Abstract
Aim
To evaluate the safety of the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor alirocumab according to diabetes mellitus status.
Methods
Safety data from 14 trials (8–104‐week durations) were analysed by treatment (alirocumab or placebo/ezetimibe control) and diabetes status (yes/no, defined by medical history). Adverse event data were assessed using descriptive statistics and Cox models.
Results
Of the 5234 trial participants, 1554 (29.7%) had diabetes. Overall, treatment‐emergent adverse events were similar in the alirocumab and control groups, except for more frequent local injection site reactions with alirocumab. Fewer people with diabetes experienced local injection site reactions [alirocumab, 3.5%, control, 2.9%; hazard ratio 1.24 (95% CI 0.68–2.25)] than those without diabetes [alirocumab, 7.5%; control, 4.9%; hazard ratio 1.51 (95% CI 1.13–2.01)]. Those with diabetes reported a greater number of serious adverse events (alirocumab, 19.4%; control, 19.7%) than those without diabetes (alirocumab, 14.5%; control, 13.5%). In people with diabetes, major adverse cardiac events occurred in 2.7% of alirocumab‐treated people [control, 3.3%; hazard ratio 0.74 (95% CI 0.41–1.35)]; in those without diabetes, 1.8% of alirocumab‐treated people had major adverse cardiac events [control, 1.7%; hazard ratio 0.95 (95% CI 0.56–1.62)]. Overall, no increase in HbA1c or fasting plasma glucose vs control treatment groups was observed, regardless of diabetes status.
Conclusion
This pooled analysis across 14 trials demonstrated similar safety for alirocumab vs control treatment, irrespective of diabetes status, except for more frequent local injection site reactions with alirocumab. People with diabetes reported fewer local injection site reactions than those without diabetes.
What's new?
People with diabetes are at high cardiovascular risk and may require additional lipid‐lowering beyond statins. PCSK9 inhibitors provide additional LDL cholesterol reductions, but their safety has not been fully evaluated by diabetes status.
Our pooled analysis of 14 phase 2/3 trials is the largest safety assessment of the PCSK9 inhibitor alirocumab in terms of study participant numbers (n = 5234), comparing those with vs without diabetes at baseline over 8–104 weeks of treatment.
Our findings show comparable safety for alirocumab vs control, irrespective of diabetes status, except for more frequent local injection site reactions with alirocumab; people with diabetes reported fewer local injection site reactions than those without. No clinically significant changes in glycaemic variables (fasting plasma glucose and HbA1c) were observed in people with or without diabetes, regardless of treatment with alirocumab or control.
What's new?
People with diabetes are at high cardiovascular risk and may require additional lipid‐lowering beyond statins. PCSK9 inhibitors provide additional LDL cholesterol reductions, but their safety has not been fully evaluated by diabetes status.
Our pooled analysis of 14 phase 2/3 trials is the largest safety assessment of the PCSK9 inhibitor alirocumab in terms of study participant numbers (n = 5234), comparing those with vs without diabetes at baseline over 8–104 weeks of treatment.
Our findings show comparable safety for alirocumab vs control, irrespective of diabetes status, except for more frequent local injection site reactions with alirocumab; people with diabetes reported fewer local injection site reactions than those without. No clinically significant changes in glycaemic variables (fasting plasma glucose and HbA1c) were observed in people with or without diabetes, regardless of treatment with alirocumab or control.
Introduction
People with Type 1 or Type 2 diabetes mellitus and elevated LDL cholesterol are at increased risk of cardiovascular disease 1. Alirocumab is a fully human monoclonal antibody to proprotein convertase subtilisin/kexin type 9 (PCSK9) that is approved in over 60 countries 2, including the USA and countries in the European Union, for the management of people with heterozygous familial hypercholesterolaemia or clinical atherosclerotic cardiovascular disease (in the USA), or primary hypercholesterolaemia (in the European Union) and with elevated LDL cholesterol levels on maximally tolerated doses of statins and/or other lipid‐lowering therapies, including ezetimibe 3, 4. In phase 3 ODYSSEY clinical studies, alirocumab significantly reduced LDL cholesterol levels from baseline at week 24 in people with hypercholesterolaemia, both alone and in combination with maximally tolerated statin, with or without other lipid‐lowering therapies 5, 6, 7, 8, 9, 10, 11, 12, 13.
In sub‐analyses by diabetes status in phase 3 ODYSSEY trials, the LDL cholesterol‐lowering efficacy of alirocumab from baseline to week 24 was generally similar in people with and without diabetes 14, 15. The efficacy of alirocumab in those with diabetes has subsequently been confirmed vs placebo in insulin‐treated people with Type 1 and Type 2 diabetes in the phase 3b ODYSSEY DM‐INSULIN trial (NCT02585778) 16 and vs usual care in people with Type 2 diabetes and mixed dyslipidaemia in the phase 3b/4 ODYSSEY DM‐DYSLIPIDEMIA study (NCT02642159) 17.
The safety results for alirocumab in people with diabetes were similar to those for the control groups in the above‐mentioned sub‐analyses and clinical trials 14, 15, 16, 17. These findings are consistent with the overall safety profile of alirocumab in a pooled analysis of 14 ODYSSEY trials, the largest safety assessment of alirocumab in terms of study participant numbers (n = 5234) 18. The pooled safety analysis focused on adverse events (AEs) of special interest categories that were predefined based on identified, potential and theoretical risks for the new PCSK9 inhibitor drug class 18. Results indicated a higher incidence of local injection site reactions in individuals who had received alirocumab vs the control groups, with a similar incidence of musculoskeletal, neurological, neurocognitive, ophthalmological and hepatic events, and AEs related to diabetes/diabetes complications, between alirocumab and control groups 18. However, findings from a recent Mendelian randomization study, which showed an association between PCSK9 genetic variants that mimic the effects of PCSK9 inhibitors and an increased risk of diabetes, have raised new concerns about the safety of PCSK9 inhibitor therapy in people with diabetes 19. To provide additional evidence regarding these concerns, we evaluated the safety of alirocumab according to diabetes status using the same pool of 14 ODYSSEY trials mentioned above.
