Abstract
Aim
To evaluate the changes in renal endpoints in type 2 diabetes patients treated with dapagliflozin versus other glucose‐lowering medications in routine clinical practice.
Materials and Methods
DARWIN‐T2D was a retrospective study conducted at 46 outpatient diabetes clinics in Italy. An automated software collected data on 17 285 patients who received dapagliflozin, glucagon‐like peptide‐1 receptor agonists, dipeptidyl peptidase‐4 inhibitors, or gliclazide, 6751 of whom had a follow‐up visit. We analysed changes in albumin excretion rate (AER) and estimated glomerular filtration rate (eGFR).
Results
Patients who received dapagliflozin (n = 473) were younger, more obese, and had a poorer glucose control than those who received a comparator (n = 2973). After ~6 months, median (interquartile range) AER declined by 37%, from 19.5 (7.5–78.2) to 13.2 (6.5–45.0) mg/g (P < 0.0001) in the dapagliflozin group and did not change in the comparator group. After adjusting for confounders, therapy with dapagliflozin versus comparators was associated with an AER reduction of 26.4 ± 13.1 mg/g (P = 0.045), and eGFR (mL/min/1.73 m2) diminished by 1.1 ± 0.5 (P = 0.049) in the dapagliflozin group and by 0.6 ± 9.1 (P = 0.002) in the comparator group (P = 0.35 between groups). No patient treated with dapagliflozin versus four patients treated with comparators experienced a doubling of serum creatinine.
Conclusions
The antiproteinuric effect of dapagliflozin is confirmed here for the first time by real‐world data. Despite a mild decline in eGFR, there was no evidence of clinically relevant worsening in renal function.
Keywords: antidiabetic drug, dapagliflozin, database research, diabetic nephropathy, type 2 diabetes
1. INTRODUCTION
The effects of glucose‐lowering medications (GLM) on cardiovascular and renal outcomes have become a major issue influencing therapeutic choices for type 2 diabetes (T2D).1 Regulatory agencies require that, in addition to lowering blood glucose, diabetes medications have a safe cardiovascular profile. During the last 3 years, a few new GLM have proved capable of protecting patients with T2D and high cardiovascular risk from major adverse cardiovascular events (MACE). Among these, the sodium‐glucose co‐transporter 2 inhibitors (SGLT2i) empagliflozin and canagliflozin,2, 3 as well as the glucagon‐like peptide‐1 receptor agonists (GLP‐1RA) liraglutide and semaglutide4, 5 reduced the risk of MACE (cardiovascular death, non‐fatal myocardial infarction or stroke). Strikingly, these drugs also showed evidence of renal protection, by reducing albuminuria and/or slowing the progression of chronic kidney disease (CKD).2, 4, 6, 7
In phase III randomized controlled trials (RCTs), treatment with SGLT2i reduced albumin excretion rate (AER),8 and slowed the decline in estimated glomerular filtration rate (eGFR) over time.9 The mechanisms mediating such effects likely result from modulation of the tubular‐glomerular feedback8 and are independent from background therapy with angiotensin converting enzyme (ACE) inhibitors.10
CKD is highly prevalent in T2D and is a strong accelerator of cardiovascular risk.11 In the longitudinal Italian Renal Insufficiency and Cardiovascular Events (RIACE) study, higher AER and lower eGFR, even in the normal range, identified individuals with increased mortality.12 Other observational studies and meta‐analyses show that an excess cardiovascular morbidity and mortality is linked to impaired renal function.13, 14 Therefore, it has been hypothesized that kidney protection contributes to cardiovascular protection in patients treated with SGLT2i.15, 16
However, data from RCTs may suffer from a limited external transferability and their findings need to be reproduced in clinical practice.17 So‐called “real‐world studies” use data accumulated during routine clinical practice to challenge results obtained in RCTs, address broader patient populations, and find predictors of clinical response.18 In the present study, we retrospectively analysed the effectiveness of the SGLT2i dapagliflozin on renal endpoints (AER and eGFR) in an Italian clinical care setting.
2. METHODS
2.1. Study design and aims
The DApagliflozin Real World evIdeNce in Type 2 Diabetes (DARWIN‐T2D) was a retrospective multicentre study performed at 46 diabetic specialist outpatient clinics in Italy. Details of the rationale and design of the study have been published previously.19 Briefly, the study aimed to describe the baseline clinical characteristics of patients who received a new prescription of dapagliflozin, a dipeptidyl peptidase‐4 (DPP‐4) inhibitor, a long‐acting GLP‐1RA (liraglutide or once‐weekly exenatide), or gliclazide in Italian routine clinical practice, and to retrospectively evaluate the change in effectiveness parameters at the first available follow‐up after 3‐12 months. An automatic software program extracted data from the same electronic chart system at all centres. The following data were collected: demographics, anthropometrics, diabetes duration, blood pressure, smoking status, fasting plasma glucose, HbA1c, lipid profile, liver enzymes, AER, eGFR (CKD‐EPI), history of complications, concomitant and previous GLM. AER values reported in charts as mg/l or mg/min (normal value 0‐20) were multiplied by 1.5 to convert to mg/g creatinine or mg/24 h equivalent (normal value 0‐30), assuming a standard urinary volume of 1500 mL.
The primary results of the study have been published previously20: although dapagliflozin was initially channelled to difficult‐to‐treat patients, it provided significant benefits with regard to the control of glucose, body weight, and blood pressure that were in line with findings from RCTs. Owing to the massive channelling bias, it was not possible to perform a propensity score matched comparison between the groups of patients who initiated dapagliflozin and those who initiated comparators.
In the present sub‐analysis of the DARWIN‐T2D study, we aimed to evaluate the effects of dapagliflozin on renal endpoints (the change from baseline in AER and eGFR) and to compare them with the effects of other GLM (combined GLP‐1RA, DPP‐4 inhibitors, or gliclazide).
2.2. Patient selection
Because the primary study aim was to describe the baseline clinical characteristics of patients who received dapagliflozin in clinical practice, the follow‐up visit was available for only a fraction of patients. We anticipated that data on AER and eGFR would only be available at both visits for some of the patients with a follow‐up visit because the guidelines do not necessarily recommend checking renal function at short intervals in all patients. We thus identified patients for whom AER was available at both visits, those for whom eGFR was available at both visits, and those for whom both AER and eGFR were available at both visits. We calculated the change in AER and eGFR as the difference from baseline to follow‐up. We also calculated the changes in HbA1c, body weight and blood pressure in the subgroup of patients with available data for AER and/or eGFR at both visits.
