Abstract
Aim
The primary objective of this study was to compare blood glucose (BG) excursions between East Asian and Caucasian patients with type 2 diabetes mellitus (T2DM) who were injection‐naive, had inadequate glycemic control with oral antihyperglycemic medications, and who required initiation with injectable therapy.
Methods
This retrospective pooled analysis included individual patient data from completed clinical trials (Insulin lispro injection/dulaglutide development programs, first patient visit ≥1997). All included patients were ≥18 years, were East Asian or Caucasian, and had data for self‐monitored BG at baseline. The primary outcome, BG excursion at baseline (least‐squares mean, standard error), was compared between patient groups using an analysis of covariance with race as the fixed effect. Independent covariates included baseline body weight, baseline HbA1c, age, and duration of T2DM.
Results
Caucasian (n = 6779) and East Asian (n = 1638) patients from 21 trials were included. BG excursions were significantly higher for East Asian than Caucasian patients at breakfast (4.03 [0.075] vs 2.59 [0.045] mmol/L), lunch (3.37 [0.080] vs 1.43 [0.049] mmol/L), and dinner (3.16 [0.080] vs 1.74 [0.047] mmol/L) (P < 0.001 adjusted analyses). Similar findings were observed for the unadjusted analyses. At each time point, postprandial BG was significantly higher for East Asian than Caucasian patients (with adjusted and unadjusted analyses).
Conclusion
These findings suggest that BG excursion and postprandial BG are higher among East Asian patients with T2DM than Caucasian patients. In addition, these findings may help clinicians select appropriate treatments for East Asian patients with T2DM who require injection therapy.
Keywords: blood glucose excursion; diabetes mellitus, type 2; East Asian
1. INTRODUCTION
High glycemic variability is common among patients with type 2 diabetes mellitus (T2DM)1 and is a key contributor to complications associated with inadequate glycemic control.2, 3 The assessment of postprandial blood glucose (BG) for management of hyperglycemia in patients with T2DM is well recognized, and guidelines now recommend targeting both pre‐ and postprandial BG to maintain effective glycemic control.3 However, ethnic differences in the glycemic response and pathophysiology of T2DM may have implications for how T2DM is managed among different populations and for treatment outcomes.4, 5, 6, 7
The prevalence of T2DM in Asian populations has increased rapidly in recent years and is associated with high levels of morbidity and mortality.8 This increase is partly due to changes in lifestyles and diet, earlier onset of T2DM among these populations, and differences in insulin secretion and resistance compared with Caucasian populations.4, 5, 6, 7 In addition, evidence from studies in healthy subjects suggests that the carbohydrate‐rich traditional Asian diet contributes to hyperglycemia9 and that postprandial hyperglycemia in response to the same glycemic load is higher in Asians than in Caucasians.4 Consequently, studies conducted in Asian patients with T2DM have shown postprandial BG to be important for management of hyperglycemia in patients on oral antihyperglycemic medication (OAM)10 or insulin.11 Despite this, few studies have directly compared BG profiles between Asian and Caucasian patients with T2DM.12
Findings from a post hoc subgroup analysis of data from a large clinical trial (PARADIGM) of insulin‐naive patients with T2DM13 suggest that East Asian patients who have not started injectable therapy have higher postprandial BG excursions than Caucasian patients.12 However, this study was not designed to assess the possible effect of race or ethnicity on BG profiles before insulin initiation and statistical analysis of the differences in postprandial BG excursion between ethnic groups was not reported.
The primary objective of this study was to compare BG excursions between East Asian and Caucasian patients with T2DM who were injection‐naive, had inadequate glycemic control with OAMs, and who required initiation with injectable therapy. To address this objective, we conducted a retrospective pooled analysis of individual patient data from clinical trials including East Asian and/or Caucasian patients from two Eli Lilly sponsored programs. In addition, we conducted a systematic review of the peer‐reviewed literature with published summary data to identify studies reporting baseline BG or BG excursion data for patients who met the inclusion criteria.
2. MATERIALS AND METHODS
2.1. Pooled analysis
2.1.1. Eligibility criteria
In this retrospective post hoc analysis, individual patient data were pooled from completed clinical trials from the Eli Lilly insulin lispro injection (Humalog®) or dulaglutide (Trulicity™) development program. All randomized and nonrandomized clinical trials were eligible for inclusion if they met the following criteria: first patient visit was on or after 1997; enrolled male or female adult (≥18 years) patients with T2DM who were injection‐naive (or had completed a sufficient washout period per individual study requirements); had inadequate glycemic control with OAMs, and required initiation with injectable therapy; and had baseline data for self‐monitored blood glucose (SMBG). As only baseline data were included in the analyses, there were no restrictions on study interventions or treatment duration.
