Abstract
The introduction of new therapeutic agents in chronic lymphocytic leukemia (CLL) and follicular lymphoma (FL), including the new kinase inhibitor idelalisib, has changed the therapeutic landscape of these diseases. However, the use of idelalisib is associated with a peculiar profile of side effects, which require an optimization of the current approach to prophylaxis and supportive treatment. Moving from the recognition that the abovementioned issue represents an unmet need in CLL and FL, a multidisciplinary panel of experts was convened to produce a consensus document aiming to provide practical recommendations for the management of the side effects during idelalisib therapy for CLL and FL. The present publication represents a consensus document from a series of meetings held during 2017. The Panel generated clinical key questions using the criterion of clinical relevance through a Delphi process and explored 4 domains, ie, diarrhea/colitis, transaminitis, pneumonitis, and infectious complications. Using the consensus method, the Panel was able to shape recommendations which may assist hematologist to minimize adverse events and guarantee adherence to treatment in patients with CLL and FL candidate to receive idelalisib.
Keywords: adverse events, chronic lymphocytic leukemia, follicular lymphoma, idelalisib
1. INTRODUCTION
Idelalisib is a phosphatidylinositol 3‐kinase delta (PIK3δ) inhibitor1, 2, 3, 4 with significant activity in patients with relapsed or refractory (R/R) CLL5 and indolent non‐Hodgkin lymphoma (NHL).6 This agent was tested in combination with rituximab in a head‐to‐head comparison against placebo and rituximab in a trial enrolling heavily pretreated CLL patients. A significant advantage of progression‐free survival and survival was documented in the experimental arm, and the study was stopped early owing to overwhelming efficacy.7
Likewise, idelalisib single agent showed efficacy in alkylator‐refractory indolent B‐NHL, including high‐risk follicular lymphoma with early relapse after chemoimmunotherapy, with a median duration of response exceeding the median duration of response in the group of patients who had a response to the most recent therapy.8, 9
However, drug‐related adverse reactions (ADRs) led to dose reductions and discontinuations,10, 11 immune‐mediated disorders, and infections being the leading cause of treatment interruption.12, 13
There is evidence that adherence to treatment is important to delay disease progression in R/R patients treated with drugs targeting B cell receptor pathway.14, 15 In view of these considerations, a multidisciplinary panel of experts convened to provide recommendations for the diagnostic workup and management of the complications arising during treatment with idelalisib. The present publication represents a consensus document generated through email correspondence and during meetings held in 2017.
2. DESIGN AND METHODS
Two chairmen (AC and PLZ) appointed a panel of 7 experts (hereafter called the Panel). A clinician with expertise in clinical epidemiology (GB) assured the methodological correctness of the process. During an initial meeting, the Panel agreed on the areas of major concern on the ADRs associated with idelalisib, by generating and rank‐ordering clinical key questions using the criterion of clinical relevance through a Delphi process.16 The candidate key questions that ranked highest formed the set of questions of the present document (Table S1 supplementary material). During a second meeting, the Panel examined the state of knowledge regarding idelalisib complications in CLL and FL. Each panelist drafted statements that addressed the key questions. Subsequently, each panelist scored his agreement with the statements made by other panelists and provided suggestions for rephrasing during 2 consensus conferences. During the consensus meetings, participants were first asked to comment in round‐robin fashion on their preliminary votes and then to propose a new vote. If at least a ≥80% consensus on the statement was not achieved, the choices were discussed and a second vote taken. If a ≥80% consensus was still not attained, the issue was declared undecidable, and no further attempt was made.
3. RESULTS
3.1. Pharmacokinetics and drug‐drug interactions of idelalisib
Idelalisib is mainly metabolized not only by aldehyde oxidase to GS‐563117 but also by cytochrome P450 (CYP) 3A4 and UGT1A4. The time to plasma C max is 1.5 hours; the elimination half‐life is 8 hours, and metabolites are excreted in the feces (78%) and urine (15%) with an oral clearance of 15 L/hour.17 Severe renal impairment does not affect idelalisib pharmacokinetics (PK) significantly,18 while severe hepatic impairment increases drug exposure by 60%.19 The PK of digoxin, a P‐gp substrate, and of rosuvastatin, a OATP1B1/1B3 substrate, are not affected by idelalisib,20 while GS‐563117 is a strong inhibitor of CYP3A4 and increases the exposure to midazolam by 5‐fold17; the use of CYP3A4‐metabolized drugs should therefore be avoided or closely monitored. Strong CYP3A4 inhibitors (ie, ketoconazole) and inducers (ie, rifampin) may increase or decrease the area under the curve (AUC) of idelalisib by 75% to 80%; their concomitant administration should therefore be discouraged.17 Drugs with potential drug‐drug interactions (DDIs) with idelalisib are reported in Table 1. The therapeutic index (TI) of a drug and associated therapeutic range of plasma concentrations affect DDIs. Beta‐lactam antibiotics, benzodiazepines, and most antiepileptic and antihypertensive drugs have high TI and wide therapeutic range of drug concentrations, while anticancer cytotoxic drugs, warfarin, digoxin, and lithium have low TI and narrow therapeutic range of drug levels, and DDIs are more likely to occur.21
Table 1.
Metabolic substrates with potential DDI with idelalisib, a drug metabolized primarily by aldehyde oxidase and CYP3A4
| Metabolizing Enzyme | Sensitive Substratesa |
|---|---|
| Aldehyde oxidase | Allopurinol, famciclovir, lenvatinib, mercaptopurine, methotrexate, pyrazinamide, zaleplon, zonisamide |
| CYP3A4 | Alfentanil, alprazolam, aprepitant, atorvastatin, avanafil, budesonide, budesonide, colchicine, darunavir, dasatinib, dronedarone, eletriptan, everolimus, felodipine, fentanyl, ibrutinib, indinavir, lovastatin, lurasidone, maraviroc, midazolam, nisoldipine, pimozide, quetiapine, rilpivirine, rivaroxaban, saquinavir, sildenafil, simvastatin, sirolimus, tacrolimus, tadalafil, ticagrelor, tipranavir, tolvaptan, triazolam, vardenafil |
Sensitive substrates are drugs that demonstrate an increase in AUC of ≥5‐fold with strong inhibitors of a given metabolic pathway in clinical DDI studies.
ADRs may increase in the presence of comorbidities (ie, liver dysfunction, hypoalbuminemia), plasma protein displacement by NSAIDs, or if a drug is metabolized by a single metabolic pathway (ie, midazolam/CYP3A4) rather than multiple enzymes (ie, diazepam is metabolized by CYP3A4, 2C9, 2C19, and 2B6). Finally, the availability of therapeutic drug monitoring (TDM) facilitates the optimal management of DDIs by adjusting the drug dose based on plasma levels. Table 1 reports the substrates of aldehyde oxidase and CYP3A4 while a strategy for the DDI management and ADR minimization is summarized in Table 2.