Methods
Study design
The purpose of the present analysis was to evaluate the safety of alirocumab vs control (placebo or ezetimibe) in people with hypercholesterolaemia with Type 1 or Type 2 diabetes vs those without diabetes. Type 1 or Type 2 diabetes at baseline was defined according to medical history. Individual trial participant data from 14 double‐blind, randomized clinical trials (four phase 2 trials 20, 21, 22, 23 and 10 phase 3 trials 5, 6, 7, 8, 9, 10, 11, 12, 13) were analysed in four pools according to diabetes status at baseline (yes/no), and according to whether participants were randomized to receive alirocumab or control.
This analysis uses a similar design to the previously published overall safety analysis of 14 alirocumab trials 18, results from which were included in the licensing application for alirocumab to the regulators. Details of trials included in the present analysis and the study design of the present analysis have been published previously 18. Briefly, double‐blind treatment durations of the trials were 8–104 weeks. Alirocumab dose and background statin use varied according to each trial. In eight phase 3 trials, the starting dose of alirocumab was 75 mg every 2 weeks, and was increased to 150 mg every 2 weeks at week 12 if prespecified LDL cholesterol levels were not achieved at week 8. Two phase 3 trials used the 150‐mg dose every 2 weeks throughout. Phase 2 studies evaluated a range of alirocumab doses vs placebo on stable background statin; this safety analysis included only individuals who had received the approved 75‐mg and 150‐mg doses every 2 weeks. All study protocols of individual trials were approved by the appropriate institutional review boards, and all study participants provided informed written consent.
Safety assessments
All AEs were recorded by the investigators, regardless of seriousness or potential relationship to alirocumab, and were coded using the Medical Dictionary of Regulatory Activities version 18.0. AEs were defined as treatment‐emergent AEs (TEAEs) if they developed, worsened or became serious during the period between the first and last dose of study treatment, plus 10 weeks to account for the potential prolonged effect of alirocumab.
An AE of special interest was an AE (serious or non‐serious) that needed to be proactively monitored, documented and managed in the prespecified manner described in the study protocols. If the investigator thought it necessary, participants were referred to a specialist for further testing. Categories for AEs of special interest were defined based on identified, potential and theoretical risks for the new drug class. The following AEs of special interest were prespecified in the phase 3 study protocols 18: local injection site reactions; general allergic events; neurological events; and ophthalmological events. Other prespecified categories of interest, including TEAEs related to hepatic disorders, neurocognitive disorders and diabetes, were analysed in the same way as the other AEs of special interest, but were not specifically defined as AEs of special interest in the protocols. In this analysis, the definitions used for AEs of special interest in phase 3 trials were applied to the data from the phase 2 trials.
Major adverse cardiac events (MACE), defined as coronary heart disease death, non‐fatal myocardial infarction, fatal or non‐fatal ischaemic stroke, or unstable angina requiring hospitalization, were adjudicated by a central Clinical Events Committee. MACE were adjudicated only in phase 3 trials.
Laboratory values, including fasting plasma glucose (FPG) and fasting HbA1c levels over time, were assessed by a central laboratory, as previously described 18.
Statistical analyses
The safety population was defined as all randomized people who received at least one full or partial dose of study treatment.
Any TEAE that was not prespecified but occurred commonly (by preferred term in ≥5% in any treatment group), AEs of special interest categories and MACE confirmed by adjudication were analysed using a predefined approach, including incidence and event rate per 100 person‐years in each treatment group (calculated as number of people with an event divided by total person‐years up to the first event or the end of the TEAE follow‐up period). To assess the consistency of treatment effect across studies, hazard ratios (HRs) and 95% CIs were calculated for AEs of special interest and adjudicated MACE using a Cox model that included the treatment group for each study. In addition, the study‐by‐treatment interaction was tested in a separate Cox model including the study, treatment and the study‐by‐treatment interaction. Other TEAEs were summarized using descriptive statistics.
To assess potential differences in glycaemic variables for alirocumab vs control, mixed‐effect model repeat measurement was used, assuming heterogeneous Toeplitz covariance structure 24 (HbA1c) or unstructured covariance structure (FPG).
Results
Baseline characteristics
Among 5234 study participants analysed, 1554 (29.7%) had diabetes, of whom 1524 (98.1%) had Type 2 diabetes, 28 (1.8%) had Type 1 diabetes and two (0.1%) had diabetes for which the type was not specified. At baseline, mean (sd) HbA1c levels in those with diabetes were 52 (11) mmol/mol [6.9 (1.1)%] and 52 (12) mmol/mol [6.9 (1.1)%] for the alirocumab and control groups, respectively, vs 38 (4) mmol/mol [5.7 (0.4)%] and 38 (5) mmol/mol [5.7 (0.4)%], respectively, for those without diabetes (Table 1). Baseline mean (sd) FPG levels in the alirocumab and control groups, respectively, were 7.5 (2.3) mmol/l (135.1 [41.3] mg/dl) and 7.5 (2.4) mmol/l (135.9 [43.9] mg/dl) in those with diabetes, vs 5.5 (0.7) mmol/l (99.2 [13.1] mg/dl) and 5.5 (0.8) mmol/l (98.8 [13.8] mg/dl) in those without diabetes (Table 1). Overall, 23.5% of those with diabetes were on insulin.
Table 1.
