Abstract
Tolerance induction through simultaneous hematopoietic stem cell and renal transplantation has shown promising results, but it is hampered by the toxicity of preconditioning therapies and graft‐versus‐host disease (GVHD). Moreover, renal function has never been compared to conventionally transplanted patients, thus, whether donor‐specific tolerance results in improved outcomes remains unanswered. We collected follow‐up data of published cases of renal transplantations after hematopoietic stem cell transplantation from the same donor and compared patient and transplant kidney survival as well as function with caliper‐matched living‐donor renal transplantations from the Austrian dialysis and transplant registry. Overall, 22 tolerant and 20 control patients were included (median observation period 10 years [range 11 months to 26 years]). In the tolerant group, no renal allograft loss was reported, whereas 3 were lost in the control group. Median creatinine levels were 85 μmol/l (interquartile range [IQR] 72‐99) in the tolerant cohort and 118 μmol/l (IQR 99‐143) in the control group. Mixed linear‐model showed around 29% lower average creatinine levels throughout follow‐up in the tolerant group (P < .01). Our data clearly show stable renal graft function without long‐term immunosuppression for many years, suggesting permanent donor‐specific tolerance. Thus sequential transplantation might be an alternative approach for future studies targeting tolerance induction in renal allograft recipients.
Keywords: bone marrow/hematopoietic stem cell transplantation, clinical research/practice, kidney (allograft) function/dysfunction, kidney transplantation/nephrology, tolerance: clinical
Short abstract
A long‐term follow‐up analysis shows stable renal graft function without immunosuppression and no graft loss in patients receiving renal transplantation after hematopoietic stem cell transplantation from the same donor.
Abbreviations
- aGVHD
acute graft‐versus‐host disease
- AIC
Akaike Information Criterion
- ALL
acute lymphoid leukemia
- AML
acute myeloid leukemia
- AZA
azathioprine
- BMT‐NP
bone marrow transplantation nephropathy
- CML
chronic myeloid leukemia
- CNI
calcineurin inhibitor
- CYA
cyclosporin A
- DBD
donor after brain death
- DSA
donor‐specific antibody
- ESRD
end‐stage renal disease
- GN
glomerulonephritis
- GVHD
graft‐versus‐host disease
- HSCT
hematopoietic stem cell transplantation
- HUS
hemolytic uremic syndrome
- IS
immunosuppression
- MMF
mycophenolate mofetil
- NK
not known
- N
no
- Nr
number
- OEDTR
Austrian Dialysis and Transplant Registry
- RT
renal transplantation
- SCD
sickle cell disease
- SMD
standardized mean difference
- TAC
tacrolimus
- TTP
thrombotic thrombocytopenic purpura
- Yr
years
- Y
yes
1. INTRODUCTION
Renal transplantation (RT) is the treatment of choice for eligible patients with end‐stage renal disease (ESRD). Whereas short‐ and intermediate‐term outcomes of renal allografts have been improved due to fewer early rejections and eventually through better standardized immunosuppressive treatment strategies, evidence that these effects have significantly enhanced long‐term transplant kidney survival is still missing.1, 2 Therefore, the induction of tolerance is considered as the ultimate goal in the field of transplantation. Currently, the only clinically successful way to induce donor‐specific tolerance is to establish full or mixed chimerism through hematopoietic stem cell transplantation (HSCT).3
Since the concept of chimerism leading to selective donor‐specific tolerance has been developed in the past century by Billingham, Medawar, and colleagues,4 substantial progress has been made in terms of preconditioning and the understanding of relevant pathways involved herein.3 However, despite promising results in murine studies, its widespread use has been hampered by the cytotoxic preconditioning, which is currently required for bone marrow transplantation. Clinically, the first evidence of tolerance through HSCT was delivered by Sayegh et al in 1991, who described a 30‐year‐old man, diagnosed with acute myelomonocytic leukemia and a 14‐year‐old girl with acute nonlymphocytic leukemia. Both received HSCTs from their HLA‐identical brothers. After a few years and gradual deterioration of their renal function, the original stem cell donors agreed to also donate a kidney. Except for small maintenance doses of steroids, no immunosuppression was given in the further course and transplant kidney function remained stable.5 Based on this success more case reports followed, including HLA‐mismatched donors, who successfully donated renal transplants after HSCT.6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21
Due to a full donor chimerism in these patients, a robust state of tolerance for the donated organ is expected. Nevertheless, most of these patients received at least short‐term immunosuppression after transplantation. This was done not only to prevent renal allograft rejection but also to prevent graft‐versus‐host disease (GVHD), which theoretically could be triggered by solid organ transplantation.22, 23 However, even though the concept of tolerance induction through HSCT was established more than 2 decades ago, long‐term follow‐up data from patients after sequential transplantation of hematopoietic stem cells and renal allografts from the same donor are mostly monocentric and limited by small patient numbers or a short follow‐up.10, 18, 24 Moreover, due to the limited patient numbers, comparisons to conventionally transplanted patients were not feasible and so far, uncertainty remains whether tolerance truly improves renal allograft survival.
In this study we aimed at analyzing the long‐term outcome of patients after sequential HSCT and RT from the same donor by comparing renal allograft function and patient survival with a matched cohort of conventionally transplanted live donor kidney recipients from the Austrian Dialysis and Transplant Registry (OEDTR).
2. METHODS
2.1. Systematic literature research
To analyze the long‐term outcome of patients after sequential HSCT and RT from the same donor, we conducted a systematic literature search in PubMed and the Cochrane database. In addition, the references of all relevant papers were manually reviewed for further reports. Studies describing combined HSCT and RT were not included because of different clinical conditions (mixed chimerism vs full chimerism, patients with ESRD but without preexisting malignant comorbidity) and different study designs.
2.2. Data retrieval
First, corresponding authors of the papers identified in the literature search were contacted and asked for follow‐up data. Data were fully anonymized, or anonymization was performed immediately after reception. Patients were included in the analysis if the follow‐up period was longer than originally described in the case report or additional information concerning patient or renal graft survival was available. Some authors reported that further, unpublished patients with previous HSCT from the same donor were transplanted. These patients were also included in our analysis. The analysis was conducted in accordance with the guidelines of good clinical practice and approved by the ethics committee of the Medical University of Vienna (EK # 1218/2017 and 1535/2012).
