Abstract
BTI320 is a proprietary fractionated mannan polysaccharide being studied for attenuation of postprandial glucose excursion. The apparent blood glucose‐lowering effect of this compound is effective in lowering postprandial hyperinsulinemia, participating in the metabolic regulation of other lipid molecules; the consequence of this activity is yet to be validated with BTI320 with respect to the risk of cardiovascular disease. The primary objective of the study was to determine the postprandial glucose and insulin responses to 3 test meals containing rice alone or consumed with BTI320 (study A) or 3 test meals (SpriteTM) alone or consumed with BTI320 (study B). Twenty overweight but otherwise healthy volunteers, 4 female and 6 male (mean age 29 years, BMI 27–28 kg/m2) in study A and 6 female and 4 male (mean age 32 years, BMI 25‐32 kg/m2) in study B participated in the BTI320 evaluations. Standardized postprandial response methodology was utilized. In study A the addition of 6‐ and 12‐g BTI320 tablets reduced postprandial glucose responses to white rice by 19% and 32% and reduced postprandial insulin responses by 16% and 24%, respectively (P ≤ .05). In study B 2.6 and 5.2 g BTI320 reduced the glycemic index by 10% and 14%, respectively, and led to 14% and 18% decreases in the insulinemic index of the soft drink (P ≤ .05). These 2 studies demonstrated that the consumption of BTI320 before carbohydrate food or sugary beverage significantly reduced postprandial glucose levels and insulin responses to that meal or beverage in a dose‐dependent manner.
Keywords: BTI320, obesity, glycemia, insulinemia, postprandial
More than 2 decades of research has confirmed that the effect of food on blood glucose levels cannot be accurately predicted on the basis of the type and amount of carbohydrates. The rate at which carbohydrate is digested and released into the bloodstream is influenced by many food factors, such as its physical form, its fat, protein, and fiber content, and the chemical structure of its carbohydrate.1, 2 For these reasons, Jenkins and colleagues at the University of Toronto developed the glycemic index (GI) in order to rank equal carbohydrate portions of different foods according to the extent to which they increase blood glucose levels after being ingested.3 The GI is expressed as the percentage increase in blood glucose produced by a specific amount (50 g) of available carbohydrates in a test food compared to the same amount of available carbohydrate from a reference food such as glucose. Similarly, the insulin response to foods varies with the composition of the food, and it may or may not be proportional to the glucose response.4, 5
BTI320 (also known as Sugardown® or PAZ320) is an over‐the‐counter chewable dietary supplement that supports healthy blood sugar by predominately suppressing postprandial glucose excursion, slowing down the rate of glucose excursion, as well as reducing the absolute amount absorbed, thereby preventing hyperglycemia without the risk of hypoglycemia.6, 7 Nuclear magnetic resonance spectroscopy showed that BTI320 consists of 2 types of galactomannan: galactomannan‐α and ‐β in a 1:4 ratio with other constituents including sorbitol. This combination of BTI320 is identified with an inhibitory activity on carbohydrate‐hydrolyzing enzymes and is able to reduce postprandial blood glucose level by reducing available blood glucose for the intestine to absorb.
Only a few studies have been published to date that show measured postprandial blood glucose together with insulin responses.8, 9, 10, 11, 12, 13 We present data from 2 studies describing the postprandial glucose and insulin responses following test meals containing 50 g carbohydrates, alone and with the investigative compound BTI320 (Boston Therapeutics, Inc, Lawrence, MA). Although these 2 studies have not been pooled because of the differences in doses of BTI320 and the index foods, the results from both studies are similar, demonstrating that BTI320 attenuates the postprandial glucose excursions of high‐carbohydrate meals in obese, otherwise healthy subjects.
Methods
Study Subjects
Both experimental protocols were approved by the University of Sydney Human Research Ethics Committee. The studies were conducted at the University of Sydney, Australia. All eligible subjects signed an informed consent form before beginning any study‐related procedures.
Two single‐center, open‐label, prospective, crossover studies were conducted, each with 10 overweight but otherwise healthy volunteers (ClinicalTrials.gov Identifier NCT03375398 and NCT03374501). These studies evaluated the postprandial glucose and insulin responses of test meals containing 50 g of available carbohydrates consumed alone or with BTI320. In study A, 3 and 6 chewable BTI320 tablets were equivalent to 6 and 12 g of mannan polysaccharide, respectively. In study B, 2 and 4 BTI320 tablets were equivalent to 2.6 and 5.2 g of mannan polysaccharide, respectively.
