Abstract
Objective
To assess the audiological and long‐term medical and technical follow‐up outcomes of an active middle ear implant.
Methods
This was a retrospective medical chart analysis of all patients provided with an active middle ear implant in a tertiary academic medical referral center between September 1, 1998, and July 31, 2015. Main outcome measures were medical and technical complications, revisions, reimplantations, explantations, coupling approaches, mean time of use, pre‐ and postoperative hearing thresholds, functional hearing gain across frequencies (250–4,000 Hz), and Freiburg monosyllablic word test at 65 dB.
Results
One hundred and three patients were identified. Fifteen were implanted bilaterally (n = 118 Vibrant Soundbridge devices [MED‐EL, Innsbruck, Austria]). Seventy‐seven devices were implanted for sensorineural and 41 for mixed and conductive hearing loss. Patients used the implant for 6.7 years (range 0.7 months–17.9 years) on average. Ninety‐one patients (77.12%) were using the device at the end of the observation period. An overall complication rate of 16.1% was observed. The revision and explantation rates were higher for devices implanted between 2004 and 2006. The device failure rate was 3.4%. Audiological evaluation showed significant hearing gains for both hearing loss patient groups.
Conclusion
This long‐term follow‐up reveals the reliability of the active middle ear implant in a single center. Overall complication rate and device failure rate are acceptable. The complication rate was higher during implementation of alternative coupling approaches. The audiological benefit was satisfactory in patients with all hearing loss types. The majority of implanted patients used the implant at the end of the observation period.
Level of Evidence
4 Laryngoscope, 129:477–481, 2019
Keywords: Active middle ear implant, hearing loss, long‐term outcome
INTRODUCTION
Active middle ear implants (AMEI), including the Vibrant Soundbridge (VSB) (MED‐EL, Innsbruck, Austria), have been used for hearing rehabilitation for more than two decades. They had been firstly intended to treat patients with moderate‐to‐severe sensorineural hearing loss.1 Originally, the floating mass transducer (FMT) was connected the to the long process of the incus.2 Alternative coupling approaches including the round,3, 4 the oval window,5 or even the promontory bone6 extended the range of indications over recent years. Therefore, patients with mixed/conductive hearing loss could also be treated with this type of active middle ear implant.1, 2, 3, 4, 5, 6 To date, a few studies focused on long‐term audiological or surgical outcomes. Maier et al.7 reported on audiological outcomes in 104 patients and 122 devices implanted in a single center. A study group led by Sterkers et al.8 reported on 125 patients implanted with the VSB, analyzing patient satisfaction using self‐assessment scales postoperatively. Zwartenkot et al.9 assessed long‐term medical and technical follow‐up outcomes of different middle ear hearing implants, including 92 VSB devices. That was the first study reporting on implant survival and implant loss per follow‐up year of the VSB. However, audiological benefit was not assessed in this study. Previous studies focused either on technical outcome, quality of life, or on long‐term audiological results. There are no studies reporting on both the audiological outcome after vibroplasty and the medical and technical long‐term performance of the AMEI in a complete cohort of patients in a single center. Long‐term data is scare regarding implant reliability. There are only few long‐term data on revision and explantation rates due technical or medical reasons. Therefore, the aim of this study was to analyze all consecutive patients who underwent an implantation of the AMEI in a tertiary referral center between September 1, 1998, and July 31, 2015. Long‐term medical and technical outcomes including the mean time of use, type of coupling approach, medical and technical complications, revisions, reimplantations, and explantations were assessed. Postoperative audiological benefit as well as safety of the implant regarding the inner ear function was evaluated.