Baseline characteristics according to baseline diabetes status in the pool of 14 phase 2/3 trials (safety population)
| People with diabetes N = 1554 | People without diabetes N = 3680 | |||
|---|---|---|---|---|
| Control n = 558 | Alirocumab n = 996 | Control n = 1336 | Alirocumab n = 2344 | |
| Age, years | 61.7 (9.6) | 62.1 (9.5) | 58.9 (11.2) | 58.3 (11.6) |
| Male, n (%) | 313 (56.1) | 587 (58.9) | 837 (62.6) | 1476 (63.0) |
| BMI, kg/m2 | 32.8 (6.0) | 32.3 (6.4) | 29.0 (5.0) | 29.0 (5.1) |
| Atherosclerotic cardiovascular disease*, n (%) | 346 (62.0) | 637 (64.0) | 912 (68.3) | 1640 (70.0) |
| Chronic kidney disease†, n/N (%) | 79/546 (14.5) | 149/979 (15.2) | 80/1246 (6.4) | 170/2203 (7.7) |
| Heterozygous familial hypercholesterolemia†, n/N (%) | 57/546 (10.4) | 90/979 (9.2) | 404/1246 (32.4) | 787/2203 (35.7) |
| Hypertension, n (%) | 492 (88.2) | 887 (89.1) | 766 (57.3) | 1324 (56.5) |
| Statins, n (%) | 524 (93.9) | 941 (94.5) | 1117 (83.6) | 2079 (88.7) |
| High‐intensity statins‡, n (%) | 239 (45.7) | 451 (47.9) | 720 (64.7) | 1315 (63.3) |
| Any other lipid‐lowering therapy, n (%) | 127 (22.8) | 231 (23.2) | 443 (33.2) | 770 (32.8) |
| Anti‐hyperglycaemic agent, n (%) | 459 (82.3) | 828 (83.1) | 0 | 0 |
| Injectable anti‐hyperglycaemic, n (%) | 132 (23.7) | 265 (26.6) | 0 | 0 |
| Insulin, n (%) | 123 (22.0) | 242 (24.3) | 0 | 0 |
| LDL cholesterol | ||||
| mmol/l | 3.0 (1.1) | 3.0 (1.0) | 3.4 (1.3) | 3.4 (1.3) |
| mg/dl | 115.9 (41.8) | 115.7 (37.9) | 130.6 (50.3) | 130.0 (49.8) |
| Non‐HDL cholesterol | ||||
| mmol/l | 3.8 (1.2) | 3.9 (1.1) | 4.1 (1.4) | 4.1 (1.4) |
| mg/dl | 148.3 (47.9) | 149.3 (43.9) | 159.0 (55.3) | 157.5 (53.5) |
| Triglycerides, median (Q1:Q3) | ||||
| mmol/l | 1.6 (1.2:2.3) | 1.7 (1.2:2.3) | 1.4 (1.0:2.0) | 1.4 (1.0:1.9) |
| mg/dl | 144.0 (107.1:201.0) | 147.0 (108.0:207.0) | 122.1 (89.4:175.0) | 120.0 (87.6:167.3) |
| HDL cholesterol | ||||
| mmol/l | 1.2 (0.3) | 1.2 (0.3) | 1.3 (0.4) | 1.3 (0.4) |
| mg/dl | 47.4 (12.3) | 46.9 (11.9) | 51.0 (13.8) | 51.3 (14.2) |
| FPG | ||||
| mmol/l | 7.5 (2.4) | 7.5 (2.3) | 5.5 (0.8) | 5.5 (0.7) |
| mg/dl | 135.9 (43.9) | 135.1 (41.3) | 98.8 (13.8) | 99.2 (13.1) |
| HbA1c | ||||
| mmol/mol | 52 (12) | 52 (11) | 38 (5) | 38 (4) |
| % | 6.9 (1.1) | 6.9 (1.1) | 5.7 (0.4) | 5.7 (0.4) |
| Duration of diabetes, years | 9.1 (8.7) | 9.3 (8.4) | 0 | 0 |
| Duration of total exposure to study treatment, weeks | 58.4 (31.1) | 62.4 (29.2) | 58.4 (31.3) | 63.6 (30.0) |
FPG, fasting plasma glucose.
Values are mean (sd), unless otherwise specified.
*Atherosclerotic coronary heart disease, ischaemic stroke or peripheral arterial disease. † n/N = number of people in phase 3 studies only. ‡High‐intensity statins were defined as atorvastatin 40 to 80 mg, rosuvastatin 20 to 40 mg or simvastatin 80 mg daily.
Study references: phase 2 trials [DFI111565 (NCT01288443), DFI11566 (NCT01288469), CL‐1003 (NCT01266876), DFI12361 (NCT01812707)]; phase 3 trials [LONG TERM (NCT01507831), HIGH FH (NCT01617655), COMBO I (NCT01644175), FH I (NCT01623115), FH II (NCT01709500), COMBO II (NCT01644188), OPTIONS I (NCT01730040), OPTIONS II (NCT01730053), MONO (NCT01644474), and ALTERNATIVE (NCT01709513)].
Baseline characteristics, including age, BMI and mean baseline lipid levels, were similar in the alirocumab and control groups within the pools of people with and without diabetes (Table 1). Generally, compared to those without diabetes, people with diabetes were slightly older, and had higher BMI and more hypertension, and lower LDL cholesterol and higher triglyceride levels (Table 1). Heterozygous familial hypercholesterolaemia and chronic kidney disease were only reported in the phase 3 studies; in this population, 9.6% of people with diabetes had heterozygous familial hypercholesterolaemia (vs 34.5% without diabetes), and 15.0% of people with diabetes had chronic kidney disease (vs 7.2% without diabetes). Overall, 63.3% of those with diabetes had atherosclerotic cardiovascular disease (vs 69.3% without diabetes), and 44.4% of those with diabetes were on high‐intensity statins (vs 55.3% without diabetes). The duration of total exposure to alirocumab was 62.4 weeks for people with diabetes, and 63.6 weeks for those without diabetes (vs 58.4 weeks for control for both diabetes subgroups).
Safety
The overall incidences of TEAEs, serious AEs, deaths and discontinuations were similar in the alirocumab and control groups (Table 2). Overall, serious AE incidence was higher in people with diabetes (alirocumab, 19.4%; control, 19.7%) vs those without diabetes [alirocumab, 14.5%; control, 13.5%; P < 0.0001 (Table 2)]; however, no pattern was observed in terms of individual specific serious AE frequency, and no difference in incidence was observed in alirocumab‐treated people vs controls.