2.3. Statistical analysis
Normality of continuous variables was tested with the Kolmogorov‐Smirnov test. Non‐normal variables were log‐transformed before statistical analysis. Normal variables are presented as mean ± standard deviation, whereas non‐normal variables are given as median (interquartile range [IQR]). Categorical variables are summarized using percentages. Differences between the two groups of patients who received dapagliflozin or comparator GLM were analysed using the unpaired Student's t test for continuous variables or the chi‐squared test for categorical variables. Owing to the large number of comparisons, the Bonferroni correction was used to adjust for alpha inflation because of multiple testing. Significance of the change from baseline in AER and eGFR was tested using the paired Student's t test or, if necessary, the Wilcoxon rank test. We also calculated the percentage of patients in the various categories of AER (normo‐, micro‐, macro‐albuminuria) at baseline and at follow‐up. The significance of moving along AER categories was tested using the Wilcoxon rank test. A multiple regression analysis was used to adjust the effects of dapagliflozin versus those of comparator GLM on renal endpoints. Covariates were selected as those being significantly different between the two groups after Bonferroni correction. Variables with missing data were handled with a multiple imputation (MI) procedure using the Monte Carlo Markov Chain (MCMC) method and n = 20 imputations. The Pearson's r coefficient was used to test linear correlations between the change in AER (log‐transformed) or eGFR and clinical characteristics, as well as the change in other effectiveness variables. In addition, to detect clinical response patterns, we used random forests (RF) and partial least squares (PLS) algorithms with change in AER (log‐transformed) as the dependent variable (see the File S1 for this article for further details). The statistical significance level was set at 0.05. SPSS ver. 23 and R ver. 3.5.0 were used.
3. RESULTS
3.1. Characteristics of study patients
Of the 2484 patients identified at baseline and who started dapagliflozin, 830 had an available follow‐up visit during the observation period. Of these, 497 (60.0%) had at least one renal endpoint available both at the baseline and follow‐up visits; n = 273 patients had valid AER data at both visits, n = 393 patients had valid eGFR values at both visits, and n = 169 patients had both endpoints available at both visits. Of the 14 801 patients in the comparator group who were evaluated at baseline, 5921 had a follow‐up visit and 2973 (50.2%) had AER and/or eGFR available at both visits (n = 2277 for eGFR, n = 1380 for AER, n = 684 for both) (Figure 1).
Figure 1.
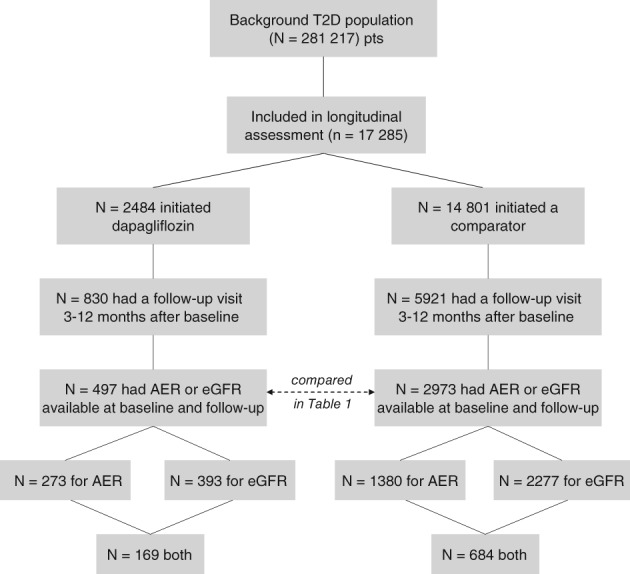
Study flowchart. T2D, type 2 diabetes; AER, albumin excretion rate; eGFR, estimated glomerular filtration rate
Clinical characteristics of the two groups are compared in Table 1: variables that remained significantly different after Bonferroni correction were age, BMI, diastolic blood pressure, fasting plasma glucose, baseline HbA1c, triglycerides, concomitant use of metformin, insulin, and diuretics, and prevalence of microangiopathy. Extended data are available in Table S1 (see the File S1 for this article).
Table 1.
Comparison of baseline characteristics between the two groups
| Dapagliflozin (n = 497) | Comparators (n = 2973) | ||||
|---|---|---|---|---|---|
| % Available | Value | % Available | Value | P | |
| Age, y | 100.0 | 60.5 ± 9.3 | 100.0 | 67.3 ± 9.0 | <0.001a |
| Sex: Male, % | 100.0 | 61.0 | 100.0 | 59.8 | 0.625 |
| Current smoking, % | 22.3 | 23.4 | 22.3 | 20.1 | 0.426 |
| Diabetes duration, y | 100.0 | 12.5 ± 8.4 | 99.9 | 11.6 ± 7.8 | 0.012 |
| BMI, kg/m2 | 93.2 | 32.8 ± 5.8 | 91.3 | 29.9 ± 5.3 | <0.001a |
| SBP, mm Hg | 73.8 | 139.6 ± 18.3 | 78.3 | 137.8 ± 18.7 | 0.087 |
| DBP, mm Hg | 73.6 | 81.2 ± 10.3 | 78.2 | 78.7 ± 9.0 | <0.001a |
| FPG, mg/dL | 92.8 | 173.9 ± 52.3 | 91.2 | 157.1 ± 38.3 | <0.001a |
| HbA1c, % | 99.0 | 8.6 ± 1.3 | 98.8 | 7.9 ± 1.0 | <0.001a |
| Total cholesterol, mg/dL | 79.5 | 174.4 ± 40.6 | 79.3 | 170.1 ± 37.2 | 0.038 |
| HDL cholesterol, mg/dL | 77.9 | 46.5 ± 13.5 | 76.8 | 48.0 ± 13.5 | 0.035 |
| Triglycerides, mg/dL | 79.9 | 166.3 ± 133.3 | 78.1 | 147.0 ± 90.3 | <0.001a |
| LDL cholesterol, mg/dL | 73.4 | 95.8 ± 32.1 | 75.0 | 92.9 ± 31.3 | 0.104 |
| SGOT, U/L | 48.1 | 25.9 ± 20.1 | 48.6 | 23.0 ± 12.8 | 0.003 |
| SGPT, U/L | 53.1 | 33.0 ± 20.2 | 49.7 | 27.3 ± 17.6 | <0.001a |
| eGFR, ml/min/1.73 m2 | 83.1 | 87.8 ± 16.4 | 82.5 | 79.5 ± 18.7 | <0.001a |
| AER, mg/g | 63.2 | 104.9 ± 342.7 | 58.6 | 76.2 ± 261.3 | 0.089 |
| Associated GLM | |||||
| Insulin | 99.8 | 55.4 | 100.0 | 15.2 | <0.001a |
| Metformin | 99.8 | 99.2 | 100.0 | 79.7 | <0.001a |
| Other medications | |||||
| Antiplatelet | 87.3 | 48.6 | 86.7 | 54.6 | 0.022 |
| Statin | 87.3 | 65.0 | 98.4 | 59.2 | 0.023 |
| ACEi/ARBs | 87.3 | 72.6 | 86.7 | 71.3 | 0.577 |
| CCB | 87.3 | 25.1 | 98.4 | 20.1 | 0.016 |
| Betablockers | 87.3 | 31.6 | 98.4 | 26.7 | 0.032 |
| Diuretics | 87.3 | 9.7 | 86.7 | 23.2 | <0.001a |
| Complications | |||||
| Microangiopathy | 100.0 | 41.9 | 93.2 | 32.6 | <0.001a |
| Macroangiopathy | 86.5 | 32.8 | 83.3 | 36.8 | 0.115 |
Data are expressed as mean ± standard deviation, or as % where appropriate. Per cent of available data is reported for all variables.