MATERIALS AND METHODS
Patients
This retrospective study comprised all consecutive patients who were implanted with the AMEI at a tertiary academic medical center between September 1, 1998, and July 31, 2015. Indication for implantation was sensorineural, conductive, or mixed hearing loss; no sufficient benefit from a conventional hearing device; or recurrent infections of the external ear canal caused by hearing aids. A computer tomography scan of the temporal bone was performed preoperatively in all patients. All patients were implanted by two senior coauthors (w.g. and w‐d.b.). Audiological measurements were performed in a sound‐isolated room with audiometers used in the clinical routine. The measurements were performed preoperatively, followed by a measurement after activation—and at 2, 3, and 6 months postoperatively. For this study, we used measurements done after 6 months. If the sixth month data was not available, earlier measurements were used for analysis. Free‐field audiometry was measured at 250; 500; 1,000; 2,000; 3,000; and 4,000 Hz. Functional hearing gain (FHG) was calculated comparing postoperative unaided free‐field thresholds to thresholds with the AMEI. Speech perception in quiet was measured using the Freiburger monosyllablic word test at 65 dB, a test for adult German‐speaking patients. Postoperative unaided and thresholds with the AMEI were compared.
Medical chart analysis of all consecutive patients was performed to evaluate all surgical complications as well as revision rates during the follow‐up period. All coupling approaches, reimplantations, and explantations were analyzed. Main outcome parameters were audiological results; long‐term data on time of use; and medical and technical complications including revision, reimplantation, explantation rates, and implant survival. The study was approved by the institutional review board (approval number 1952/2017).
Statistical Analysis
The statistical analysis was performed with the Statistical Program of Social Sciences (SPSS, version 23.0; SPSS, Inc, Chicago, IL). The level of statistical significance is set at 0.05, two‐tailed. In order to compare unaided and AMEI‐aided thresholds in free‐field audiometry and in the Freiburger monosyllablic word test, a paired t test was utilized. Descriptive analysis was performed determining the mean and standard deviation (SD).
RESULTS
Patients
A total of 103 patients were identified. Fifteen were implanted bilaterally (n = 118 VSB devices). The mean follow‐up period was 7.9 years (range 1.1 months–17.9 years). Patients used the AMEI for 6.7 years (range 0.7 months–17.9 years) on average. Ninety‐one patients (77.12%) were users at the end of observation period.
Surgical Results
Patient demographics including types of hearing loss and data on coupling approaches are shown in Table 1. Data regarding revision surgery with explantations or revisions due to medical and technical indications are presented in Table 2. Medical or technical complications occurred in 19 patients (16.1%). Twelve patients (10.17%) were explanted and not re‐implanted with another implant. Device failure occurred in four patients (3.39%). Although still implanted, two patients were nonusers at the end of the observation period due to their personal subjective dissatisfaction with the implant function. Figure 1 shows the percentage of AMEI implantations that were either explanted or revised per 3‐year periods. An increase in the explantation/revision rate is observed for devices implanted between 2004 and 2006. The rate remained stable for other time periods. Frequency of complications for individual coupling approaches are presented in Table 3.
Table 1.
Patients Demographics and Characteristics.
| Hearing Loss | Mean Age, Years | Male/Female, n | Male/Female, % | FMT Coupling | Total | |
|---|---|---|---|---|---|---|
| Sensorineural | 50.4 | 41/36 | 53.2/46.8 | Long process | 73 | 77/65.3 |
| Short process | 4 | |||||
| Mixed and conductive | 43.9 | 22/19 | 53.7/46.3 | RW | 19 | 41/34.7 |
| OW | 8 | |||||
| Stapes | 9 | |||||
| Promontorium | 5 | |||||
| Total | 48.3 | 63/55 | 53.4/46.6 | 118/100 |
FMT = floating mass transducer; n = number of cases; OW = oval window; RW = round window.
Table 2.
Device Explantations and Revision Surgeries.
| Indication | Complication | Type of Surgery | n | Total | % |
|---|---|---|---|---|---|
| Medical | Infection | Explantation | 4 | 14 | 11.9 |
| Wire extrusion in the outer ear canal | Explantation | 3 | |||
| Pain complaints | Explantation | 3 | |||
| FMT displacement | FMT repositioning | 4 | |||
| Technical | Device failure | Explantation | 2 | 5 | 4.2 |
| Device failure | Reimplantation | 2 | |||
| Poor benefit (unknown reason) | Explantation | 1 | |||
| Progressive hearing loss | Explantation/CI implantation | 13 | 13 | 11 | |
| No second surgery | 86 | 86 | 72.9 | ||
| Total | 118 | 100 |
CI = cochlear implant; FMT = floating mass transducer; n = number of cases.