Table 2.
Safety characteristics according to diabetes status at baseline in the pool of 14 phase 2/3 trials (safety population)
| People with diabetes N = 1554 | People without diabetes N = 3680 | |||
|---|---|---|---|---|
| n (%) | Control n = 558 | Alirocumab n = 996 | Control n = 1336 | Alirocumab n = 2344 |
| TEAEs | 431 (77.2) | 781 (78.4) | 1030 (77.1) | 1818 (77.6) |
| Serious TEAEs | 110 (19.7) | 193 (19.4) | 181 (13.5) | 341 (14.5) |
| TEAEs leading to death | 7 (1.3) | 9 (0.9) | 15 (1.1) | 13 (0.6) |
| TEAEs leading to discontinuation | 43 (7.7) | 87 (8.7) | 94 (7.0) | 145 (6.2) |
AE, adverse event; TEAE, treatment‐emergent adverse event.
As shown in Fig. 1, the most commonly reported TEAEs by preferred term in people with and without diabetes were nasopharyngitis (diabetes: alirocumab, 9.2%, control, 8.1%; non‐diabetes: alirocumab, 11.1%, control, 10.7%) and upper respiratory tract infection (diabetes: alirocumab, 7.2%, control, 9.0%; non‐diabetes: alirocumab, 6.6%, control, 6.5%). TEAEs that occurred with preferred term ≥5% in any treatment group by primary system organ class and preferred term, stratified by diabetes status, are shown in Table S1.
Figure 1.
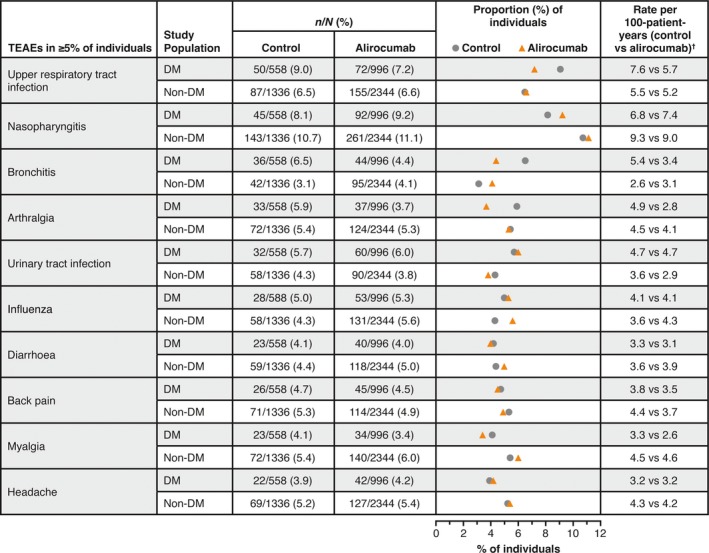
Treatment‐emergent adverse events (TEAEs) in ≥5% of people with and without diabetes in the pool of 14 phase 2/3 trials (safety population). n/N = number of study participants with at least one event. †Number of people with an event per person‐year, calculated as number of people with an event divided by total person‐years. For people with an event, number of person‐years is calculated up to the date of the first event; for those without an event, it corresponds to the length of the TEAE period. DM, diabetes mellitus.
The incidence rates of AEs of special interest are shown in Fig. 2. A significantly higher incidence of local injection site reactions was seen with alirocumab vs control in people without diabetes [alirocumab, 7.5%, control, 4.9%; HR 1.51 (95% CI 1.13–2.01)]. The incidence of local injection site reactions was lower in people with diabetes, with no significant differences between treatment arms [alirocumab, 3.5%, control, 2.9%; HR 1.24 (95% CI 0.68–2.25)]. In those with diabetes, rates of local injection site reactions in people receiving concomitant injectable therapies at baseline were 2.0% [8/397 participants; alirocumab, 1.9% (5/265 participants); control, 2.3% (3/132 participants)] vs 3.7% in those not receiving concomitant injectable therapies at baseline [43/1157 participants; alirocumab, 4.1% (30/731 participants); control, 3.1% (13/426 participants)]. Most local injection site reactions were mild in intensity (Fig. 3). Other AEs of special interest generally occurred at similar rates in the alirocumab and control groups in both diabetes and non‐diabetes subgroups (Fig. 2). Further details of AEs of special interest, including specific events in ≥0.5% of study participants, stratified by diabetes status, are shown in Table S2.
Figure 2.
