Abstract
Background
Previous studies found evidence that dispositional optimism is related to lower pain sensitivity. Recent findings suggest that temporarily increasing optimism by means of imagining a positive future may also have pain‐alleviating effects.
Objectives
The present experiment was designed to investigate conditioned pain modulation (CPM) as a potential underlying mechanism of this pain‐alleviating effect of induced optimism.
Methods
For this purpose, 45 healthy participants were randomized into an optimistic or neutral imagery condition. Additionally, participants completed questionnaires on dispositional optimism, pain catastrophizing and pain expectations. CPM was assessed by delivering a series of five heat pain stimuli on the nondominant hand before and during immersion of the dominant hand in water of 5°C for 70 s.
Results
A clear CPM effect was found, that is heat pain reports were lower during simultaneous cold water stimulation. Although the optimism manipulation successfully increased optimism, it did not affect pain ratings or CPM. Post hoc analyses indicated that dispositional optimism was not associated with the magnitude of CPM, but pain catastrophizing and pain expectations did significantly correlate with the CPM effect.
Conclusion
Pain‐specific but not general cognitions appear to influence endogenous pain modulation.
Significance
Conditioned pain modulation is not the underlying mechanism of the pain‐alleviating effects of induced optimism. However, pain‐specific cognitions including pain catastrophizing and pain expectations affect endogenous pain modulation which should be taken into account in treatment and CPM research.
1. INTRODUCTION
Dispositional optimism, defined as global expectations that everything will turn out well, was found to be associated with lower pain sensitivity and better adaptation to acute and chronic pain (Geers, Wellman, Helfer, Fowler, & France, 2008; Hanssen, Vancleef, Vlaeyen, & Peters, 2014; Sobol‐Kwapinska, Bąbel, Plotek, & Stelcer, 2016; Wright et al., 2011). Even though optimism is considered a trait‐like characteristic that is relatively stable over time (Scheier & Carver, 1985), optimism can be temporarily increased by means of positive imagery (Peters, Flink, Boersma, & Linton, 2010). Previous studies demonstrated that inducing optimism through imagery led to lower pain intensity ratings during the cold pressor test (Hanssen, Peters, Vlaeyen, Meevissen, & Vancleef, 2013) and counteracted the negative effects of pain on executive functioning (Boselie, Vancleef, & Peters, 2017; Boselie, Vancleef, Smeets, & Peters, 2014) and well‐being (Boselie, Vancleef, & Peters, 2018). These studies suggest that optimism may be causally related to lower pain sensitivity.
One mechanism by which optimism could affect pain sensitivity is through top‐down modulation of subcortical and spinal nociceptive input, that is by activating descending pain inhibitory pathways. Conditioned pain modulation (CPM), or the “pain‐inhibits‐pain” phenomenon is frequently used as a measure of the inhibitory capacity of an individual's endogenous pain modulation system (Kennedy, Kemp, Ridout, Yarnitsky, & Rice, 2016). Within the CPM paradigm, two nociceptive stimuli are applied simultaneously to different body parts. The more intense and/or longer lasting (conditioning) stimulus usually reduces the perceptual intensity of the other (test) stimulus. The magnitude of this reduction is assumed to reflect the efficacy of an individual's endogenous pain inhibiting system.
To our knowledge, only one study has examined the association between optimism and CPM, and found that higher optimism was related to enhanced CPM (Goodin et al., 2013). The role of specific, pain‐related, expectations has been studied more often for their effect on CPM (Bjørkedal & Flaten, 2012; Cormier, Piché, & Rainville, 2013; Goffaux, Redmond, Rainville, & Marchand, 2007). Expecting pain relief during application of the conditioning stimulus increases CPM, whereas expecting hyperalgesia decreases CPM. It can be speculated that optimists hold more benign pain‐specific expectations and expect lower levels of pain and/or more pain relief through the conditioning stimulus. Moreover, optimists show less pain catastrophizing (Bargiel‐Matusiewicz & Krzyszkowska, 2009; Hanssen et al., 2013; Sinclair, 2001) and CPM seems to be lower in high pain catastrophizers (Goodin et al., 2009; Rhudy, Maynard, & Russell, 2007; Weissman‐Fogel, Sprecher, & Pud, 2008). Thus, both pain expectations and pain catastrophizing could mediate the effects of optimism on CPM.
This study aimed at examining whether enhanced CPM could explain the effect of optimism on pain. Following previous studies, we induced a temporary optimistic state by having participants imagine a positive future. CPM was compared between participants in the optimistic imagery condition and a neutral imagery condition. We hypothesized that participants in the optimism condition would show a more prominent CPM effect compared to participants in the control condition. We further hypothesized that the effect of optimism on CPM would be mediated by more benign pain expectations and/or by lower levels of pain catastrophizing.
2. METHODS
2.1. Participants
A power calculation was performed based on the primary hypothesis of reduced pain ratings during CPM compared to baseline. Assuming a medium effect size (f = 0.25) and a correlation of 0.5 between the pre‐ and postassessment, to obtain a power of 0.90 with α = 0.05 a total of 46 participants were needed. Participants were recruited through posters at Maastricht University, and through online participant platforms. Inclusion criteria were proficiency of the Dutch language and age between 18 and 35 years. People were not eligible if they had prior experience with the imagery exercises or the cold pressor test, or if they were suffering from any chronic or acute pain syndrome, cardiovascular disease or the Raynaud syndrome. Informed consent was obtained from each participant at the beginning of the experiment. Participation was rewarded with course credit or a gift voucher to the value of €7.50.
The final sample consisted of 45 students (40 females) from Maastricht University aged 18–26 years (M = 21.6, SD = 2.1).