2.1.2. Baseline and analysis variables
The analyses included subpopulations of East Asian and Caucasian patients identified by race/ethnicity as reported in the case report forms. Patients were classified as East Asian if the reported race/ethnicity was East Asian or Asian, and where the patient was located in China, Japan, the Republic of Korea, Hong Kong, or Taiwan. Patients were classified as Caucasian if the reported race/ethnicity was White.
Data extracted from patient‐level records included baseline data for age (years), gender, body weight (kg), body mass index (kg/m2), duration of diabetes (years), glycated haemoglobin (HbA1c, %), and breakfast, lunch, dinner, and daily average preprandial BG, postprandial BG, and BG excursion based on finger stick SMBG (mmol/L) measurements. If BG excursions were not reported, these were derived from preprandial and postprandial BG measurements.
The primary outcome was BG excursion defined as the difference between preprandial and postprandial BG at breakfast. Secondary outcomes included BG excursion at lunch and dinner, and the daily average BG excursion.
2.1.3. Statistical analysis
The full analysis set included all eligible randomized patients or all eligible enrolled patients from nonrandomized studies. Mean BG excursion at baseline was compared between East Asian and Caucasian patients using an analysis of covariance. The model included race as the fixed effect and baseline body weight, baseline HbA1c, age, and duration of T2DM as covariates. There was no imputation for missing BG measurements. Data were reported as mean (standard deviation) or least‐squares mean (standard error of the mean). Statistical analyses were conducted using SAS® Version 9.4 (SAS Institute, Cary, NC, USA).
2.2. Systematic review
The methodology for the systematic review is reported in the Supplementary Appendix.
3. RESULTS
3.1. Pooled analysis of clinical trials
3.1.1. Study selection and patient population
Of the 37 clinical trials identified, 21 met the eligibility criteria (Table 1). A total of 6779 Caucasian patients and 1638 East Asian patients from the 21 trials met the criteria for inclusion and their data were pooled for the analyses. As all clinical trials were part of two Eli Lilly clinical development programs, the trials had similar procedures and eligibility criteria. All procedures for the clinical trials were conducted in accordance with the ethical standards at each site and the relevant Declaration of Helsinki at the time the studies were conducted. Informed consent was obtained from all trial participants.
Table 1.
Clinical studies included in the pooled analysis
| Eli Lilly Identifier CT.gov Number | Key Inclusion Criteria at Enrolment | Analysis Groups (N) Caucasian = 6779 East Asian = 1638 | Patient Characteristics at Baseline (Before Treatment with Study Drug), Mean (SD) | ||||
|---|---|---|---|---|---|---|---|
| HbA1c criteria | Previous treatment | Age (yr) | Body weight (kg) | HbA1c (%) | Diabetes Duration (yr) | ||
|
H9X‐JE‐GBDQ NCT01468181 |
7.0‐11.0% | Stable dose (≥8 wk) of SU, BG, TZD, α‐GI, or glinide monotherapy for ≥3 mo | EA: 394 | EA: 57.4 (10.96) | EA: 71.4 (13.26) | EA: 8.5 (1.11) | EA: 7.7 (6.29) |
|
H9X‐JE‐GBDY NCT01584232 |
7.0‐10.0% | Stable dose (≥8 wk) SU or BG | EA: 361 | EA: 56.8 (10.92) | EA: 71.0 (13.71) | EA: 8.0 (0.85) | EA: 8.8 (6.41) |
|
H9X‐MC‐GBCF NCT00734474 |
7.0‐9.5% or >8.0‐9.5% (diet & exercise) | Diet and exercise, met, OAM, or met + OAM |
C: 613 EA: 178 |
C: 56.2 (9.46) EA: 52.1 (10.31) |
C: 93.5 (16.05) EA: 74.0 (10.39) |
C: 8.1a (1.06) EA: 8.2 (1.12) |
C: 7.1 (5.13) EA: 6.9 (5.09) |
|
H9X‐MC‐GBCJ NCT00630825 |