Table 2.
General strategy to minimize the clinical impact of DDIs and associated ADRs of idelalisib
| Clinical Condition | Frequency/Severity of ADRs | Action Required |
|---|---|---|
| Inhibition of metabolism of a drug with low TI and single CYP‐dependent metabolic pathway (ie, idelalisib vs cyclosporine, tacrolimus, fentanyl, sunitinib) | High/potentially severe | Intensive TDM, if available, and dose reduction; if unavailable, choose alternative drug |
| Inhibition of metabolism of a drug with intermediate TI and multiple CYP‐dependent metabolic pathways involved (ie, idelalisib vs carbamazepine, phenytoin, etoricoxib) | Low/moderate | Clinical evaluation; dose reduction usually not required |
| Inhibition of metabolism of a drug with high TI and single or multiple CYP‐dependent metabolic pathways involved (ie, idelalisib vs diazepam, paroxetine, trazodone, codeine) | Low/none | None |
3.2. Diarrhea/cholitis
Diarrhea is one of the most common ADRs that can eventually lead to idelalisib dose reduction and/or discontinuation, and the majority of the cases have no identifiable infectious pathogen.8, 22, 23, 24, 25 PI3K inhibition has been associated with immune dysregulation resulting in inhibition of regulatory T cells and consequent damage by CD8+ cytotoxic T cells.22, 25, 26 These events may result in either an inflammatory, ischemic, or a mixed histologic pattern.
Enteric budesonide has been a commonly used treatment for severe diarrhea or colitis, resulting in a relatively shorter time to resolution12 with a mean time to resolution of 12.1 days (range, 1‐35 days).12 This drug should not be used for chronic suppression of symptoms associated with ongoing use of idelalisib.
3.3. Recommendations
3.3.1. General assessment (Table 3)
Table 3.
General assessment of diarrhea/colitis
| Patient Evaluation | Laboratory Testing |
|---|---|
|
Physical examination ○ Fever ○ Dizziness ○ Abdominal pain/cramping/peritonism ○ Signs of dehydration Type and time of onset of diarrhea ○ No. of stools ○ Composition of stools ○ Timing of stools Travel history Identification of potential causative agents: ○ Drug profile ○ Dietary profile |
Stool workup Occult fecal blood Stool toxin test for C. difficile Stool cultures ○ Salmonella ○ Escherichia coli ○ Campylobacter Blood count, electrolytes, creatinine, blood urea nitrogen Colonoscopy (in selected cases) |
In patients with CLL or FL who are candidate to treatment with idelalisib, a careful collection of history of inflammatory bowel diseases or microscopic colitis, and of functional gastrointestinal disorders, is recommended.
The evaluation of the presence of sequelae of gastrointestinal surgery is also recommended.
In patients with unexplained and/or persistent gastrointestinal disorders, gastroenterological referral is recommended.
Any chronic enterocolitis uncontrolled by therapy advises against the use of idelalisib.
The Panel agreed that no prophylaxis for the diarrhea or colitis in patients candidate to idelalisib is indicated.
3.3.2. Management (Figures 1 and 2)
Figure 1.

The issue of diarrhea/colitis: assessment of patients assigned to treatment with idelalisib ± rituximab as first option
Figure 2.
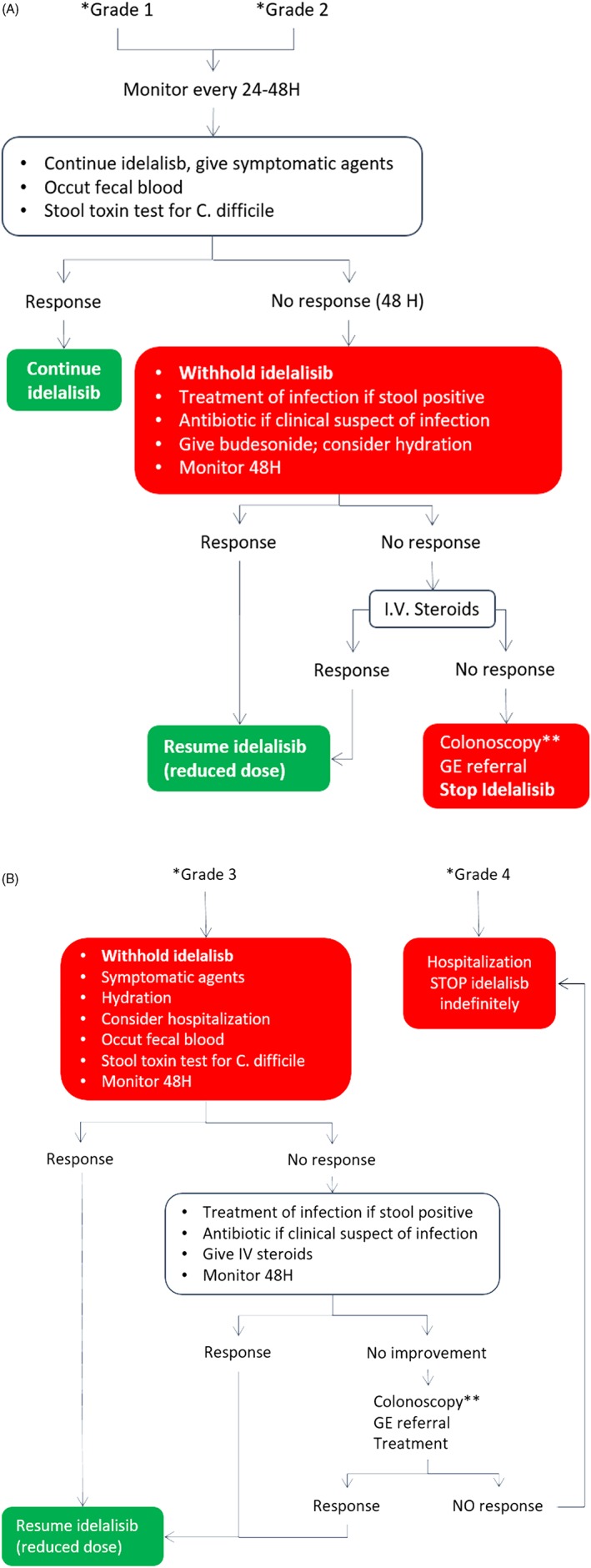
Management of idelalisib‐induced diarrhea. (*grade 1 = stool increase <4/day over baseline; grade 2 = stool increase 4‐6; grade 3 = stool increase ≥7, hospitalization required; grade 4 = life threatening; **always if bleeding)
Patients treated with idelalisib should be clinically monitored for the appearance of diarrhea or symptoms of colitis.
Patients with CLL or FL, in whom diarrhea appears during treatment with idelalisib, should be investigated to rule out an infection cause.