2.2. Pain Stimulation
A Thermo‐Sensory Analyzer (TSA; Medoc Advanced Medical Systems, Durham, NC) was used for applying contact heat. A 30 × 30 mm Peltier thermode was attached to the ventral forearm of participants’ nondominant hand. Heat pain thresholds were determined with the “Limits”‐program of the TSA software in which the temperature of the thermode rises steadily from 36°C with the speed of 1°C/s. Participants were instructed to click on a mouse as soon as the stimulus was considered painful. This was repeated seven times. The temperature of the last five trials was averaged to calculate the pain threshold. For the test stimulus, the individual pain threshold was increased by 3°C with a maximum temperature of 51°C. The “Ramp and Hold” program was used to apply the test stimuli. Starting at 36°C, temperature increased with 6°C/s to reach the individual maximum temperature, which was presented for 1 s, and then decreased again by 10°C/s to 36°C. Five stimuli were given with a variable interstimulus interval of 5–7 s. The cold pressor test (CPT) served as the conditioning stimulus. Participants submerged their dominant hand in a water bath that was maintained at a constant temperature of 5°C. A Plexiglas tank of 36 × 30 × 15 cm (W × L × D) with an open heating bath circulator and an immersion cooler was used (Julabo ED‐19A; Julabo Seelbach, Germany). A plastic tank containing water at 22°C was positioned next to the Plexiglas tank and served to standardize prestimulation temperature of participants’ hands (immersion of 1 min).
2.3. Optimism manipulation
Participants were randomly assigned to the optimism or control condition following a computerized randomization procedure. Participants in the experimental condition were instructed to think and write about a future in which everything went well and all their dreams would be fulfilled. After writing for 15 min, they visualized this future as vividly as possible for another 5 min. This so‐called best possible self (BPS) exercise has repeatedly been shown to temporarily increase optimism (Boselie et al., 2014; Hanssen et al., 2013; Peters et al., 2010). In the control condition, participants wrote about and visualized a typical day (TD) according to the same procedure.
2.4. Measures
2.4.1. Pain catastrophizing scale (PCS)
The PCS is a 13‐item questionnaire that measures catastrophic thoughts and feelings about pain (Sullivan, Bishop, & Pivik, 1995). Participants indicate on a 5‐point Likert scale ranging from 0 = not at all to 4 = always to what extent statements, such as “I become afraid that the pain may get worse”, apply to them. Higher scores reflect more catastrophic thinking. The psychometric properties of this instrument are good, with high internal consistency (α = 0.92) and moderate test–retest stability (0.73; Lamé, Peters, Kessels, Van Kleef, & Patijn, 2008). Internal consistency in this study was α = 0.89. In addition to the standard trait version of the PCS questionnaire, a situational version of the PCS (S‐PCS) was used (Campbell et al., 2010). The instructions of the PCS were adjusted in such a way that all items referred to the experience of the experimental pain stimuli. In this study, the internal consistency was high (α = 0.95).
2.4.2. Life‐orientation test (LOT‐R)
Baseline levels of optimism were assessed by means of the LOT‐R (Scheier, Carver, & Bridges, 1994). The questionnaire contains three positive and three negative statements (e.g., “If something can go wrong for me, it will”) and four filler items that are rated on a 5‐point Likert scale with the anchors 1 (strongly disagree) to 5 (strongly agree). High scores indicate higher levels of optimism. The psychometric properties of the LOT‐R have been found to be satisfactory and suitable for measuring dispositional optimism in healthy individuals (Glaesmer et al., 2012). In this study, the Cronbach's alpha for the total scale was 0.74 indicating a satisfactory internal consistency.
2.4.3. Future expectations (FEX)
The FEX (Hanssen et al., 2013) assesses expectations for 10 positive and 10 negative future outcomes such as “You will have health problems”, “People will find you dull and boring” or “You will get a lot of satisfaction out of life” tapping into five domains (general, health, personal, professional and social). This measure was used as a manipulation check of the optimism manipulation as previous studies found the optimism induction to increase state optimism (Boselie et al., 2014, 2017; Hanssen et al., 2013). Participants rated the likelihood of these outcomes on 7‐point Likert scales ranging from 1 = not likely at all to occur to 7 = extremely likely to occur, resulting in two total scores, one for the positive and one for the negative statements. In this study, Cronbach's α was 0.84 for the positive subscale and 0.83 for the negative subscale.
2.4.4. Positive and negative affect schedule (PANAS)
The PANAS is a self‐report measure assessing positive and negative affect (Watson, Clark, & Tellegen, 1988). Participants indicate on a 5‐point Likert scale (1 = not at all, 5 = very much) to what extent the 20 words (e.g., inspired, restless, guilty) describe their current emotional state. It has good convergent and discriminant validity (Crawford & Henry, 2004) and both scales had a high internal consistency in this study (positive affect: α = 0.84; negative affect: α = 0.85). Previous studies found the BPS manipulation to also increase positive affect (Boselie et al., 2014, 2017; Hanssen et al., 2013; Sheldon & Lyubomirsky, 2006) and therefore the PANAS was used as a second manipulation check in this study.
2.4.5. Expected and experienced pain intensity ratings
Before each pain induction, participants completed a visual analogue scale (VAS) asking “How painful do you expect the heat/cold stimulus to be?” (0 = no pain at all to 100 = extreme pain). After each pain induction, the actual experienced pain intensity was measured with the same VAS. In addition, before CPM expected pain change was assessed using the following question: “Do you think that the perception of the heat stimulus changes when the other hand is immersed in the cold water? Please express your expectation as + x per cent (for increased pain intensity) or – x per cent (for decreased pain intensity) or 0 (for no change of pain intensity)”.
2.4.6. Quality of writing and imagery
To check for possible qualitative differences between the BPS and TD exercises, participants were asked to answer the following two questions on VAS scales ranging from 0 to 100: “How well could you imagine yourself in the situation you described in your writing?” (not at all – extremely well) and “How vivid were the pictures you imagined?” (not vivid at all – very vivid).
2.5. Procedure
An overview of the procedure is presented in Figure 1. Participants were told that they would participate in a study on the effects of visualization on their thoughts and feelings while experiencing hot and cold nociceptive stimuli. Upon arrival at the laboratory, participants signed an informed consent form. First, the heat pain threshold was determined. Next, five trials of the test stimulus (threshold + 3°C) were given. Participants rated expected painfulness once before the five trials and experienced pain after each trial on a VAS. Subsequently, participants filled out the LOT‐R, PCS, FEX and PANAS on a computer after which they completed either the BPS or the TD writing and imagery assignment. The FEX and PANAS were filled out once more as manipulation check.
Figure 1.
