Abstract
Aim
We aimed to compare modifiable cardiometabolic risk factors among treatment‐seeking adolescents with obesity in Italy, Germany and Norway.
Methods
This retrospective, registry‐based, cross‐sectional cohort study included 2,327 (59% girls) 12–18 year‐old adolescents with obesity from three tertiary care outpatient clinics in Europe, between 1999 and 2015. The prevalence of cardiometabolic risk factors was compared between clinics, and multivariate logistic regression models including gender, age, waist circumference and body mass index were used to assess the associations between population and cardiometabolic risk.
Results
In total, 1,396 adolescents (60% girls) from Italy, 654 (58% girls) from Germany and 277 (51% girls) from Norway were included. The mean ± SD age was 15.2 ± 1.6 years, body mass index 38.8 ± 6.5 kg/m2 and body mass index standard deviation score 3.21 ± 0.43. The prevalence of elevated nonhigh‐density lipoprotein‐cholesterol in Norway, Germany and Italy was 60%, 54% and 45%, while the prevalence of high systolic or diastolic blood pressure (≥130 or ≥85 mmHg) were 15%, 46% and 66%, respectively.
Conclusion
Cardiometabolic risk factors among treatment‐seeking adolescents with obesity from Italy, Germany and Norway differed across the populations in this study, which might imply that preventive clinical work should reflect such differences.
Keywords: Adolescence, Cardiometabolic risk factors, Dyslipidemia, Metabolic syndrome, Obesity
Abbreviations
- BMI
Body mass index
- BMI SDS
Body mass index standard deviation score
- BP
Blood pressure
- HDL‐C
High‐density lipoprotein‐cholesterol
- LDL‐C
Low‐density lipoprotein‐cholesterol
- SD
Standard deviation
Key notes.
Cardiometabolic risk factors might differ across populations; we investigated 2,327 treatment‐seeking adolescents with obesity from Italy, Germany and Norway.
The prevalence of elevated nonhigh‐density lipoprotein‐cholesterol was highest among Norwegians (60%) and lowest among Italians (45%), while high blood pressure was most common in the German (66%) and lowest in the Norwegian (15%) cohort.
Our findings suggest that preventive clinical work should reflect such differences in cardiometabolic risk factors across populations.
Introduction
Childhood obesity has reached epidemic proportions worldwide 1. During childhood and adolescence, obesity in general, and abdominal obesity in particular, is associated with increased levels of cardiovascular risk factors, such as serum triglycerides, low‐density lipoprotein‐cholesterol (LDL‐C) and decreased high‐density lipoprotein‐cholesterol (HDL‐C). Being obese is also associated with dysglycaemia and high blood pressure. All these factors are independent risk factors for cardiovascular disease 2. An unhealthy diet has been associated with obesity, dyslipidemia, dysglycaemia and high blood pressure, whilst weight loss, healthy diets and physical activity may improve these risk factors 3. In addition, geographic, genetic, cultural and lifestyle differences may influence the cardiometabolic effects of severe obesity 4, 5.
During the last decade, it has been suggested that elevated nonhigh‐density lipoprotein‐cholesterol (non HDL‐C), containing all the atherogenic particles, may be a stronger predictor of morbidity and mortality from cardiovascular disease than elevated LDL‐C 6. Nonetheless, elevated LDL‐C is a major causal risk factor for cardiovascular disease in adults, and it has been demonstrated that lowering LDL‐C reduces the risk of cardiovascular disease 7. In a clinical report from 2017, the American Academy of Pediatrics recommended shifting the focus in youths from metabolic syndrome, a cluster of metabolic risk factors 8, to single specific risk factors, such as hypertension and LDL‐C 9.
We aimed to assess regional differences in south, middle and north of Europe in the prevalence of specific cardiometabolic risk factors; defined as elevated levels of LDL‐C, non HDL‐C and triglycerides, dysglycaemia, elevated blood pressure and metabolic syndrome, in treatment‐seeking Italian, German and Norwegian adolescents with moderate to severe obesity. We hypothesised that the prevalence of specific cardiometabolic risk factors among adolescents seeking treatment for obesity differed between these countries.
Materials and methods
Design, setting and participants
This study was a collaborative project between three tertiary obesity clinics in Italy, Germany and Norway, comparing three registry‐based cohorts of adolescents (12–18 years) with obesity using a retrospective, cross‐sectional design. The investigators agreed on prespecified research questions (hypotheses), which could be tested by using the available data.
The patients were referred to the obesity clinics by primary care paediatricians or general practitioners. Adolescents with moderate to severe obesity 10, and with complete data on age, gender, body mass index (BMI), LDL‐C, non HDL‐C and components of metabolic syndrome 8; waist circumference, systolic and diastolic blood pressure (systolic BP and diastolic BP), triglycerides, HDL‐C and fasting plasma glucose recorded, were included into the study.