Eligibility criteria included: age 25–65 years, nonsmokers, body mass index (BMI) > 25 kg/m2, normal dietary habits, low to moderate physical activity, ability to fast for ≥10 hours before each test session, ability to refrain from eating a legume‐based evening meal or drinking alcohol the day before each test session, ability to consume each test meal within 12 minutes, not taking medications likely to interfere with absorption and metabolism, and ability to provide informed consent. Subjects were excluded from the study if they followed a restrictive diet such as a low‐caloric, low‐carbohydrate, or vegan diet. Other exclusion criteria were physical or mental illness, food allergy or food intolerance, regular use of prescriptive medication (other than contraceptive medication), pregnancy or lactation (women), participation in another clinical trial, and use of general anesthesia in the mouth. Eligibility criteria were the same in both studies except that the age (18–65 years) and BMI (>25 kg/m2 for white subjects and >23 kg/m2 for Asian subjects) criteria were different in study B.
In the first study (study A), subjects were given rice‐based test meals served in fixed portions containing 50 g of available carbohydrates, alone or with BTI320 (6 g or 12 g). In the second study (study B), subjects were given test meals consisting of SpriteTM soft drink served in fixed portions containing 50 g of available carbohydrates, alone or with BTI320 (2.6 g or 5.2 g). Glycemic and insulinemic responses were compared to a reference food (glucose solution, GI value = 100) that was prepared by dissolving 51.4 g glucose in 250 mL warm water (Glucodin® powder, Boots Health Care Company, North Ryde, NSW, Australia). Safety assessments, including clinical laboratory results, vital sign measurements, physical examinations, and adverse events, were collected throughout the study period until follow‐up.
Study Test Meals
Each rice‐based test meal was served to a subject in a fixed portion containing 50 g of available carbohydrates. All 3 test meals consisted of the same portion of cooked Jasmine rice (Sun Rice® Jasmine Fragrant Rice, Ricegrowers Limited, NSW, Australia) served with 250 mL water. Two of the test meals also included the consumption of either 6 g or 12 g BTI320 tablets before the test meal. The macronutrient contents of each test meal were 4.6 g protein, 0.3 g fat, 0.4 g fiber, and 932 kJ energy. The primary ingredients of each BTI320 tablet were 2 g mannan polysaccharide and 1.5 g sorbitol.
Similarly, each soft drink‐based test meal was served to a subject in a fixed portion containing 50 g of available carbohydrates. All 3 test meals consisted of the same portion of SpriteTM soft drink (The Coca‐Cola Company, Australia). Two of the test meals also included the consumption of either 2.6 g or 5.2 g BTI320 tablets. The reference food (glucose solution) was also given to study participants. The macronutrient contents of each test meal were 50 g sugar and 876 kJ energy; the reference food was 50 g sugar and 800 kJ energy. The primary ingredients of each BTI320 tablet were 1.3 g mannan polysaccharide and 2.3 g sorbitol.
Procedures
Following the screening period and compliance with the inclusion and exclusion criteria, for each test meal session, subjects reported to the Research Center after fasting for 10 hours and consumed equal‐carbohydrate portions of test foods containing 50 g of available carbohydrate. Finger‐prick blood samples were obtained at 10 minutes (–10) and immediately (0) before ingestion and at 15, 30, 45, 60, 90, and 120 minutes after the meals.
In both studies the meal and test compound were assessed on duplicate occasions. In study A the 3 rice test meals were consumed on 2 separate occasions; thus, each subject completed 6 separate test sessions. The 6 test meals were randomly presented to the subjects. For example, test meals were administered to subject 1 in the following treatment periods: (1) rice, (2) rice + 6 g BTI320, (3) rice + 12 g BTI320, (4) rice, (5) rice + 6 g BTI320, and (6) rice + 12 g BTI320. In study B the reference food and the 3 soft drink test meals were each consumed by the 10 subjects on 2 separate occasions; therefore, each subject completed 8 separate test sessions. The reference food was consumed at the first and last test sessions, and the 6 soft drink sessions were conducted in random order. For example, test meals were administered to subject S1194 in the following treatment periods: (1) glucose alone (reference), (2) Sprite™ and 5.2 g BTI320, (3) Sprite™ alone, (4) Sprite™ alone, (5) Sprite™ and 2.6 g BTI320, (6) Sprite™ and 2.6 g BTI320, (7) Sprite™ and 5.2 g BTI320, and (8) glucose alone (reference). In all cases in both studies, BTI320 was administered at least 10 minutes before the ingestion of rice or Sprite™.
Determination and Measurement of Plasma Glucose and Insulin Concentrations
Each blood sample was collected into a 1.5‐mL plastic microcentrifuge tube containing 10 IU of the anticoagulant heparin sodium salt (Grade II, Sigma Chemical Company, Castle Hill, NSW, Australia). Immediately after collection, the blood sample was mixed with the anticoagulant by gently inverting the tube and then centrifuged at 12,500g for 0.5–1 minute at room temperature. The plasma was immediately transferred into a labeled, uncoated plastic microcentrifuge tube and then stored at –20°C until analyzed.