Abbreviations: ACEi, angiotensin‐converting enzyme inhibitors; AER, albumin excretion rate; ARBs, angiotensin receptor blockers; BMI, body mass index; CCB, calcium channel blockers; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; GLM, glucose‐lowering medications; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SBP, systolic blood pressure; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase.
Statistically significant after Bonferroni correction.
To evaluate selection bias, we compared the 3470 patients with and the 3281 patients without follow‐up data for renal endpoints: variables with a standardized difference > 0.1 (indicating imbalance) were age, diabetes duration, LDL cholesterol and statin use, baseline eGFR, and microangiopathy (Table S2).
3.2. Change in renal endpoints during therapy with dapagliflozin versus comparators
The change in AER was examined in 273 patients who received dapagliflozin and in 1380 patients who received a comparator (Table 2). During therapy with dapagliflozin, median (IQR) AER declined from 19.5 (7.5‐78.2) to 13.2 (6.5‐45.0) mg/g (P < 0.0001), equivalent to a 37% reduction (Figure 2A). The average ± SEM AER change was −39.3 ± 14.8 mg/g. Significant decreases in AER occurred only in patients with micro‐ or macroalbuminuria, but not in patients with normoalbuminuria at baseline (Figure 2B,D).
Table 2.
Change in AER in the various subgroups of patients
| Dapagliflozin | Comparators | |||||
|---|---|---|---|---|---|---|
| Subgroup | Baseline | Follow‐up | Change | Baseline | Follow‐up | Change |
| All patients, n | 273 | 273 | 273 | 1380 | 1380 | 1380 |
| Mean ± SEM | 105.0 ± 20.3 | 65.8 ± 10.4a | −39.3 ± 14.8b | 76.3 ± 6.6 | 70.4 ± 6.1 | −5.9 ± 4.1 |
| Median (IQR) | 19.5 (7.5; 78.1) | 13.2 (6.5; 45.0)a | −2.1 (−26.4; 2.3)b | 14.9 (7.2; 41.3) | 12.3 (7.0; 34.4) | −0.5 (−9.6; 4.3) |
| By baseline albuminuria | ||||||
| Normoalbuminuria, n | 164 | 164 | 164 | 951 | 951 | 951 |
| Mean ± SEM | 10.5 ± 0.6 | 13.3 ± 1.9 | 2.8 ± 1.8 | 10.6 ± 0.2 | 14.9 ± 1.1a | 4.3 ± 1.1 |
| Median (IQR) | 8.4 (5.5; 15.2) | 8.8 (4.8; 13.3) | 0.0 (−3.0; 2.7) | 8.9 (5.1; 15.3) | 8.8 (4.9; 51.3) | 0.0 (−3.0; 3.9) |
| Microalbuminuria, n | 93 | 93 | 93 | 356 | 356 | 356 |
| Mean ± SEM | 108.6 ± 7.0 | 84.7 ± 14.1 | −23.8 ± 13.6 | 90.6 ± 3.4 | 90.1 ± 7.8 | −0.5 ± 6.9 |
| Median (IQR) | 90.0 (52.1; 143.9) | 49.4 (21.6; 89.1)a | −35.3 (−75.9; −3.3)b | 66.7 (42.0; 121.2) | 43.5 (20.1; 93.4) | −17.7 (−46.7; 7.1) |
| Macroalbuminuria, n | 16 | 16 | 16 | 73 | 73 | 73 |
| Mean ± SEM | 1054.0 ± 243.6 | 493.1 ± 107.2a | −560.9 ± 204.0b | 861.5 ± 78.9 | 697.5 ± 76.4a | −164.0 ± 66.7 |
| Median (IQR) | 590.8 (480.2; 1228.5) | 379.1 (172.4; 661.7)a | −265.4 (−786.0; −113.2) | 630.6 (389.6; −1082.0) | 519.0 (318.0; −858.0)a | −184.5 (−343.5; −95.0) |
| By ACEi/ARBs therapy | ||||||
| No ACEi/ARBs, n | 68 | 68 | 68 | 345 | 345 | 345 |
| Mean ± SEM | 44.5 ± 9.4 | 24.5 ± 4.3a | −20.1 ± 7.4 | 47.5 ± 10.1 | 32.7 ± 4.4 | −14.7 ± 8.0 |
| Median (IQR) | 11.4 (6.5; 50.1) | 10.8 (6.0; 27.0)a | −1.5 (−8.1; 2.0) | 10.7 (6.8; 25.9) | 10.5 (6.4; 23.0) | −0.5 (−6.0; 3.0) |
| ACEi/ARBs, n | 179 | 179 | 179 | 872 | 872 | 872 |
| Mean ± SEM | 132.8 ± 30.2 | 83.5 ± 15.1a | −49.3 ± 22.4b | 96.4 ± 9.6 | 93.2 ± 9.4 | −3.2 ± 5.7 |
| Median (IQR) | 23.7 (8.5; 103.7) | 15.8 (8.1; 66.8)a | −3.0 (−31.9; 3.0)b | 16.9 (7.4; 56.5) | 13.4 (7.1; 45.1) | −0.7 (−12.4; 4.6) |
| By baseline eGFR | ||||||
| eGFR >90 mL/min/1.73 m2, n | 97 | 97 | 97 | 293 | 293 | 293 |
| Mean ± SEM | 136.5 ± 45.5 | 82.8 ± 23.8 | −53.7 ± 33.6 | 58.5 ± 9.1 | 49.6 ± 8.8 | −8.9 ± 8.0 |
| Median (IQR) | 19.0 (7.4; −75.0) | 12.0 (7.5; −48.8)a | −2.1 (−27.0; −2.7) | 15.2 (6.8; 47.3) | 12.0 (6.0; 32.4) | −1.0 (−12.3; 4.5) |
| eGFR ≤90 mL/min/1.73 m2, n | 92 | 92 | 92 | 566 | 566 | 566 |
| Mean ± SEM | 115.6 ± 34.3 | 69.2 ± 15.0 | −46.4 ± 25.6b | 96.9 ± 12.5 | 94.2 ± 12.3 | −2.8 ± 6.8 |
| Median (IQR) | 25.1 (8.1; −123.7) | 18.5 (7.1; −70.1)a | −2.7 (−30.7; −2.0)b | 15.8 (6.5; 48.9) | 13.0 (6.0; 39.2) | −0.7 (−10.6; 4.6) |
Values of AER are reported both as mean ± SEM (with p values from paired Student's t test) and as median and IQR (with P values from Wilcoxon rank test). ACEi, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers.