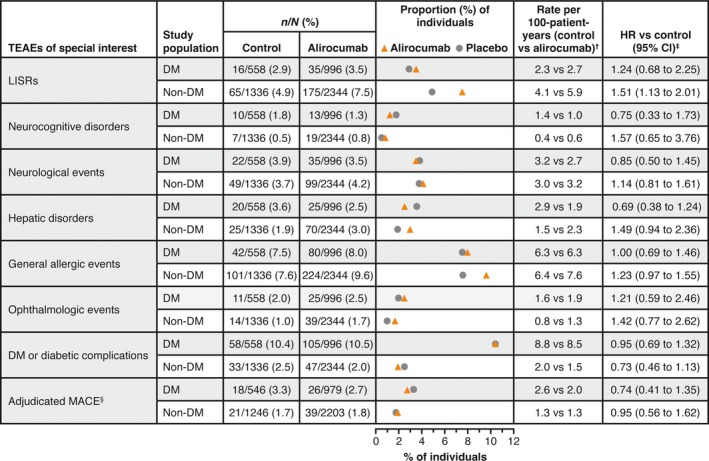
Adverse events of special interest according to diabetes status at baseline in the pool of 14 phase 2/3 trials (safety population). †Calculated as number of people with an event divided by total person‐years. For people with an event, the number of person‐years is calculated up to the date of the first event; for those without an event, it corresponds to the length of the treatment‐emergent adverse event (TEAE) period. ‡Calculated using a Cox model stratified on the study. §Major adverse cardiac events (MACE) were defined as coronary heart disease death, non‐fatal myocardial infarction, ischaemic stroke or unstable angina requiring hospitalization, and were adjudicated by a central Clinical Events Committee only in phase 3 trials. Local injection site reactions (LISRs) were selected using an electronic case report form‐specific tick box on the adverse event page in phase 3 studies and phase 2 study DFI12361, selected using Medical Dictionary of Regulatory Activities (MedDRA) high‐level term ‘injection site reaction’ in the other phase 2 studies. Neurocognitive disorder: events selected using a custom MedDRA query, based on the five following high‐level group terms: deliria (including confusion); cognitive and attention disorders and disturbances; dementia and amnestic conditions; disturbances in thinking and perception; and mental impairment disorders. Neurological events: standardized MedDRA queries ‘demyelination’ (broad and narrow), ‘peripheral neuropathy’ (broad and narrow), and ‘Guillain–Barre syndrome’ (broad and narrow), excluding the following preferred terms: ‘acute respiratory distress syndrome’, ‘asthenia’, ‘respiratory arrest’ and ‘respiratory failure’. Hepatic disorder: standardized MedDRA query ‘hepatic disorder’. General allergic events: selected using a custom MedDRA query with standardized MedDRA query ‘hypersensitivity’ (broad and narrow), excluding the following preferred terms linked to LISRs: (‘infusion site dermatitis’, ‘infusion site hypersensitivity’, ‘infusion site rash’, ‘infusion site urticaria,’ ‘injection site dermatitis’, ‘injection site hypersensitivity’, ‘injection site rash’, ‘injection site urticaria’ and ‘vasculitis’. Ophthalmologic events: standardized MedDRA queries ‘optic nerve disorders’ (broad and narrow), ‘retinal disorders’ (narrow) and ‘corneal disorders’ (narrow). Diabetes or diabetic complications: high‐level group term ‘diabetes complications’, high‐level term ‘diabetes mellitus’ and high‐level term ‘carbohydrate tolerance analyses (including diabetes)’, excluding preferred term ‘blood glucose decreased’. In study participants with diabetes at baseline, terms such as ‘diabetes mellitus’ indicate a worsening of the condition or loss of glycaemic control. DM, diabetes mellitus; HR, hazard ratio; TEAE, treatment‐emergent adverse event.
Figure 3.
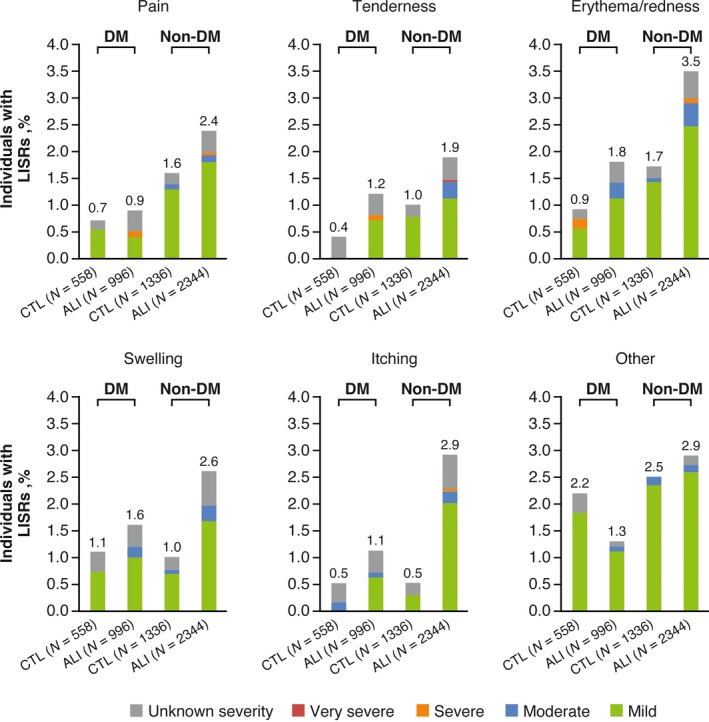
Comparison of local injection site reactions (LISRs) according to diabetes status at baseline in the pool of 14 phase 2/3 trials (safety population). The category of LISRs included those judged to be related to the injection of study treatment by the investigator and not attributable to another injectable agent. If the investigator or study participant recognized any LISRs and/or signs of local intolerability at the injection site, this was to be treated and followed up as per the investigator's medical judgement, and recorded on a special case report form. Local injection site reactions were graded by severity and characterized by related signs and symptoms such as (but not limited to) redness and pain. Drug reactions and/or LISRs considered to be allergic (or that had an allergic component) were reported under the category of general allergic events. Adverse events with cutaneous involvement and with obvious allergic origin or LISRs that expanded/worsened were to be evaluated by a dermatologist as soon as possible 18. ALI, alirocumab; CTL, control; DM, diabetes mellitus.
Adjudicated MACE (in phase 3 trials only) were reported in 2.7% of alirocumab‐treated people with diabetes [3.3% of controls; HR 0.74 (95% CI 0.41–1.35)], and in 1.8% of alirocumab‐treated people without diabetes [1.7% of controls; HR 0.95 (95% CI 0.56–1.62); Fig. 2].
Overall, no changes in median HbA1c and FPG measurements were observed over time for up to 104 weeks with alirocumab, regardless of diabetes status (Fig. 4). There were no differences in least squares mean HbA1c and FPG for alirocumab vs control (P values comparing alirocumab and control by diabetes status at each time >0.05), except for one timepoint showing a small but significant difference for alirocumab vs control [week 12 least squares mean HbA1c in people without diabetes: 38.0 (alirocumab) and 38.3 (control); P=0.0338 (Figs S1 and S2)].
Figure 4.