Daily monitoring of the diarrhea grade according to Common Terminology Criteria for Adverse Events National Cancer Institute is recommended.
The patients should be advised to withhold idelalisib in the case of a diarrhea grade 3 (7 stools or higher over baseline), or grade 2 (>4 stools) persistent for more than 2 days.
In the presence of bloody diarrhea and in cases unresponsive to treatment, colonoscopy and gastroenterological referrals are advised.
3.3.3. Treatment
In the presence of diarrhea (any grade), patients should stop lactulose‐containing products, alcohol, and high osmolar supplements. They should also increase liquid intake (plus electrolytes) and should take light and frequent meals.
For grade 1 or grade 2 diarrhea, idelalisib can be continued and symptomatic therapy should be initiated just after the collection of samples (stools, blood) for the evaluation of a possible infectious cause. In grade 1 or 2 diarrhea of infective etiology, idelalisib should be continued and antibiotic therapy started.
In grade 3 or grade 4 diarrhea, and in grade 1 or grade 2 diarrhea nonresponding after 2 days of symptomatic and/or antibiotic therapy, idelalisib should be discontinued. If diarrhea remains unresolved after 48 hours, steroids should be started (budesonide, 3 mg × 3 qd) after the exclusion of any infective etiology.
Budesonide should be continued until complete resolution of diarrhea.
Bacterial decontamination is not recommended, while probiotic administration can be added to the general management strategy.
IV fluid supplements should be given to any grade 3 or 4 diarrhea and in grade 2 according to the clinical condition.
IV steroids are recommended after colonoscopy in the presence of hemorrhagic colitis and in cases unresponsive to budesonide.
In patients who have interrupted idelalisib, the drug may be resumed when the patient experiences a return to the basal intestinal evacuation attitudes. The Panel agreed that idelalisib should be resumed at a lower dose than the initial one (eg, 100 mg twice daily).
Concomitant use of budesonide for a limited period should be considered in the case of diarrhea recurrence after patient's clinical assessment.
3.4. Transaminitis
An elevation of alanine transaminase (ALT) and aspartate transaminase (AST) blood levels of any grade frequently occurs (35%‐50%) during treatment with idelalisib (grade ≥3 in 14%‐16% of patients).12, 24
The pattern of liver injury is hepatocellular, with more severe elevations in the transaminases compared with alkaline phosphatase and bilirubin. Transaminase elevation tends to quickly remit after withdrawal of the drug, but it may rapidly recur upon re‐exposure to the drug if not managed properly (dose reduction).
In most cases, the transaminitis is neither because of viral reactivation nor to DDIs. Viral reactivation and hepatitis have not been described in anti‐HBc‐positive patients or in HCV‐positive and/or HBsAg‐positive carriers treated according the current clinical practice.27, 28, 29 Transaminitis is rather suggestive of a liver injury secondary to an adaptive immune response elicited by the on‐target inhibition of PI3Kδ in regulatory T cells,30, 31 as suggested by the occurrence of hepatocellular damage when using other drugs belonging to the same class.32 An activated T cell infiltrate is seen on liver biopsy specimens of patients who experienced idelalisib‐related persistent hepatitis.31 Hepatotoxicity is more prevalent in younger (<65 years), previously untreated patients who have a more intact immune system than older R/R subjects.33 Moreover, transaminitis usually responds to steroids and is prominent when idelalisib is given in combination with immunomodulatory agents, such as lenalidomide.30, 34, 35
Because immune‐mediated hepatotoxicity occurs rather frequently under idelalisib, a pretreatment test of HCV and HBV serological status should be guaranteed for every patient. The most recent guidelines recommended prophylaxis against HBV reactivation with lamivudine in every patient with anticore reactivity‐receiving regimens including rituximab.36
3.5. Recommendations
3.5.1. General assessment (Figure 3)
Figure 3.

The issue of transaminitis: assessment of patients assigned to treatment with idelalisib ± rituximab as first option. (UNL, upper normal limit; DAA, directly acting antivirals; *>5 UNL because of a histologically proved CLL‐dependent or FL‐dependent liver disease)
In patients with CLL or FL candidate to treatment with idelalisib, careful evaluation of the past history of chronic hepatitis is recommended.
All patients should undergo HCV and HBV screening by anti‐HCV, HBsAg, anti‐HBc, and anti‐HBs.
In patients with anti‐HCV positivity, quantitative HCV‐RNA should be assayed. In patients with HBsAg or anti‐HBc positivity, HBV‐DNA should be assayed.
In patients with history of chronic hepatitis and/or anti‐HCV or HBsAg positivity, hepatological referral is advised to define the need for an antiviral treatment. The hepatological referral is not required in isolated anti‐HBc‐positive/HBV‐DNA‐negative patients.
The patients with anti‐HBc positivity must receive universal prophylaxis with lamivudine if treated with rituximab.
The Panel agreed that no cutoff value of increased transaminases is per se a contraindication to the use of idelalisib; however, a careful monitoring is mandatory in the patients with liver enzyme elevation before starting treatment with idelalisib.
A history of chronic liver disease, a value of transaminases >3 the upper normal limit (UNL), or >5 UNL because of a histologically proved CLL‐dependent or FL‐dependent liver disease indicate the need of hepatological referral prior to start idelalisib.
The presence of a decompensated cirrhosis advises against the use idelalisib.
Patients with liver disease candidate to therapy with idelalisib should maintain their basal treatment of chronic hepatitis or infection with directly acting antivirals (DAA) with special attention to drug‐drug interaction.
All CLL and FL patients treated with idelalisib should be monitored clinically for the appearance of transaminitis. Monitoring of the following parameters is recommended: AST, ALT, GGT, APH, and bilirubin.
Transaminases should be checked biweekly in the first 3 months of therapy, monthly up to the sixth month of therapy, and then as clinically indicated.
3.5.2. Management (Figure 4)
Figure 4.
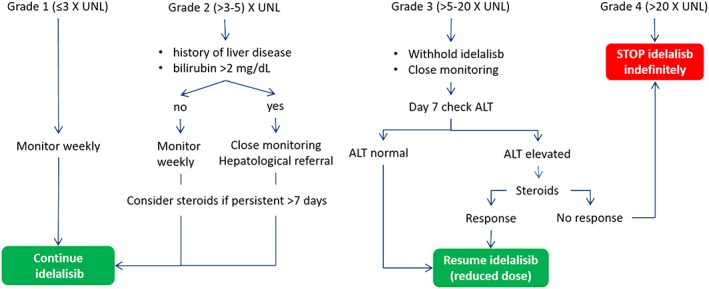
Management of transaminitis. (UNL, upper normal limit; ALT, alanine aminostransferase)
In grades 1 and 2, transaminitis idelalisib can be continued with careful monitoring (weekly).