P < 0.05 versus baseline.
P < 0.05 versus comparators.
Figure 2.

Change in AER during therapy with dapagliflozin or comparators. Values of AER at baseline (pre) and at follow‐up (post), along with the change from baseline (right panel), are shown for A, all patients, B, patients with baseline normoalbuminuria, C, microalbuminuria or D, macroalbuminuria. Columns' height in histograms indicate median value, whereas bars indicate the interquartile range. *P < 0.05 for the indicated comparison
In patients who received a comparator, overall AER did not change significantly from baseline to follow‐up (average ± SEM change −5.9 ± 4.1 mg/g; P = 0.156), although it declined in those with baseline macroalbuminuria. The change in AER was significantly greater during therapy with dapagliflozin than with comparators, especially for patients with micro/macroalbuminuria at baseline (Table 2). During therapy with dapagliflozin, AER declined significantly both in patients with and in those without ongoing therapy with ACE inhibitors (ACEi) or angiotensin receptor blockers (ARBs), while AER did not change significantly in the comparator group, irrespective of therapy with ACEi/ARBs. The change from baseline in AER in patients on ACEi/ARBs was significantly greater during therapy with dapagliflozin than comparators. Dapagliflozin reduced AER irrespectively of baseline eGFR (< or > 90 mL/min/1.73 m2) and significantly more than comparators in patients with eGFR <90 mL/min/1.73 m2 (Table 2).
After adjusting for baseline differences between the two groups with a multiple regression analysis, therapy with dapagliflozin remained associated with a significantly greater reduction in AER than therapy with comparators (−26.4 ± 13.1 mg/g; P = 0.045) (Table 3).
Table 3.
Results of the multiple regression analysis
| Variable | B ± SEM | P |
|---|---|---|
| Dapagliflozin (vs. comparators) | −26.42 ± 13.14 | 0.045 |
| Age, y | 0.39 ± 0.57 | 0.497 |
| BMI, kg/m2 | 0.49 ± 0.86 | 0.564 |
| Diastolic blood pressure, mm Hg | −0.36 ± 0.47 | 0.444 |
| Fasting plasma glucose, mg/dL | −0.45 ± 0.18 | <0.001 |
| HbA1c, % | −4.14 ± 5.02 | 0.410 |
| Triglycerides, mg/dL | 0.02 ± 0.04 | 0.585 |
| SGPT, U/L | 0.12 ± 0.24 | 0.618 |
| eGFR, mL/min/1.73 m2 | 0.33 ± 0.29 | 0.251 |
| Insulin use (yes vs. no) | −2.80 ± 11.4 | 0.806 |
| Metformin use (yes vs. no) | 24.50 ± 13.27 | 0.065 |
| Diuretic use (yes vs. no) | −7.19 ± 11.16 | 0.520 |
| Microangiopathy (yes vs. no) | −17.61 ± 8.80 | 0.045 |
Change in AER was the dependent variable, whereas covariates were selected as variables that remained different between the two groups after Bonferroni correction (see Table 1).
According to AER categories, 12.8% of patients in the dapagliflozin group and 10.9% in the comparator group regressed to a lower AER class, whereas 4.4% in the dapagliflozin group and 8.3% in the comparator group progressed to a higher class, for a net improvement of 8.4% in the dapagliflozin group versus 2.6% in the comparator group (P < 0.001) (Figure S1).
The change in eGFR was analysed in 393 patients in the dapagliflozin group and 2277 patients in the comparator group. During therapy with dapagliflozin, mean ± SD eGFR declined from 87.5 ± 16.3 to 86.5 ± 17.8 mL/min/1.73 m2 (P = 0.049), with a reduction of 1.1 mL/min/1.73 m2. During therapy with a comparator, eGFR declined from 79.2 ± 18.8 to 78.6 ± 19.1 mL/min/1.73 m2 (P = 0.002), equivalent to a reduction of 0.6 mL/min/1.73 m2. The change from baseline in eGFR between the two groups was not significantly different (P = 0.35). No patient in the dapagliflozin group versus 4 patients in the comparator group exhibited a doubling of serum creatinine; no patient in the dapagliflozin group versus 11 patients in the comparator group exhibited a decline of >40% in eGFR (both not significantly different between groups).
3.3. Overall effectiveness of dapagliflozin and comparators
Effectiveness of dapagliflozin and comparators on glucose control, blood pressure and body weight has already been described in the total cohort of patients with a follow‐up examination.20 In the present subgroups, HbA1c declined by 0.8 ± 1.2% in the dapagliflozin group (n = 490) and 0.6 ± 1.1% in the comparator group (n = 2899); body weight decreased by 2.9 ± 3.5 kg in the dapagliflozin group (n = 456) and 0.6 ± 3.3 kg in the comparator group (n = 2631); systolic blood pressure declined by 3.3 ± 17.5 mm Hg in the dapagliflozin group (n = 336) and 0.2 ± 19.4 mm Hg in the comparator group (n = 1972).