All blood samples collected from each individual subject throughout the entire study were analyzed within the same assay run using internal controls (CFAS, Precinorm S, and Precinorm U, Boehringer Mannheim, Australia). Plasma glucose concentrations were measured in duplicate using a Roche/Hitachi 912® automatic spectrophotometric centrifugal analyzer (Boehringer Mannheim GmbH, Mannheim, Germany) employing the glucose hexokinase/glucose‐6‐phosphate dehydrogenase enzymatic assay (Boehringer Mannheim Australia, Castle Hill, NSW, Australia). Plasma insulin concentrations were measured using a solid‐phase antibody‐coated tube radioimmunoassay kit (Diagnostic Products Corporation, Los Angeles, California). The final insulin concentration of each plasma sample was calculated by converting the radioactive counts observed, using a calibration curve created by standards of known insulin concentrations.
Statistical Analyses
The average value of the 2 duplicate plasma glucose concentrations recorded for each blood sample was used as the mean blood glucose concentration for each of the 8 time points of each 2‐hour test session. For each subject, the incremental area under the 120‐minute plasma glucose response curve (AUCGLUCOSE) for each test meal was calculated using the trapezoidal rule with the baseline, fasting value truncated at 0.14
The baseline value was the average concentration of the 2 fasting blood samples (–10 and 0 minutes). The AUCGLUCOSE measured the blood glucose response after consumption of a test meal relative to the blood glucose levels produced by an equivalent amount (50 g) of reference food. The AUCGLUCOSE was calculated by the area beneath the curve above the fasting level only; thus, any negative area below the fasting level was not considered in the computations. The AUC insulin response (AUCINSULIN) curve for each subject's test meal was calculated using the same method.
For each subject in study B, in addition to the postprandial AUCGLUCOSE and AUCINSULIN values, the GI and insulinemic index (II) were determined. The GI is a value assigned to food that indicates the effect of food on blood glucose levels.3 The II quantifies the postprandial insulin response to an isoenergetic portion of a test food in comparison to a reference food. The use of reference food to calculate GI and II values reduces the effect of natural differences (eg, body weight, lifestyle, metabolism) between the subjects.
The GI and II values were calculated by dividing the average plasma AUC value for a test food by the average plasma AUC value for the equal‐carbohydrate portion of the reference food and multiplying by 100:
Repeated‐measures analysis of variance (ANOVA) was used to determine whether there were any significant differences among the mean AUCGLUCOSE and AUCINSULIN responses of the 3 rice test meals (study A), and among the mean GI and II responses of the soft drink test meals (study B). If a statistically significant treatment effect was found, a post hoc multiple‐comparisons test was performed in order to identify the specific significant differences. All statistical testing was 2‐sided and performed at the .05 significance level. Differences were determined to be statistically significant if the calculated P value was ≤.05. Continuous variables were summarized with mean, standard deviation, standard error of the mean, median, minimum, and maximum. Statistical tests in study A were done using Statview (version 4.02, Abacus Concepts Inc, Berkeley, California), and in study B using PASW (version 21.0, SPSS Inc, Chicago, Illinois).
Results
Ten healthy, nonsmoking, overweight or obese subjects voluntarily participated in each of the 2 studies. The demographic characteristics are presented in Table 1.
Table 1.
Demographics
| Study A | Study B | ||
|---|---|---|---|
| Demographic | N = 10 | N = 10 | |
| Age (y) | Mean (SD) | 29.2 (3.3) | 32.4 (10.9) |
| Range | 25.6–36.8 | 18.7–55.6 | |
| Sex | Male | 6 (60%) | 4 (40%) |
| Female | 4 (40%) | 6 (60%) | |
| BMI (kg/m2) | Mean (SD) | 27.3 (1.1) | 27.4 (2.6) |
| Range | 25.5–28.7 | 25.3–32.3 |
BMI indicates body mass index.
Plasma Glucose Response
The change in plasma glucose from the fasting baseline value for the 10 subjects in study A is presented as the average 120‐minute incremental plasma glucose response curves in Figure 1. The control meal (rice alone) produced the highest peak plasma glucose concentration (mean ± SEM; 144.4 ± 4.7 mg/dL) at 30 minutes and the greatest overall glycemic response (mean AUCGLUCOSE 2695 ± 336 mg·min/dL). The overall glycemic response produced by the 2 test meals containing rice + BTI320 was similar throughout the 120‐minute experimental period. Both rice + BTI320 test meals produced a steady rise in plasma glucose to a lower peak response at 30 minutes compared with the control meal, followed by a gradual decline in glucose response between 30 and 120 minutes after ingestion of the test meal. The rice + 12 g BTI320 meal produced a smaller plasma glucose concentration at each time point, resulting in a lower overall glycemic response compared to the rice + 6 g BTI320 meal and the control meal.