Figure 1.
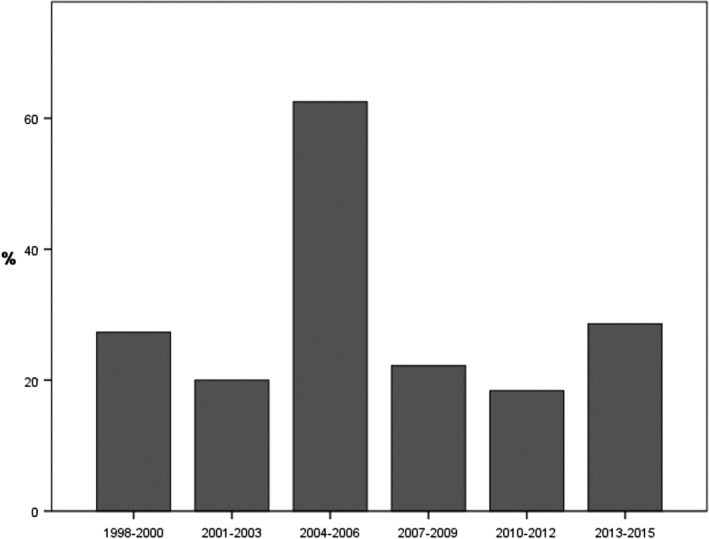
Percentage of Vibrant Soundbridge (MED‐EL, Innsbruck, Austria) implantations requiring an explantation or revision per 3 years.
Table 3.
Complications for Individual FMT Coupling Approaches.
| Hearing loss | FMT coupling | Complications, n | Total | Complications, % |
|---|---|---|---|---|
| Sensorineural | Long process | 7 | 73 | 9.6 |
| Short process | 0 | 4 | 0 | |
| Mixed | RW | 10 | 19 | 52.6 |
| OW | 2 | 8 | 25 | |
| Stapes | 0 | 9 | 0 | |
| Promontorium | 0 | 5 | 0 |
FMT = floating mass transducer; OW = oval window; RW = round window.
Implant Survival
Survival analysis was studied by summing up all individual follow‐up data. Nonusers were not considered as lost to follow‐up. Figure 2 shows the Kaplan‐Meier graph of the result per implant. Average implant loss for technical defects was one per 158 years of follow‐up. It was calculated adding up the total duration of follow‐up. Nonusage of the implant was not considered as implant loss.
Figure 2.
