadoterate meglumine with other GBCAs.
In conclusion, the efficacy results of our study in conjunction with an acceptable and transient AE profile indicate that gadoterate meglumine is a safe and effective GBCA when intravenously administered in adults and children for MRI examination.
References
- 1. Tielbeek JA, Ziech ML, Li Z, et al. Evaluation of conventional, dynamic contrast enhanced and diffusion weighted MRI for quantitative Crohn's disease assessment with histopathology of surgical specimens. Eur Radiol 2014;24:619–629. [DOI] [PubMed] [Google Scholar]
- 2. Montant P, Sigovan M, Revel D, Douek P. MR imaging assessment of myocardial edema with T2 mapping. Diagn Interv Imaging 2015;96:885–890. [DOI] [PubMed] [Google Scholar]
- 3. Burt JR, Zimmerman SL, Kamel IR, Halushka M, Bluemke DA. Myocardial T1 mapping: techniques and potential applications. Radiographics 2014;34:377–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alili C, Pages E, Curros Doyon F, Perrochia H, Millet I, Taourel P. Correlation between MR imaging ‐ prognosis factors and molecular classification of breast cancers. Diagn Interv Imaging 2014;95:235–242. [DOI] [PubMed] [Google Scholar]
- 5. Sillou S, Poirée S, Millischer AE, Chapron C, Hélénon O. Urinary endometriosis: MR imaging appearance with surgical and histological correlations. Diagn Interv Imaging 2015;96:373–381. [DOI] [PubMed] [Google Scholar]
- 6. Fukunaga T, Fujii S, Inoue C, et al. Accuracy of semiquantitative dynamic contrast‐enhanced MRI for differentiating type II from type I endometrial carcinoma. J Magn Reson Imaging 2015;41:1662–1668. [DOI] [PubMed] [Google Scholar]
- 7. Okigawa T, Utsunomiya D, Tajiri S, et al. Incidence and severity of acute adverse reactions to four different gadolinium‐based MR contrast agents. Magn Reson Med Sci 2014;13:1–6. [DOI] [PubMed] [Google Scholar]
- 8. Hunt CH, Hartman RP, Hesley GK. Frequency and severity of adverse effects of iodinated and gadolinium contrast materials: retrospective review of 456,930 doses. AJR Am J Roentgenol 2009;193:1124–1127. [DOI] [PubMed] [Google Scholar]
- 9. Prince MR, Zhang H, Zou Z, Staron RB, Brill PW. Incidence of immediate gadolinium contrast media reactions. AJR Am J Roentgenol 2011;196:W138–W143. [DOI] [PubMed] [Google Scholar]
- 10. Forsting M, Palkowitsch P. Prevalence of acute adverse reactions to gadobutrol‐‐a highly concentrated macrocyclic gadolinium chelate: review of 14,299 patients from observational trials. Eur J Radiol 2010;74:e186–e192. [DOI] [PubMed] [Google Scholar]
- 11. Ishiguchi T, Takahashi S. Safety of gadoterate meglumine (Gd‐DOTA) as a contrast agent for magnetic resonance imaging: results of a post‐marketing surveillance study in Japan. Drugs R D 2010;10:133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maurer M, Heine O, Wolf M, Durmus T, Wagner M, Hamm B. Tolerability and diagnostic value of gadoteric acid in the general population and in patients with risk factors: results in more than 84,000 patients. Eur J Radiol 2012;81:885–890. [DOI] [PubMed] [Google Scholar]
- 13. Meyer D, Schaefer M, Bonnemain B. Gd‐DOTA, a potential MRI contrast agent: current status of physiological knowledge. Invest Radiol 1988;23(Suppl 1):S232–235. [DOI] [PubMed] [Google Scholar]
- 14. Herborn CU, Honold E, Wolf M, et al. Clinical safety and diagnostic value of the gadolinium chelate gadoterate meglumine (Gd‐DOTA). Invest Radiol 2007;42:58–62. [DOI] [PubMed] [Google Scholar]
- 15. Deray G, Rouviere O, Bacigalupo L, et al. Safety of meglumine gadoterate (Gd‐DOTA)‐enhanced MRI compared to unenhanced MRI in patients with chronic kidney disease (RESCUE study). Eur Radiol 2013;23:1250–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Le Mignon MM, Chambon C, Warrington S, Davies R, Bonnemain B. Gd‐DOTA. Pharmacokinetics and tolerability after intravenous injection into healthy volunteers. Invest Radiol 1990;25:933–937. [PubMed] [Google Scholar]
- 17. Oudkerk M, Sijens PE, Van Beek EJ, Kuijpers TJ. Safety and efficacy of dotarem (Gd‐DOTA) versus magnevist (Gd‐DTPA) in magnetic resonance imaging of the central nervous system. Invest Radiol 1995;30:75–78. [DOI] [PubMed] [Google Scholar]
- 18.MedDRA Distribution File Format Document Version 18.0. Available at: http://www.meddra.org/sites/default/files/guidance/file/dist_file_format_18_0_english.pdf. Accessed on May, 2016.