Figure 1.
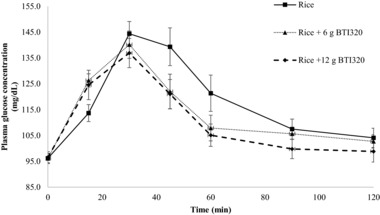
Change in glucose response at 120 minutes following the rice test meal for low‐dose and high‐dose BTI320 compared with no intervention (mean ± standard error).
The mean ± SEM of the incremental area under the 120‐minute plasma glucose response curve was 2695 ± 336, 2170 ±199, and 1835 ± 218 mg·min/dL for subjects receiving rice alone, rice + 6 g BTI320, and rice + 12 g BTI320, respectively (Table 2). One‐factor repeated‐measures ANOVA indicated that the differences in AUCGLUCOSE values were statistically significant (P ≤ .05), and post hoc pairwise comparisons using the Fisher least significant difference test showed that the mean AUCGLUCOSE response for the rice‐alone group was significantly greater than the mean AUCGLUCOSE responses for the rice + 6 g BTI320 (P ≤ .05) and the rice + 12 g BTI320 groups (P ≤ .05). The mean AUCGLUCOSE response for the rice + 12 g BTI320 group was also found to be significantly lower than the mean AUCGLUCOSE response for the rice + 6 g BTI320 group (P ≤ .05).
Table 2.
Mean and SEM Incremental Areas Under the 2‐Hour Glucose and Insulin Response Curves for the Test Foods
| Glucose (mg·min/dL) | Insulin (pmol·min/L) | ||||
|---|---|---|---|---|---|
| Test Food | Mean | SEM | Mean | SEM | |
| Study A | Rice | 2695 | 336 | 11 336 | 2530 |
| Rice + 6 g BTI320 | 2170 | 199 | 9546 | 2262 | |
| Rice + 12 g BTI320 | 1835 | 218 | 8590 | 1982 | |
| Study B | Reference food: Glucose 50 | 3300 | 393 | 18 500 | 2830 |
| Sprite™ | 2194 | 370 | 11 764 | 1729 | |
| Sprite™ + 2.6 g BTI320 | 1963 | 321 | 10 428 | 1636 | |
| Sprite™ + 5.2 g BTI320 | 1861 | 306 | 10 161 | 1948 | |
All tests were repeated twice in each subject. SEM indicates standard error of the mean.
The change in plasma glucose concentrations from the fasting baseline level for the 10 subjects in study B is shown in Figure 2. The reference glucose produced the highest peak plasma glucose concentration (160.8 ± 6.5 mg/dL) at 30 minutes and the greatest overall AUCGLUCOSE of 3300 ± 393 mg·min/dL. Plasma glucose levels following the consumption of the reference food were greater than those produced by all 3 test foods at each time point during the first 105 minutes, after which the plasma glucose level continued to fall below the baseline level.
Figure 2.
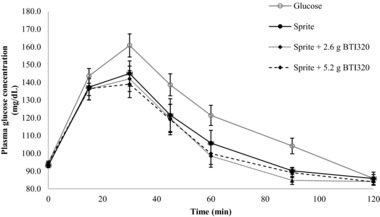
Change in glucose response at 120 minutes following the Sprite™ test meal for low‐dose and high‐dose BTI320 compared with no intervention (mean ± standard error).
The overall glycemic response produced by the 3 test foods was similar throughout the 120‐minute experimental period. However, the magnitude of the responses differed among the test foods. All 3 Sprite™ test foods showed a relatively rapid rise to a peak plasma glucose response at 30 minutes followed by a steady decline in glucose concentration between 30 and 120 minutes. Among the test foods, the Sprite™ + 5.2 g BTI320 group resulted in the lowest peak concentration and smallest overall glycemic response during the experimental period.
The mean ± SEM (range) of GI values (calculated relative to the reference food) for the 3 test meals were 64 ± 3 (range 47–91) for Sprite™ alone, 58 ± 3 (range 31–88) for Sprite™ + 2.6 g BTI320, and 55 ± 3 (range 39–81) for Sprite™ + 5.2 g BTI320. Differences in GI values were found to be statistically significant among test groups (P ≤ .05). The GI value of the reference food (glucose solution) was significantly greater than the mean GI values of all 3 test foods (P ≤ .05). The addition of 5.2 g BTI320 to the Sprite™ test food produced a significant reduction in GI (P ≤ .05) compared with Sprite™ alone. No significant reduction in GI was observed for the Sprite™ + 2.6 g BTI320 group compared to the Sprite™ consumed alone (P > .05).