In patients with history of chronic hepatitis and grade 2 transaminitis and/or bilirubin >2 mg/dL close monitoring, careful evaluation of the liver function and hepatological referral is recommended.
In the presence of an increase of transaminases over 5 times the UNL (grade 3), idelalisib should be temporarily discontinued.
The presence of transaminase elevation 20 times over the UNL (grade 4) should prompt permanent discontinuation of idelalisib.
In patients who have discontinued idelalisib because of grade 3 transaminitis (>5 and <20 UNL), idelalisib should be reassumed at a lower dose of 100 mg twice daily when a return to the basal ALT values occurs. The Panel agreed that in the case of a pressing need of therapy for CLL or FL, idelalisib can be reassumed at values of ALT equal or lower than 3 times the UNL.
In the absence of other causes, transaminitis greater than 5 times the normal level, persisting without a significant decline after 7 days from the drug withdrawal, a steroid‐based treatment should be given.
A transaminitis less than 5 times the normal level should be monitored without an immediate steroid‐based treatment. In this case, corticosteroid treatment may be considered in persisting transaminitis and after a new patient's clinical assessment.
The steroid optimal dose should be no less than 1 mg/kg prednisolone or equivalent.
3.6. Pneumonitis
Pneumonia occurred in 6% to 22% of patients treated with idelalisib, and noninfectious pneumonitis was diagnosed in 2% to 5% of patients, with few fatal cases.5, 7, 8, 12, 37, 38 The clinical profile of noninfectious pneumonitis because of idelalisib is characterized by an acute/subacute onset, although in a series including 5 patients, the time between appearance of symptoms and diagnosis was 3 to 9 weeks.37, 39 Cough, dyspnea, and fever are the main symptoms. Chest X‐ray shows usually bilateral infiltrates. High‐resolution CT scan documents diffuse ground glass opacities with a peribronchial distribution, alveolar consolidations, and even pleural effusion. Bronchoalveolar lavage (BAL) showed an increase of lymphocytes, and histopathologic examination of lung biopsy showed subacute hypersensitivity pneumonitis or organizing pneumonia.40
Smoking history, chronic obstructive lung disease, previous radiation treatment, and idiopathic pulmonary fibrosis are the most relevant conditions associated with a significant increase of drug‐induced lung injury.41, 42 Pulmonary function tests represent a sensitive tool for detecting lung disorders even in asymptomatic subjects.43
The clinical and laboratory profiles of drug‐induced lung injury are not specific. Characteristic features of drug‐induced lung injury on high‐resolution CT scan, such as ground glass attenuation and/or perilobular pattern, may be consistent with the clinical hypothesis but are not pathognomonic. Therefore, the final diagnosis should be corroborated, by BAL which may show a negative microbiological profile and lymphocytosis at cytological examination.44 Interestingly, the diagnostic role of rapid on‐site cytologic examination of bronchoalveolar lavage in acute lung injury, which may provide results in a few hours, was documented.45 The utility of the combination of BAL and transbronchial lung biopsy (feasible only in patients with platelets>50 000/mL and no coagulation defects) allowed a diagnostic yield around 80%, even in patients with respiratory failure.46 The evaluation of LDH, peripheral blood oxygen saturation, and pulmonary function tests including CO diffusion index are currently used to stratify the lung injury severity.47
High‐dose steroids (up to 500 mg methylprednisolone iv qd) may be used in patients with drug‐induced acute lung injury.42 Multiple methylprednisolone pulses were used in cases of idelalisib‐related pneumonitis with rapid respiratory deterioration.37 Respiratory support in patients with acute lung injury and respiratory failure because of idelalisib need to be optimized to ensure adequate gas exchange while minimizing the risks of ventilator‐induced lung injury.48 Noninvasive ventilation delivered by helmet appeared to be associated with significant reduction in intubation rates and 90‐day mortality in a randomized clinical trial enrolling patients with acute respiratory distress syndrome.49
3.6.1. Recommendations (Figure 5)
Figure 5.
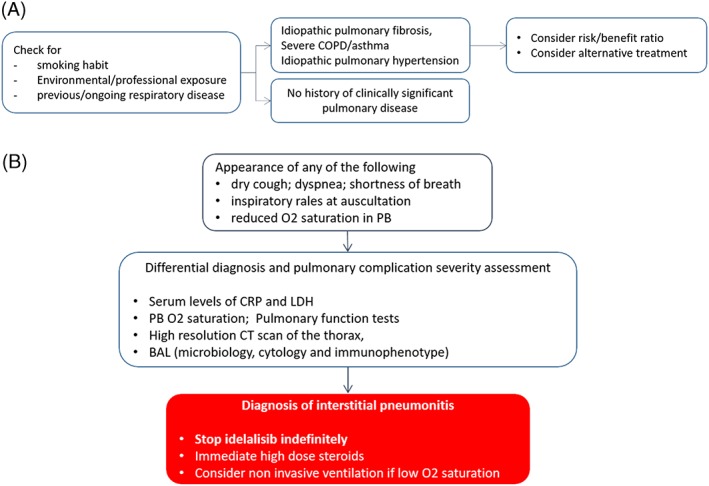
The issue of pneumonitis: management algorithm for patients assigned to treatment with idelalisib ± rituximab as first option (UNL, upper normal limit; PB, peripheral blood; COPD, chronic obstructive pulmonary disorder; CRP, C‐reactive protein; BAL, bronchoalveolar lavage; *>5 UNL because of a histologically proved CLL‐dependent or FL‐dependent liver disease; DAA, directly acting antivirals)
In CLL and FL patients candidate to treatment with idelalisib, careful evaluation of smoking habit, history of respiratory disease, history of environmental, or professional exposure is recommended.
The Panel agreed that there are no absolute contraindications to idelalisib.
Idiopathic pulmonary fibrosis, severe COPD/asthma, and idiopathic pulmonary hypertension represent relative contraindications to idelalisib.
In these cases, the individual risk/benefit should be evaluated, and patients should be informed that idelalisib may increase the risk of new pulmonary complications and may cause worsening of the pulmonary disease.
No data on the efficacy of prophylaxis in patients candidate to idelalisib are available so far.
Interstitial pneumonitis should be suspected in the presence of dry cough, dyspnea, shortness of breath, inspiratory rales at auscultation, or reduction O2 saturation in peripheral blood.
With appearance of these pulmonary symptoms, the first goal of the diagnostic workup is the differential diagnosis between an infectious pulmonary complication and interstitial pneumonitis.
High‐resolution CT scan of the thorax and BAL (with microbiological, cytological, and immunophenotypic analysis) are the necessary highly informative examinations.
Transbronchial lung biopsy may represent a useful tool for a positive diagnostic criterion of interstitial pneumonitis in selected cases with a difficult‐to‐diagnose condition and without contraindications to this procedure.