>7.0‐10.5% | Any combination of 2 of: SU, BG, TZD, DPP‐IV inhibitors | C: 151 | C: 59.9 (10.83) | C: 98.9 (17.23) | C: 8.1a (0.88) | C: 8.7 (6.93) |
|
H9X‐MC‐GBDA NCT01064687 |
1 OAM: 7.0‐11.0% 2/3 OAM: 7.0‐10.0% |
≤3 OAMs | C: 728 | C: 56.7 (9.65) | C: 99.0 (18.82) | C: 8.1 (1.37) | C: 8.7 (5.40) |
|
H9X‐MC‐GBDB NCT01075282 |
1 OAM: 7.0‐11.0% 2/3 OAM: 7.0‐10.0% |
≤3 OAMs |
C: 571 EA: 43 |
C: 58.5 (9.08) EA: 53.9 (8.66) |
C: 91.4 (17.77) EA: 71.0 (10.96) |
C: 8.1 (0.96) EA: 8.6 (1.22) |
C: 9.5 (6.08) EA: 9.3 (6.12) |
|
H9X‐MC‐GBDE NCT01624259 |
7.0‐10.0% | Diet and exercise, stable dose (≥3 mo) met (≥1500 mg/day) | C: 515 | C: 57.3 (9.35) | C: 94.8 (18.73) | C: 8.0 (0.78) | C: 7.2 (5.48) |
|
H9X‐MC‐GBDG NCT01769378 |
7.0‐9.5% | Stable dose SU (≥50% max dose) for ≥3 mo | C: 250 | C: 58.4 (9.80) | C: 86.6 (17.06) | C: 8.4 (0.71) | C: 7.6 (4.94) |
|
H9X‐MC‐GBDN NCT01149421 |
7.0‐9.5% | ≥1 OAM for ≥1 mo | C: 608 | C: 57.0 (10.30) | C: 92.9 (19.75) | C: 7.9 (0.75) | C: 8.5 (5.93) |
|
F3Z‐CR‐IOPH NCT00548808 |
7.0‐11.0% | OAMs without insulin injection for ≥3 mo |
C: 144 EA: 103 |
C: 60.0 (8.84) EA: 56.2 (8.25) |
C: 82.0 (16.44) EA: 66.6 (10.70) |
C: 8.8 (1.03) EA: 8.7 (1.00) |
C: 11.7 (6.34) EA: 9.8a (4.69) |
|
F3Z‐CR‐IOQI NCT01773473 |
7.0‐11.0% | SU, BG, TZD, α‐GI, glinide, or DPP‐IV inhibitor monotherapy or combination |
C: 29 EA: 374 |
C: 54.0 (8.08) EA: 56.7 (9.98) |
C: 79.8 (11.50) EA: 68.6 (12.19) |
C: 9.0 (1.07) EA: 8.5 (1.09) |
C: 10.6 (6.90) EA: 9.4 (6.20) |
|
F3Z‐EW‐S020 NCT00664534 |
7.0‐11.0% | Met and ≥1 other OAM (SU or TZD) without insulin for ≥3 mo | C: 331 | C: 55.0 (8.64) | C: 84.0 (14.74) | C: 9.1a (1.35) | NR |
|
F3Z‐JE‐IOPU NCT00971997 |
7.5‐11.0% | OAMs (≥3 mo); without insulin for <6 mo | EA: 135 | EA: 60.3 (10.21) | EA: 66.6 (14.05) | EA: 8.3 (0.80) | EA: 11.4 (7.11) |
| F3Z‐MC‐IOHIb | HbA1c 1.2 X ULN | OAM (>6 mo) and SU (max dose) and met (500‐2550 mg) for ≥1 mo | C: 92 | C: 56.7 (8.25) | C: 82.9 (14.90) | C: 9.4 (1.45) | C: 10.0 (7.46) |
| F3Z‐MC‐IOHMb | HbA1c 1.2 X ULN | OAM (>6 mo) and SU (max dose) for ≥1 mo | C: 145 | C: 67.9 (4.88) | C: 78.0 (12.25) | C: 9.9a (1.40) | C: 11.9 (7.57) |
| F3Z‐MC‐IOMYb | HbA1c >125% X ULN within 4 weeks of study entry | Single OAM (met or second generation SU ≥3 mo) with the last ≥30 days at max dose | C: 531 | C: 59.0 (8.83) | C: 83.6 (15.12) | C: 9.1a (1.44) | C: 7.6 (5.48) |
|
F3Z‐MC‐IOND NCT00036504 |
None | OAMs without insulin (30 days) | C: 78 | C: 56.2 (9.73) | C: 94.6 (18.35) | C: 8.7a (1.19) | C: 8.8 (6.74) |
|
F3Z‐MC‐IOOX NCT00377858 |
7.5‐12.0% | OAM without insulin and ≥2 of: met 1500 mg/day, SU 1/2 max dose, TZD 30 mg/day pioglitazone or 4 mg/day rosiglitazone |
C: 293 EA: 50 |
C: 61.6 (9.59) EA: 56.1 (9.10) |
C: 83.9 (15.45) EA: 66.5 (10.42) |
C: 9.3 (1.17) EA: 8.8 (0.87) |
C: 12.1 (6.89) EA: 12.8 (10.05) |
| F3Z‐US‐IOMNb | ≥8% | OAM and 1700 mg/day met for ≥3 mo | C: 336 | C: 55.2 (10.03) | C: 97.4a (19.54) | C: 9.4a (1.48) | C: 9.0a (6.06) |
|
F3Z‐US‐IONWb
Jacober et al. Diab Metab Obes 2006;8:448‐455 |
HbA1c 1.2‐2.0 X ULN | ≥2 OAMs of different classes in combination for ≥2 mo | C: 45 | C: 56.2 (9.59) | C: 98.4 (18.39) | C: 9.3a (1.28) | C: 8.9 (5.02) |
|
F3Z‐US‐IOOV NCT00279201 |
HbA1c 1.2‐2.0 X ULN | ≥2 OAMs for ≥3 mo | C: 1319 | C: 58.8 (9.45) | C: 93.5 (19.88) | C: 8.9a (1.17) | C: 9.8 (6.06) |
Abbreviations: α‐GI, alpha‐glucosidase inhibitor; BG, biguanide; C, Caucasian; CT.gov, ClinicalTrials.gov; DPP‐IV, dipeptidyl peptidase IV; EA, East Asian; HbA1c, glycated haemoglobin; max, maximum; met, metformin; mo, month; NR, not reported; OAM, oral antihyperglycemic medication; SD, standard deviation; SU, sulfonylurea medication; TZD, thiazolidinedione; ULN, upper limit of normal; US, United States; yr, year; wk, week.