The Italian cohort consisted of 1396 (60% female) adolescents referred to the Division of Auxology, Istituto Auxologico Italiano, Verbania, between 2010 and 2013.
The German cohort consisted of 654 (58% female) adolescents referred to Adipositas Reha‐Klinik Insula, Bischofswiesen, Oberbayern, in the period 1999 to 2008.
The Norwegian cohort included 277 adolescents (51% female), referred to the Morbid Obesity Centre, Vestfold Hospital Trust, Tønsberg, from 2009 to 2015.
Data sources and measurements
An overview is presented in Table S1. Height was measured to the nearest 0.1 cm using a Harpenden stadiometer (Holtain, Pembrokeshire, UK) in Italy and Germany, and a Heightronic Digital Stadiometer (QuickMedical, Washington, DC, USA) in Norway. Weight (kg) was measured by a Salus electronic scale (Salus Vandoni Srl, Gaggiano, Italy) in the Italian and German cohorts. In the Norwegian cohort, weight was measured using a Tanita BC‐418 bioimpedance body composition analyser (Tanita Corp., Tokyo, Japan). Waist circumference was measured using a standardised anthropometric tape, measuring the circumference at the midpoint between the iliac crest and the lower part of the lateral rib cage (cm). BMI was calculated as weight (kg)/height (m)2, and converted to age‐ and sex‐adjusted BMI standard deviation score (BMI SDS) according to the reference from the International Obesity Task Force 10. BMI was also classified into overweight, obesity or severe obesity according to the International Obesity Task Force definitions; BMI corresponding to a BMI of ≥25–29, ≥30–34 or ≥35 kg/m2 at age 18 10.
Blood pressure measurements
The Italian cohort measured systolic and diastolic BP twice on the dominant arm in the sitting position with an aneroid sphygmomanometer (Tema Certus, Milan, Italy), by using appropriate sized cuffs after the patient had rested for at least 15 minutes. The mean values were calculated and rounded to the nearest 5 mmHg value.
The German adolescents had their BP measured twice aneroidly (Bosch und Sohn boso medicus control, Juningen, Germany) in a sitting position at least five minutes after arriving in the medical department, using large cuff sizes on the mid‐upper dominant arm. The mean values were calculated.
In Norway, BP was measured using a Dinamap ProCare digital oscillometric device (GE Healthcare, Buckinghamshire, UK). BP measurements were performed in the sitting position using appropriate sized cuffs (the mid‐upper arm circumference was measured) four times on the dominant arm, and the average of the three last measurements was calculated.
Metabolic measurements
Venous blood samples were collected after an overnight fast. In Italy and Germany, the analyses were done using Cobas Hitachi enzymatic colorimetric assays (Roche Diagnostics Gmbh, Mannheim, Germany). In Norway, the analyses were performed immediately thereafter by layered dry‐slide chemical methods with photometric reflection and potentiometric detection principles using Vitros Microslide Technology (Ortho‐Clinical Diagnostics, Buckinghamshire, UK).
Definition of cardiometabolic risk factors and MetS
LDL‐C was calculated using the Friedewald equation; LDL‐C = total cholesterol – HDL‐C – triglycerides/2.2. Non HDL‐C (mmol/L) was calculated as total‐cholesterol minus HDL‐C. Participants were classified as having elevated values (yes/no) of LDL‐C and non HDL‐C according to thresholds of ≥2.8 mmol/L and ≥3.1 mmol/L 11. Metabolic syndrome was defined according to the International Diabetes Federation criteria 8. The first criterion (mandatory) is abdominal obesity, waist circumference ≥90th percentile or adult cut‐off if lower 12. In addition, at least two of the other four criteria regarding BP, triglycerides, HDL‐C and fasting plasma glucose had to be fulfilled. Participants were classified as having elevated BP if they had systolic BP ≥130 or diastolic BP ≥85 mmHg and elevated plasma glucose if fasting plasma glucose ≥5.6 mmol/L. High triglycerides and low HDL‐C were defined as ≥1.7 mmol/L or <1.03 mmol/L (<1.29 mmol/L for females ≥16 years), respectively 8.