Because there was no correlation between the changes in HbA1c and systolic blood pressure (SBP) (r = 0.04; P = 0.100), in an exploratory analysis we selected patients who experienced a below median response in HbA1c and an above median response in SBP: in 31 patients who received dapagliflozin, AER declined by 35%, from median (IQR) 23.7 (6.7; 137.3) to 16.9 (6.8; 80.4) (P = 0.09), while in 162 patients who received comparators, AER tended to increase by 23% (P = 0.13).
3.4. Predictors of improvement in AER
To detect predictors of AER decline in patients who received dapagliflozin, we first analysed linear correlations: the change in AER was inversely related to change in eGFR (r = −0.17; P = 0.024; n = 169), but not to the change in other efficacy variables (HbA1c, blood pressure and body weight). Thus, we used two complementary non‐linear approaches: random forests and PLS (Table S3). Variables detected by RF were, in order of importance, HDL, LDL, diastolic blood pressure, triglycerides, total cholesterol, SBP and disease duration. According to PLS, only baseline AER, HbA1c and diastolic blood pressure had a significant impact.
4. DISCUSSION
In this real‐world study, T2D patients initiating dapagliflozin experienced a significant reduction in AER at the first follow‐up visit, on average ~6 months after baseline. Remarkably, a similar effect was not observed with comparator GLM. While a mild and marginally significant decline in eGFR was observed during dapagliflozin use, there was no evidence of clinically meaningful impairment in renal function. These results are extremely consistent with findings from phase III RCTs and confirm that dapagliflozin exerts beneficial effects on the kidney. Because renal protection can contribute to cardiovascular protection, we argue that these data lend support to the potential cardiovascular efficacy of dapagliflozin. While awaiting for results of the dedicated cardiovascular outcome trial,21 registry studies indicate that dapagliflozin can protect from cardiovascular events similarly to other SGLT2i.22
In RCTs, dapagliflozin consistently reduced AER by 36‐40%8, 9 irrespective of therapy with ACE inhibitors or ARBs.10 In our study, AER declined by 37% in patients taking, and by 45% in patients not taking ACEi/ARBs. The antiproteinuric effects of dapagliflozin are probably largely independent of glucose control, as patients who experienced a decline in SBP with no decline in HbA1c showed an AER reduction quantitatively similar to the entire cohort, while the same was not observed in patients taking other GLM. This observation is consistent with the supposed direct renal effect of SGLT2i via reactivation of the tubular‐glomerular feedback.23
Owing to the non‐randomized nature of the comparison between treatments, the analysis of AER response predictors was performed only in the dapagliflozin group. The change in eGFR emerged as the sole linear predictor, but it explained <3% of AER variation. Non‐linear approaches identified blood pressure, lipid profile, HbA1c and disease duration as variables that impacted the ability of dapagliflozin to lower AER. While triglycerides have already been associated with diabetic nephropathy,24, 25, 26 it is remarkable that cholesterol levels modulated the renal effects of SGLT2i.
In RCTs, dapagliflozin therapy was associated with an initial drop in eGFR, followed by eGFR stabilization, such that, after 2 years of treatment, eGFR was significantly higher than in placebo‐treated patients.27 The mild decline in eGFR we observe at ~6 months after initiation of dapagliflozin is consistent with RCTs and the change in eGFR during dapagliflozin was not significantly different than during therapy with comparators. Our data are also particularly reassuring on the risk that SGLT2i may acutely worsen renal function. Postmarketing pharmacovigilance has identified cases of acute kidney injury (AKI) in patients taking SGLT2i,28, 29 probably because of alterations of renal haemodynamics.30, 31 On the contrary, an observational study comparing 377 SGLT2i users with 377 matched non‐users did not find any association between SGLT2i and AKI.32 In our survey, we found no case of doubling of serum creatinine among 393 patients initiated on dapagliflozin, also suggesting that dapagliflozin did not precipitate AKI over the ~6‐month follow‐up.
Interpretation of our results must take into consideration the study's limitations. First, AER was derived from a single measure and had to be converted from different units of measure. This is, however, commonly carried out in clinical practice and validated in epidemiological research.33 Second, patients who received comparator GLM in the DARWIN‐T2D trial were significantly different to those who received dapagliflozin. As already noted, because of the massive channelling of dapagliflozin to difficult‐to‐treat patients, a propensity score matching was inefficient and adjustment was performed using multiple regression. Thus, the degree of evidence that can be inferred from this observational study is not comparable to that of an RCT. Third, follow‐up duration was short (~6 months), only allowing an analysis of acute changes in renal endpoints. While the effects of dapagliflozin on AER are expected to be rapid,8 a longer observation will be needed to evaluate benefits over time and trends in eGFR. The fact that AER did not decline in the comparator group must also be interpreted in view of the short follow‐up, because GLP‐1RA and DPP‐4 inhibitors may take longer to reduce AER.34, 35 Finally, the sample size was small: less than 60% of patients had AER and/or eGFR available at both visits. This was not surprising, because eGFR and AER are not routinely checked at short intervals in all patients, but it suggests that selection bias cannot be ruled out and generalizability should be considered with caution.
In summary, this is the first real‐world study confirming that a short‐term treatment with the SGLT2i dapagliflozin reduces albuminuria and does not cause a clinically meaningful worsening in renal function. Although longer and larger studies may be needed to establish whether these benefits persist over time in the real world, our study supports the validity and importance of registry interrogation to determine transferability of RCT data to the clinical setting.
Supporting information
File S1. Online supplement
Table S1. Baseline clinical characteristics of study patients. Patients are divided according to the availability of AER and/or eGFR at both visits. Percent of available data is reported for all variable. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL, high density lipoprotein; LDL low density lipoprotein; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase; AER, albumin excretion rate; eGFR, estimated glomerular filtration rate; ACEi, angiotensin converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CCB, calcium channel blockers; PAD, peripheral arterial disease; TIA, transient ischemic attack.
Table S2. Analysis of selection bias. Comparison of clinical characteristics between patients with and without data on renal outcomes at follow‐up. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL, high density lipoprotein; LDL low density lipoprotein; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase; AER, albumin excretion rate; eGFR, estimated glomerular filtration rate; ACEi, angiotensin converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CCB, calcium channel blockers. P‐values for the comparison between groups are shown. However, statistical significance based on p‐values largely depends on sample size and does not inform on the magnitude of the difference. Thus, to better evaluate clinical meaningfulness of the differences, we calculated the standardized difference (D). A value of D ≤ 0.10 is usually indicative of a good match between groups.