Baseline data are not available for all patients.
Clinical trial identifier not available as the trial was conducted prior to requirements for clinical trial registration.
All patients had T2DM, were injection‐naive, and had inadequate glycemic control with OAMs at enrollment according to the study inclusion criteria. For most studies, the inclusion criteria required patients to have HbA1c >7% at baseline (Table 1). Patients had been diagnosed with T2DM for an average of 8.9 years and had mean HbA1c levels >7% at baseline (Table 2). However, there were significant differences between the East Asian and Caucasian groups. Compared with the Caucasian group, there was a significantly higher proportion of men in the East Asian group, and patients were significantly younger, with significantly lower body weight, body mass index, and HbA1c levels (Table 2).
Table 2.
Baseline characteristics of patients included in the pooled analysis
| Variablea | Caucasian Patients (N = 6779) | East Asian Patients (N = 1638) | P Valueb |
|---|---|---|---|
| Mean age, yr | 57.9 (9.64) | 56.6 (10.49) | <0.001 |
| Male, n (%) | 3687 (54.4) | 1024 (62.5) | <0.001 |
| Body weight, kg | 91.6 (18.69) | 70.1 (12.79) | <0.001 |
| BMI, kg/m2 | 32.2 (5.33) | 26.0 (3.63) | <0.001 |
| Duration of T2DM, yr | 8.9 (6.05) | 8.9 (6.44) | 0.885 |
| HbA1c, % | 8.6 (1.26) | 8.4 (1.04) | <0.001 |
Abbreviations: BMI, body mass index; HbA1c, glycated haemoglobin; T2DM, type 2 diabetes mellitus; yr, year.
All data are mean (standard deviation) unless otherwise noted.
Fisher exact test for categorical measures, ANOVA model (response = race) for continuous measures.
3.1.2. Blood glucose
BG excursions were highest after breakfast in both populations when adjusted for baseline body weight, baseline HbA1c, age, and duration of T2DM, and were significantly higher for East Asian patients than for Caucasian patients (Figure 1, Table 3). In addition, BG excursions were significantly higher for East Asian patients than Caucasian patients at lunch, and dinner, and for the daily average (Figure 1). Similar findings were observed for the unadjusted analyses (Table 3).
Figure 1.
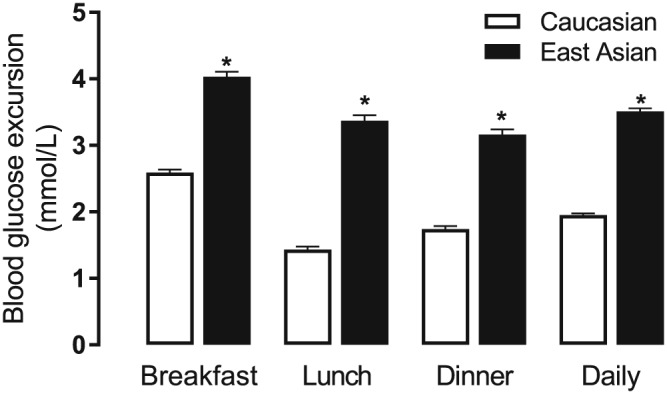
Pooled analysis of blood glucose excursion for East Asian and Caucasian injection‐naive patients with inadequate glycemic control after oral antihyperglycemic medication. Data are reported as the adjusted least‐squares mean difference between postprandial and preprandial blood glucose at each time point. Error bars denote standard error of the mean. * Blood glucose excursions between East Asians and Caucasians were significantly different at each time point (P < 0.001, adjusted ANCOVA)
Table 3.