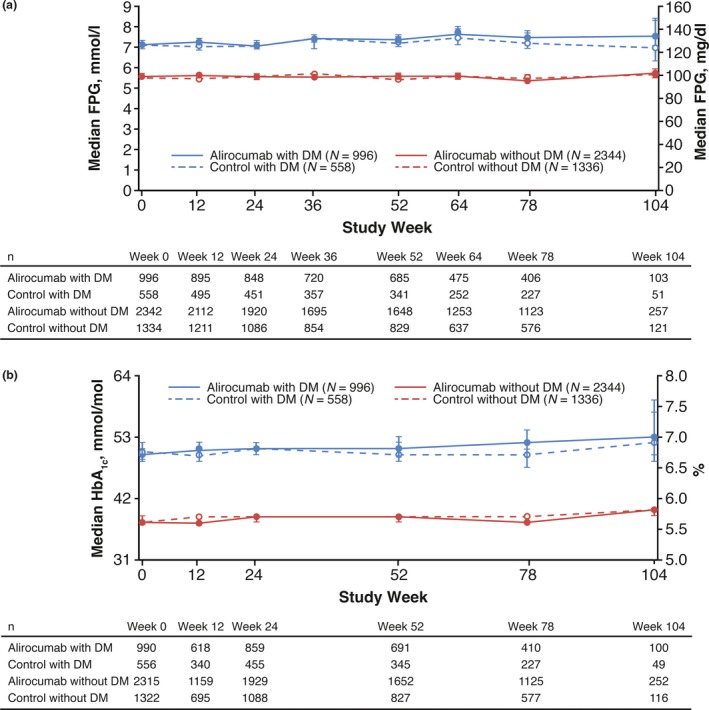
Median (a) fasting plasma glucose (FPG) and (b) HbA1c over time in people with and without diabetes in the pool of 14 phase 2/3 trials (safety population). Error bars indicate 95% CI values. Approximate values of FPG in mmol/l and HbA1c in mmol/mol are shown on the left‐hand y‐axis in panels (a) and (b), respectively. For HbA1c, the following formula was used to convert % to mmol/mol units: HbA1c in mmol/mol = 10.93*(HbA1c in %) – 23.5. DM, diabetes mellitus.
Discussion
The present pooled safety analysis across 14 phase 2 and three ODYSSEY trials of treatment duration ranging from 8 to 104 weeks showed that, irrespective of diabetes status, alirocumab safety was similar to that of control. The exception was the higher rates of local injection site reactions (the majority of which were mild in nature) with alirocumab treatment vs control in those with and without diabetes. These findings are consistent with previous findings in the overall ODYSSEY study population 18. Similar overall safety findings were also demonstrated in insulin‐treated participants with diabetes in the DM‐INSULIN study 16 and in those with Type 2 diabetes and mixed dyslipidaemia in the DM‐DYSLIPIDEMIA study (both 24 weeks’ treatment duration) 17. Additionally, similar safety results were shown with evolocumab, another PCSK9 inhibitor, in diabetes sub‐analyses of the evolocumab PROFICIO studies 25, 26 including the cardiovascular outcomes FOURIER trial 27. The lower rates of local injection site reactions that were observed in people with diabetes compared with people without diabetes, were previously seen in the diabetes sub‐analyses of five placebo‐controlled phase 3 trials (52–78 weeks’ treatment duration) and the ezetimibe‐controlled COMBO II trial (104 weeks’ treatment duration) 14, 15. This difference may simply be attributable to a greater familiarity with injectable therapies and/or fingerstick blood glucose testing in people with diabetes compared with those without; in the present analysis, ~25% of study participants in the diabetes group were receiving injectable antihyperglycaemic drugs. Other AEs of special interest generally occurred at similar rates between alirocumab and control in both the diabetes and non‐diabetes subgroups.
Consistent with previous results that did not find an association between alirocumab treatment and impaired glycaemic control 14, 15, 17, 28, 29, including at very‐low LDL cholesterol levels of <25 or <15 mg/dl (<0.6 or <.4 mmol/l) 30, the present pooled safety analysis showed that alirocumab did not affect FPG and HbA1c levels over time for up to 104 weeks of treatment in people with and without diabetes. Analyses of evolocumab PROFICIO trials with 48−52 weeks of follow‐up and the diabetes sub‐analysis of the FOURIER trial with 168 weeks of follow‐up also did not show changes in FPG or HbA1c levels with evolocumab treatment 25, 27, 31.
Adjudicated MACE rates from the present analysis are similar to the rates seen in the post hoc analysis of the ODYSSEY LONG TERM trial (alirocumab, 1.7% vs placebo, 3.3%) 10. As expected, and consistent with results from the FOURIER diabetes sub‐analysis 27, MACE rates were higher in those with diabetes (compared with people without diabetes), which is consistent with their expected higher baseline cardiovascular risk.
This safety assessment by diabetes status was conducted in the largest dataset for alirocumab available to date (N = 5234), representing a more robust and heterogenous study population than the populations assessed in previous sub‐analyses of alirocumab by diabetes status, such as the post hoc analysis of the COMBO II study (n = 720) 15 and the pooled analysis of five placebo‐controlled ODYSSEY trials (n = 3499) 14. Nevertheless, these data are limited by the overall study durations, and by differences among the studies pooled (such as durations and populations). Although the studies included in the present analysis had follow‐up durations of up to 2 years, a longer‐term effect of PCSK9 inhibition on glycaemic control cannot be excluded, and studies of longer duration are required. The present analysis included people with Type 1 and Type 2 diabetes, who may differ in their demographic and clinical characteristics, although the proportion of people with Type 1 diabetes included was small (1.8% of the overall diabetes population). Furthermore, the studies included in this analysis were not designed to determine the effect of alirocumab on cardiovascular events. Such data will be provided by the long‐term cardiovascular outcomes trial for alirocumab (ODYSSEY OUTCOMES; NCT01663402) in 18 924 study participants (~29% of whom had diabetes) randomized 1–12 months after acute coronary syndrome, with a median follow‐up of 2.8 years; the primary results for the OUTCOMES trial are now available (Steg et al., American College of Cardiology 2018; unpublished). Data on diabetes and prediabetes variables (reported by investigators and determined by serial HbA1c and FPG measurements) from OUTCOMES are expected to be reported at a later date.