Table S3. Determinants of the change in AER. The Random Forest (RF) procedure selected variables on the basis of the percentage increment in mean square error (%IncMSE). We created decision trees and their errors for 500 bootstrapped sub‐datasets and compared them with the error obtained permuting values of each variable: the higher the %IncMSE the greater the importance of each variable (1). Partial least squares (PLS) regression, also known as projection to latent structures, is an extension of the principal component analysis, and was run to identify variables that most contributed to the change in AER. A variable importance in projection (VIP) of 0.8 or higher and with a 95% jacknifed confidence interval not crossing zero was chosen to select the variables that most affected the outcome (2). VIPs and %IncMSEs from individual imputed datasets were pooled as described by Rubin (3). Variables marked with * are considered to be the most significant contributors to change in AER. AER, albumin excretion rate; LDL, low density lipoproteins; HDL, high density lipoproteins; TC, total cholesterol; TG, triglycerides; FPG, fasting plasma glucose; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; %IncMSE, percent increase in mean square error; VIP, variable importance in projection.
Figure S1. Change in albuminuria category. For patients who received dapagliflozin or comparators, we calculated the percentages of patients who exhibited a progression (+) or a regression (−) in AER category for the entire cohort (all) and by baseline albuminuria class. *P < 0.05 from chi square test.
ACKNOWLEDGMENTS
We wish to thank Alessia Russo, Italian Diabetes Society, for the invaluable technical support. The study was supported by the Italian Diabetes Society, through a grant from AstraZeneca. The external sponsor had no role in study design, data analysis and interpretation, and decision to publish.
Composition of the DARWIN‐T2D database
Agostino Consoli and Gloria Formoso (Dipartimento di Medicina e Scienze dell'Invecchiamento ‐ Università Degli studi G. D'Annunzio di Chieti‐Pescara); Giovanni Grossi (Ospedale San Francesco di Paola ‐ Azienda Sanitaria Provinciale di Cosenza); Achiropita Pucci (Azienda Sanitaria Provinciale di Cosenza); Giorgio Sesti and Francesco Andreozzi (Azienda Ospedaliero Universitaria di Catanzaro); Giuseppe Capobianco (Azienda Sanitaria Locale Napoli 2 Nord); Adriano Gatti (Ospedale San Gennaro dei Poveri ‐ Azienda Sanitaria Locale Napoli 1 Centro); Riccardo Bonadonna, Ivana Zavaroni and Alessandra Dei Cas (Azienda Ospedaliero Universitaria di Parma); Giuseppe Felace (Ospedale di Spilimbergo ‐ Azienda per l'Assistenza Sanitaria n.5 Friuli Occidentale); Patrizia Li Volsi (Ospedale di Pordenone ‐ Azienda per l'Assistenza Sanitaria n.5 Friuli Occidentale); Raffaella Buzzetti and Gaetano Leto (Ospedale Santa Maria Goretti ‐ Azienda Sanitaria Locale di Latina); Gian Pio Sorice (Fondazione Policlinico Universitario A. Gemelli, Roma); Paola D'Angelo (Ospedale Sandro Pertini ‐ Azienda Sanitaria Locale Roma 2); Susanna Morano (Azienda Ospedaliera Universitaria Policlinico Umberto I, Roma); Antonio Carlo Bossi (Ospedale di Treviglio ‐ Azienda Socio Sanitaria Territoriale Bergamo Ovest); Edoardo Duratorre (Ospedale Luini Confalonieri di Luino ‐ Azienda Socio Sanitaria Territoriale Sette Laghi); Ivano Franzetti (Ospedale Sant'Antonio Abate di Gallarate ‐ Azienda Socio Sanitaria Territoriale Valle Olona); Paola Silvia Morpurgo (Ospedale Fatebenefratelli ‐ Azienda Socio Sanitaria Territoriale Fatebenefratelli Sacco); Emanuela Orsi (Fondazione IRCCS Ca’ Granda ‐ Ospedale Maggiore Policlinico di Milano); Fabrizio Querci (Ospedale Pesenti Fenaroli di Alzano Lombardo ‐ Azienda Socio Sanitaria Territoriale Bergamo Est); Massimo Boemi† and Federica D'Angelo (Presidio Ospedaliero di Ricerca INRCA‐IRCCS di Ancona); Massimiliano Petrelli (Azienda Ospedaliero Universitaria Ospedali Riuniti di Ancona); Gianluca Aimaretti and Ioannis Karamouzis (Azienda Ospedaliero Universitaria Maggiore della Carità di Novara); Franco Cavalot (Azienda Ospedaliero Universitaria San Luigi Gonzaga, Orbassano); Giuseppe Saglietti† (Ospedale Madonna del Popolo di Omegna ‐ Azienda Sanitaria Locale Verbano Cusio Ossola); Giuliana Cazzetta (Casa della Salute, Ugento ‐ Distretto Socio Sanitario Gagliano del Capo ‐ Azienda Sanitaria Locale di Lecce); Silvestre Cervone (Presidio ospedaliero San Marco in Lamis ‐ Distretto Socio Sanitario San Marco in Lamis ‐ Azienda Sanitaria Locale di Foggia); Eleonora Devangelio (Distretto Socio Sanitario di Massafra ‐ Azienda Sanitaria Locale di Taranto); Olga Lamacchia (Azienda Ospedaliero Universitaria Ospedali Riuniti di Foggia); Salvatore Arena (Ospedale Umberto I ‐ Azienda Sanitaria Provinciale di Siracusa); Antonino Di Benedetto (Azienda Ospedaliera Universitaria Policlinico G. Martino di Messina); Lucia Frittitta (Azienda Ospedaliera di Rilievo Nazionale e di Alta Specializzazione Garibaldi di Catania); Carla Giordano (Azienda Universitaria Policlinico Paolo Giaccone di Palermo); Salvatore Piro (Azienda Ospedaliera di Rilievo Nazionale e di Alta Specializzazione Garibaldi di Catania); Manfredi Rizzo, Roberta Chianetta and Carlo Mannina (Azienda Universitaria Policlinico Paolo Giaccone di Palermo); Roberto Anichini (Ospedale San Jacopo di Pistoia ‐ Azienda USL Toscana Centro); Giuseppe Penno (Azienda Ospedaliero Universitaria Pisana); Anna Solini (Azienda Ospedaliera Universitaria Pisana); Bruno Fattor (Comprensorio Sanitario di Bolzano ‐ Azienda Sanitaria della Provincia Autonoma di Bolzano); Enzo Bonora and Massimo Cigolini (Azienda Ospedaliero Universitaria Integrata di Verona); Annunziata Lapolla and Nino Cristiano Chilelli (Complesso Socio Sanitario Ai Colli ‐ Azienda ULSS n.6 Euganea); Maurizio Poli (Ospedale Girolamo Fracastoro di San Bonifacio ‐ Azienda ULSS n.9 Scaligera); Natalino Simioni and Vera Frison (Ospedale di Cittadella ‐ Azienda ULSS n.6 Euganea); Carmela Vinci (Azienda ULSS n.4 Veneto Orientale).