Ethical considerations
Parents or legal tutors of the participants signed a written informed consent. The study was approved by the Ethical Committee of Istituto Auxologico Italiano, IRCCS, Milan, Italy (ref. 01C630), the local Ethical Committee at Adipositas Reha Klinik Insula, Bischofswiesen, Oberbayern, Germany and by the Regional Committee for Medical and Health Research Ethics of South‐East Norway (2015/2415). Within the Norwegian cohort, adolescents and their parents provided written informed consent allowing inclusion into the Vestfold Childhood Obesity Registry upon engaging in treatment (Regional Ethics Committee approval number S‐08742c 2008/19081). Written informed consent was provided from all participants, and the study was performed in accordance with the Declaration of Helsinki.
Statistical analyses
Crude differences between groups regarding continuous and categorical variables were assessed using one‐way ANOVA or chi‐square test. In addition, mean standardised differences, Cohen`s d, were calculated.
Comparisons between the cohorts in regards to the prevalence of elevated LDL‐C and non HDL‐C levels, metabolic syndrome and its components were performed using chi‐square tests. In addition, univariate and multivariate logistic regression models were used to estimate possible associations between nationality – Italian or German, using Norwegian as the reference category due to the lowest prevalence metabolic syndrome – and the main outcomes, elevated LDL‐C and non HDL‐C, and metabolic syndrome and its components. The final multivariate analysis was adjusted for gender, using girls as the reference category, age (years), waist circumference (cm) and BMI (kg/m2).
The goodness of fit was tested using the Hosmer and Lemeshow test. This study is considered exploratory; therefore, no correction for multiple testing was performed and p‐values <0.05 were considered statistically significant. All analyses were performed with SPSS version 21.0 (IBM Corp., Armonk, New York, USA).
Results
Complete datasets for analysis were available from 98%, 91% and 72% of the Italian, Norwegian and German cohorts, respectively (Fig. 1). Demographic, clinical and biochemical characteristics are shown in Table 1. The mean age differed slightly between the cohorts (14.9–15.3 years), and more than half (59%) of the total cohort were female, with the lowest proportion (51%) in the Norwegian cohort. The German adolescents had higher mean measures of BMI‐SDS and waist circumference when compared with their Italian and Norwegian counterparts and nearly all adolescents fulfilled the metabolic syndrome criterion for abdominal obesity. The proportions of adolescents with BMI above the IOTF‐35 threshold of severe obesity were 74% in the Italian, 85% in the German and 83% in the Norwegian cohort. Mean systolic and diastolic BP values were highest in the German cohort (133 and 81 mmHg), and lowest in the Norwegian cohort (116 and 61 mmHg). The mean values of fasting plasma glucose, total‐cholesterol, HDL‐C and triglycerides differed significantly between the cohorts; effect sizes ranging from 0.1 to 0.8 for differences (Table 1), with the lowest mean value found in the Italian cohort. The mean LDL‐C levels did not differ among the cohorts.
Figure 1.

The exclusion process for the Italian, German and Norwegian cohorts. *One person can be missing more than one type of data value.
Table 1.
Demographic, clinical and biochemical characteristics of the adolescents
| Italy (n = 1,396) | Germany (n = 654) | Norway (n = 277) | Total (n = 2,327) | p value | Nor vs It | Nor vs Ger | It vs Ger | |
|---|---|---|---|---|---|---|---|---|
| Mean (SD) or n (%) | Standardised mean difference | |||||||
| Demographic data | ||||||||
| Gender, female (%) | 840 (60.2) | 382 (58.4) | 142 (51.3) | 1,364 (58.6) | 0.023 | |||
| Age, years | 15.3 (1.6) | 15.2 (1.5) | 14.9 (1.7) | 15.2 (1.6) | <0.001 | 0.31 | 0.24 | 0.08 |