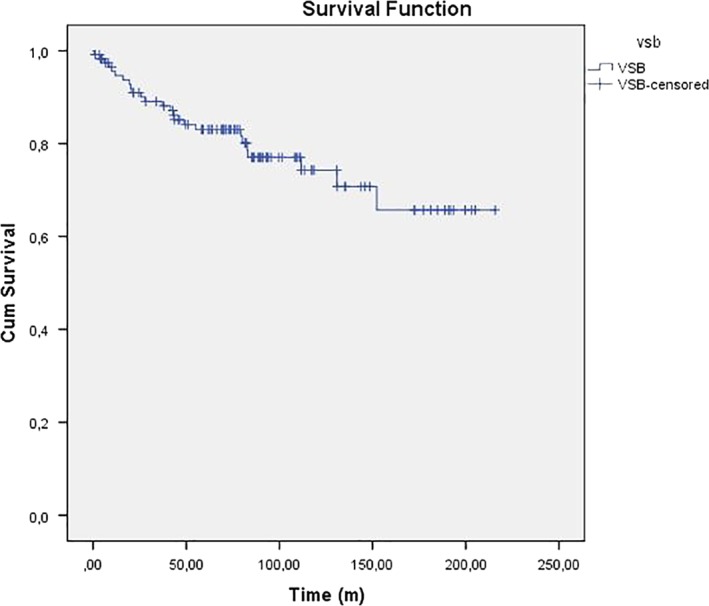
Kaplan‐Meier implant survival graph. m, months; VSB = Vibrant Soundbridge (MED‐EL, Innsbruck, Austria). [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
Audiological Data
The mean difference between preoperative and unaided postoperative bone conduction thresholds was less than 5 dB at each frequency for both the sensorineural and the conductive/mixed hearing loss patient group, which is clinically insignificant. The average FHG was 15.4 dB (± 8.4 dB SD) (P = .0001). Table 4 shows FHG for different types of hearing loss. Hearing gains in patients with sensorineural and mixed/conductive hearing loss across frequencies are shown in Figure 3 and Figure 4, respectively. The highest mean FHG was observed at 2,000 Hz (26 dB), followed by hearing gains at 1,000 and 3,000 Hz (both 20.8 dB). Lowest hearing gains are shown at 4,000; 500; and 250 Hz (16.3, 8.1, and 0.5 dB, respectively). Mean hearing gains were statistically significant at each frequency (P = .0001), except at 250 Hz (P = .783). AMEI‐aided Freiburg monosyllablic word test measurements showed a mean improvement of 25.6% (± 15.7%) at 65 dB for all patients (P = .0001). Freiburg monosyllablic word test improvements for patients with sensorineural and mixed/conductive hearing loss were 22.2 dB (± 15.8 dB) and 32.0 dB (± 13.2 dB), respectively. On average, improvements were statistically significant for both patient groups (P = .0001, P = .0001).
Table 4.
Mean FHG in Free‐Field Audiometry in Different Hearing Loss Types.
| Type of Hearing Loss | Mean FHG (dB) | P Value |
|---|---|---|
| Sensorineural | 13.1 ± 8.9 | < .0001 |
| Mixed/conductive | 20.9 ± 8.8 | < .0001 |
dB = decibel; FHG = functional hearing gain.
Figure 3.
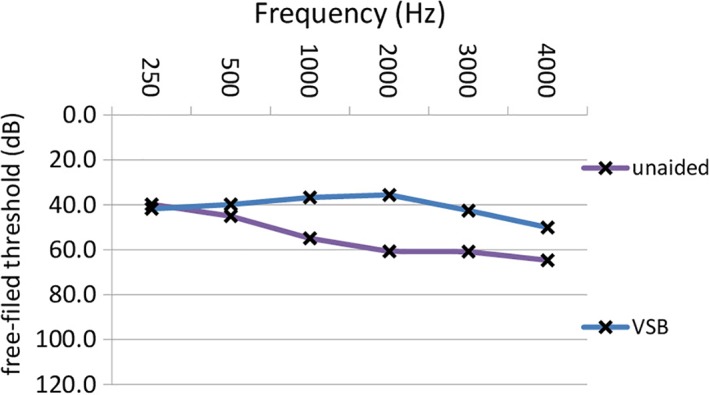
Mean postoperative unaided and thresholds with the VSB in free‐field audiometry across frequencies in patients with sensorineural hearing loss. unaided: postoperative thresholds without the VSB; VSB: postoperative thresholds with the VSB. dB = decibel; Hz = Hertz; VSB = Vibrant Soundbridge (MED‐EL, Innsbruck, Austria). [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
Figure 4.