- 19. Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast‐enhanced magnetic resonance imaging. J Am Soc Nephrol 2006;17:2359–2362. [DOI] [PubMed] [Google Scholar]
- 20. Broome DR, Girguis MS, Baron PW, Cottrell AC, Kjellin I, Kirk GA. Gadodiamide‐associated nephrogenic systemic fibrosis: why radiologists should be concerned. AJR Am J Roengenol 2007;188:586–592. [DOI] [PubMed] [Google Scholar]
- 21. Khurana A, Runge VM, Narayanan M, Greene JF Jr, Nickel AE. Nephrogenic systemic fibrosis: a review of 6 cases temporally related to gadodiamide injection (ommniscan). Invest Radiol 2007;42:139–145. [DOI] [PubMed] [Google Scholar]
- 22. Thomsen HS. Nephrogenic systemic fibrosis: a serious adverse reaction to gadolinium ‐ 1997‐2006‐2016. Part 2. Acta Radiol 2016;57:643–648. [DOI] [PubMed] [Google Scholar]
- 23. Perez‐Rodriguez J, Lai S, Ehst BD, Fine DM, Bluemke DA. Nephrogenic systemic fibrosis: incidence, associations, and effect of risk factor assessment‐‐report of 33 cases. Radiology 2009;250:371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology 2007;243:148–157. [DOI] [PubMed] [Google Scholar]
- 25. Amet S, Launay‐Vacher V, Clément O, et al. Incidence of nephrogenic systemic fibrosis in patients undergoing dialysis after contrast‐enhanced magnetic resonance imaging with gadolinium‐based contrast agents: the Prospective Fibrose Nephrogénique Systémique study. Invest Radiol 2014;49:109–115. [DOI] [PubMed] [Google Scholar]
- 26. Wang Y, Alkasab TK, Narin O, et al. Incidence of nephrogenic systemic fibrosis after adoption of restrictive gadolinium‐based contrast agent guidelines. Radiology 2011;260:105–111. [DOI] [PubMed] [Google Scholar]
- 27. Balassy C, Roberts D, Miller SF. Safety and efficacy of gadoteric acid in pediatric magnetic resonance imaging: overview of clinical trials and post‐marketing studies. Pediatr Radiol 2015;45:1831–1841. [DOI] [PubMed] [Google Scholar]
- 28. Nacif MS, Arai AE, Lima JA, Bluemke DA. Gadolinium‐enhanced cardiovascular magnetic resonance: administered dose in relationship to United States Food and Drug Administration (FDA) guidelines. J Cardiovasc Magn Reson 2012;14:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kallen AJ, Jhung MA, Cheng S, et al. Gadolinium‐containing magnetic resonance imaging contrast and nephrogenic systemic fibrosis: a case‐control study. Am J Kidney Dis 2008;51:966–975. [DOI] [PubMed] [Google Scholar]
- 30. Zou Z, Ma L. Nephrogenic systemic fibrosis: review of 408 biopsy‐confirmed cases. Indian J Dermatol 2011;56:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]