In conclusion, the present pooled analysis across 14 phase 2 and three trials of 8 to 104 weeks’ duration showed that the safety of alirocumab was similar to that of control treatment, irrespective of diabetes status. The exception was the higher rates of local injection site reactions with alirocumab vs control treatment, generally mild in intensity, which were lower in those with vs those without diabetes.
Funding sources
This analysis and the ODYSSEY trials were funded by Sanofi and Regeneron Pharmaceuticals, Inc. The sponsor was involved in the study design, collection, analysis and interpretation of data, as well as data checking of information provided in the manuscript.
Competing interests
The authors report the following: L.A.L.: personal fees from Esperion, and grants and personal fees from Amgen, AstraZeneca, Kowa, The Medicines Company, Merck, Regeneron Pharmaceuticals, Inc. and Sanofi, outside the submitted work; F.J.T.: speaker's bureau and consultant/advisory board fees from AstraZeneca, Amgen, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Janssen Pharmaceuticals, Merck Sharpe & Dohme, Novartis Pharmaceuticals Co., Novo Nordisk, Sanofi and Regeneron Pharmaceuticals, Inc.; D.G.K.: served as a consultant for Regeneron Pharmaceuticals, Inc. and Sanofi; M.B.‐B. and A.L.: employees of and shareholders in Sanofi. J.M.: contractor for Sanofi; R.S.: employee of and shareholder in Regeneron Pharmaceuticals, Inc.; and P.H.J.: Chief Science Officer for the National Lipid Association and consultant/on advisory panels for Amgen, Merck, Sanofi and Regeneron Pharmaceuticals, Inc.
Supporting information
Figure S1. HbA1c over time in people with (A and B) and without diabetes mellitus (C and D) according to treatment status (safety population).
Figure S2. Fasting plasma glucose over time in people with (A and B) and without diabetes mellitus (C and D) according to treatment status (safety population).
Table S1. Treatment‐emergent adverse events that occurred with preferred term ≥5% in any treatment group by primary system organ class and preferred term, by diabetes status (safety population).
Table S2. Further details on adverse events of special interest, including specific events in ≥0.5% of participants (safety population).
Acknowledgements
Medical writing assistance and editorial support was provided by Grace Shim, PhD of Prime (Knutsford, UK), supported by Sanofi and Regeneron Pharmaceuticals, Inc. according to Good Publication Practice guidelines. The sponsor was involved in the study design, collection, analysis and interpretation of data, as well as data checking of information provided in the manuscript. The authors were responsible for all content and editorial decisions, and received no honoraria related to the development of this publication.
Diabet. Med. 35, 1742–1751 (2018)
An abbreviated version of this work was presented as a poster at the American College of Cardiology congress in Washington, DC, 2017: Leiter LA, Tinahones FJ, Karalis DG, Bujas‐Bobanovic M, Letierce A, Mandel J, Samuel R, Jones PH. Alirocumab safety in individuals with and without diabetes mellitus: pooled data from 14 ODYSSEY trials. J Am Coll Cardiol. 2017;69:1674.
References
- 1. Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12‐yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993; 16: 434–444. [DOI] [PubMed] [Google Scholar]
- 2. Regeneron Pharmaceuticals, Inc . Regeneron and Sanofi Announce Plans to Make Praluent® (alirocumab) More Accessible and Affordable for Patients with the Greatest Health Risk and Unmet Need. Available at http://investor.regeneron.com/releasedetail.cfm?releaseid=1060465. Last accessed 29 March 2018.
- 3. US Food and Drug Administration . Praluent prescribing information (US). Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125559s001lbl.pdf. Last accessed 7 August 2017.
- 4. European Commission . Praluent summary of product characteristics (EC). Available at http://ec.europa.eu/health/documents/community-register/2015/20150923132812/anx_132812_en.pdf. Last accessed 9 Sep 2016.
- 5. Bays H, Gaudet D, Weiss R, Ruiz JL, Watts GF, Gouni‐Berthold I et al Alirocumab as Add‐On to Atorvastatin Versus Other Lipid Treatment Strategies: ODYSSEY OPTIONS I Randomized Trial. J Clin Endocrinol Metab 2015; 100: 3140–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cannon CP, Cariou B, Blom D, McKenney JM, Lorenzato C, Pordy R et al Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur Heart J 2015; 36: 1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ginsberg HN, Rader DJ, Raal FJ, Guyton JR, Baccara‐Dinet MT, Lorenzato C et al Efficacy and Safety of Alirocumab in Patients with Heterozygous Familial Hypercholesterolemia and LDL‐C of 160 mg/dl or Higher. Cardiovasc Drugs Ther 2016; 30: 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kastelein JJ, Ginsberg HN, Langslet G, Hovingh GK, Ceska R, Dufour R et al ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J 2015; 36: 2996–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kereiakes DJ, Robinson JG, Cannon CP, Lorenzato C, Pordy R, Chaudhari U et al Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: The ODYSSEY COMBO I study. Am Heart J 2015; 169: 906–915.e13. [DOI] [PubMed] [Google Scholar]
- 10. Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M et al Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372: 1489–1499. [DOI] [PubMed] [Google Scholar]
- 11. Roth EM, Taskinen MR, Ginsberg HN, Kastelein JJ, Colhoun HM, Robinson JG et al Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double‐blind, randomized Phase 3 trial. Int J Cardiol 2014; 176: 55–61. [DOI] [PubMed] [Google Scholar]
- 12. Farnier M, Jones P, Severance R, Averna M, Steinhagen‐Thiessen E, Colhoun HM et al Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular‐risk patients: The ODYSSEY OPTIONS II randomized trial. Atherosclerosis 2016; 244: 138–146. [DOI] [PubMed] [Google Scholar]