Conflicts of interest
G. P. F. received grant support, lecture or advisory board fees from AstraZeneca, Boehringer‐Ingelheim, Eli Lilly, Novo Nordisk, Sanofi, Genzyme, Abbott, Novartis, Merck Sharp & Dohme. A. S. received research grants from Astra Zeneca and served as advisory board member for Boehringer‐Ingelheim and Eli‐Lilly. G. P. reported receiving personal fees from Astra‐Zeneca, Boehringer‐Ingelheim, Eli‐Lilly, and Merck‐Sharp & Dohme. A. G. received grant support or lecture fees from Novo Nordisk, Takeda, AstraZeneca. R. A. received research grants, lecture or advisory board fees from Merck Sharp & Dome, AstraZeneca, Novartis, Boeringher‐Ingelheim, Sanofi, Takeda, Janssen, Novo Nordisk, Eli Lilly, J.J and Mundi Pharma. S. D. P. received research support from AstraZeneca, Boheringer‐Ingelheim, Merck Sharpe & Dohme, Novartis Pharmaceutical Co., and lecture and advisory board fees from Abbott, AstraZeneca, Boheringer‐Ingelheim, Eli Lilly & Co, GlaxoSmithKline, Laboratoires Servier, Hamni Pharmaceuticals, Merck Sharpe & Dohme, Novartis Pharmaceutical Co., Novo Nordisk, Sanofi, and Takeda Pharmaceuticals. A. A. received research grants, lecture or advisory board fees from Merck Sharp & Dome, AstraZeneca, Novartis, Boeringher‐Ingelheim, Sanofi, Mediolanum, Janssen, and Novo Nordisk. M. L. M. declares no conflict of interest.
Author contributions
Study design: G. P. F., A. S., S. D. P. and A. A. Data collection and analysis: G. P. F., A. S., A. G., R. A., G. P. and M. L. M. Manuscript writing: G. P. F., A. S. and A. A. Manuscript revision: A. S., S. D. P. and A. A. All authors approved the final version of the manuscript.
Fadini GP, Solini A, Manca ML, et al. Effectiveness of dapagliflozin versus comparators on renal endpoints in the real world: A multicentre retrospective study. Diabetes Obes Metab. 2019;21:252–260. 10.1111/dom.13508
Funding information The study was supported by the Italian Diabetes Society, through a grant from AstraZeneca.
REFERENCES
- 1. Avogaro A, Fadini GP, Sesti G, Bonora E, Del Prato S. Continued efforts to translate diabetes cardiovascular outcome trials into clinical practice. Cardiovasc Diabetol. 2016;15:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644‐657. [DOI] [PubMed] [Google Scholar]
- 3. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 4. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 5. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mann JFE, Orsted DD, Brown‐Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839‐848. [DOI] [PubMed] [Google Scholar]
- 7. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323‐334. [DOI] [PubMed] [Google Scholar]
- 8. Dekkers CCJ, Petrykiv S, Laverman GD, Cherney DZ, Gansevoort RT, Heerspink HJL. Effects of the SGLT‐2 inhibitor dapagliflozin on glomerular and tubular injury markers. Diabetes Obes Metab. 2018;20:1988‐1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petrykiv SI, Laverman GD, de Zeeuw D, Heerspink HJL. The albuminuria‐lowering response to dapagliflozin is variable and reproducible among individual patients. Diabetes Obes Metab. 2017;19:1363‐1370. [DOI] [PubMed] [Google Scholar]
- 10. Heerspink HJ, Johnsson E, Gause‐Nilsson I, Cain VA, Sjostrom CD. Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin‐angiotensin blockers. Diabetes Obes Metab. 2016;18:590‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hamada S, Gulliford MC. Multiple risk factor control, mortality and cardiovascular events in type 2 diabetes and chronic kidney disease: a population‐based cohort study. BMJ Open. 2018;8:e019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Penno G, Solini A, Bonora E, et al. Defining the contribution of chronic kidney disease to all‐cause mortality in patients with type 2 diabetes: the renal insufficiency and cardiovascular events (RIACE) Italian multicenter study. Acta Diabetol. 2018;55:603‐612. [DOI] [PubMed] [Google Scholar]
- 13. Cea Soriano L, Johansson S, Stefansson B, Rodriguez LA. Cardiovascular events and all‐cause mortality in a cohort of 57,946 patients with type 2 diabetes: associations with renal function and cardiovascular risk factors. Cardiovasc Diabetol. 2015;14:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fox CS, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end‐stage renal disease in individuals with and without diabetes: a meta‐analysis. Lancet. 2012;380:1662‐1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perrone‐Filardi P, Avogaro A, Bonora E, et al. Mechanisms linking empagliflozin to cardiovascular and renal protection. Int J Cardiol. 2017;241:450‐456. [DOI] [PubMed] [Google Scholar]
- 16. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752‐772. [DOI] [PubMed] [Google Scholar]
- 17. Frieden TR. Evidence for health decision making ‐ beyond randomized, controlled trials. N Engl J Med. 2017;377:465‐475. [DOI] [PubMed] [Google Scholar]
- 18. Patel A, Billot L. Reality and truth: balancing the hope and the hype of real‐world evidence. Circulation. 2017;136:260‐262. [DOI] [PubMed] [Google Scholar]
- 19. Fadini GP, Zatti G, Consoli A, Bonora E, Sesti G, Avogaro A. Rationale and design of the DARWIN‐T2D (DApagliflozin real world evIdeNce in type 2 diabetes): a multicenter retrospective nationwide Italian study and crowdsourcing opportunity. Nutr Metab Cardiovasc Dis. 2017;27:1089‐1097. [DOI] [PubMed] [Google Scholar]
- 20. Fadini GP, Zatti G, Baldi I, et al. Use and effectiveness of dapagliflozin in routine clinical practice: an Italian multicentre retrospective study. Diabetes Obes Metab. 2018;20:1781‐1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Raz I, Mosenzon O, Bonaca MP, et al. DECLARE‐TIMI 58: Participants' baseline characteristics. Diabetes Obes Metab. 2018;20:1102‐1110. [DOI] [PubMed] [Google Scholar]
- 22. Birkeland KI, Jorgensen ME, Carstensen B, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium‐glucose co‐transporter‐2 inhibitors versus other glucose‐lowering drugs (CVD‐REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2017;5:709‐717. [DOI] [PubMed] [Google Scholar]