Plasma Insulin Response
The change in plasma insulin from the fasting baseline value for subjects in study A is presented in Figure 3. Consistent with the glycemic result, the control food (rice alone) produced an increase in plasma insulin concentration and the largest overall plasma insulin response (AUCINSULIN 11,336 ± 2530 pmol·min/L). The overall shape and magnitude of the insulinemic response curves produced by the 2 test meals (rice + BTI320) were similar throughout the experimental period. Both meals produced a steady rise in plasma insulin concentration to a peak response at 30 minutes followed by a gradual decline in insulin concentration between 30 and 120 minutes. Similar to the corresponding glycemic response curves, both the peak and overall insulin response were lower in the rice + 12 g BTI320 compared to the rice + 6 g BTI320.
Figure 3.
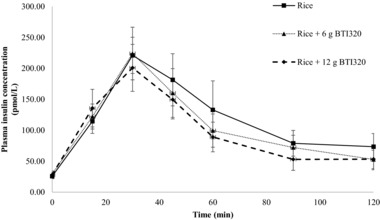
Change in insulin response at 120 minutes following the rice test meal for low‐dose and high‐dose BTI320 compared with no intervention (mean ± standard error).
The mean ± SEM of the incremental area under the 2‐hour plasma insulin response curve (AUCINSULIN) are listed in Table 2 (P ≤ .05). One‐factor repeated‐measures ANOVA indicated that the differences in AUCINSULIN values were statistically significant (P ≤ .05); post hoc pairwise comparisons using the Fisher least significant difference test showed that the mean AUCINSULIN response for the rice‐alone group was significantly greater than the mean insulin responses for the rice + 6 g BTI320 group (P ≤ .05) and the rice + 12 g BTI320 group (P ≤ .05).
The change in plasma insulin concentrations from the fasting baseline level for the 10 subjects in study B is shown in Figure 4. Specifically, the reference glucose produced a rapid rise in plasma insulin concentrations, with the greatest response at 30 minutes. The overall insulinemic response for the test foods was relatively similar, with a steady rise in insulin response to a moderate peak concentration at 30 minutes followed by a gradual decline in plasma insulin level between 30 and 120 minutes. Similar to their glycemic responses, the Sprite™ + 5.2 g BTI320 test food produced the lowest overall insulin response among the test foods.
Figure 4.
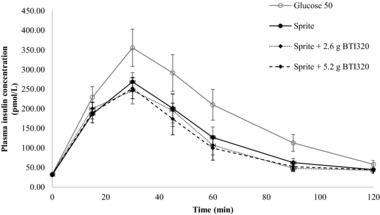
Change in insulin response at 120 minutes following the Sprite™ test meal for low‐dose and high‐dose BTI320 compared with no intervention (mean ± standard error).
The mean ± SEM (range) of II values (calculated relative to the reference food) for the 3 test meals were 67 ± 3 (45–96) for Sprite™ alone, 57 ± 3 (41–80) for Sprite™ + 2.6 g BTI320, and 55 ± 3 (37–78) for Sprite™ + 5.2 g BTI320. II values were found to be statistically significant (P ≤ .05) among the treatment groups. The II value of the reference food was significantly greater than the mean II values of the 3 Sprite™ + BTI320 groups. The addition of 2.6 g or 5.2 g BTI320 produced a significant reduction in II compared with Sprite™ alone (each P ≤ .05).
The insulinemic response curves produced by the 2 test foods with BTI320 were relatively proportional to their corresponding glycemic responses as well as the reference food and the test food without BTI320 (Figure 5).
Figure 5.
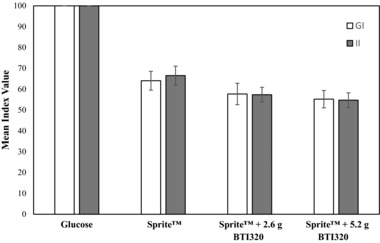
Mean and standard error of the mean glucose index (GI) and insulinemic index (II) values for the 3 test foods and the reference food (glucose).
Adverse Events
No serious adverse effects were reported or observed in either study, and none of the subjects prematurely discontinued the study. Two subjects in study A reported minor gastrointestinal discomfort following an experimental session containing the highest dose (12 g) of BTI320 tablets. These subjects reported mild stomach pain and/or diarrhea.
Discussion
BTI320, a novel nonsystemic, nontoxic carbohydrate‐based compound designed to reduce postprandial glucose excursions, has been studied in obese (but otherwise healthy) subjects and high‐risk subjects with prediabetes. The investigative compound works by blocking carbohydrate‐hydrolyzing enzymes involved in the breakdown of complex carbohydrates into glucose in the gastrointestinal tract, thus reducing the glucose and insulin spikes postprandially. Current science suggests that attenuation of these spikes reduces the risk of diabetic complications.15, 16, 17, 18, 19 Positive results from a recent clinical study indicated that given the ease of administration and high levels of tolerance, BTI320 has the potential to be used as an adjunct to lifestyle modification in high‐risk individuals. Thus, it is tempting to speculate that the chronic administration of BTI320 may lead to diminution or retardation of the development of complications in overweight subjects.