2.3. Requested parameters
Basic demographic and clinical characteristics were extracted from the reports: gender, relation of donor, age at each transplantation, indication for HSCT, cause of ESRD, HLA‐mismatch, immunosuppressive regimen after RT, creatinine at each follow‐up (μmol/l), episodes of GVHD, incidence of rejection of the renal allograft and date of last follow‐up.
2.4. Control group of conventionally transplanted living‐donor recipients
To compare the long‐term patient and transplant kidney‐survival of sequentially with conventionally transplanted patients, data from recipients of renal transplants were extracted from the OEDTR.25 The OEDTR was established by the Austrian Society of Nephrology in 1970 and has almost complete follow‐up. The registry comprises data extracted from the original medical records, which were created by the responsible physician at the time of a patient's follow‐up visit. All recorded ESRD patients who received their first single‐organ kidney transplant from a living donor between 1990 and 2012, were eligible for this study and followed up until 2014 (N = 724). To achieve a more balanced analysis of the long‐term renal allograft function, we excluded patients who lost their renal allograft within the first 3 months (Figure 1). Because this general patient population differs from the population of tolerant patients due to their medical history, we decided to match patients from each group according to immunologic and clinical key characteristics (Table 1). Caliper‐matching criteria were chosen reflecting the previously described influence of those parameters on long‐term transplant kidney survival and the availability of patient information common in the literature data and the OEDTR.26, 27, 28 We aimed at a 1:1 matching because of the limited pool of potential matching candidates from the OEDTR once all criteria, especially optimal HLA match, and missing data were taken into account. Patients were selected from the OEDTR without replacement, such that each control patient occurred only once. Only if for a given sequential patient no other suitable matching partner was found in the registry data, we allowed that a single individual from the OEDTR acted as partner for more than one sequential patient. The rationale for this was the extraction of a subgroup from the OEDTR that is as close to the group of literature patients as possible while still retaining reasonable variability within the selected subgroup. Differences between the groups for several key characteristics are quantified as standardized mean differences (SMDs), which express the difference of the means between the groups in units of the pooled standard deviation.
Figure 1.
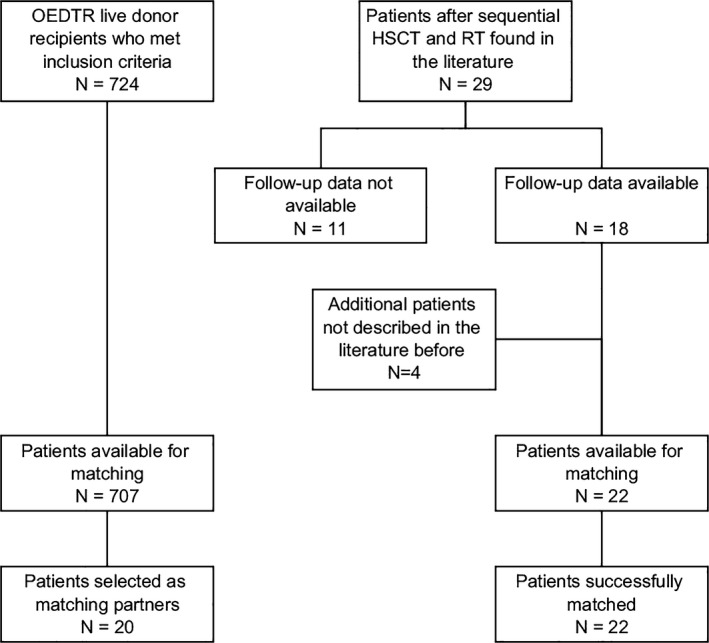
Flow chart illustrating data retrieval and further patient selection for the matching procedure
Table 1.
Patient characteristics compared between sequential and conventional group
| Variable | Tolerant patients | Conventional RT | |||
|---|---|---|---|---|---|
| Matched | SMD | Pre‐matching | SMD | ||
| Number of patients | 22 | 20 | — | 707 | — |
| Age at RT* (y) | 33 (14) | 33 (15) | 4% | 39 (17) | 33% |
| Age of kidney donor* (y) | 46 (13) | 47 (11) | 5% | 49 (11) | 27% |
| Sex match at renal transplantation* (matched) | 12 (55%) | 12 (60%) | 10% | 268 (38%) | 23% |
| Sex (female) | 10 (46%) | 8 (40%) | 10% | 241 (34%) | 35% |
| Overall serum creatinine after RT (μmol/l) | 85 (72, 99) | 118 (99, 143) | — | 142 (115, 178) | — |
| Median total follow‐up time (y) | 10 (7, 22) | 11 (10, 14) | — | 7 (4, 13) | — |
| Mean number of mismatched HLA antigens at RT* | 0 (0) | 0.3 (0.5) | 84% | 2.9 (1.6) | 172% |
| Mean number of mismatched HLA antigens at HSCT | 1 (1.3) | — | |||
| Age at HSCT (y) | 23 (17, 30) | — | |||
| Time between HSCT and RT (y) | 5 (3, 9) | — | |||
| Median IS free amount of follow‐up (%) | 90 (59, 100) | Patients are treated with immunosuppression throughout follow‐up | |||
Patient characteristics compared between the tolerant and conventional groups. Standardized mean differences were computed to quantify the difference between the groups before and after matching. Variables noted by * were used in the matching procedure. Data presented by mean (standard deviation), median (first quartile, third quartile), or absolute frequency (relative frequency).
The main analyses presented here used a matching based on the variables that were available for each patient. By varying the size of the control group and allowing more matches for each tolerant patient and drawing patients with and without replacement from the OEDTR, we checked how sensitive the results from statistical analysis were to the choice of the control group. Furthermore, results were compared to analyses done on matchings based on patients with complete data only.
2.5. Statistical analysis
Metric variables are expressed as mean and standard deviation or median and range (interquartile or absolute). Categorical variables are expressed as absolute and relative frequencies. Transplant kidney and patient survival are depicted using Kaplan‐Meier graphs. Due to the low number of events overall as well as crossing Kaplan‐Meier curves, we used the Uno c‐index to compare the matched tolerant and conventional group.29 It can be interpreted as a conditional probability that for any pair of tolerant and conventional patients, the risk of an event (graft loss or death) is higher for the control patient. Longitudinal measurements of serum creatinine levels were investigated using a linear mixed model in which the group status is entered as main variable of interest. We used a random intercept and random slope to accommodate the patient‐specific trajectories of the creatinine levels. Interaction terms with follow‐up time, nonlinear effects of time using natural splines and different random effect structures were assessed by means of the Akaike information criterion (AIC) to improve model fit. Normality of residuals was checked to ensure validity of the model. Other variables such as recipient's age at RT, HLA‐mismatch (set to 0 for HSCT patients), sex match between donor and recipient, and donor age were included in the model to assess whether differences in patient characteristics between the tolerant and conventional group were adequately controlled for by the matching procedure. All tests performed were two‐sided and a P < .05 was considered statistically significant. Data management and analysis was performed by SAS software (SAS Institute Inc., Cary, USA) and the R statistical software (R Core Team, Vienna, Austria).