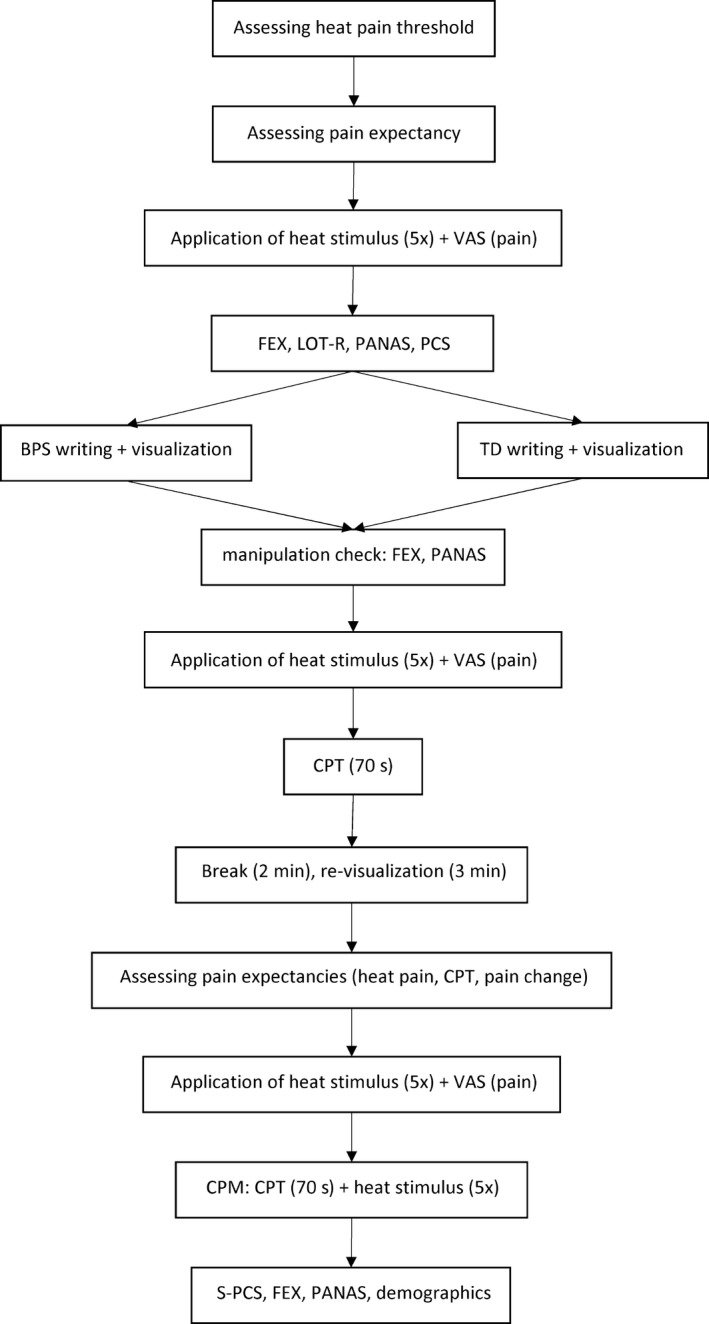
Experimental procedure
To examine whether the optimism manipulation affected heat and CPT pain ratings per se, another five trials of the test stimulus were given followed by a first administration of the CPT. Before the CPT, participants first immersed their dominant hand into water at room temperature for 1 min. Then, they immersed their hand into water of 5°C for 70 s. Pain ratings were obtained at 20, 40, 60 and 70 s. Participants who withdrew their hand from the water before 70 s. had passed were asked for a pain rating at the moment of withdrawal. After a 2‐min break, participants were instructed to re‐visualize their BPS or TD for 3 min to ensure that the required state would still be present during the CPM procedure. Subsequently, the CPM paradigm was introduced. First, expected pain intensity ratings were acquired for both the test stimulus and the conditioning stimulus, and expected pain change during CPM was assessed. Five trials of the test stimulus were then administered alone, and pain ratings were obtained after each heat stimulus. Next, the combined test and conditioning stimulus phase started. Ten seconds after participants had inserted their hand in the cold water, another five trials of the test stimulus were delivered, with pain ratings obtained after each trial. In addition, participants rated CPT pain at 70 s. Finally, participants filled in the S‐PCS, a demographics questionnaire, and again the FEX and PANAS to check whether the effects of the manipulation persisted. Participants were compensated and thanked for their participation.
2.6. Statistical analysis
Prior to data analysis, data were checked for normality, outliers and missing values. The only missing data were pain ratings during cold water immersion that occurred due to premature withdrawal. Three participants did not complete the 70‐s immersion of the first CPT (range 40–43 s). Immediately upon hand withdrawal, these participants provided pain ratings that were imputed at every subsequent missing value of the immersion phase. Two CPT ratings of the CPM phase were missing, one due to premature withdrawal (53 s) and the other due to omission to record the score, and were replaced by the participants’ final ratings of the previous CPT phase.1 Four mean heat pain ratings were obtained (before manipulation, immediately after manipulation, before CPT and during CPT) by averaging the five ratings of each individual trial within these series. Magnitude of the CPM effect was calculated as the difference in heat pain ratings during and before CPT.
Randomization checks with regard to age, FEX, LOT‐R, PANAS and PCS were performed by means of independent samples t tests. Two ANCOVAs for FEX positive and negative, respectively, with time as within‐subject factor (immediate postvisualization, at the end on the experiment), group allocation (BPS, TD) as between‐subject factor, and FEX baseline as covariate were used as manipulation checks. Similar analyses were performed for positive and negative affect (PANAS). Potential group differences in terms of quality of writing and visualization were tested by independent samples t tests.
Differences in heat pain ratings (before CPM induction) between the conditions were assessed with a repeated‐measures ANOVA with mean heat pain score before versus immediately after the BPS/TD manipulation as within‐subject factor and condition (BPS, TD) as between‐subject factor. Differences in CPT pain ratings between the conditions were tested by means of a repeated‐measures ANOVA on the pain scores at 20, 40, 60 and 70 s as the within‐subject variable and condition as between‐subject variable.