Serum levels of C‐reactive protein (CRP) and LDH, peripheral blood O2 saturation, and pulmonary function tests including DLCO measurement are complementary tests for the differential diagnosis and are useful for the severity assessment.
In patients with a diagnosis of interstitial pneumonitis, idelalisib should not be resumed.
Immediate high‐dose steroids is the recommended therapy of interstitial pneumonitis.
Noninvasive ventilation should be considered even when there is a mild reduction in oxygen saturation.
3.7. Infectious complications
Clinical trials assessing the efficacy of idelalisib outside of the licensed indications in CLL and indolent NHL reported an unexpected high rate of serious adverse events (AE) and increased mortality.50, 51, 52 The excess of deaths were mainly associated to infections, namely Pneumocystis jiroveci pneumonia (PJP) and human cytomegalovirus (HCMV)‐related disease. Pneumocystis jiroveci pneumonia is a relatively easily preventable disease, and virtually no cases were reported during idelalisib treatment in patients receiving specific concomitant chemoprophylaxis.53
Furthermore, preexisting lung diseases such as chronic obstructive pulmonary disease (COPD), bronchiectasias, and asthma as well as smoking have been associated with an increased risk of PJP and bacterial pneumonia, thus making a careful collection of patients' history necessary.54
Vaccinations against S. pneumoniae and influenza should be offered to all patients and caregivers. Although there is no evidence of correlation between B cell receptor inhibitors and tuberculosis (TB), screening for latent TB and consequent suppressive therapy for infected subjects was indicated.55
Cytomegalovirus viremia should be regularly monitored, and a pre‐emptive treatment strategy should be pursued when the viremia predefined cutoff has been reached, while maintaining the patient on idelalisib. The management principles should be extrapolated from already‐published guidelines on anti‐CD52 use in CLL and from the guidelines on the management of HCMV in solid organ transplantation.56, 57
On the other hand, bacterial pneumonia in patients treated with idelalisib does not have a significantly higher incidence than in patients with any type of indolent B cell malignancies receiving active treatment, nor is characterized by distinctive severity patterns. For this reason, there is no specific need of preventive measures.
3.7.1. Recommendations (Figure 6)
Figure 6.
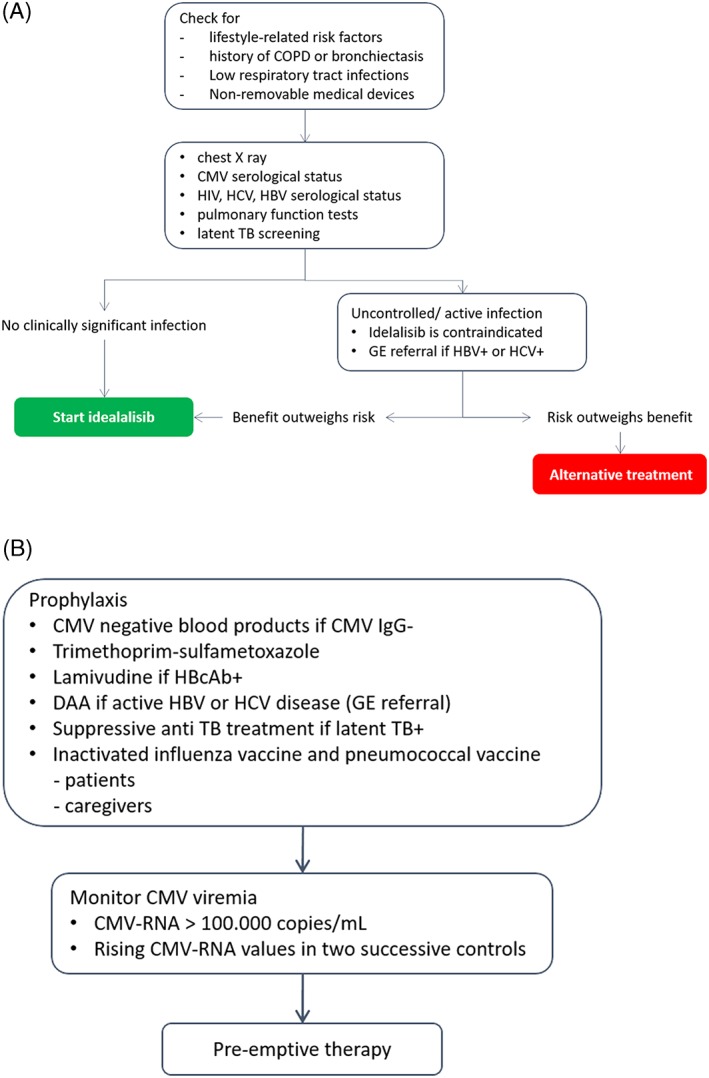
The issue of infectious complications: management algorithm for patients assigned to treatment with idelalisib ± rituximab as first option. (UNL, upper normal limit; PB, peripheral blood; BAL, bronchoalveolar lavage; GE, gastroenterologist; TB, tubercolosis; *>5 UNL because of a histologically proved CLL‐dependent or FL‐dependent liver disease; DAA, directly acting antivirals)
In CLL and FL patients candidate to treatment with idelalisib, careful evaluation of history of repeated infective episodes, history of COPD or bronchiectasis, presence low respiratory tract infections, and presence of nonremovable medical devices is recommended.
Information about lifestyle‐related factors predisposing to infection should be collected.
Evaluation of chest X‐ray, CMV serological status, HIV, HCV, HBV serological status, pulmonary function tests, and latent TB screening (Table 4 and Figure 7) is recommended.
In CLL or FL patients, any uncontrolled infection contraindicates the use of idelalisib.
Prophylaxis of infectious episodes in patients receiving idelalisib should be attained by using CMV‐negative blood products in CMV IgG‐negative patients.
Trimethoprim‐sulfametoxazole is indicated during treatment and up to 2 to 6 months after discontinuation in all patients as a prophylaxis for PJP (Table 5)58, 59
For patients with a diagnosis of latent TB, suppressive anti‐TB treatment is recommended (Table 6).60
Any persistent episode of fever should be monitored looking for CMV DNA testing.
In all patients (sero‐positive and sero‐negative), CMV viremia should be regularly monitored at least on a monthly basis.
Pre‐emptive therapy is recommended for value of CMV‐RNA ≥ 100 000 copies/mL or rising values in 2 successive controls.
There is no evidence that the immunoglobulin replacement therapy patients treated with idelalisib should be modified with respect to that used in CLL or FL patients.
In CLL patients treated with idelalisib, inactivated influenza vaccine and pneumococcal vaccine are mandatory for every patient, relative, and caregiver.
Table 4.