Pooled analysis of blood glucose profiles and excursion for East Asian and Caucasian injection‐naive patients with inadequate glycemic control after oral antihyperglycemic medication
| Time Point | Unadjusted Preprandial BGa, mmol/L | Adjusted Preprandial BGb, mmol/L | Unadjusted Postprandial BGa, mmol/L | Adjusted Postprandial BGb, mmol/L | Unadjusted BG Excursiona, mmol/L | Adjusted BG Excursionb, mmol/L | |||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) | LS mean (SE) | N | Mean (SD) | LS mean (SE) | N | Mean (SD) | LS mean (SE) | |
| Morning | |||||||||
| Caucasian | 3791 | 9.92 (2.806) | 9.76 (0.035) | 3733 | 12.49 (3.689) | 12.32 (0.051) | 3728 | 2.60 (2.495) | 2.59 (0.045) |
| East Asian | 1430 | 9.20 (2.228) | 9.61 (0.060) | 1432 | 13.22 (3.390) | 13.63 (0.085) | 1430 | 4.02 (2.757) | 4.03 (0.075) |
| P valuec | <0.001 | 0.048 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| Midday | |||||||||
| Caucasian | 3524 | 9.95 (3.435) | 9.82 (0.047) | 3490 | 11.36 (3.480) | 11.23 (0.051) | 3473 | 1.44 (2.449) | 1.43 (0.049) |
| East Asian | 1426 | 9.48 (3.059) | 9.82 (0.078) | 1425 | 12.97 (3.392) | 13.19 (0.082) | 1425 | 3.48 (3.006) | 3.37 (0.080) |
| P valuec | <0.001 | 0.960 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| Evening | |||||||||
| Caucasian | 3766 | 10.02 (3.345) | 9.88 (0.046) | 3742 | 11.77 (3.501) | 11.60 (0.049) | 3729 | 1.78 (2.408) | 1.74 (0.047) |
| East Asian | 1426 | 9.53 (3.150) | 9.85 (0.078) | 1419 | 12.65 (3.493) | 12.97 (0.083) | 1417 | 3.14 (3.112) | 3.16 (0.080) |
| P valuec | <0.001 | 0.767 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| Daily | |||||||||
| Caucasian | 3747 | 9.98 (2.877) | 9.82 (0.034) | 3699 | 11.90 (3.220) | 11.74 (0.040) | 3684 | 1.96 (1.524) | 1.95 (0.028) |
| East Asian | 1427 | 9.41 (2.431) | 9.80 (0.058) | 1425 | 12.95 (2.923) | 13.28 (0.068) | 1424 | 3.55 (1.791) | 3.51 (0.048) |
| P valuec | <0.001 | 0.727 | <0.001 | <0.001 | <0.001 | <0.001 |
Abbreviations: BG, blood glucose; HbA1c, glycated haemoglobin; LS least‐squares; SD, standard deviation; SE, standard error of the mean.
ANCOVA with race as a fixed effect.
Adjusted for baseline body weight, age, duration of type 2 diabetes mellitus, and baseline HbA1c.
Differences between East Asian and Caucasian groups.
BG profiles were significantly different between East Asian and Caucasian patients (Figure 2, Table 3). In the unadjusted analysis, preprandial BG was significantly lower and postprandial BG was significantly higher for East Asian patients than for Caucasian patients at all time points (Figure 2A). The differences in preprandial BG at lunch, dinner, and for the daily preprandial average between East Asian and Caucasian patients were not significant when the analyses were adjusted for baseline body weight, baseline HbA1c, age, and duration of T2DM. However, differences between East Asian and Caucasian patients in all postprandial BG levels remained significant after adjustment for these factors (Figure 2B, Table 3).
Figure 2.
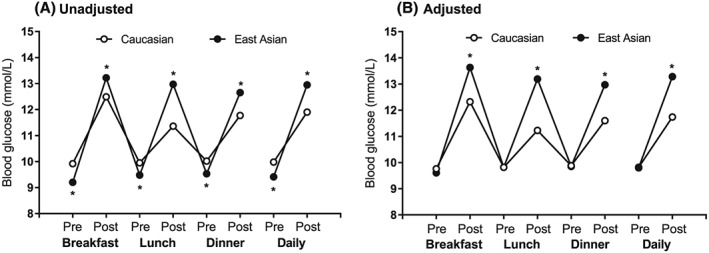
Pooled analysis of blood glucose profiles for East Asian and Caucasian injection‐naive patients with inadequate glycemic control after oral antihyperglycemic medication. A, Unadjusted mean preprandial and postprandial blood glucose at each time point. B, Adjusted least‐squares mean preprandial and postprandial blood glucose at each time point. Error bars are not visible as they are within the symbols for each data point. Unadjusted preprandial and postprandial blood glucose and adjusted postprandial blood glucose were significantly different between Caucasians and East Asians at all time points (*P < 0.001, ANCOVA); adjusted prebreakfast blood glucose was significantly higher in Caucasians than in East Asians (P = 0.048)
3.2. Systematic review of the literature
Five publications met the eligibility criteria for the systematic review; four studies reported findings in Caucasian populations14, 15, 16, 17 and one study12 reported findings separately for Caucasian and/or East Asian populations. There was a tendency for higher BG excursions at breakfast, lunch, and dinner in East Asian patients than in Caucasian patients (Supplementary Table S1). However, there was a high level of variability in BG excursions within each study and between each of the study populations. Meta analyses were not conducted because there were insufficient data to compare East Asian with Caucasian populations. Details of the search results are reported in the Supplementary Appendix.