The hypothesis that induced optimism would lead to a more prominent CPM effect was tested with a repeated‐measures ANOVA with mean heat pain scores before and during the CPT as within‐subject factor and condition as between‐subject factor. In case of a significant effect of induced optimism, mediation analyses according to the method of Baron and Kenny (Baron & Kenny, 1986) were performed with CPM condition as predictor, the CPM effect as dependent variable and pain expectations or situational pain catastrophizing as the mediator in separate regression analyses.
3. RESULTS
Twenty‐three participants (four men) were assigned to the BPS condition and 22 (one man) to the TD condition. As the results did not change when controlling for sex, or when omitting male participants, analyses for the whole sample are reported. The randomization check revealed that the groups did not differ significantly on age or any of the baseline questionnaires, except for FEX negative which was lower in the BPS group (t(43) = 2.077; p = 0.044).
3.1. Manipulation check
The FEX and PANAS scores at the three time points in the two conditions are shown in Table 1. The ANCOVA for the positive FEX scores showed a significant condition effect (F = 7.395; p = 0.009) and no condition × time interaction (F = 0.107; p = 0.745), indicating that participants in the BPS condition had significantly higher positive FEX scores than participants in the TD condition at both postmanipulation time points. Similarly, for the negative FEX scores, only the condition effect reached significance (F = 9.299; p = 0.004; condition × time interaction: F = 1.315; p = 0.258). To verify that the increase in optimism in the BPS condition lasted until the end of the experiment, we additionally carried out a repeated‐measures ANOVA within the BPS condition with planned contrasts between the three time points. The positive FEX score was significantly larger at both postmanipulation time points compared to the premanipulation time point (immediately postmanipulation: F = 22.931, p < 0.001; end of experiment: F = 5.768, p = 0.025). Moreover, the final positive FEX score was not significantly different from the score immediately postmanipulation (F = 1.685, p = 0.208). Similarly, for the negative FEX score, the repeated‐measures ANOVA within the BPS condition indicated lower scores at both postmanipulation time points (resp. F = 17.527, p < 0.001 and F = 7.330, p = 0.013) and no difference between the postmanipulation time points (F = 3.570, p = 0.072). Thus, optimism effects were maintained throughout the experimental session.
Table 1.
Means and standard deviations (SD) of FEX and PANAS scores throughout the experiment
| Means (SD) | ||||||
|---|---|---|---|---|---|---|
| Premanipulation | Postmanipulation | Postrevisualization | ||||
| BPS | TD | BPS | TD | BPS | TD | |
| FEX‐Pos | 53.65 (5.81) | 52.77 (7.41) | 56.30 (5.90) | 53.23 (7.94) | 55.52 (6.71) | 52.77 (7.10) |
| FEX‐Neg | 28.00 (7.37) | 32.73 (7.89) | 23.69 (6.19) | 31.95 (8.91) | 25.17 (6.71) | 32.45 (8.91) |
| PA | 30.87 (4.69) | 28.5 (7.37) | 33.65 (5.56) | 26.91 (7.69) | 31.39 (6.75) | 27.00 (8.09) |
| NA | 13.65 (4.55) | 14.27 (4.49) | 12.30 (2.87) | 13.59 (4.25) | 13.43 (3.91) | 13.55 (3.90) |
BPS: best possible self; FEX‐Neg: Future Expectations positive scale; FEX‐Pos: Future Expectations positive scale; NA: negative affect scale (PANAS); PA: positive affect scale (PANAS); TD: typical day.
For PA, the ANCOVA indicated a significant condition effect (F = 8.342, p = 0.006) and a trend towards a condition × time interaction (F = 13.478, p = 0.068). The ANOVA with planned contrasts within the BPS condition demonstrated that whereas positive affect increased immediately after the manipulation (F = 6.424, p = 0.019), this effect had disappeared at the end of the experimental session (F = 0.214, p = 0.648). Paralleling previous studies using the BPS (Boselie et al., 2014; Hanssen et al., 2013), there was no significant conditions effect on NA (F = 0.342; p = 0.562). The quality of imagination did not significantly differ between the BPS and the TD group (81.5 vs. 84.2; p = 0.476). Similarly, there was no significant difference in the vividness of imagination (70.96 vs. 72.45; p = 0.802).
3.2. Effects of the optimism manipulation on pain intensity
Heat pain ratings for the four assessment periods are displayed separately for the two conditions in Table 2. To assess the effects of the optimism manipulation on heat pain intensity, the mean heat pain rating before (premanipulation) and after (postmanipulation) the BPS/TD manipulation was compared by means of a repeated‐measures ANOVA. The time effect was significant (F(1,43) = 10.19, p = 0.003), indicating an increase in pain ratings over time, but there was no time × condition interaction (F(1,43) = 0.39, p = 0.538).
Table 2.
Pain ratings for each assessment period per condition
| Premanipulation | Postmanipulation | Pre‐CPM | During CPM | |
|---|---|---|---|---|
| BPS | 59.82 (12.26) | 63.17 (13.29) | 60.73 (14.05) | 48.98 (18.76) |
| TD | 63.28 (19.80) | 65.55 (20.56) | 63.46 (20.73) | 52.05 (27.54) |
BPS: best possible self; TD: typical day.
Pain rating for the first CPT (i.e., when given alone) was examined by means of a repeated‐measures ANOVA with ratings at 20, 40, 60 and 70 s as the dependent variable and condition as the independent variable. A significant time effect was found (F(3,41) = 20.53, p < 0.001), showing that pain increased with time in the water. There was no significant condition effect (F(3,41) = 1.72, p = 0.179) nor a time × condition interaction (F(1,43) = 2.49, p = 0.620). Mean CPT ratings at 20, 40, 60 and 70 s were, respectively, 69.8, 79.1, 83.5 and 85.4 in the BPS condition and 68.9, 75.9, 81.4 and 81.6 in the TD condition. Thus, the optimism manipulation did not affect heat pain or CPT ratings.
3.3. Effects of the optimism manipulation on CPM
As can be seen from Table 2, heat pain ratings were lower during than before CPT in both conditions. The mean CPM effect was −11.58. A repeated‐measures ANOVA revealed a significant effect of Time (F(1,43) = 34.378, p < 0.001), indicating that CPM was successfully induced, but a nonsignificant condition × time interaction (F(1,43) = 0.007, p = 0.932.). Thus, the manipulation did not affect CPM. Because of the absence of a condition effect on CPM, no mediation analysis was performed.