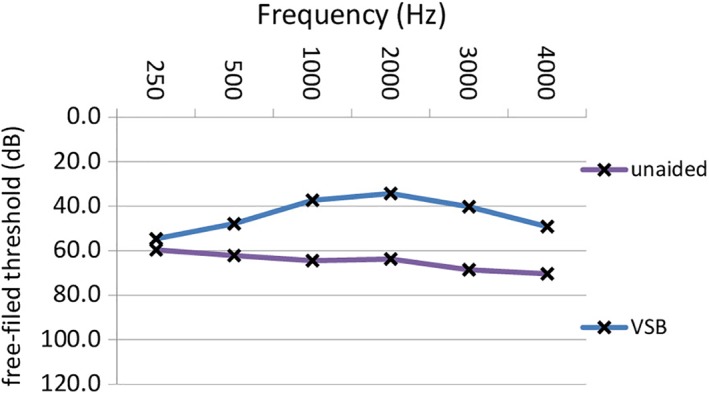
Mean postoperative unaided and thresholds with the VSB in free‐field audiometry across frequencies in patients with mixed/conductive hearing loss. unaided: postoperative thresholds without the VSB; VSB: postoperative thresholds with the VSB. dB = decibel; Hz = Hertz; m = months; VSB = Vibrant Soundbridge (MED‐EL, Innsbruck, Austria). [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
DISCUSSION
This study provides the evaluation of the AMEI regarding the long‐term surgical and technical follow‐up as well as the short‐term audiological benefit. We report on a complete cohort of patients who were provided with the VSB in a single center. Furthermore, this article provides data on audiological outcomes, types of coupling approach, mean time of use, medical and technical complications, revisions, reimplantations, and explantations in a complete patient cohort after vibroplasty.
Long‐term evaluation showed that 77% of patients used the implant at the time of the last clinical check‐up in our center. Similar results were shown by Mosnier et al.10 In their study, 77% of patients used the VSB after an average 8‐year follow‐up period.
Due to of the large distance to the hospital, some of our patients chose to follow up in another institution. Five patients have been lost to follow‐up in the first 5 months. The mean follow‐up period of our study was 7.9 years, which is comparable to studies analyzing AMEI devices with the longest follow‐up periods.8, 10
A total of 10.17% of our patients required an explantation without implantation of another implant. One study group reported a higher explantation rate of 18.5%.9 Three of 12 explantations were required because the VSB wire was protruded in the outer ear canal and pulled out accidently by a physician. In all cases, this resulted in an emergency explantation. Complication was reported in 2 different studies for the VSB and in 1 study for MET (Cochlear Ltd, Sydney, Australia). Pointing out that the implant wire can protrude into the outer ear canal might thus be essential for patients and their treating physicians. This could contribute in preserving the implant with a revision surgery.
The rate of technical complications in our cohort was low compared to literature: device failure occurred in four patients (3.39%), which is less than 7% reported by Zwartenkot et al.9 The medical complication rate was comparable to other studies regarding the VSB. Three patients (2.5%) were explanted because of an infection. Lassaletta et al.11 noted an infection rate of 8.3% in their study population. Floating mass transducer displacement occurred in 3.39% of implanted patients. Zwartenkot et al.9 reported on 1% of patients with the same complications. Furthermore, they presented 3.2% of patients who had to be explanted due to growing pain complaints. In our study population, 2.5% of patients required an explantation due to the same issue. Furthermore, they reported a mean implant loss of one per 74 follow‐up years. It was higher than the implant loss of one per 158 follow‐up years reported in our study.
The same study group showed an increase in the complication rate during an earlier 3‐year period in which a new experimental transcanal approach technique was introduced. However, frequency of complications had decreased with the years. In our institution, we observe an increase of the complication rate from 2004 to 2006, and a significantly higher complication rate for devices coupled onto the round window. Between 1998 and late 2002, the middle ear hearing implant system was classically crimped onto the long process of the incus.13 This original standard surgical procedure caused low revision rates only. Between 2004 and 2006, new experimental coupling procedures were implemented that evaluated the efficacy and safety of round window vibroplasty.4 The procedure included enlargement of the facial recess and exposure of the round window niche. If necessary, the lip of the round window was drilled to expose the round window membrane, followed by positioning of the FMT onto the round window membrane. During the first experimental procedures, the FMT was initially covered with the thin layer of temporal muscle fascia and fibrin glue. Five patients had to be revised because the fascia was resorbed over time (in within 1 year) and the FMT lost connection to the round window membrane with deterioration of hearing outcome. After 2006, fixation was thus performed with Tuttoplast (Tutogen Medical, Neunkirchen am Brand, Germany) with fibrin glue to optimize stabilization of the FMT. Evaluation shows that this coupling approach was safe and effective with stable complication rates (Fig. 1.) Additionally, different titanium couplers were introduced. This facilitated the fixation of the FMT, which might have contributed to more stable outcomes. The complication rate remained stable in other time periods.