4. DISCUSSION
This study is the first to compare BG profiles of injection‐naive East Asian patients and Caucasian patients with T2DM and an inadequate glycemic response to OAMs using a pooled analysis of patient‐level data. Overall, this large, retrospective pooled analysis of 8417 injection‐naive patients with T2DM demonstrated higher postprandial BG levels and greater BG excursions in East Asian patients compared with Caucasian patients following breakfast, lunch, and dinner. These findings were evident even after adjusting for patient body weight, baseline HbA1c, age, and duration of T2DM. Evidence from the peer‐reviewed literature was limited and, of the five studies retrieved, only one study reported BG profiles of East Asian patients.12 However, findings from the literature were generally supportive of the pooled analysis. Overall, this study supports evidence from studies conducted in Taiwan and Japan10, 11 and a post hoc subgroup analysis of patients from China and the Republic of Korea12 that postprandial BG is an important treatment target among East Asian patients with T2DM.
Given the complex pathophysiology of T2DM, the reasons for the difference in BG profiles between East Asian and Caucasian populations are likely to be multifactorial and related to both lifestyle and diet, and to ethnic differences in insulin resistance and the capacity to secrete insulin.7 Asian populations have higher glycemia in response to the same carbohydrate load4 and higher daily intake of carbohydrate compared with Caucasian populations.9, 10 Greater insulin resistance is observed in Asian populations, possibly because of higher levels of visceral fat per body weight or waist circumference compared with Caucasian populations.5, 18 Also the capacity to secrete insulin appears to have a more predominant role in T2DM among Asian populations compared with Caucasians, particularly in those who are of lean body weight.7, 19, 20, 21 In this study, East Asian patients were of lower body weight than their Caucasian counterparts. However, the differences in BG profiles remained statistically significant after adjustment for body weight (as well as baseline HbA1c, age, and duration of T2DM) in the analyses.
Differences in BG profiles among patients with T2DM of different races and ethnicities may have implications for how T2DM is managed and for treatment outcomes. Patients in Asia have earlier onset and longer duration of T2DM and are at a higher risk of microvascular complications, particularly renal disease, compared with Caucasians.5, 22, 23 Analysis of BG profiles among Caucasian patients with T2DM who were insulin‐naive has shown that the contribution of postprandial BG to excess hyperglycemia decreases relative to fasting BG in poorly controlled patients.24 In contrast, several studies have shown that targeting postprandial BG is as important as targeting preprandial BG for management of hyperglycemia among East Asian patients who are poorly controlled on OAMs, before receiving injectable treatment10 and among those on insulin.10, 11 Analysis of Asian patients with T2DM who were insulin‐naive has shown that the contribution of postprandial BG to 4‐hour excess hyperglycemia after meals and 24‐hour excess hyperglycemia is similar to the contribution of preprandial BG/fasting BG, respectively, in poorly controlled patients (HbA1c >7%).10 In addition, findings from a retrospective post hoc analysis of patients with T2DM of different ethnicities showed that Asian patients had a greater need for, and higher doses of, mealtime insulin than non‐Asian patients on basal insulin glargine plus prandial insulin lispro.12 Together with the current study, these findings suggest that postprandial BG is an important treatment target in East Asian populations and suggests that East Asian patients may have a greater and earlier need of therapies. Indeed, assessment of treatment patterns from noninterventional studies has shown that use of premixed insulins in Asian populations is widespread, with approximately one‐third of Japanese patients commencing insulin with a premixed insulin25 and approximately two‐thirds of Chinese patients on OAMs using a premixed insulin.26
The main strength of this study was the large number of patients available for analysis from multiple clinical studies with similar eligibility criteria and procedures. Significant differences in BG profiles and excursions between the East Asian and Caucasian groups were observed in the unadjusted analyses and after analyses were adjusted to take into account differences in baseline characteristics between the groups. However, interpretation of the findings should take into account the retrospective nature of the analyses and that the included studies were not designed to compare BG profiles of patients from different ethnicities. As this was a post‐hoc pooled analysis, our intention was to show whether there were differences in BG profiles between the two ethnic groups. We did not match the baseline characteristics and demographics between East Asian and Caucasian patients. Therefore, it was impossible to explore the reasons for the differences observed in this study. Although the included clinical studies were very similar in design, the interpretation of the findings should take into account that data were pooled data from two clinical trial programs that were conducted at different times, and that included patients with various OAM treatment regimens, and patients from different Caucasian populations. The systematic review of the literature confirmed the rationale for conducting a pooled analysis and showed that there is limited information in the peer‐reviewed literature on postprandial BG before commencing injectable treatment. Moreover, there was a high degree of variability in the data available from the peer‐reviewed literature; only one study included data on BG excursion at baseline and only one study reported on BG excursions in East Asian patients.