3.4. Post hoc analyses: the role of dispositional optimism, trait pain catastrophizing, and pain expectations
In line with the results of previous studies, we explored whether dispositional optimism and/or trait pain catastrophizing were associated with the magnitude of CPM. In addition, because of the presumed role of pain expectations, we also explored the association of expected heat pain during CPM and expected pain relief with the magnitude of CPM. Pearson's correlations between scores on the LOT‐R, the PCS, expected heat pain and expected pain relief and the CPM effect were calculated. Dispositional optimism (r = −0.177, p = 0.245) and expected pain relief (r = −0.199, p = 0.189) were not significantly correlated with the CPM effect. However, trait catastrophizing (r = 0.413, p = 0.005) and expected heat pain (r = 0.375, p = 0.011) showed a significant positive correlation with the CPM effect. Thus, higher pain catastrophizing and higher expected heat pain during CPM were associated with less reduction in heat pain scores during the conditioning stimulus. Figures 2a,b show the scatterplots of these associations.
Figure 2.

(a) The correlation between trait pain catastrophizing and the magnitude of the CPM effect (r = 0.413, p = 0.005). (b) The correlation between expected heat pain during CPT and the magnitude of the CPM effect (r = 0.375, p = 0.011)
4. DISCUSSION
The present study was set up to examine the effects of induced optimism on CPM as a potential underlying mechanism of the reduced pain sensitivity that was previously reported in optimistic individuals and after optimism induction. Although the manipulation of optimism was successful, no effect on sensitivity for heat or cold pain or on CPM was found. Post hoc analysis showed that there was neither an association of dispositional optimism with the CPM effect. Thus, no evidence was found that optimism could activate or enhance endogenous pain inhibitory mechanisms. However, we did find evidence that pain‐specific cognitions may influence these endogenous pain inhibitory mechanisms. The post hoc analyses showed that both pain expectancies and trait pain catastrophizing were associated with the magnitude of the CPM effect.
One potential explanation for the absence of an (induced and dispositional) optimism effect on CPM could be that we used a very potent CPM paradigm. The paradigm with CPT pain as the conditioning stimulus and heat pain as the test stimulus generally has large effects possibly resulting from the activation of several underlying inhibitory circuits. Such a strong CPM paradigm might be especially sensitive for decreases of fully activated inhibition but insensitive for further increases because of ceiling effects. Thus, with this paradigm, it might be easier to uncover decreases in CPM effects due to catastrophizing than to find increases due to optimism. Another possibility is that optimism is generally ineffective in potentiating descending inhibition, and that its pain‐reducing effects rely on other mechanisms. So far, only one study found an association between dispositional optimism and increased CPM (Goodin et al., 2013), and only after adjusting the analyses for sex, ethnicity, depressive symptoms and pain catastrophizing. Simple correlational analyses did not reveal a significant association between dispositional optimism and magnitude of CPM in that study either. Adjusting our analyses for sex and pain catastrophizing by including these variables in a multiple regression analysis together with dispositional optimism did not change the results and only yielded a significant effect for pain catastrophizing (data not shown). It may be speculated that global cognitions relating to all life domains as is the case in optimism, have less direct relevance for, and less effect on, nociceptive control circuits compared to more proximal pain‐related cognitions.
The motivation for the present study was derived from the Hanssen et al. (2013) study that showed that inducing optimism by means of the BPS can lower cold pressor pain sensitivity. However, we could not replicate the pain‐reducing effect of the BPS intervention in this study. Neither heat pain ratings nor CPT pain ratings at baseline (i.e., when given alone, before the CPM induction) differed between the TD and BPS condition. One difference with the previous study was that in the present study, the experimental session lasted longer and several successive pain stimuli were applied. One could argue that this may have eliminated the effect of the BPS, reducing optimism to its baseline level in the course of the session. We tried to counter this by including a short re‐visualization after the first cold pressor test, before the CPM procedure started. The manipulation check demonstrated that state optimism remained significantly elevated until the end of the experimental session while mood effects declined, paralleling the findings of a previous study (Peters, Vieler, & Lautenbacher, 2016). Hence, whether and under what circumstances induced optimism affects pain sensitivity requires further investigation.
The finding that higher levels of pain catastrophizing are associated with reduced CPM is in line with the results of two previous studies (Rhudy et al., 2007; Weissman‐Fogel et al., 2008). If indeed people with high levels of pain catastrophizing have a less efficient pain inhibitory system, this may be one of the reasons why they are at increased risk of developing persistent pain. Pain catastrophizing is one of the main prognostic factors of pain persistence after an acute pain episode (e.g., Wertli et al., 2014) and after surgical procedures (Jackson, Tian, Wang, Iezzi, & Xie, 2016; Sobol‐Kwapinska et al., 2016). Less efficient enodogenous pain inhibition because of pain catastrophizing may lead to high pain intensity in the acute phase of injury, which can have a sensitizing influence on the nociceptive system (Chapman & Vierck, 2017). Indeed, less efficient CPM has previously been found related to higher levels of clinical pain (Vaegter & Graven‐Nielsen, 2016; Yarnitsky, 2015) and increased risk of persistent pain after surgery (Ossipov, Morimura, & Porreca, 2014; Petersen, Graven‐Nielsen, Simonsen, Laursen, & Arendt‐Nielsen, 2016; Yarnitsky et al., 2008). Future studies should examine whether targeting pain catastrophizing by means of cognitive behavioural therapy could reinstal efficient descending pain inhibition (cf. Seminowicz et al., 2013; Salomons, Moayedi, Erpelding, & Davis, 2014; Shpaner et al., 2014), and thereby reduce the risk of persistent pain.
It should be noted that the association between pain catastrophizing and CPM has not consistently been reported (Cormier et al., 2013; Horn‐Hofmann, Priebe, Schaller, Görlitz, & Lautenbacher, 2016; King et al., 2013; Nahman‐Averbuch, Sprecher, Jacob, & Yarnitsky, 2016) with one study even finding enhanced CPM with higher levels of pain catastrophizing (Granot et al., 2008). Whether or not pain catastrophizing affects endogenous pain inhibition may depend on the specific CPM protocol used (Granot et al., 2008; Nahman‐Averbuch, Nir, Sprecher, & Yarnitsky, 2016). As noted above, we have used a particularly strong CPM paradigm, which might facilitate finding an inhibitory effect.