Screening of latent tuberculosis
| Key Points |
|---|
|
1. Any patient should be asked about symptoms of TB before being tested for LTBI. 2. Chest radiography can be done if efforts are intended also for active TB case finding. 3. Individuals with TB symptoms or any radiological abnormality should be investigated further for active TB and other conditions. 4. Every asymptomatic individual has to be tested for latent TB, with Mantoux tuberculin skin test (TST) or interferon‐gamma release assay (IGRA) test. 5. Either TST or IGRA test can be used to test for LTBI in high‐income and upper middle‐income countries with estimated TB incidence less than 100 per 100 000. |
Figure 7.
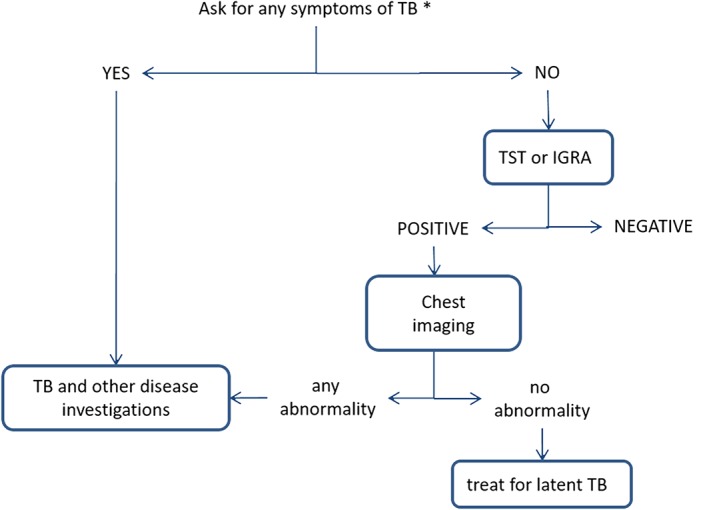
Algorithm for tuberculosis screening. (*any symptoms of TB include any 1 of cough, hemoptysis, fever, night sweats, weight loss, chest pain, shortness of breath, fatigue; TST, tubercolin skin test; IGRA, interferon‐gamma release assays)
Table 5.
| Dose | |
|---|---|
| First‐line option | |
| Trimethoprim‐sulfamethoxazole | 160 + 800 mg (one DS tablet) orally, daily or 160 + 800 mg (one DS tablet) orally, 3 times a week |
| Second‐line options (if trimethoprim‐sulfamethoxazole is contraindicated, allergy or hypersensitivity to sulfa‐drugs is documented) | |
| Atovaquone 1 | 500 mg orally, daily with a high‐fat meal |
| Pentamidine | 300 mg inhaled through nebulizer, every 4 weeks (administered through a jet nebulizer producing a droplet size of 1‐2 μ) |
| Dapsone | 100 mg orally, daily |
Table 6.
Treatment of latent tubercolosis60
| Options | Dose per Body Weight | Standard Daily Dose |
|---|---|---|
| 6‐month isoniazid | 5 mg/kg | 300 mg |
| 3‐4‐month isoniazid plus rifampicin | I 5 mg/R 10 mg/kg | I 300 mg/R 600 mg |
| 9‐month isoniazid | 5 mg/kg | 300 mg |
| 3‐month regimen of weekly rifapentine + isoniazid | NA | I 900 mg/RF 900 mg |
Abbreviations: I, isoniazid; R, rifampicin; RF, rifapentin; NA, not applicable.
4. DISCUSSION
Idelalisib is an effective drug, having a role in treatment algorithms of CLL and FL.61, 62 A drug‐specific profile of adverse event was recently confirmed in 2 trials showing grade ≥3 diarrhea in 20% of the patients, pneumonia in 14%, Pneumocystis jirovecii pneumonia in 5%, sepsis in 6%,63 increased transaminase level in 21%, pneumonitis in 1.4%, with discontinuations because of AE or ADRs in 27% of the patients.64
Expert panel opinions has provided guidance on the management of ADRs,12, 24 and this position paper grew out of the realization that a multidisciplinary panel of experts in hematology, clinical pharmacology, pneumology, gastroenterology, and infectious disease is required to provide health care professionals with updated recommendations12, 24 allowing for the optimization of treatment with this PIK3δ inhibitor. In this analysis, the diagnostic workup including referrals to specialists before idelalisib treatment was described, and new measures for prevention and management of diarrhea/colitis, transaminitis, pneumonitis, and infections were formulated (Figures 1, 2, 3, 4, 5, 6, 7), along with the description of clinically meaningful pharmacologic interactions.
Drug‐drug interactions are frequently associated with ADRs and are predictable on the basis of the knowledge of metabolic pathways involved. Because the incidence of ADRs and hospitalization rate related to DDIs is still high,65 we proposed here some practical measures which may assist the treating physician in minimizing potentially dangerous interactions.
The lack of clinical studies on the efficacy of screening, of prophylaxis, and treatment of AE and ADRs forced the Panel to use the methods of consensus for shaping the recommendations of this work. The recommendations proposed here apply to all the patients with CLL and FL with a clear indication to start treatment with idelalisib and were prepared taking into consideration that adherence to treatment is very important to get the maximum benefit from continuous treatment and that switch to other mechanism‐based treatment may achieve disease control in the majority of patients with intolerance to ibrutinib or idelalisib.11
This and other reports providing guidance on how to optimize outcome with novel mechanism‐based treatment24, 66, 67 highlight that a number of issues with treatment‐emergent ADRs require further investigation, with special reference to the incidence with longer follow‐up and management in the real‐world setting.68
CONFLICT OF INTEREST
Funding of the project was provided from at‐arm's‐length contribution from Gilead (Italy). The funding source had no role in identifying statements, abstracting data, synthesizing results, or preparing the manuscript or in the decision to submit the manuscript for publication.
Supporting information
Table S1. Clinical key‐questions defined by the expert panel using the criterion of clinical relevance.