In conclusion, findings from this retrospective pooled analysis of individual patient data showed significantly higher postprandial BG excursions in East Asian patients with T2DM who were injection‐naive and had inadequate glycemic control with OAMs compared with Caucasian patients. These findings have clinical implications for the effect of ethnicity on the BG profiles in patients with T2DM and suggest that there should be greater emphasis on the control of BG excursions in East Asian patients. In addition, these findings may help clinicians select appropriate treatments for East Asian patients with T2DM who require injection therapy.
CONFLICT OF INTEREST
J.N.H. is an employee of Eli Lilly and Company. P.F.L. was an employee of Eli Lilly and Company at the time of manuscript preparation. L.N.J. has received consulting and lecture fees from Eli Lilly and Company, Bristol‐Myers Squibb, Novartis, Novo Nordisk, Merck, Bayer, Takeda, Sanofi, Roche and Boehringer Ingelheim, and has received research grants from Roche and Sanofi. X.M.Z. has no conflicts of interest to declare.
FUNDING SUPPORT
This study was sponsored by Eli Lilly and Company, manufacturer/licensee of several injectable insulins and GLP‐1 agonists. Medical writing assistance was provided by Serina Stretton, PhD, CMPP and Rebecca Lew, PhD, CMPP of ProScribe—Envision Pharma Group, and was funded by Eli Lilly and Company. ProScribe's services complied with international guidelines for Good Publication Practice (GPP3).
ROLE OF THE SPONSOR
Eli Lilly and Company was involved in the study design, data collection, data analysis, and preparation of the manuscript.
ROLE OF CONTRIBUTORS
All authors participated in the drafting, critical revision, and approval of the final version of the manuscript. All authors participated in the interpretation of the study results. X.M.Z., J.N.H., and L.N.J were involved in the conception of the study and the study design, and P.F.L. was involved in data collection and conducted the statistical analyses.
Supporting information
Table S1 Eligible clinical studies retrieved from the systematic review of the literature
Zhang XM, Li PF, Hou JN, Ji LN. Blood glucose profiles in East Asian and Caucasian injection‐naive patients with type 2 diabetes inadequately controlled on oral medication: a pooled analysis. Diabetes Metab Res Rev. 2018;34:e3062 10.1002/dmrr.3062
Contributor Information
Jia Ning Hou, Email: hou_jia_ning@lilly.com.
Li Nong Ji, Email: jiln@bjmu.edu.cn.
REFERENCES
- 1. Bonora E, Corrao G, Bagnardi V, et al. Prevalence and correlates of post‐prandial hyperglycaemia in a large sample of patients with type 2 diabetes mellitus. Diabetologia. 2006;49(5):846‐854. [DOI] [PubMed] [Google Scholar]
- 2. Kovatchev BP. Metrics for glycaemic control—from HbA1c to continuous glucose monitoring. Nat Rev Endocrinol. 2017;13(7):425‐436. [DOI] [PubMed] [Google Scholar]
- 3. International Diabetes Foundation . 2011. Guideline for Management of PostMeal Glucose in Diabetes. https://www.idf.org/our‐activities/advocacy‐awareness/resources‐and‐tools/82:management‐of‐postmeal‐glucose.html. Accessed Sep 21, 2017.