Additionally, we found the expected painfulness of the heat stimuli during simultaneous cold pressor pain to be associated with the CPM effect. This concurs with other studies showing that pain expectations correlate with the magnitude of CPM (Cormier et al., 2013; Goffaux et al., 2007; Keltner et al., 2006). Robust associations between pain expectations and actual pain experiences have been reported in both clinical pain populations and pain‐free volunteers (Peerdeman, Van Laarhoven, Peters, & Evers, 2016) and it may be proposed that this is at least partly explained through endogenous pain modulation (Ploghaus, Becerra, Borras, & Borsook, 2003). Expectancy manipulations, such as placebo and nocebo induction, could clarify whether the impact on pain is (partly) mediated through strengthening or weakening the efficiency of descending pain modulation as measured by CPM (Eippert, Finsterbusch, Bingel, & Büchel, 2009).
This study has several limitations. First, pain catastrophizing and dispositional optimism were measured after the first series of heat pain stimuli had been applied. Despite the PCS and LOT‐R being considered trait measures and thus relatively stable, it cannot be ruled out that the recent pain experience might have affected their scores. Second, the homogeneity of the young and healthy sample may have led to ceiling effects in CPM as their pain inhibitory capacity was already high which might explain why we did not find any effects of optimism on CPM. Third, the sample mainly consisted of female participants and results may not be generalizable to males. Sex differences in the magnitude of CPM have been reported, with women typically having less efficient CPM than men (Goodin et al., 2009; Granot et al., 2008). Future studies could examine whether sex effects are reduced when using a strong CPM paradigm such as in the present study. Moreover, the effect of expectations on CPM magnitude should be replicated in a mixed sex sample because at least one study found that induced expectations only affected the magnitude of CPM in women (Bjørkedal & Flaten, 2012).
To conclude, the results of the current study provide evidence that pain‐specific cognitions but not generalized optimistic cognitions affect the CPM effect. Future studies should examine whether less efficient endogenous pain modulation could explain the prognostic role of pain catastrophizing and pain expectancies in the development of persistent pain and whether this can be curtailed by intervening on these psychological variables.
CONFLICTS OF INTEREST
None declared.
AUTHORS’ CONTRIBUTIONS
All authors have contributed substantially to the content of the manuscript. MLP, MMH, FO and SL contributed to the design of the study. JT and FO acquired the data. JT, MMH and MLP analysed and interpreted the results. JT drafted the article, MLP and MMH critically revised it and SL and FO reviewed and commented on it. All authors have given approval of the manuscript.
Traxler J, Hanssen MM, Lautenbacher S, Ottawa F, Peters ML. General versus pain‐specific cognitions: Pain catastrophizing but not optimism influences conditioned pain modulation. Eur J Pain. 2019;23:150–159. 10.1002/ejp.1294
Note
The participant withdrawing her hand prematurely received one of five test stimuli without concurrent cold pressor pain. Omitting this participant from the analyses did not affect the results.
REFERENCES
- Bargiel‐Matusiewicz, K. , & Krzyszkowska, A. (2009). Dispositional optimism and coping with pain. European Journal of Medical Research, 14, 271–274. 10.1186/2047-783X-14-S4-271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron, R. M. , & Kenny, D. A. (1986). The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology, 51, 1173–1182. 10.1037/0022-3514.51.6.1173 [DOI] [PubMed] [Google Scholar]
- Bjørkedal, E. , & Flaten, M. A. (2012). Expectations of increased and decreased pain explain the effect of conditioned pain modulation in females. Journal of Pain Research, 5, 289–300. 10.2147/JPR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boselie, J. J. , Vancleef, L. M. , & Peters, M. L. (2017). Increasing optimism protects against pain‐induced impairment in task‐shifting performance. Journal of Pain, 18, 446–455. 10.1016/j.jpain.2016.12.007 [DOI] [PubMed] [Google Scholar]
- Boselie, J. J. M. , Vancleef, L. M. G. , & Peters, M. L. (2018). Filling the glass: Effects of a positive psychology intervention on executive task performance in chronic pain patients. European Journal of Pain, 22(7), 1268–1280. 10.1002/ejp.1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boselie, J. J. , Vancleef, L. M. , Smeets, T. , & Peters, M. L. (2014). Increasing optimism abolishes pain‐induced impairments in executive task performance. Pain, 155, 334–340. 10.1016/j.pain.2013.10.014 [DOI] [PubMed] [Google Scholar]
- Campbell, C. M. , Kronfli, T. , Buenaver, L. F. , Smith, M. T. , Berna, C. , Haythornthwaite, J. A. , & Edwards, R. R. (2010). Situational versus dispositional measurement of catastrophizing: Associations with pain responses in multiple samples. Journal of Pain, 11, 443–453. 10.1016/j.jpain.2009.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, C. R. , & Vierck, C. J. (2017). The transition of acute postoperative pain to chronic pain: An integrative overview of research on mechanisms. Journal of Pain, 18, 351–359. [DOI] [PubMed] [Google Scholar]
- Cormier, S. , Piché, M. , & Rainville, P. (2013). Expectations modulate heterotopic noxious counter‐stimulation analgesia. Journal of Pain, 14, 114–125. 10.1016/j.jpain.2012.10.006 [DOI] [PubMed] [Google Scholar]