2.6. Data retrieval and patient characteristics in sequential group
After examination of all search results, 18 relevant reports (published 1991‐2017) were found, describing 29 patients with sequential renal and hematopoietic stem cell transplantation (Figure 1).5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 24 Authors of 15 reports sent follow‐up data for a total of 18 patients. Despite intensive efforts, data from the other reports were not available because patients were lost to follow‐up soon after the transplantation or publication. Four patients (N7, N8, N19, N21), not described in the literature before, were included. Patient N19 was transplanted and followed‐up at our clinic. The median observation period was 10 years (range 11 months to 26 years). Demographic, clinical, and transplant‐associated parameters are summarized and compared to the conventional group in Table 1. A more detailed description of all tolerant patients is shown in Tables 2 and 3. Except for one patient, all indications for HSCT were of hematologic origin. Two patients received their renal transplants from unrelated but fully HLA‐matched donors. Compared to the conventional group, the tolerant patients did not receive any immunosuppressive treatment during >90% of the whole follow‐up period.
Table 2.
Detailed demographic, clinical, and transplant‐associated patient findings
| No. | Sex | Reason for HSCT | Donor | HLA mismatch | Cause of ESRD | Age (y) | IS after RT | IS‐free (y) | |
|---|---|---|---|---|---|---|---|---|---|
| At RT | Donor | ||||||||
| 1 | M | Wiskott‐Aldrich Syndrome | Mother | Two mismatches | Mesangial proliferative GN | 18 | 42 | Glucocorticoids, CyA | 24 |
| 2 | F | ALL | Brother | HLA‐identical | HUS | 28 | 25 | None, later AZA and Glucocorticoids | 0 |
| 3 | F | Aplastic anemia | Father | Three mismatches | Radiation nephritis | 14 | NK | Glucocorticoids, CyA | 21 |
| 4 | F | CML | Sister | Two mismatches | BMT‐NP | 37 | 35 | None | 19 |
| 5 | F | AML | Brother | HLA‐identical | HUS | 40 | NK | Glucocorticoids and AZA | NK |
| 6 | M | AML | Sister | HLA‐identical | BMT‐NP | 39 | 33 | None | 10 |
| 7 | M | Morbus Hodgkin | Brother | HLA‐identical | BMT‐NP | 48 | 51 | None | 4 |
| 8 | F | AML | Unrelated | HLA‐identical | BMT‐NP | 29 | NK | None | 9 |
| 9 | F | CML | Sister | HLA‐identical | Diabetes type 1 | 37 | NK | None | 17 |
| 10 | M | CML | Sister | HLA‐identical | Unknown | 64 | 56 | None | 11 |
| 11 | M | Lymphoma | Sibling | HLA‐identical | HUS/TTP | 59 | 67 | MMF, Glucocorticoids | 5 |
| 12 | F | ALL | Mother | One mismatch | BMT‐NP | 30 | 57 | MMF, Glucocorticoids | 5 |
| 13 | M | AML | Father | Two mismatches | CNI‐associated | 21 | 45 | Tac, MMF, Glucocorticoids | 9 |
| 14 | M | AML | Sister | HLA‐identical | TTP | 28 | 26 | Tac, MMF | 3 |
| 15 | F | Comb. immune‐deficiency syndrome | Mother | Three mismatches | Loss of first renal allograft due to infection | 22 | 61 | CyA, Glucocorticoids | 6 |
| 16 | M | Idiopathic aplastic anemia | Sister | HLA‐identical | Chronic nephangiosclerosis | 41 | 52 | Glucocorticoids | 9 |
| 17 | F | AML | Unrelated | HLA‐identical | Complication of aGvHD treatment | 17 | 46 | Tac, Glucocorticoids | 9 |
| 18 | M | Polymorphic rhabdomyosarkoma | Father | Two mismatches | BMT‐NP | 21 | 43 | Glucocorticoids | 3 |
| 19 | M | ALL | Father | Three mismatches | BMT‐NP | 25 | 46 | Glucocorticoids | 1 |
| 20 | M | B‐cell non‐Hodgkin lymphoma | Sister | HLA‐identical | Acute and chronic rejection of 1. transplant kidney | 30 | 22 | Glucocorticoids | 13 |
| 21 | M | Aplastic anemia | Sister | HLA‐identical | CNI toxicity | 54 | 63 | Single Dose Glucocorticoid | 1,5 |
| 22 | F | Sickle cell disease | Mother | Three mismatches | Focal segmental glomerulosclerosis due to SCD | 28 | 56 | Glucocorticoid, MMF, Tacrolimus | 0,2 |
By some authors the cause of ESRD was described as bone marrow transplant nephropathy (BMT‐NP). In the literature BMT‐NP is defined as chronic renal disease presenting within 100 days after BMT. It includes chemo‐irradiation–associated nephropathies caused by the conditioning therapies in the absences of other nephrotoxins.14, 40
Table 3.