- 4. Dickinson S, Colagiuri S, Faramus E, Petocz P, Brand‐Miller JC. Postprandial hyperglycemia and insulin sensitivity differ among lean young adults of different ethnicities. J Nutr. 2002;132(9):2574‐2579. [DOI] [PubMed] [Google Scholar]
- 5. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301(20):2129‐2140. [DOI] [PubMed] [Google Scholar]
- 6. Ma RC, Chan JC. Type 2 diabetes in east Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281(1):64‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hulman A, Simmons RK, Brunner EJ, et al. Trajectories of glycaemia, insulin sensitivity and insulin secretion in South Asian and white individuals before diagnosis of type 2 diabetes: a longitudinal analysis from the Whitehall II cohort study. Diabetologia. 2017;60(7):1252‐1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet. 2010;375(9712):408‐418. [DOI] [PubMed] [Google Scholar]
- 9. Zuniga YL, Rebello SA, Oi PL, et al. Rice and noodle consumption is associated with insulin resistance and hyperglycaemia in an Asian population. Br J Nutr. 2014;111(06):1118‐1128. [DOI] [PubMed] [Google Scholar]
- 10. Wang JS, Tu ST, Lee IT, et al. Contribution of postprandial glucose to excess hyperglycaemia in Asian type 2 diabetic patients using continuous glucose monitoring. Diabetes Metab Res Rev. 2011;27(1):79‐84. [DOI] [PubMed] [Google Scholar]
- 11. Shimizu H, Uehara Y, Okada S, et al. Contribution of fasting and postprandial hyperglycemia to hemoglobin A1c in insulin‐treated Japanese diabetic patients. Endocr J. 2008;55(4):753‐756. [DOI] [PubMed] [Google Scholar]
- 12. Ji L, Min KW, Oliveira J, Lew T, Duan R. Comparison of efficacy and safety of two starting insulin regimens in non‐Asian, Asian Indian, and East Asian patients with type 2 diabetes: a post hoc analysis of the PARADIGM study. Diabetes Metab Syndr Obes. 2016;9:243‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bowering K, Reed VA, Felicio JS, Landry J, Ji L, Oliveira J. A study comparing insulin lispro mix 25 with glargine plus lispro therapy in patients with type 2 diabetes who have inadequate glycaemic control on oral anti‐hyperglycaemic medication: results of the PARADIGM study. Diabet Med. 2012;29(9):e263‐e272. [DOI] [PubMed] [Google Scholar]
- 14. Lalic NM, Micic D, Antic S, et al. Effect of biphasic insulin aspart on glucose and lipid control in patients with type 2 diabetes mellitus. Expert Opin Pharmacother. 2007;8(17):2895‐2901. [DOI] [PubMed] [Google Scholar]
- 15. Kapitza C, Forst T, Coester HV, Poitiers F, Ruus P, Hincelin‐Méry A. Pharmacodynamic characteristics of lixisenatide once daily versus liraglutide once daily in patients with type 2 diabetes insufficiently controlled on metformin. Diabetes Obes Metab. 2013;15(7):642‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kann PH, Wascher T, Zackova V, et al. Starting insulin therapy in type 2 diabetes: twice‐daily biphasic insulin Aspart 30 plus metformin versus once‐daily insulin glargine plus glimepiride. Exp Clin Endocrinol Diabetes. 2006;114(09):527‐532. [DOI] [PubMed] [Google Scholar]
- 17. Franek E, Haluzik M, Canecki Varzic S, et al. Twice‐daily insulin degludec/insulin aspart provides superior fasting plasma glucose control and a reduced rate of hypoglycaemia compared with biphasic insulin aspart 30 in insulin‐naive adults with type 2 diabetes. Diabet Med. 2016;33(4):497‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kadowaki T, Sekikawa A, Murata K, et al. Japanese men have larger areas of visceral adipose tissue than Caucasian men in the same levels of waist circumference in a population‐based study. Int J Obes (Lond). 2006;30(7):1163‐1165. [DOI] [PubMed] [Google Scholar]
- 19. Staimez LR, Weber MB, Ranjani H, et al. Evidence of reduced beta‐cell function in Asian Indians with mild dysglycemia. Diabetes Care. 2013;36(9):2772‐2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fukushima M, Usami M, Ikeda M, et al. Insulin secretion and insulin sensitivity at different stages of glucose tolerance: a cross‐sectional study of Japanese type 2 diabetes. Metabolism. 2004;53(7):831‐835. [DOI] [PubMed] [Google Scholar]
- 21. Chan WB, Tong PC, Chow CC, et al. The associations of body mass index, C‐peptide and metabolic status in Chinese type 2 diabetic patients. Diabet Med. 2004;21(4):349‐353. [DOI] [PubMed] [Google Scholar]
- 22. Li J, Dong Y, Wu T, Tong N. Differences between western and Asian type 2 diabetes patients in the incidence of vascular complications and mortality: a systematic review of randomized controlled trials on lowering blood glucose. J Diabetes. 2016;8(6):824‐833. [DOI] [PubMed] [Google Scholar]
- 23. Lanting LC, Joung IM, Mackenbach JP, et al. Ethnic differences in mortality, end‐stage complications, and quality of care among diabetic patients: a review. Diabetes Care. 2005;28(9):2280‐2288. [DOI] [PubMed] [Google Scholar]
- 24. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26(3):881‐885. [DOI] [PubMed] [Google Scholar]
- 25. Freemantle N, Balkau B, Danchin N, et al. Factors influencing initial choice of insulin therapy in a large international non‐interventional study of people with type 2 diabetes. Diabetes Obes Metab. 2012;14(10):901‐909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ji LN, Lu JM, Guo XH, et al. Glycemic control among patients in China with type 2 diabetes mellitus receiving oral drugs or injectables. BMC Public Health. 2013;13(1):602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Eligible clinical studies retrieved from the systematic review of the literature