- Crawford, J. R. , & Henry, J. D. (2004). The positive and negative affect schedule (panas): Construct validity, measurement properties and normative data in a large non‐clinical sample. British Journal of Clinical Psychology, 43, 245–265. 10.1348/0144665031752934 [DOI] [PubMed] [Google Scholar]
- Eippert, F. , Finsterbusch, J. , Bingel, U. , & Büchel, C. (2009). Direct evidence for spinal cord involvement in placebo analgesia. Science, 326, 404 10.1126/science.1180142 [DOI] [PubMed] [Google Scholar]
- Geers, A. L. , Wellman, J. A. , Helfer, S. G. , Fowler, S. L. , & France, C. R. (2008). Dispositional optimism and thoughts of well‐being determine sensitivity to an experimental pain task. Annals of Behavioral Medicine, 36, 304–313. 10.1007/s12160-008-9073-4 [DOI] [PubMed] [Google Scholar]
- Glaesmer, H. , Rief, W. , Martin, A. , Mewes, R. , Brähler, E. , Zenger, M. , & Hinz, A. (2012). Psychometric properties and population‐based norms of the life orientation test revised (lot‐r). British Journal of Health Psychology, 17, 432–445. 10.1111/j.2044-8287.2011.02046.x [DOI] [PubMed] [Google Scholar]
- Goffaux, P. , Redmond, W. J. , Rainville, P. , & Marchand, S. (2007). Descending analgesia–when the spine echoes what the brain expects. Pain, 130, 137–143. 10.1016/j.pain.2006.11.011 [DOI] [PubMed] [Google Scholar]
- Goodin, B. R. , Kronfli, T. , King, C. D. , Glover, T. L. , Sibille, K. , & Fillingim, R. B. (2013). Testing the relation between dispositional optimism and conditioned pain modulation: Does ethnicity matter? Journal of Behavioral Medicine, 36, 165–174. 10.1007/s10865-012-9411-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodin, B. R. , Mcguire, L. , Allshouse, M. , Stapleton, L. , Haythornthwaite, J. A. , Burns, N. , … Edwards, R. R. (2009). Associations between catastrophizing and endogenous pain‐inhibitory processes: Sex differences. Journal of Pain, 10, 180–190. 10.1016/j.jpain.2008.08.012 [DOI] [PubMed] [Google Scholar]
- Granot, M. , Weissman‐Fogel, I. , Crispel, Y. , Pud, D. , Granovsky, Y. , Sprecher, E. , & Yarnitsky, D. (2008). Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (dnic) paradigm: Do conditioning stimulus painfulness, gender and personality variables matter? Pain, 136, 142–149. 10.1016/j.pain.2007.06.029 [DOI] [PubMed] [Google Scholar]
- Hanssen, M. M. , Peters, M. L. , Vlaeyen, J. W. , Meevissen, Y. M. , & Vancleef, L. M. (2013). Optimism lowers pain: Evidence of the causal status and underlying mechanisms. Pain, 154, 53–58. 10.1016/j.pain.2012.08.006 [DOI] [PubMed] [Google Scholar]
- Hanssen, M. M. , Vancleef, L. M. , Vlaeyen, J. W. , & Peters, M. L. (2014). More optimism, less pain! The influence of generalized and pain‐specific expectations on experienced cold‐pressor pain. Journal of Behavioral Medicine, 37, 47–58. 10.1007/s10865-012-9463-8 [DOI] [PubMed] [Google Scholar]
- Horn‐Hofmann, C. , Priebe, J. A. , Schaller, J. , Görlitz, R. , & Lautenbacher, S. (2016). Lack of predictive power of trait fear and anxiety for conditioned pain modulation (cpm). Experimental Brain Research, 234, 3649–3658. 10.1007/s00221-016-4763-9 [DOI] [PubMed] [Google Scholar]
- Jackson, T. , Tian, P. , Wang, Y. , Iezzi, T. , & Xie, W. (2016). Toward identifying moderators of associations between presurgery emotional distress and postoperative pain outcomes: A meta‐analysis of longitudinal studies. Journal of Pain, 17, 874–888. 10.1016/j.jpain.2016.04.003 [DOI] [PubMed] [Google Scholar]
- Keltner, J. R. , Furst, A. , Fan, C. , Redfern, R. , Inglis, B. , & Fields, H. L. (2006). Isolating the modulatory effect of expectation on pain transmission: A functional magnetic resonance imaging study. Journal of Neuroscience, 26, 4437–4443. 10.1523/JNEUROSCI.4463-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, D. L. , Kemp, H. I. , Ridout, D. , Yarnitsky, D. , & Rice, A. S. (2016). Reliability of conditioned pain modulation: A systematic review. Pain, 157, 2410–2419. 10.1097/j.pain.0000000000000689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, C. D. , Goodin, B. , Kindler, L. L. , Caudle, R. M. , Edwards, R. R. , Gravenstein, N. , … Fillingim, R. B. (2013). Reduction of conditioned pain modulation in humans by naltrexone: An exploratory study of the effects of pain catastrophizing. Journal of Behavioral Medicine, 36, 315–327. 10.1007/s10865-012-9424-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamé, I. E. , Peters, M. L. , Kessels, A. G. , Van Kleef, M. , & Patijn, J. (2008). Test—retest stability of the pain catastrophizing scale and the tampa scale for kinesiophobia in chronic pain over a longer period of time. Journal of Health Psychology, 13, 820–826. 10.1177/1359105308093866 [DOI] [PubMed] [Google Scholar]
- Nahman‐Averbuch, H. , Nir, R.‐R. , Sprecher, E. , & Yarnitsky, D. (2016). Psychological factors and conditioned pain modulation: A meta‐analysis. Clinical Journal of Pain, 32, 541–554. 10.1097/AJP.0000000000000296 [DOI] [PubMed] [Google Scholar]
- Nahman‐Averbuch, H. , Sprecher, E. , Jacob, G. , & Yarnitsky, D. (2016). The relationships between parasympathetic function and pain perception: The role of anxiety. Pain Practice, 16, 1064–1072. 10.1111/papr.12407 [DOI] [PubMed] [Google Scholar]