Detailed clinical patient and follow‐up findings
| No. | Follow‐up (y) | Creatinine at last follow‐up (μmol/l) | GVHD after RT | Death | Ref. | Note |
|---|---|---|---|---|---|---|
| 1 | 26 | 133.0 | N | N | 6 | |
| 2 | 24 | 97.0 | N | N | 12 | Diagnosis of radiation induced pulmonary fibrosis in 2000; lung transplantation in 2011 |
| 3 | 23 | 79.7 | N | N | 13 | HCV cirrhosis (Child‐Pugh A), Diabetes mellitus type 2 |
| 4 | 19 | 106.1 | N | N | 14 | |
| 5 | 13 | NK | N | Y | 14 | Died of unknown cause |
| 6 | 10 | 106.2 | N | N | 14 | |
| 7 | 5 | 68.1 | N | Y | NP | Died of unknown cause, 11 y after RT |
| 8 | 9 | 76,9 | NK | N | NP | |
| 9 | 17 | 80.0 | N | Y | 7 | Died of unknown cause, 20 y after RT |
| 10 | 12 | 79.0 | N | Y | 8 | Died 12 y after RT with functioning transplant kidney, cause of death presumed cardiac |
| 11 | 10 | 131.0 | N | N | 9 | |
| 12 | 10 | 69.9 | N | N | 9 | |
| 13 | 9 | 81.0 | NK | N | 15 | |
| 14 | 6 | 97.2 | N | N | 11 | |
| 15 | 6 | 87.6 | N | N | 16 | Second RT, successful pregnancy 4 y after second renal transplantation |
| 16 | 10 | 80 | Y | N | 17 | HCV cirrhosis (Child‐Pugh A), manifestation of GVHD: cutaneous |
| 17 | 11 | 48.0 | Y | N | 18 | Manifestation of GVHD: cutaneous and musculoskeletal: scleroderma of lower and upper extremity, skin flare, avascular necrosis of knees, hips, shoulders, ankles; diagnosis of squamous cell carcinoma of the palate in 2016, currently in remission |
| 18 | 4 | 99.1 | N | N | 19 | |
| 19 | 1 | 93.8 | N | N | NP | |
| 20 | 22 | 53.0 | Y | N | 24 | Manifestation of GVHD: increase of liver function impairment (first seen after HSCT) |
| 21 | 2 | 106.0 | N | N | NP | |
| 22 | 1 | 96.0 | Y | N | 21 | Manifestation of GVHD: oral mucosa (mild) |
2.7. Matching of the OEDTR control group
All 22 patients could be included into the matched comparison. We identified 707 patients from the OEDTR who met our inclusion criteria. Twenty patients were selected as matching partners for the tolerant group (Table 1). Causes of ESRD in these patients were vascular nephropathy (N = 1), glomerulonephritis (N = 5), and 14 patients were labeled as “other” cause of ESRD. Four patients had type 2 diabetes, and 2 of them developed diabetes after transplantation. The matching procedure reduced almost all SMDs into acceptable ranges. The SMD in the HLA mismatches remained high even after matching, which is due to the lack of mismatches in the group of sequentially transplanted patients.
2.8. Maintenance immunosuppression
Withdrawal of immunosuppression in the tolerant group: Seven patients did not receive any immunosuppression after renal transplantation. All other patients received at least short‐term immunosuppression initially after RT (see Table 2), which was successfully withdrawn in the later course. At the time of the last follow‐up, 20 of 21 patients (records not available in one patient) were free of immunosuppression. Patient N2 successful withdrew IS after RT but developed a radiation‐induced pulmonary fibrosis in the later course and IS including steroids and azathioprine had to be reinitiated. In 2011, a successful lung transplantation was performed and tacrolimus was added. Maintenance IS in the control group was given as per the local center standards, that is, calcineurin inhibitor (CNI), mycophenolate mofetil (MMF), and steroids in all patients aiming at tacrolimus (TAC) troughs between 4 and 8 ng/ml after the first years.
3. RESULTS
3.1. Overall renal allograft survival and graft function in sequentially transplanted patients compared to conventional transplanted patients
No graft loss was reported in the tolerant group, whereas 3 (15%) were lost in the control group (Kaplan‐Meier analysis depicted in Figure 2). The corresponding Uno c‐index comparing the 2 groups was 0.81, suggesting an improved renal graft survival in the tolerant group within the study period. The 95% confidence interval (0.67‐0.96) excluded parity (0.5), providing evidence against the hypothesis that the renal allograft survival probabilities are equal in both groups (P < .001).
Figure 2.
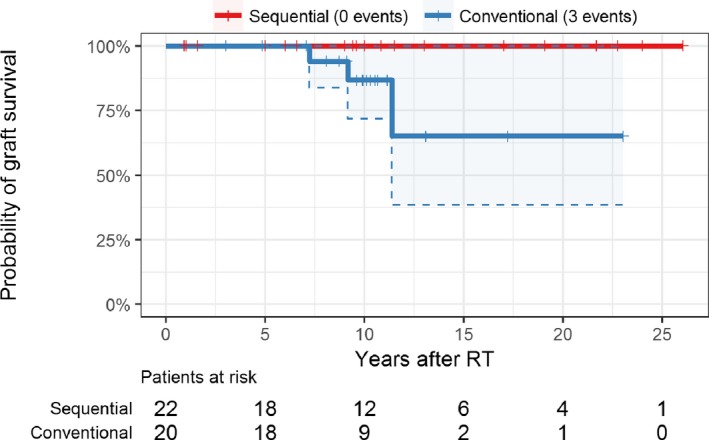
Kaplan‐Meier plot of transplant kidney survival; shaded areas and dashed lines represent 95% confidence intervals. No transplanted kidney was lost in the group of sequentially transplanted patients. RT, renal transplantation [Color figure can be viewed at wileyonlinelibrary.com]
Serum creatinine levels over the whole study period are depicted in Figure 3, median serum creatinine levels were 85 μmol/l (first quartile 72, third quartile 99) and 118 μmol/l (IQR 99‐143) for the tolerant and conventional group, respectively. In a crude comparison ignoring the longitudinal nature of the data, the median level in the conventional group was significantly higher than in the tolerant group (Mann‐Whitney U test P < .001).
Figure 3.
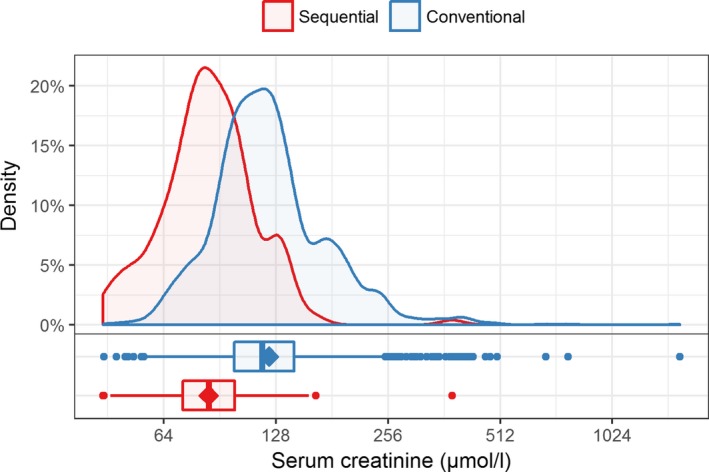
Comparison of serum creatinine levels (on log2 scaled axis) after RT between groups; the mean is indicated as diamond shape in the boxplots [Color figure can be viewed at wileyonlinelibrary.com]
Figure 4 shows results from the mixed‐model analysis of log2‐transformed serum creatinine levels, taking the study design into account. No relevant difference in the change of creatinine levels over time between the 2 groups was found (interaction with follow‐up time was not included in the models, either by checking significance or by AIC). The effect of time was found to be nonlinear (a model including spline terms of time using 3 knots showed improved AIC), with a decrease in creatinine levels shortly after transplantation. Group status was significant in the model (P = .006), providing evidence that serum creatinine levels in the tolerant group are on average 29% lower than in the conventional group. Estimated effects of group status remained stable regardless of modeling of time. After inclusion of other relevant key characteristics, group status remained significant (P = .045), whereas none of the other variables in the model had significant results. In an unmatched analysis, comparing all patients from the OEDTR with available creatinine measurements (N = 273) and the study group, the OEDTR patients exhibited a trend toward higher creatinine values over time (Figure S1).