Rates of technical and medical complication are comparable to those of other active middle ear implants. Authors presented device failure rates of 12.5%12 and 9%14 in patients implanted with the Carina implant and 28%9 in patients implanted with MET (Cochlear Ltd, Sydney, Australia). Eighteen percent of patients implanted with Carina (Cochlear Ltd, Sydney, Australia) were explanted because of an infection.14
Audiological outcome was stable and similar to that in the literature for all types of hearing loss. The bone conduction thresholds were preserved postoperatively, which indicates that integrity of the inner ear was not affected by the implantation. Mean FHG values were satisfactory in patients with types of hearing loss. The mean FHG for sensorineural hearing loss is in accordance with the literature ranging from 13.9 to 28.1 dB on average.15, 16, 17, 18 Two studies reported higher mean hearing gains in patients with conductive and mixed hearing loss (32 dB19 and 36.1 dB20). When analyzed by each frequency, significant hearing gains were reached at each frequency from 500 to 4,000 Hz. Jung et al.21 reported significant gains at 2,000 and 4,000 Hz but not at 1,000 and 3,000 Hz.
CONCLUSION
This report provides information on the long‐term performance of the VSB active middle ear implant and shows an acceptable implant reliability regarding medical and technical complication rates. Highest complication rates were observed during experimental procedures with alternative coupling approaches. The VSB provides satisfactory audiological benefits regarding the mean FHG and speech perception improvement in patients with all hearing loss types. Long‐term surgical outcome of vibroplasty for sensorineural and mixed hearing loss is comparable to those of other similar active middle ear implants with an acceptable implant survival period. The majority of patients used the implant at the end of the observation period. Because explantation rates were in a moderate range, we believe the system is safe and effective considering appropriate patient selection and adequate surgical approach.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1. Gstoettner W. First experience with implantable hearing aids in Austria In: Josef Dézsy. Medicine 2000. Dr. Peter Müller Verlag; 2000;147–160. [Google Scholar]
- 2. Luetje CM, Brackman D, Balkany TJ, et al. Phase III clinical trial results with the Vibrant Soundbridge implantable middle ear hearing device: a prospective controlled multicenter study. Otolaryngol Head Neck Surg 2002;126:97–107. [DOI] [PubMed] [Google Scholar]
- 3. Colletti V, Soli SD, Carner M, Colletti L. Treatment of mixed hearing losses via implantation of a vibratory transducer on the round window. Int J Audiol 2006;45:600–608. [DOI] [PubMed] [Google Scholar]
- 4. Baumgartner WD, Boheim K, Hagen R, et al. The vibrant Soundbridge for conductive and mixed hearing losses: European multicenter study results. Adv Otorhinolaryngol 2010;69:38–50. [DOI] [PubMed] [Google Scholar]
- 5. Verhaegen VJ, Mulder JJ, Cremers CW, Snik AF. Application of active middle ear implants in patients with severe mixed hearing loss. Otol Neurotol 2012;33:297–301. [DOI] [PubMed] [Google Scholar]
- 6. Vyskocil E, Riss D, Honeder C, et al. Vibroplasty in mixed and conductive hearing loss: comparison of different coupling methods. Laryngoscope 2014;124:1436–1443. [DOI] [PubMed] [Google Scholar]