- Ossipov, M. H. , Morimura, K. , & Porreca, F. (2014). Descending pain modulation and chronification of pain. Current Opinion in Supportive and Palliative Care, 8, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerdeman, K. J. , Van Laarhoven, A. I. , Peters, M. L. , & Evers, A. W. (2016). An integrative review of the influence of expectancies on pain. Frontiers in Psychology, 7, 1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, M. L. , Flink, I. K. , Boersma, K. , & Linton, S. J. (2010). Manipulating optimism: Can imagining a best possible self be used to increase positive future expectancies? Journal of Positive Psychology, 5, 204–211. 10.1080/17439761003790963 [DOI] [Google Scholar]
- Peters, M. L. , Vieler, J. S. , & Lautenbacher, S. (2016). Dispositional and induced optimism lead to attentional preference for faces displaying positive emotions: An eye‐tracker study. Journal of Positive Psychology, 11, 258–269. 10.1080/17439760.2015.1048816 [DOI] [Google Scholar]
- Petersen, K. K. , Graven‐Nielsen, T. , Simonsen, O. , Laursen, M. B. , & Arendt‐Nielsen, L. (2016). Preoperative pain mechanisms assessed by cuff algometry are associated with chronic postoperative pain relief after total knee replacement. Pain, 157(1), 400–1406. [DOI] [PubMed] [Google Scholar]
- Ploghaus, A. , Becerra, L. , Borras, C. , & Borsook, D. (2003). Neural circuitry underlying pain modulation: Expectation, hypnosis, placebo. Trends in Cognitive Sciences, 7, 197–200. 10.1016/S1364-6613(03)00061-5 [DOI] [PubMed] [Google Scholar]
- Rhudy, J. L. , Maynard, L. J. , & Russell, J. L. (2007). Does in vivo catastrophizing engage descending modulation of spinal nociception? Journal of Pain, 8, 325–333. 10.1016/j.jpain.2006.10.003 [DOI] [PubMed] [Google Scholar]
- Salomons, T. V. , Moayedi, M. , Erpelding, N. , & Davis, K. D. (2014). A brief cognitive‐behavioural intervention for pain reduces secondary hyperalgesia. Pain, 155, 1446–1452. 10.1016/j.pain.2014.02.012 [DOI] [PubMed] [Google Scholar]
- Scheier, M. F. , & Carver, C. S. (1985). Optimism, coping, and health: Assessment and implications of generalized outcome expectancies. Health Psychology, 4, 219–247. 10.1037/0278-6133.4.3.219 [DOI] [PubMed] [Google Scholar]
- Scheier, M. F. , Carver, C. S. , & Bridges, M. W. (1994). Distinguishing optimism from neuroticism (and trait anxiety, self‐mastery, and self‐esteem): A reevaluation of the life orientation test. Journal of Personality and Social Psychology, 67, 1063–1078. 10.1037/0022-3514.67.6.1063 [DOI] [PubMed] [Google Scholar]
- Seminowicz, D. A. , Shpaner, M. , Keaser, M. L. , Krauthamer, G. M. , Mantegna, J. , Dumas, J. A. , … Naylor, M. R. (2013). Cognitive‐behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. Journal of Pain, 14, 1573–1584. 10.1016/j.jpain.2013.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon, K. M. , & Lyubomirsky, S. (2006). How to increase and sustain positive emotion: The effects of expressing gratitude and visualizing best possible selves. Journal of Positive Psychology, 1, 73–82. 10.1080/17439760500510676 [DOI] [Google Scholar]
- Shpaner, M. , Kelly, C. , Lieberman, G. , Perelman, H. , Davis, M. , Keefe, F. J. , & Naylor, M. R. (2014). Unlearning chronic pain: A randomized controlled trial to investigate changes in intrinsic brain connectivity following cognitive behavioral therapy. NeuroImage Clinical, 5, 365–376. 10.1016/j.nicl.2014.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair, V. G. (2001). Predictors of pain catastrophizing in women with rheumatoid arthritis. Archives of Psychiatric Nursing, 15, 279–288. 10.1053/apnu.2001.28686 [DOI] [PubMed] [Google Scholar]
- Sobol‐Kwapinska, M. , Bąbel, P. , Plotek, W. , & Stelcer, B. (2016). Psychological correlates of acute postsurgical pain: A systematic review and meta‐analysis. European Journal of Pain, 20, 1573–1586. 10.1002/ejp.886 [DOI] [PubMed] [Google Scholar]
- Sullivan, M. J. , Bishop, S. R. , & Pivik, J. (1995). The pain catastrophizing scale: Development and validation. Psychological Assessment, 7, 524–532. 10.1037/1040-3590.7.4.524 [DOI] [Google Scholar]
- Vaegter, H. B. , & Graven‐Nielsen, T. (2016). Pain modulatory phenotypes differentiate subgroups with different clinical and experimental pain sensitivity. Pain, 157, 1480–1488. 10.1097/j.pain.0000000000000543 [DOI] [PubMed] [Google Scholar]
- Watson, D. , Clark, L. A. , & Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: The panas scales. Journal of Personality and Social Psychology, 54, 1063–1070. 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Weissman‐Fogel, I. , Sprecher, E. , & Pud, D. (2008). Effects of catastrophizing on pain perception and pain modulation. Experimental Brain Research, 186, 79–85. 10.1007/s00221-007-1206-7 [DOI] [PubMed] [Google Scholar]
- Wertli, M. M. , Eugster, R. , Held, U. , Steurer, J. , Kofmehl, R. , & Weiser, S. (2014). Catastrophizing—a prognostic factor for outcome in patients with low back pain: A systematic review. Spine Journal, 14, 2639–2657. 10.1016/j.spinee.2014.03.003 [DOI] [PubMed] [Google Scholar]
- Wright, M. A. , Wren, A. A. , Somers, T. J. , Goetz, M. C. , Fras, A. M. , Huh, B. K. , … Keefe, F. J. (2011). Pain acceptance, hope, and optimism: Relationships to pain and adjustment in patients with chronic musculoskeletal pain. Journal of Pain, 12, 1155–1162. [DOI] [PubMed] [Google Scholar]
- Yarnitsky, D. (2015). Role of endogenous pain modulation in chronic pain mechanisms and treatment. Pain, 156, S24–S31. 10.1097/01.j.pain.0000460343.46847.58 [DOI] [PubMed] [Google Scholar]
- Yarnitsky, D. , Crispel, Y. , Eisenberg, E. , Granovsky, Y. , Ben‐Nun, A. , Sprecher, E. , … Granot, M. (2008). Prediction of chronic post‐operative pain: Pre‐operative dnic testing identifies patients at risk. Pain, 138, 22–28. 10.1016/j.pain.2007.10.033 [DOI] [PubMed] [Google Scholar]