Figure 4.
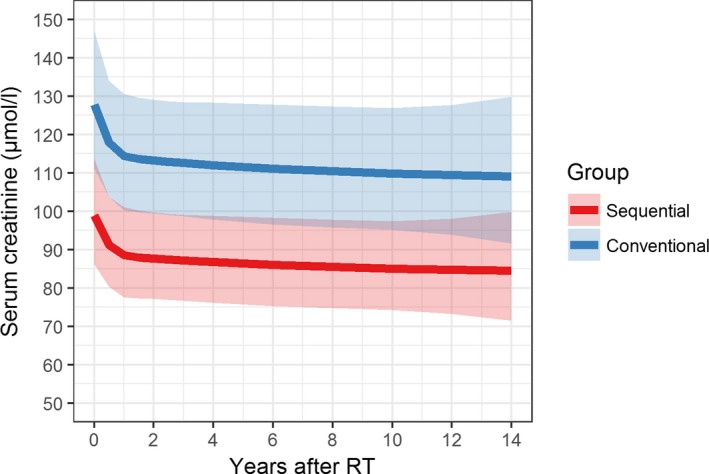
Mixed model for log2‐transformed serum creatinine levels modeling time using natural splines with 3 knots. Estimates were transformed to original scale. The shaded areas around the model estimates constitute 95% confidence intervals. The overall difference between the 2 groups was significant. No relevant difference in the change of creatinine levels over time between the 2 groups was found. Four patients from the control group had to be excluded due to missing longitudinal serum creatinine measurements. We restricted the analysis to the first 14 years after RT due to lack of serum creatinine measurements for most OEDTR patients thereafter. RT, renal transplantation [Color figure can be viewed at wileyonlinelibrary.com]
In a subset of patients further immunologic assays have been performed. Both patients had no signs of humoral immune activation (measured as donor‐ and recipient‐specific antibodies). Moreover, protocol biopsies performed one and 12 months after RT revealed no signs of allograft rejection. Notably, recipient‐derived T cells measured before RT and 1 year after remained virtually unchanged (Figure 5).
Figure 5.
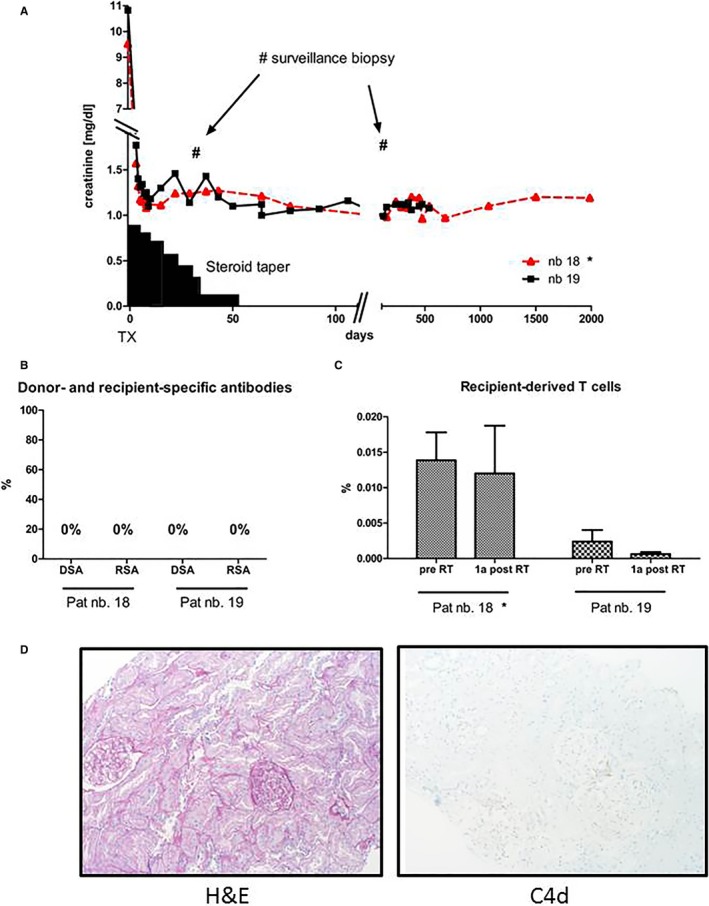
(A) Clinical course of the 2 tolerant patients transplanted at our center (pat. N18 and N19). Both patients underwent transplantation with only a short steroid taper to reduce ischemia‐reperfusion injury. No further immunosuppression was administered. (B) Humoral immune activation was assessed by measuring donor‐ and recipient‐specific antibodies. There were no signs of humoral immune activation. (C) Chimerism was assessed via real‐time PCR in separated T cells before and 1 year after RT in both patients. The proportion of recipient‐derived T cells was low and virtually didn't change over the time. (D) Protocol biopsies performed 1 month and 12 months after RT showed structurally regular renal tissue with no signs of cell‐ or antibody‐mediated rejection. Data from patient N18 have been shown previously (Schwarz 2016) with a shorter follow‐up19 [Color figure can be viewed at wileyonlinelibrary.com]
3.2. Overall patient survival in sequentially transplanted patients compared to conventionally transplanted patients
Of 22 patients, 4 deaths were reported. Patient N5 was lost to follow‐up 13 years after RT and died in the later course. The exact time of death is not recorded and the patient is therefore treated as censored at the last follow‐up visit. Patient N7 was last seen 5 years after transplantation at the treating medical center and died 11 years after renal transplantation. Patient N9 was last seen in 2012, 17 years after renal transplantation. At that time, her creatinine was in normal range. Later, no more visits to the outpatient clinic were recorded. She died in 2015. All 3 patients died of unknown causes. Patient N10 died in 2014, 12 years after RT. His presumed cause of death was of cardiac origin. Notably, the patient died with a functioning transplant kidney.