- 7. Maier H, Hinze AL, Gerdes T, et al. Long‐term results of incus vibroplasty in patients with moderate‐to‐severe sensorineural hearing loss. Audiol Neurootol 2015;20:136–146. [DOI] [PubMed] [Google Scholar]
- 8. Sterkers O, Boucarra D, Labassi S, et al. A middle ear implant, the Symphonix Vibrant Soundbridge: retrospective study of the first 125 patients implanted in France. Otol Neurotol 2003;24:427–436. [DOI] [PubMed] [Google Scholar]
- 9. Zwartenkot JW, Mulder JJ, Snik AF, Cremers CW, Mylanus EA. Active middle ear implantation: long‐term medical and technical follow‐up, implant survival, and complications. Otol Neurotol 2016;37:513–519. [DOI] [PubMed] [Google Scholar]
- 10. Mosnier I, Sterkers O, Bouccara D, et al. Benefit of the Vibrant Soundbridge device in patients implanted for 5 to 8 years. Ear Hear 2008;29:281–284. [DOI] [PubMed] [Google Scholar]
- 11. Lassaletta L, Calvino M, Sanchez‐Cuadrado I, Perez‐Mora RM, Munoz E, Gavilan J. Pros and cons of round window vibroplasty in open cavities: audiological, surgical, and quality of life outcomes. Otol Neurotol 2015;36:944–952. [DOI] [PubMed] [Google Scholar]
- 12. Bruschini L, Forli F, Passetti S, Bruschini P, Berrettini S. Fully implantable Otologics MET Carina device for the treatment of sensorineural and mixed hearing loss: audio‐otological results. Acta Otolaryngol 2010;130:1147–1153. [DOI] [PubMed] [Google Scholar]
- 13. Fisch U, Cremers CW, Lenarz T, et al. Clinical experience with the Vibrant Soundbridge implant device. Otol Neurotol 2001;22:962–972. [DOI] [PubMed] [Google Scholar]
- 14. Martin C, Deveze A, Richard C, et al. European results with totally implantable carina placed on the round window: 2‐year follow‐up. Otol Neurotol 2009;30:1196–1203. [DOI] [PubMed] [Google Scholar]
- 15. Todt I, Seidl RO, Gross M, Ernst A. Comparison of different vibrant Soundbridge audioprocessors with conventional hearing aids. Otol Neurotol 2002;23:669–673. [DOI] [PubMed] [Google Scholar]
- 16. Luetje CM, Brown SA, Cullen RD. Vibrant Soundbridge implantable hearing device: critical review and single‐surgeon short‐ and long‐term results. Ear Nose Throat J 2010;89:E9–E14. [DOI] [PubMed] [Google Scholar]
- 17. Pok SM, Schlogel M, Böheim K. Clinical experience with the active middle ear implant Vibrant Soundbridge in sensorineural hearing loss. Adv Otorhinolaryngol 2010;69:51–58. [DOI] [PubMed] [Google Scholar]
- 18. Grégoire A, Van Damme JP, Gilain C, Bihin B, Garin P. Our auditory results using the Vibrant Soundbridge on the long process of the incus: 20 years of data. Auris Nasus Larynx. 2017. doi: 10.1016/j.anl.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 19. Dumon T, Gratacap B, Firmin F, et al. Vibrant Soundbridge middle ear implant in mixed hearing loss. Indications, techniques, results. Rev Laryngol Otol Rhinol (Bord) 2009;130:75–81. [PubMed] [Google Scholar]
- 20. Schwab B, Salcher RB, Maier H, Kontorinis G. Oval window membrane vibroplasty for direct acoustic cochlear stimulation: treating severe mixed hearing loss in challenging middle ears. Otol Neurotol 2012;33:804–809. [DOI] [PubMed] [Google Scholar]
- 21. Jung J, Kim JW, Moon IS, Kim SH, Choi JY. Vibrant Soundbridge can improve the most comfortable listening level in sensorineural hearing loss: our experience with 61 patients. Clin Otolaryngol 2018;43:369–373. [DOI] [PubMed] [Google Scholar]