Cuneo A, Barosi G, Danesi R, et al. Management of adverse events associated with idelalisib treatment in chronic lymphocytic leukemia and follicular lymphoma: A multidisciplinary position paper. Hematological Oncology. 2019;37:3–14. 10.1002/hon.2540
REFERENCES
- 1. Wiestner A. Emerging role of kinase‐targeted strategies in chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2012;2012:88‐96. [DOI] [PubMed] [Google Scholar]
- 2. Lannutti BJ, Meadows SA, Herman SE, et al. CAL‐101, a p110delta selective phosphatidylinositol‐3‐kinase inhibitor for the treatment of B‐cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117(2):591‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3‐kinase‐δ inhibitor CAL‐101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116(12):2078‐2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Uno JK, Rao KN, Matsuoka K, et al. Altered macrophage function contributes to colitis in mice defective in the phosphoinositide‐3 kinase subunit p110d. Gastroenterology. 2010;139(5):1642‐1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3‐kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123(22):3390‐3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flinn IW, Kahl BS, Leonard JP, et al. Idelalisib, a selective inhibitor of phosphatidylinositol 3‐kinase‐δ, as therapy for previously treated indolent non‐Hodgkin lymphoma. Blood. 2014;123(22):3406‐3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370(11):1008‐1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gopal AK, Kahl BS, Flowers CR, et al. Idelalisib is effective in patients with high‐risk follicular lymphoma and early relapse after initial chemoimmunotherapy. Blood. 2017;129(22):3037‐3039. [DOI] [PubMed] [Google Scholar]
- 10. O'Brien SM, Lamanna N, Kipps TJ, et al. A phase 2 study of idelalisib plus rituximab in treatment‐naïve older patients with chronic lymphocytic leukemia. Blood. 2015;126(25):2686‐2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mato AR, Hill BT, Lamanna N, et al. Optimal sequencing of ibrutinib, idelalisib, and venetoclax in chronic lymphocytic leukemia: results from a multicenter study of 683 patients. Ann Oncol. 2017;28:1050‐1056. [DOI] [PubMed] [Google Scholar]
- 12. Coutré SE, Barrientos JC, Brown JR, et al. Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk Lymphoma. 2015;56(10):2779‐2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lampson BL, Kasar SN, Matos TR, et al. Idelalisib given front‐line for treatment of chronic lymphocytic leukemia causes frequent immune‐mediated hepatotoxicity. Blood. 2016;128(2):195‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. UK CLL Forum . Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: a UK and Ireland analysis of outcomes in 315 patients. Haematologica. 2016;101(12):1563‐1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown JR, Ghia P, Jones JA, et al. Outcomes of patients with relapsed and refractory chronic lymphocytic leukemia (CLL) who discontinue idelalisib treatment. J Clin Oncol. 2016;34(suppl 15). Abstract 7531 [Google Scholar]
- 16. William PL, Webb C. The Delphi technique: a methodological discussion. J Adv Nurs. 1994;19(1):180‐186. [DOI] [PubMed] [Google Scholar]
- 17. Ramanathan S, Jin F, Sharma S, Kearney BP. Clinical pharmacokinetic and pharmacodynamic profile of idelalisib. Clin Pharmacokinet. 2016;55(1):33‐45. [DOI] [PubMed] [Google Scholar]
- 18. Jin F, Robeson M, Zhou H, et al. Clinical drug interaction profile of idelalisib in healthy subjects. J Clin Pharmacol. 2015;55(8):909‐919. [DOI] [PubMed] [Google Scholar]
- 19. Jin F, Robeson M, Zhou H, Hisoire G, Ramanathan S. The pharmacokinetics and safety of idelalisib in subjects with severe renal impairment. Cancer Chemother Pharmacol. 2015;76(6):1133‐1141. [DOI] [PubMed] [Google Scholar]
- 20. Jin F, Robeson M, Zhou H, Hisoire G, Ramanathan S. The pharmacokinetics and safety of idelalisib in subjects with moderate or severe hepatic impairment. J Clin Pharmacol. 2015;55(8):944‐952. [DOI] [PubMed] [Google Scholar]
- 21. Muller PY, Milton MN. The determination and interpretation of the therapeutic index in drug development. Nat Rev Drug Discov. 2012;11(10):751‐761. [DOI] [PubMed] [Google Scholar]
- 22. Weidner AS, Panarelli NC, Geyer JT, et al. Idelalisib‐associated colitis: histologic findings in 14 patients. Am J Surg Pathol. 2015;39(12):1661‐1667. [DOI] [PubMed] [Google Scholar]
- 23. Sharman JP, Coutre SE, Furman RR, et al. Second interim analysis of a phase 3 study of idelalisib (ZYDELIG®) plus rituximab (R) for relapsed chronic lymphocytic leukemia (CLL): efficacy analysis in patient subpopulations with Del(17p) and other adverse prognostic factors. Blood. 2014;124. Abstract 330 [Google Scholar]
- 24. de Weerdt I, Koopmans SM, Kater AP, van Gelder M. Incidence and management of toxicity associated with ibrutinib and idelalisib: a practical approach. Haematologica. 2017;102(10):1629‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Louie CY, DiMaio MA, Matsukuma KE, Coutre SE, Berry GJ, Longacre TA. Idelalisib‐associated enterocolitis: clinicopathologic features and distinction from other enterocolitides. Am J Surg Pathol. 2015;39(12):1653‐1660. [DOI] [PubMed] [Google Scholar]
- 26. Yeung CCS, Hockenbery DM, Westerhoff M, et al. Pathology results of tissue biopsy during idelalisib‐associated diarrhea/colitis. Blood. 2016;128. Abstract 4391 [Google Scholar]
- 27. Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017;152(6):1297‐1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Torres HA, Hosry J, Mahale P, Economides MP, Jiang Y, Lok AS. Hepatitis C virus reactivation in patients receiving cancer treatment: a prospective observational study. Hepatology. 2018;67(1):36‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Persico M, Aglitti A, Caruso R, et al. Efficacy and safety of new direct antiviral agents in hepatitis C virus‐infected patients with diffuse large B‐cell non‐Hodgkin's lymphoma. Hepatology. 2018;67(1):48‐55. [DOI] [PubMed] [Google Scholar]
- 30. Lampson BL, Brown JR. PI3Kδ‐selective and PI3Kα/δ‐combinatorial inhibitors in clinical development for B‐cell non‐Hodgkin lymphoma. Expert Opin Investig Drugs. 2017;26(11):1267‐1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Holt MP, Ju C. Mechanisms of drug‐induced liver injury. AAPS J. 2006;8:48‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Flinn IW, O'Brien S, Kahl B, et al. Duvelisib, a novel oral dual inhibitor of PI3K‐δ,γ, is clinically active in advanced hematologic malignancies. Blood. 2018;131(8):877‐887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brown JR, Zelenetz AD, Furman RR, et al. Risk factors for grade 3/4 transaminase elevation in patients with chronic lymphocytic leukemia treated with idelalisib. Blood. 2017;130(suppl 1). Abstract 3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheah CY, Nastoupil LJ, Neelapu SS, Forbes SG, Oki Y, Fowler NH. Lenalidomide, idelalisib, and rituximab are unacceptably toxic in patients with relapsed/refractory indolent lymphoma. Blood. 2015;125(21):3357‐3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smith SM, Pitcher B, Jung SH, et al. Unexpected and serious toxicity observed with combined idelalisib, lenalidomide and rituximab in relapsed/refractory B cell lymphomas: alliance A051201 and A051202. Blood. 2014;124. Abstract 3091 [Google Scholar]
- 36. Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck‐Ytter YT, American Gastroenterological Association Institute . American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148(1):215‐219. [DOI] [PubMed] [Google Scholar]
- 37. Haustraete E, Obert J, Diab S, et al. Idelalisib‐related pneumonitis. Eur Respir J. 2016;47(4):1280‐1283. [DOI] [PubMed] [Google Scholar]
- 38. Kahl BS, Spurgeon SE, Furman RR, et al. A phase 1 study of the PI3Kdelta inhibitor idelalisib in patients with relapsed/refractory mantle cell lymphoma (MCL). Blood. 2014;123(22):3398‐3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gupta A, Hsiao CL. Idelalisib‐induced pneumonitis. BMJ Case Rep. 2016. bcr‐2016‐216343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Center for drug evaluation and research . Application number 205858Orig1s000; medical review (s): pag 65. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205858Orig1s000MedR.pdf. Accessed May 16, 2018.