Two deaths were recorded in the matched subset of conventionally transplanted patients from the OEDTR. The Uno c‐index for the corresponding Kaplan‐Meier survival curves comparing the 2 groups was 0.39, suggesting a lower mortality in the conventional group within the study period (Figure 6). However, due to the low number of events, the 95% confidence interval was wide (0.08‐0.71) and included parity (0.5), thus lacking evidence against the hypothesis that the overall survival probabilities are equal in both groups (P = .54).
Figure 6.
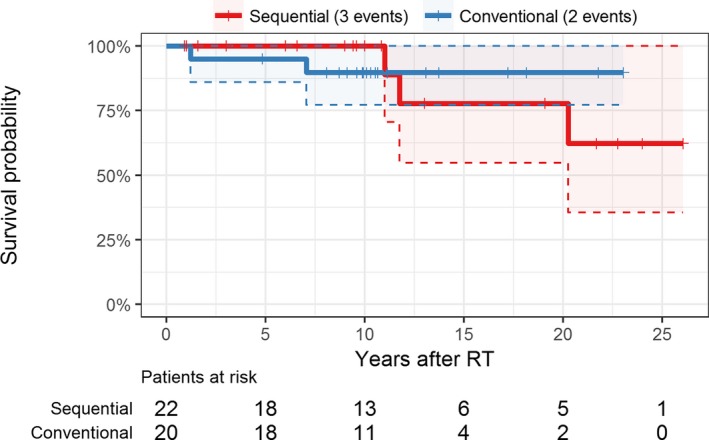
Kaplan‐Meier plot of overall patient survival; shaded areas and dashed lines represent 95% confidence intervals. Overall there were 4 deaths in the sequential group but one was treated as censored at the last follow‐up visit as the exact time of death is unknown. RT, renal transplantation [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Further notable events in the patients′ later course
After HSCT, patient N15 received a renal allograft from a fully matched deceased donor. Eight years later, the allograft was lost due to infection and a second renal transplantation was performed, in which the previous HSCT donor (mother) donated a kidney. Withdrawal of all immunosuppressive drugs was successful 10 weeks later. Four years later, the patient gave birth to a child without rejection. The patient developed HLA antibodies but they were not targeted against the allograft. Restart of immunosuppressive therapy was not needed in the further course.
Follow‐up data (exceeding the published report) regarding graft function or patient survival from one patient described by Beitinjaneh et al were not available.20 Nevertheless, the treating center reported 3 further patients not described before, who received sequential HSCT and RT. The HSCTs were performed between 1982 and 1986 (indications: acute myeloid leukemia [AML], aplastic anemia, chronic myeloid leukemia [CML]). Renal allografts were transplanted 6 to 20 years later (median 16 years). All three patients died (33, 14, and 26 years after HSCT). Due to a lack of further patient data (donor age, course of creatinine, HLA mismatch, immunosuppressive regimens), we did not include these patients in our analysis.
4. DISCUSSION
The induction of donor‐specific tolerance remains the holy grail in transplantation. Currently, the only clinically successful concept to establish donor‐specific tolerance to a renal graft is via HSCT from the same donor. However, widespread use is hampered mainly by cytotoxic preconditioning before bone marrow transplantation. Thus, few patients have been reported in the literature that have received combined HSCT and RT from the same donor solely for the purpose of tolerance induction. However, several patients have been reported who had received an HSCT for a conventional indication (eg, a hematologic malignancy) and subsequently received a kidney from the same donor. The aim of our study was to contribute long‐term data of such tolerant patients and to compare those findings with matched living donor transplant recipients under conventional IS.
Compared to previous studies, our analysis represents the largest and longest follow‐up observation of this specific patient population and includes additional patients not described in the literature before. One of our major findings is that over a total observation period of 250 patient‐years not one single allograft loss was recorded. Moreover, kidney function was significantly better compared to our matched group of conventionally living donor recipients. There are several reasons that might attribute to these results. First, in addition to receiving initial IS immediately after RT, most of the tolerant patients were successfully withdrawn from immunosuppression, thus adverse side effects and nephrotoxicity caused by conventional IS regimens were largely avoided.30 Moreover, in tolerant patients without the need of ongoing immunosuppressive therapy, adherence, which is a main risk factor for chronic allograft loss, is of no concern and the likelihood of infections might be lower.31, 32 Furthermore it must be addressed, that this highly selected subgroup of the OEDTR represents patients with comparably promising clinical parameters (young age, low HLA mismatch) and hence may not be representative of the general population of kidney transplant recipients. On the other hand, it must also be pointed out that 4 patients from the study group died in the further follow‐up period. Nevertheless, death censoring was applied within our analysis, considering that our primary aim was to evaluate the impact of tolerance on renal graft survival and taking the previous mostly severe hemato‐oncologic diseases in this special group of patients into account.
Despite significantly higher serum creatinine levels in the control group and 3 renal graft losses in the follow‐up, no relevant difference in the change of creatinine levels over time was found. The reason for this may also be the selection of the control group, representing patients with a very good outlook after transplantation. In an unmatched analysis, comparing all OEDTR patients with the sequential patients, the OEDTR patients exhibit a trend toward higher creatinine values over time. When interpreting the creatinine slopes of the control patients, one may further conclude that, in line with recent findings from Gaston et al, those graft losses could be associated with new‐onset allograft dysfunction in the later course, rather than a slow process of continuous worsening of allograft function.33 Unfortunately, clinical data from the control group were limited and more detailed information regarding the cause of the renal graft losses were not available.
So far, 3 centers in the United States reported results from their prospective trials of simultaneous HSCT and RT where IS was successfully withdrawn in a considerable number of patients.34, 35, 36, 37 Nevertheless, complications such as in part severe bacterial, viral, or fungal infections were described.35, 36 Due to these risks, the use of combined transplantations solely for the purpose of tolerance induction, is subject to considerable controversy, especially in patients who may do well on conventional immunosuppression. Our data on the other hand indicate a sustained long term benefit regarding transplant kidney function in patients after tolerance induction via hematopoietic stem cell transplantation from the same donor.
About two‐thirds of the patients in our analysis received at least short‐term immunosuppressive therapy after RT, which was, except for one patient (N2, lung transplantation), successfully withdrawn in the later course. Notably, none of the tolerant patients was taking long‐term corticosteroid treatment.