Unlike other orally administered antidiabetic drugs used for the treatment of type 2 diabetes mellitus, BTI320 is associated with gastrointestinal toxicities (eg, abdominal cramping, diarrhea). Similar to other α‐glucosidase inhibitors, the primary complaints of BTI320 were flatulence and abdominal distress, which appeared to be dose dependent.20, 21 The current studies had few adverse events, all gastrointestinal in nature, and none resulting in discontinuation from the study.
In 2016, a 16‐week phase 2, double‐blind, randomized, placebo‐controlled proof‐of‐concept study was carried out at the Chinese University of Hong Kong to examine the glucose‐lowering effects of BTI320 in 60 high‐risk Chinese subjects with prediabetes (ClinicalTrials.gov Identifier NCT02358668). The 3 treatment arms of the study were: low‐dose BTI320 (4 g 3 times daily; n = 24) and high‐dose BTI320 (8 g 3 times daily; n = 24) compared with placebo (3 times daily; n = 12) from baseline to week 4. Postprandial hyperglycemia and glycemic variability were significantly attenuated in subjects receiving low‐dose BTI320 compared to placebo. Treatment with 4 g BTI320 significantly reduced postprandial glucose AUC after 1 hour (P ≤ .05), 2 hours (P ≤ .05), and 3 hours (P ≤ .05). Reductions in postprandial glucose were observed in the high‐dose BTI320 group, albeit not reaching statistical significance. Improvements in lipids profile were also seen in the high‐dose BTI320 group, as evident by a reduction in serum triglyceride (P ≤ .05) and an increase in HDL cholesterol (P ≤ .05), both of which are biomarkers indicative of atherosclerosis retardation. Overall, the study demonstrated that BTI320 was well tolerated, with the most common side effects being abdominal distension, flatulence, and diarrhea occurring in approximately 20% to 30% of subjects in the treatment groups.
An earlier study evaluating the efficacy of BTI320 was performed by Trask and colleagues at the Dartmouth‐Hitchcock Medical Center in 24 subjects with type 2 diabetes mellitus who were treated with oral antidiabetic agents and/or insulin.6, 7 Subjects consumed a test meal without BTI320 and then ingested low‐dose (8 g) and high‐dose (16 g) BTI320 before test meals during subsequent visits. Despite absence of significant reduction in 2‐hour postprandial excursions in subjects assigned low‐dose BTI320 (the “nonresponders”), a trend in postprandial AUC reduction can be seen, suggesting that subjects may experience glycemic variability compared to placebo. The study showed that treatment with BTI320 was safe and effective in reducing postprandial glucose excursion. Because postprandial control is difficult to achieve in individuals with type 2 diabetes mellitus, results from this study suggest that the therapeutic action of BTI320 may be extended to this disease population to stall progression and improve disease management.
The 2 present studies measured the postprandial glucose and insulin responses to high‐glycemic test meals and demonstrated that the addition of BTI320 tablets significantly reduced postprandial glucose and insulin responses in a dose‐responsive manner. In the rice study the lower dose of BTI320 containing 6 g mannan polysaccharide and 4.5 g sorbitol resulted in a 19% reduction in postprandial glucose and a 16% decrease in postprandial insulin response compared to rice consumed alone. The higher dose of BTI320 containing 12 g mannan polysaccharide and 9 g sorbitol resulted in a 32% reduction in the 2‐hour glucose response and a 24% reduction in the postprandial insulin response compared with the white rice control meal.
In the soft drink (Sprite™) study the lower dose of BTI320 containing 2.6 g mannan polysaccharide and 4.6 g sorbitol produced a 10% reduction in the GI and a 14% decrease in the II. The addition of the higher dose of BTI320 containing 5.2 g mannan polysaccharide and 9.2 g sorbitol produced 14% and 18% reductions in GI and II, respectively, of the Sprite™ soft drink. A dose‐response effect was observed, such that the higher dose of BTI320 produced greater reductions in postprandial responses to the soft drink.
Importantly, these 2 studies differentiate from the aforementioned Trask studies6, 7 in that our studies were in overweight, otherwise healthy volunteers who did not have a diagnosis of type 2 diabetes mellitus and were not maintained on concomitant oral hypoglycemics or insulin. Therefore, our studies report on the effects of BTI320 in the treatment‐ and disease state–naive population in an attempt to evaluate the direct effects of BTI320 on postprandial glucose excursion, which were pronounced at varying doses of BTI320. Further, there was no suggestion of subclinical diabetic gastroparesis as in the Trask studies, and thus, no potential interference of the efficacy of BTI320. These data are of value in that BTI320 was able to regulate postprandial glucose excursion in the absence of other factors known to influence glycemic control.