- 41. Khunger M, Rakshit S, Pasupuleti V, et al. Incidence of pneumonitis with use of programmed death 1 and programmed death‐ligand 1 inhibitors in non‐small cell lung cancer: a systematic review and meta‐analysis of trials. Chest. 2017;152(2):271‐281. [DOI] [PubMed] [Google Scholar]
- 42. Bonniaud P, Georges M, Favrolt N, Camus P. Pneumopathies intrestitielles iatrogeniques. Rev Prat. 2014;64:951‐956. [PubMed] [Google Scholar]
- 43. Poletti V, Chilosi M, Olivieri D. Diagnostic invasive procedures in diffuse infiltrative lung diseases. Respiration. 2004;71(2):107‐119. [DOI] [PubMed] [Google Scholar]
- 44. Meyer KC, Raghu G, Baughman RP, et al. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. 2012;185(9):1004‐1014. [DOI] [PubMed] [Google Scholar]
- 45. Ravaglia C, Gurioli C, Casoni G, et al. Diagnostic role of rapid on‐site cytologic examination (ROSE) of broncho‐alveolar lavage in ALI/ARDS. Pathologica. 2012;104(2):65‐69. [PubMed] [Google Scholar]
- 46. Romagnoli M, Bigliazzi C, Casoni G, et al. The role of transbronchial lung biopsy for the diagnosis of diffuse drug‐induced lung disease: a case series of 44 patients. Sarcoidosis Vasc Diffuse Lung Dis. 2008;25:36‐45. [PubMed] [Google Scholar]
- 47. DesPrez K, McNeil JB, Wang C, Bastarache JA, Shaver CM, Ware LB. Oxygenation saturation index predicts clinical outcomes in ARDS. Chest. 2017;152(6):1151‐1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chiumello D, Brochard L, Marini JJ, et al. Respiratory support in patients with acute respiratory distress syndrome: an expert opinion. Crit Care. 2017;21(1):240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016;315(22):2435‐2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cheah CY, Fowler NH. Idelalisib in the management of lymphoma. Blood. 2016;128(3):331‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Greenwell IB, Ip A, Cohen JB. PI3K inhibitors: understanding toxicity mechanisms and management. Oncology (Williston Park). 2017;31:821‐828. [PubMed] [Google Scholar]
- 52. Raju S, Ghosh S, Mehta AC. Chest CT signs in pulmonary disease: a pictorial review. Chest. 2017;151(6):1356‐1374. [DOI] [PubMed] [Google Scholar]
- 53. Maertens J, Cesaro S, Maschmeyer G, et al. ECIL guidelines for preventing pneumocystis jirovecii pneumonia in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother. 2016;71(9):2397‐2404. [DOI] [PubMed] [Google Scholar]
- 54. Khodavaisy S, Mortaz E, Mohammadi F. Pneumocystis jirovecii colonization in chronic obstructive pulmonary disease (COPD). Curr Med Mycol. 2015;1:42‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dobler CC, Cheung K, Nguyen J, Martin A. Risk of tuberculosis in patients with solid cancers and haematological malignancies: a systematic review and meta‐analysis. Eur Respir J. 2017;50(2):1700157. [DOI] [PubMed] [Google Scholar]
- 56. O'Brien SM, Keating MJ, Mocarski ES. Updated guidelines on the management of cytomegalovirus reactivation in patients with chronic lymphocytic leukemia treated with alemtuzumab. Clin Lymphoma Myeloma. 2006;7(2):125‐130. [DOI] [PubMed] [Google Scholar]
- 57. Kotton CN, Kumar D, Caliendo AM, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid‐organ transplantation. Transplantation. 2013;96(4):333‐360. [DOI] [PubMed] [Google Scholar]
- 58. Cooley L, Dendle C, Wolf J, et al. Consensus guidelines for diagnosis, prophylaxis and management of pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern Med J. 2014;44(12b):1350‐1363. [DOI] [PubMed] [Google Scholar]
- 59. Cordonnier C, Alanio A, Cesaro S, et al. Pneumocystis jirovecii pneumonia: still a concern in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother. 2016;71(9):2379‐2385. [DOI] [PubMed] [Google Scholar]
- 60. Geneva: World Health Organization . Guidelines on the management of latent tuberculosis infection. 2015. [PubMed]
- 61. ESMO Guidelines Committee . Appendix 4: chronic lymphocytic leukaemia: eUpdate published online 27 June 2017 (www.esmo.org/Guidelines/Haematological‐Malignancies). Ann Oncol. 2017;28(suppl 4):iv149‐iv152. [DOI] [PubMed] [Google Scholar]
- 62. Kahl BS, Yang DT. Follicular lymphoma: evolving therapeutic strategies. Blood. 2016;127(17):2055‐2063. [DOI] [PubMed] [Google Scholar]
- 63. Jones JA, Robak T, Brown JR, et al. Efficacy and safety of idelalisib in combination with ofatumumab for previously treated chronic lymphocytic leukaemia: an open‐label, randomised phase 3 trial. Lancet Haematol. 2017;4(3):e114‐e126. [DOI] [PubMed] [Google Scholar]
- 64. Zelenetz AD, Barrientos JC, Brown JR, et al. Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: interim results from a phase 3, randomised, double‐blind, placebo‐controlled trial. Lancet Oncol. 2017;18(3):297‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dechanont S, Maphanta S, Butthum B, Kongkaew C. Hospital admissions/visits associated with drug‐drug interactions: a systematic review and meta‐analysis. Pharmacoepidemiol Drug Saf. 2014;23(5):489‐497. [DOI] [PubMed] [Google Scholar]
- 66. Gribben JG, Bosch F, Cymbalista F, et al. Optimising outcomes for patients with chronic lymphocytic leukaemia on ibrutinib therapy: European recommendations for clinical practice. Br J Haematol. 2018;180(5):666‐667. [DOI] [PubMed] [Google Scholar]
- 67. Boriani G, Corradini P, Cuneo A, et al. Practical management of ibrutinib in the real life: focus on atrial fibrillation and bleeding. Hematol Oncol. 2018. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 68. Compagno M, Wang Q, Pighi C, et al. Phosphatidylinositol 3‐kinase δ blockade increases genomic instability in B cells. Nature. 2017;542(7642):489‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical key‐questions defined by the expert panel using the criterion of clinical relevance.