- 23. Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium‐glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587‐597. [DOI] [PubMed] [Google Scholar]
- 24. Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR. Risk factors for renal dysfunction in type 2 diabetes: U.K. prospective diabetes study 74. Diabetes. 2006;55:1832‐1839. [DOI] [PubMed] [Google Scholar]
- 25. Penno G, Solini A, Zoppini G, et al. Rate and determinants of association between advanced retinopathy and chronic kidney disease in patients with type 2 diabetes: the renal insufficiency and cardiovascular events (RIACE) Italian multicenter study. Diabetes Care. 2012;35:2317‐2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Russo GT, De Cosmo S, Viazzi F, et al. Plasma triglycerides and HDL‐C levels predict the development of diabetic kidney disease in subjects with type 2 diabetes: the AMD annals initiative. Diabetes Care. 2016;39:2278‐2287. [DOI] [PubMed] [Google Scholar]
- 27. Kohan DE, Fioretto P, Johnsson K, Parikh S, Ptaszynska A, Ying L. The effect of dapagliflozin on renal function in patients with type 2 diabetes. J Nephrol. 2016;29:391‐400. [DOI] [PubMed] [Google Scholar]
- 28. Perlman A, Heyman SN, Matok I, Stokar J, Muszkat M, Szalat A. Acute renal failure with sodium‐glucose‐cotransporter‐2 inhibitors: analysis of the FDA adverse event report system database. Nutr Metab Cardiovasc Dis. 2017;27:1108‐1113. [DOI] [PubMed] [Google Scholar]
- 29. Desai M, Yavin Y, Balis D, et al. Renal safety of canagliflozin, a sodium glucose co‐transporter 2 inhibitor, in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2017;19:897‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Szalat A, Perlman A, Muszkat M, Khamaisi M, Abassi Z, Heyman SN. Can SGLT2 inhibitors cause acute renal failure? Plausible role for altered glomerular hemodynamics and medullary hypoxia. Drug Saf. 2018;41:239‐252. [DOI] [PubMed] [Google Scholar]
- 31. Hahn K, Ejaz AA, Kanbay M, Lanaspa MA, Johnson RJ. Acute kidney injury from SGLT2 inhibitors: potential mechanisms. Nat Rev Nephrol. 2016;12:711‐712. [DOI] [PubMed] [Google Scholar]
- 32. Nadkarni GN, Ferrandino R, Chang A, et al. Acute kidney injury in patients on SGLT2 inhibitors: a propensity‐matched analysis. Diabetes Care. 2017;40:1479‐1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pugliese G, Solini A, Fondelli C, et al. Reproducibility of albuminuria in type 2 diabetic subjects. Findings from the renal insufficiency and cardiovascular events (RIACE) study. Nephrol Dial Transplant. 2011;26:3950‐3954. [DOI] [PubMed] [Google Scholar]
- 34. Avgerinos I, Karagiannis T, Malandris K, et al. Glucagon‐like peptide 1 receptor agonists and microvascular outcomes in type 2 diabetes: a systematic review and meta‐analysis. Diabetes Obes Metab. 2018. [published online ahead of print 29. 10.1111/dom.13484]. [DOI] [PubMed] [Google Scholar]
- 35. Avogaro A, Fadini GP. The effects of dipeptidyl peptidase‐4 inhibition on microvascular diabetes complications. Diabetes Care. 2014;37:2884‐2894. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1. Online supplement
Table S1. Baseline clinical characteristics of study patients. Patients are divided according to the availability of AER and/or eGFR at both visits. Percent of available data is reported for all variable. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL, high density lipoprotein; LDL low density lipoprotein; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase; AER, albumin excretion rate; eGFR, estimated glomerular filtration rate; ACEi, angiotensin converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CCB, calcium channel blockers; PAD, peripheral arterial disease; TIA, transient ischemic attack.
Table S2. Analysis of selection bias. Comparison of clinical characteristics between patients with and without data on renal outcomes at follow‐up. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL, high density lipoprotein; LDL low density lipoprotein; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase; AER, albumin excretion rate; eGFR, estimated glomerular filtration rate; ACEi, angiotensin converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CCB, calcium channel blockers. P‐values for the comparison between groups are shown. However, statistical significance based on p‐values largely depends on sample size and does not inform on the magnitude of the difference. Thus, to better evaluate clinical meaningfulness of the differences, we calculated the standardized difference (D). A value of D ≤ 0.10 is usually indicative of a good match between groups.
Table S3. Determinants of the change in AER. The Random Forest (RF) procedure selected variables on the basis of the percentage increment in mean square error (%IncMSE). We created decision trees and their errors for 500 bootstrapped sub‐datasets and compared them with the error obtained permuting values of each variable: the higher the %IncMSE the greater the importance of each variable (1). Partial least squares (PLS) regression, also known as projection to latent structures, is an extension of the principal component analysis, and was run to identify variables that most contributed to the change in AER. A variable importance in projection (VIP) of 0.8 or higher and with a 95% jacknifed confidence interval not crossing zero was chosen to select the variables that most affected the outcome (2). VIPs and %IncMSEs from individual imputed datasets were pooled as described by Rubin (3). Variables marked with * are considered to be the most significant contributors to change in AER. AER, albumin excretion rate; LDL, low density lipoproteins; HDL, high density lipoproteins; TC, total cholesterol; TG, triglycerides; FPG, fasting plasma glucose; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; %IncMSE, percent increase in mean square error; VIP, variable importance in projection.
Figure S1. Change in albuminuria category. For patients who received dapagliflozin or comparators, we calculated the percentages of patients who exhibited a progression (+) or a regression (−) in AER category for the entire cohort (all) and by baseline albuminuria class. *P < 0.05 from chi square test.