However, similar to the Trask studies, our studies also highlight the importance of the glycemic index in the management of glucose excursions. The GI is a numerical value assigned to carbohydrates (on a scale from 0 to 100) based on their rate of glycemic response (ie, how slowly or how quickly the food causes an increase in blood glucose levels).22, 23, 24 Low‐GI foods (GI values ≤55) contain slowly digested carbohydrates, which in turn produce a gradual, relatively low rise in blood glucose levels. In contrast, high‐GI foods (GI values ≥70) release glucose rapidly and produce a rapid rise and fall in the blood sugar level. GI values have important health implications and are being used to construct dietary plans. A lower glycemic response often equates to a lower insulin demand and may improve long‐term blood glucose control.
Over the past decade, a growing body of research has shown that the overall glycemic impact of subject diets can influence the development of insulin resistance and the risk of associated diseases such as heart disease and diabetes mellitus, independent of the total carbohydrate content of the diet.7 To date, the available evidence suggests that diets based on low‐GI carbohydrate‐rich foods improve insulin sensitivity and blood glucose control in people with prediabetes and diabetes mellitus, reduce high blood fat levels, and may help prolong peak physical performance during endurance events.25, 26, 27, 28, 29 Because non‐insulin‐dependent diabetes mellitus and coronary heart disease continue to be major causes of illness and death in all industrialized countries, the extent to which the glycemic impact of diets influences both the onset and progression of these diseases is an issue of great importance. Nonetheless, the effects of BTI320 in this population have yet to be described, and further studies are ongoing to more fully elucidate any effects regarding diabetic‐related complications.
Conclusion
To summarize, BTI320 attenuated postprandial rise in blood glucose level as well as having a positive effect on lipid profile. The association between corresponding glycemic and insulin excursions on ingestion of the investigative compound was found to be dose dependent in obese yet otherwise healthy individuals. Because of its ease of administration and high levels of tolerance, BTI320 has the potential to be used as an adjunct to lifestyle modification to prevent glucose excursion. Further studies are ongoing to assess the influence of BTI320 on glucose excursions in type 2 diabetics and ensuing long‐term diabetic complications.
Conflict of Interest
K.K.Y.L. and C.W.R. are employees of Boston Therapeutics, Inc and Sugardown Co, Ltd. D.R.L. and C.C. are contractors of Boston Therapeutics, Inc. All authors were compensated for their work on this study. All research was funded by Boston Therapeutics, Inc.
References
- 1. Brand‐Miller JC, Wolever TMS, Colagiuri S, Foster‐Powell K. The Glucose Revolution. The Authoritative Guide to the Glycemic Index. New York: Marlowe and Company; 1999. [Google Scholar]
- 2. Truswell AS. Glycaemic index of foods. Eur J Clin Nutr. 1992;46(Suppl 2):S91–S101. [PubMed] [Google Scholar]
- 3. Jenkins DJA, Wolever TMS, Taylor RH, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–366. [DOI] [PubMed] [Google Scholar]
- 4. Holt SHA, Brand‐Miller JC, Petocz P. An insulin index of foods: the insulin demand generated by 1000‐kJ portions of common foods. Am J Clin Nutr. 1997;66:1264–1276. [DOI] [PubMed] [Google Scholar]
- 5. Ostman EM, Liljeberg‐Elmståhl HG, Björck IM. Inconsistency between glycemic and insulinemic responses to regular and fermented milk products. Am J Clin Nutr. 2001;74:96–100. [DOI] [PubMed] [Google Scholar]
- 6. Trask LE, Kasid N, Homa K, Chaidarun S. Safety and efficacy of the nonsystemic chewable complex carbohydrate dietary supplement PAZ320 on postprandial glycemia when added to oral agents or insulin in patients with type 2 diabetes mellitus. Endocrinol Pract. 2013;19:627–632. [DOI] [PubMed] [Google Scholar]
- 7. Trask LE, Chaidarun SS, Platt D, Parkin CG. Treatment with a novel galactomannan derivative reduces 2‐hour prostprandial glucose excursions in individuals with type 2 diabetes treated with oral medications and/or insulin. J Diabetes Sci Technol. 2014;8:1018–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hermansen K, Rasmussen O, Gregersen S, Larsen S. Influence of ripeness of banana on the blood glucose and insulin response in type 2 diabetic subjects. Diabetes Med. 1992;9:739–743. [DOI] [PubMed] [Google Scholar]