- 13. Moriarty PM, Thompson PD, Cannon CP, Guyton JR, Bergeron J, Zieve FJ et al Efficacy and safety of alirocumab vs ezetimibe in statin‐intolerant patients, with a statin rechallenge arm: The ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol 2015; 9: 758–769. [DOI] [PubMed] [Google Scholar]
- 14. Ginsberg HN, Farnier M, Robinson JG, Cannon CP, Sattar N, Baccara‐Dinet MT et al Efficacy and safety of alirocumab: pooled analyses of 1048 individuals with diabetes mellitus from five placebo‐controlled Phase 3 studies of at least 52 weeks duration. Circulation 2015; 132: A17070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leiter LA, Zamorano JL, Bujas‐Bobanovic M, Louie MJ, Lecorps G, Cannon CP et al Lipid‐lowering efficacy and safety of alirocumab in patients with or without diabetes: A sub‐analysis of ODYSSEY COMBO II. Diabetes Obes Metab 2017; 19: 989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leiter LA, Cariou B, Muller‐Wieland D, Colhoun HM, Del Prato S, Tinahones FJ et al Efficacy and safety of alirocumab in insulin‐treated individuals with type 1 or type 2 diabetes and high cardiovascular risk: The ODYSSEY DM‐INSULIN randomized trial. Diabetes Obes Metab 2017; 19: 1781–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ray KK, Leiter LA, Muller‐Wieland D, Cariou B, Colhoun HM, Henry RR et al Alirocumab vs usual lipid‐lowering care as add‐on to statin therapy in individuals with type 2 diabetes and mixed dyslipidaemia: The ODYSSEY DM‐DYSLIPIDEMIA randomized trial. Diabetes Obes Metab 2018; 20: 1479–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jones PH, Bays HE, Chaudhari U, Pordy R, Lorenzato C, Miller K et al Safety of Alirocumab (A PCSK9 Monoclonal Antibody) from 14 Randomized Trials. Am J Cardiol 2016; 118: 1805–1811. [DOI] [PubMed] [Google Scholar]
- 19. Ference BA, Robinson JG, Brook RD, Catapano AL, Chapman MJ, Neff DR et al Variation in PCSK9 and HMGCR and Risk of Cardiovascular Disease and Diabetes. N Engl J Med 2016; 375: 2144–2153. [DOI] [PubMed] [Google Scholar]
- 20. Roth EM, McKenney JM, Hanotin C, Asset G, Stein EA. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med 2012; 367: 1891–1900. [DOI] [PubMed] [Google Scholar]
- 21. Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R et al Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low‐density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet 2012; 380: 29–36. [DOI] [PubMed] [Google Scholar]
- 22. McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand AC, Stein EA. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol 2012; 59: 2344–2353. [DOI] [PubMed] [Google Scholar]
- 23. Teramoto T, Kobayashi M, Uno K, Takagi Y, Matsuoka O, Sugimoto M et al Efficacy and Safety of Alirocumab in Japanese Subjects (Phase 1 and 2 Studies). Am J Cardiol 2016; 118: 56–63. [DOI] [PubMed] [Google Scholar]
- 24. Littell RC, Pendergast J, Natarajan R. Modelling covariance structure in the analysis of repeated measures data. Stat Med 2000; 19: 1793–1819. [DOI] [PubMed] [Google Scholar]
- 25. Blom DJ, Koren MJ, Roth E, Monsalvo ML, Djedjos CS, Nelson P et al Evaluation of the efficacy, safety and glycaemic effects of evolocumab (AMG 145) in hypercholesterolaemic patients stratified by glycaemic status and metabolic syndrome. Diabetes Obes Metab 2017; 19: 98–107. [DOI] [PubMed] [Google Scholar]
- 26. Sattar N, Preiss D, Robinson JG, Djedjos CS, Elliott M, Somaratne R et al Lipid‐lowering efficacy of the PCSK9 inhibitor evolocumab (AMG 145) in patients with type 2 diabetes: a meta‐analysis of individual patient data. Lancet Diabetes Endocrinol 2016; 4: 403–410. [DOI] [PubMed] [Google Scholar]
- 27. Sabatine MS, Leiter LA, Wiviott SD, Giugliano RP, Deedwania P, De Ferrari GM et al Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new‐onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol 2017; 376: 1713–1722. [DOI] [PubMed] [Google Scholar]
- 28. Colhoun HM, Ginsberg HN, Robinson JG, Leiter LA, Muller‐Wieland D, Henry RR et al No effect of PCSK9 inhibitor alirocumab on the incidence of diabetes in a pooled analysis from 10 ODYSSEY Phase 3 studies. Eur Heart J 2016; 37: 2981–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leiter LA, Müller‐Wieland D, Baccara‐Dinet MT, Letierce A, Samuel R, Cariou B. Efficacy and safety of alirocumab in people with prediabetes vs those with normoglycaemia at baseline: a pooled analysis of 10 phase III ODYSSEY clinical trials. Diabet Med 2018; 35: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Robinson JG, Rosenson RS, Farnier M, Chaudhari U, Sasiela WJ, Merlet L et al Safety of Very Low Low‐Density Lipoprotein Cholesterol Levels With Alirocumab: Pooled Data From Randomized Trials. J Am Coll Cardiol 2017; 69: 471–482. [DOI] [PubMed] [Google Scholar]
- 31. Sattar N, Toth PP, Blom DJ, Koren MJ, Soran H, Uhart M et al Effect of the Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitor Evolocumab on Glycemia, Body Weight, and New‐Onset Diabetes Mellitus. Am J Cardiol 2017; 120: 1521–1527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. HbA1c over time in people with (A and B) and without diabetes mellitus (C and D) according to treatment status (safety population).
Figure S2. Fasting plasma glucose over time in people with (A and B) and without diabetes mellitus (C and D) according to treatment status (safety population).
Table S1. Treatment‐emergent adverse events that occurred with preferred term ≥5% in any treatment group by primary system organ class and preferred term, by diabetes status (safety population).
Table S2. Further details on adverse events of special interest, including specific events in ≥0.5% of participants (safety population).