Most patients received IS for the prevention of a potential GVHD episode. Manifestations of a GVHD were reported in 4 patients (N16, N17, N20, N22). They included skin and musculoskeletal lesions, increased liver enzymes, or mild restrictive lung disease. Thus, considering the risk of even at least temporary IS, we think there is enough evidence to support minimalization of IS after kidney transplantation in GVHD‐free and tolerant patients. Notably, a steroid taper around RT seems to be beneficial, mainly to reduce ischemia‐reperfusion injury which is a well‐known cause for initial‐transplant kidney dysfunction.38, 39
There are some limitations in our study that need to be addressed when interpreting the results. First, a publication bias cannot be ruled out. It might be that unsuccessful cases were not reported in the literature and therefore not included in this study. However, this seems to be unlikely, given the novelty of this hypothetical issue, the rarity of an available donor for both HSCT and RT when needed, and the limited number of centers with the infrastructure and experience to perform sequential transplantations.
Despite frequent communications with the authors or their successors, we were not able to gather outcome data of all 29 published patients. We tried to minimize this limitation by including further patients that have been sequentially transplanted but have not been published in the literature. Furthermore, due to the retrospective design of our study and the long period between the publication of the first cases and our analysis, the quantity of collectable data is limited and varied strongly between different patients. We therefore focused our analysis on basic parameters such as renal transplant function and patient survival. Unfortunately, this also implies that tests for donor specific antibodies (DSAs) or chimerism persistence are missing. Hence, we added supplemental material describing the lack of DSAs, good transplant kidney function, as well as a virtually unchanged proportion of recipient‐derived T cells in the 2 sequentially transplanted patients from our center.
Furthermore, even if publication bias is ignored, the statistical power of this study to accurately estimate differences between tolerant patients and conventionally transplanted patients is limited due to the relatively low number of available patients.
In conclusion, our data with greater numbers and lengthier follow‐up than previously available indicate that tolerant patients after HSCT maintain a better function of their transplant kidney than comparable patients under conventional immunosuppression. Furthermore, overall renal graft and patient survival was at least comparable, and allowing for limitations of the analysis, perhaps superior to a matched living donor cohort. These data lend support to efforts currently underway to utilize co‐transplantation of donor hematopoietic stem cells to improve outcome in larger numbers of renal transplant patients.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting information
ACKNOWLEDGMENTS
We want to thank Claudine Helg, Eric P Cohen, Bram Vasudev, Christian Koenecke, and Toshiaki Tanaka for their support with the data collection. We also want to thank the administrators and all contributors of the Austrian Dialysis and Transplant Registry.
Eder M, Schwarz C, Kammer M, et al. Allograft and patient survival after sequential HSCT and kidney transplantation from the same donor—A multicenter analysis. Am J Transplant. 2019;19:475–487. 10.1111/ajt.14970
Michael Eder, Christoph Schwarz, Thomas Wekerle, and Rainer Oberbauer contributed equally.
[The copyright line for this article was changed on 17 July, 2018, after original online publication]
REFERENCES
- 1. Keith DS, Vranic G, Nishio‐Lucar A. Graft function and intermediate‐term outcomes of kidney transplants improved in the last decade: analysis of the United States kidney transplant database. Transplant Direct. 2017;3(6):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lamb KE, Lodhi S, Meier‐Kriesche HU. Long‐term renal allograft survival in the United States: a critical reappraisal. Am J Transplant. 2011;11(3):450‐462. [DOI] [PubMed] [Google Scholar]
- 3. Pilat N, Wekerle T. Transplantation tolerance through mixed chimerism. Nat Rev Nephrol. 2010;6(10):594‐605. [DOI] [PubMed] [Google Scholar]
- 4. Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172(4379):603‐606. [DOI] [PubMed] [Google Scholar]
- 5. Sayegh MH, Fine NA, Smith JL, Rennke HG, Milford EL, Tilney NL. Immunologic tolerance to renal allografts after bone marrow transplants from the same donors. Ann Intern Med. 1991;114(11):954‐955. [DOI] [PubMed] [Google Scholar]
- 6. Jacobsen N, Taaning E, Ladefoged J, Kristensen JK, Pedersen FK. Tolerance to an HLA‐B, DR disparate kidney allograft after bone‐marrow transplantation from same donor. Lancet. 1994;343(8900):800. [DOI] [PubMed] [Google Scholar]
- 7. Sellers MT, Deierhoi MH, Curtis JJ, et al. Tolerance in renal transplantation after allogeneic bone marrow transplantation‐6‐year follow‐up. Transplantation. 2001;71(11):1681‐1683. [DOI] [PubMed] [Google Scholar]
- 8. Ravanan R, Dudley CR, Smith RM, Burton CJ, Lear PA, Unsworth DJ. Use of skin grafting to demonstrate tolerance before kidney transplantation without immunosuppression in the recipient of a previous bone marrow transplant. Transplantation. 2005;79(3):375‐376. [DOI] [PubMed] [Google Scholar]
- 9. Tanaka T, Ishida H, Shirakawa H, Amano H, Nishida H, Tanabe K. Renal transplantation after myeloablative and non‐myeloablative hematopoietic cell transplantation from the same donor. Int J Urol. 2007;14(11):1044‐1045. [DOI] [PubMed] [Google Scholar]
- 10. Hamawi K, De Magalhaes‐Silverman M, Bertolatus JA. Outcomes of renal transplantation following bone marrow transplantation. Am J Transplant. 2003;3(3):301‐305. [DOI] [PubMed] [Google Scholar]
- 11. Alvarez S, Boltansky A, Alfaro J, et al. Unresponsiveness to a kidney graft after a fully matched allogenic bone marrow transplantation combined with low‐dose tacrolimus therapy: a case report. Transpl Proc. 2011;43(6):2344‐2346. [DOI] [PubMed] [Google Scholar]
- 12. Helg C, Chapuis B, Bolle JF, et al. Renal transplantation without immunosuppression in a host with tolerance induced by allogeneic bone marrow transplantation. Transplantation. 1994;58(12):1420‐1422. [PubMed] [Google Scholar]