- 9. Ito Y, Mizukuchi A, Kise M, et al. Postprandial blood glucose and insulin responses to pre‐germinated brown rice in healthy subjects. J Med Invest. 2005;52:159–164. [DOI] [PubMed] [Google Scholar]
- 10. Ostman E, Granfeldt Y, Persson L, Björck I. Vinegar supplementation lowers glucose and insulin responses and increases satiety after a bread meal in health subjects. Eur J Clin Nutr. 2005;59:983–988. [DOI] [PubMed] [Google Scholar]
- 11. Shukla A, Iliescu RG, Thomas CE, Aronne LJ. Food order has a significant impact on postprandial glucose and insulin levels. Diabetes Care. 2015;38:e98–e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Augustin LSA, Chiavaroli L, Campbell J, et al. Post‐prandial glucose and insulin responses of hummus alone or combined with a carbohydrate food: a dose‐response study. Nutr J. 2015;15:13 10.1186/s12937-016-0129-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frid AH, Nilsson M, Holst JJ, Björck IME. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am J Clin Nutri. 2005;82:69–75. [DOI] [PubMed] [Google Scholar]
- 14. Tai MM. A mathematical model for the determination of total area under glucose tolerance and other metabolic curves. Diabetes Care. 1994;17:152–154. [DOI] [PubMed] [Google Scholar]
- 15. Brownlee M, Hirsch I. Glycemic variability: a hemoglobin A1C‐independent risk factor for diabetic complications. JAMA. 2006;295:1707–1708. [DOI] [PubMed] [Google Scholar]
- 16. Fang FS, Li ZB, Li CL, Tian H, Li J, Cheng XL. Influence of glycemic variability on the HbA1c level in elderly male patients with type 2 diabetes. Intern Med. 2012;51:3109–3113. [DOI] [PubMed] [Google Scholar]
- 17. Suh S, Kim JH. Glycemic variability: how do we measure it and why is it important? Diabetes Metab J. 2015;39:273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rodbard D. Glycemic variability: measurement and utility in clinical medicine and research—one viewpoint. Diabetes Technol Ther. 2011;13:1077–1080. [DOI] [PubMed] [Google Scholar]
- 19. Jung HS. Clinical impliactions of glucose variability: chronic complications of diabetes. Endocrinol Metab. 2015;30:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. BelHadj S, Hentati O, Elfeki A, Hamden K. Inhibitory activities of Ulva lactuca polysaccharides on digestive enzymes related to diabetes and obesity. Arch Physiol Biochem. 2013;119:81–87. [DOI] [PubMed] [Google Scholar]
- 21. Scheen AJ. Is there a role for alpha‐glucosidase inhibitors in the prevention of type 2 diabetes mellitus. Drugs. 2003;63:933–951. [DOI] [PubMed] [Google Scholar]
- 22. Monro JA, Shaw M. Glycemic impact, glycemic glucose equivalents, glycemic index, and glycemic load: definitions, distinctions, and implications. Am J Clin Nutr. 2008;87:237S–243S. [DOI] [PubMed] [Google Scholar]
- 23. Barclay AW, Petocz P, McMillan‐Price J, et al. Glycemic index, glycemic load, and chronic disease risk—a meta‐analysis of observational studies. Am J Clin Nutr. 2008;87(3):627–637. [DOI] [PubMed] [Google Scholar]
- 24. Brand‐Miller JC, Stockmann K, Atkinson F, Petocz P, Denyer G. Glycemic index, postprandial glycemia, and the shape of the curve in healthy subjects: analysis of a database of more than 1000 foods. Am J Clin Nutr. 2009;89(1):97–105. [DOI] [PubMed] [Google Scholar]
- 25. Brand‐Miller JC. The importance of glycemic index in diabetes. Am J Clin Nutr. 1994;59(suppl 1):747S–752S. [DOI] [PubMed] [Google Scholar]
- 26. Bhupathiraju SN, Tobias DK, Malik VS, et al. Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta‐analysis. Am J Clin Nutri. 2014;100(1):218–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Durkalec‐Michalski K, Zawieja EE, Zawieja BE, Jurkowska D, Buchowski MS, Jeszka J. Effects of low versus moderate glycemic index diets on aerobic capacity in endurance runners: three‐week randomized controlled crossover trial. Nutrients. 2018;10(3):370 10.3390/nu10030370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goff LM, Cowland DE, Hooper L, Frost GS. Low glycaemic index diets and blood lipids: a systematic review and meta‐analysis of randomised controlled trials. Nutr Metab Cardiovasc Dis. 2013;23(1):1–10. [DOI] [PubMed] [Google Scholar]
- 29. Schwingshackl L, Hoffmann G. Long‐term effects of low glycemic index/load vs. high glycemic index/load diets on parameters of obesity and obesity‐associated risks: a systematic review and meta‐analysis. Nutr Metab Cardiovasc Dis. 2013;23(8):699–706. [DOI] [PubMed] [Google Scholar]