- 13. Sorof JM, Koerper MA, Portale AA, Potter D, DeSantes K, Cowan M. Renal transplantation without chronic immunosuppression after T cell‐depleted, HLA‐mismatched bone marrow transplantation. Transplantation. 1995;59(11):1633‐1635. [PubMed] [Google Scholar]
- 14. Butcher JA, Hariharan S, Adams MB, Johnson CP, Roza AM, Cohen EP. Renal transplantation for end‐stage renal disease following bone marrow transplantation: a report of six cases, with and without immunosuppression. Clin Transplant. 1999;13(4):330‐335. [DOI] [PubMed] [Google Scholar]
- 15. Fangmann J, Kathrin Al‐Ali H, Sack U, et al. Kidney transplant from the same donor without maintenance immunosuppression after previous hematopoietic stem cell transplant. Am J Transplant. 2011;11(1):156‐162. [DOI] [PubMed] [Google Scholar]
- 16. Vondran FW, Eiermann T, Thaiss F, Schwinzer R, Nashan B, Koch M. In vitro and in vivo proof of tolerance after two‐step haploidentical bone marrow and kidney transplantation of the same donor. Transplantation. 2012;93(6):e23‐e25. [DOI] [PubMed] [Google Scholar]
- 17. Garrouste C, Socie G, Heng AE. Kidney transplantation after previous hematopoietic stem cell transplant: need of immunosuppressive treatment? Transpl Int. 2014;27(9):e92‐e93. [DOI] [PubMed] [Google Scholar]
- 18. Younge J, Duffner UA, Bunchman T, Abdel‐Mageed A. Ten year follow‐up for a patient postmatched unrelated donor bone marrow transplant followed by same donor kidney transplant: normal renal function without immunosuppression. Transplantation. 2015;99(9):e162. [DOI] [PubMed] [Google Scholar]
- 19. Schwarz C, Lawitschka A, Bohmig GA, et al. Kidney transplantation with corticosteroids alone after haploidentical HSCT from the same donor. Transplantation. 2016;100(10):2219‐2221. [DOI] [PubMed] [Google Scholar]
- 20. Beitinjaneh A, Burns LJ, Majhail NS. Solid organ transplantation in survivors of hematopoietic cell transplantation: a single institution case series and literature review. Clin Transplant. 2010;24(4):E94‐E102. [DOI] [PubMed] [Google Scholar]
- 21. Knuppel E, Medinger M, Stehle G, et al. Haploidentical hematopoietic bone marrow transplantation followed by living kidney transplantation from the same donor in a sickle cell disease patient with end‐stage renal failure. Ann Hematol. 2017;96(4):703‐705. [DOI] [PubMed] [Google Scholar]
- 22. Dey B, Sykes M, Spitzer TR. Outcomes of recipients of both bone marrow and solid organ transplants. A review. Medicine (Baltimore). 1998;77(5):355‐369. [DOI] [PubMed] [Google Scholar]
- 23. Sharma A, Armstrong AE, Posner MP, et al. Graft‐versus‐host disease after solid organ transplantation: a single center experience and review of literature. Ann Transplant. 2012;17(4):133‐139. [DOI] [PubMed] [Google Scholar]
- 24. Koenecke C, Hertenstein B, Schetelig J, et al. Solid organ transplantation after allogeneic hematopoietic stem cell transplantation: a retrospective, multicenter study of the EBMT. Am J Transplant. 2010;10(8):1897‐1906. [DOI] [PubMed] [Google Scholar]
- 25. Heinze G, Kainz A, Hörl WH, Oberbauer R. Mortality in renal transplant recipients given erythropoietins to increase haemoglobin concentration: cohort study. BMJ. 2009;339:b4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ashby VB, Leichtman AB, Rees MA, et al. A kidney graft survival calculator that accounts for mismatches in age, sex, HLA, and body size. Clin J Am Soc Nephrol. 2017;12(7):1148‐1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Massie AB, Leanza J, Fahmy LM, et al. A risk index for living donor kidney transplantation. Am J Transplant. 2016;16(7):2077‐2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Milner J, Melcher ML, Lee B, et al. HLA matching trumps donor age: donor‐recipient pairing characteristics that impact long‐term success in living donor kidney transplantation in the era of paired kidney exchange. Transplant Direct. 2016;2(7):e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Uno H, Cai T, Pencina MJ, D'Agostino RB, Wei LJ. On the C‐statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30(10):1105‐1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349(24):2326‐2333. [DOI] [PubMed] [Google Scholar]
- 31. Wiebe C, Nevins TE, Robiner WN, Thomas W, Matas AJ, Nickerson PW. The synergistic effect of class II HLA epitope‐mismatch and nonadherence on acute rejection and graft survival. Am J Transplant. 2015;15(8):2197‐2202. [DOI] [PubMed] [Google Scholar]
- 32. Nankivell BJ, Kuypers DR. Diagnosis and prevention of chronic kidney allograft loss. Lancet. 2011;378(9800):1428‐1437. [DOI] [PubMed] [Google Scholar]
- 33. Gaston RS, Fieberg A, Hunsicker L, et al. Late graft failure after kidney transplantation as the consequence of late versus early events. Am J Transplant. 2017;18:1158‐1167. [DOI] [PubMed] [Google Scholar]
- 34. Kawai T, Sachs DH, Sprangers B, et al. Long‐term results in recipients of combined HLA‐mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant. 2014;14(7):1599‐1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leventhal J, Abecassis M, Miller J, et al. Tolerance induction in HLA disparate living donor kidney transplantation by donor stem cell infusion: durable chimerism predicts outcome. Transplantation. 2013;95(1):169‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scandling JD, Busque S, Shizuru JA, et al. Chimerism, graft survival, and withdrawal of immunosuppressive drugs in HLA matched and mismatched patients after living donor kidney and hematopoietic cell transplantation. Am J Transplant. 2015;15(3):695‐704. [DOI] [PubMed] [Google Scholar]
- 37. Mahr B, Granofszky N, Muckenhuber M, Wekerle T. Transplantation tolerance through hematopoietic chimerism: progress and challenges for clinical translation. Front Immunol. 2017;8:1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kumar S, Allen DA, Kieswich JE, et al. Dexamethasone ameliorates renal ischemia‐reperfusion injury. J Am Soc Nephrol. 2009;20(11):2412‐2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cavaille‐Coll M, Bala S, Velidedeoglu E, et al. Summary of FDA workshop on ischemia reperfusion injury in kidney transplantation. Am J Transplant. 2013;13(5):1134‐1148. [DOI] [PubMed] [Google Scholar]
- 40. Cohen EP, Lawton CA, Moulder JE, Becker CG, Ash RC. Clinical course of late‐onset bone marrow transplant nephropathy. Nephron. 1993;64(4):626‐635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