| Anthropometric data | ||||||||
| Weight, kg | 103.9 (21.2) | 118.1 (24.0) | 109.2 (22.1) | 108.5 (23.0) | <0.001 | 1.15 | 1.83 | 2.96 |
| Height, m | 1.64 (0.09) | 1.71 (0.09) | 1.68 (0.09) | 1.67 (0.09) | <0.001 | 0.13 | 0.10 | 0.23 |
| BMI, kg/m2 | 38.1 (6.1) | 40.4 (7.1) | 38.6 (6.0) | 38.8 (6.5) | <0.001 | 0.20 | 0.69 | 0.90 |
| BMI SDS, IOTF reference | 3.15 (0.43) | 3.31 (0.43) | 3.23 (0.37) | 3.21 (0.43) | <0.001 | 0.12 | 0.12 | 0.24 |
| Waist circumference, cm | 114.6 (14.8) | 122.4 (15.9) | 112.0 (12.7) | 116.5 (15.4) | <0.001 | 0.68 | 2.62 | 1.97 |
| BMI classification according to IOTF a | ||||||||
| Overweight (%) | 5 (0.4) | 8 (1.2) | – | 13 (0.6) | <0.001 | |||
| Obesity (%) | 357 (25.6) | 88 (13.5) | 48 (17.3) | 493 (21.2) | ||||
| Severe obesity (%) | 1,034 (74.1) | 558 (85.3) | 229 (82.7) | 1,821 (78.3) | ||||
| Cardiometabolic risk factors | ||||||||
| Systolic BP | 126 (12) | 133 (17) | 116 (15) | 127 (15) | <0.001 | 2.75 | 4.03 | 1.86 |
| Diastolic BP | 78 (8) | 81 (13) | 61 (7) | 77 (11) | <0.001 | 5.40 | 5.25 | 0.96 |
| Fasting plasma glucose, mmol/L | 4.3 (0.4) | 4.8 (0.8) | 4.9 (0.7) | 4.5 (0.6) | <0.001 | 0.83 | 0.11 | 0.63 |
| Total‐cholesterol, mmol/L | 4.2 (0.8) | 4.4 (0.9) | 4.5 (0.8) | 4.3 (0.8) | <0.001 | 0.33 | 0.11 | 0.22 |
| HDL‐cholesterol, mmol/L | 1.1 (0.3) | 1.2 (0.3) | 1.2 (0.3) | 1.2 (0.3) | 0.026 | 0.19 | 0.00 | 0.19 |
| LDL‐cholesterol, mmol/L | 2.7 (0.7) | 2.7 (0.8) | 2.6 (0.7) | 2.7 (0.8) | 0.290 | 0.12 | 0.11 | 0.00 |
| Non HDL‐cholesterol, mmol/L | 3.1 (0.8) | 3.3 (0.8) | 3.3 (0.8) | 3.1 (0.8) | <0.001 | 0.22 | 0.00 | 0.22 |
| Triglycerides, mmol/L | 1.1 (0.5) | 1.4 (0.7) | 1.5 (0.8) | 1.2 (0.6) | <0.001 | 0.53 | 0.12 | 0.40 |
| Elevated non HDL‐cholesterol, % (95% CI)b | 44.9 (42.3–47.6) | 54.4 (50.5–58.3) | 59.6 (53.7–65.5) | 49.3 (47.2–51.4) | <0.001 | |||
| Elevated LDL‐cholesterol, % (95% CI)b | 42.6 (40.4–45.3) | 40.4 (36.5–44.2) | 40.4 (34.4–46.3) | 41.7 (39.7–43.8) | 0.56 | |||
| Elevated BP, % (95% CI) | 46.0 (43.3–48.7) | 65.7 (62.0–69.5) | 15.2 (10.9–19.5) | 47.9 (45.8–49.9) | <0.001 | |||
| Metabolic syndrome, % (95% CI) | 26.4 (24.0–28.7) | 39.6 (35.9–43.4) | 24.2 (19.1–29.2) | 29.8 (28.0–31.7) | <0.001 | |||
Statistics are ANOVA for continuous and chi‐square test for categorical variables.
Classification of body mass index according to the International Obesity Task Force.
Elevated LDL‐C (low‐density lipoprotein‐cholesterol) is defined as values ≥2.8 mmol/L, elevated non HDL‐C (non‐high‐density lipoprotein‐cholesterol) is defined as values ≥3.1 mmol/L.
The proportions of adolescents with elevated LDL‐C (42%) did not differ significantly among the cohorts. The prevalence of elevated non HDL‐C was highest in Norway (60%) and lowest in Italy (45%). The prevalence of metabolic syndrome was higher in the German cohort (40%) than in the Italian and Norwegian cohorts (26% and 24%, respectively) (Table 1). The proportions of adolescents fulfilling the glucose, triglyceride and blood pressure components of metabolic syndrome were 0.4%, 10.0% and 46% (Italy), 6.4%, 24.0% and 65.7% (Germany) and 3.6%, 33.9% and 15.2% (Norway; Table S2).
Table 2 and Figure 2 show the unadjusted (model 1) and adjusted (models 2–5) odds ratios (ORs) for elevated LDL‐C and non HDL‐C levels, elevated blood pressure and metabolic syndrome according to nationality. As compared with the Norwegian cohort, the Italian adolescents had significantly lower unadjusted odds for elevated non HDL‐C (OR: 0.6, 95% CI: 0.4–0.7) and increased odds for elevated BP (4.8, 95% CI: 3.4–6.7), both which remained nearly unchanged after multivariate adjustments for gender, age, waist circumference and BMI. The German adolescents had ten‐fold higher unadjusted odds for elevated BP (OR: 10.7, 95% CI: 7.5–15.5) compared with the Norwegian cohort, which remained nearly unchanged after multivariate adjustments.
Table 2.
Multivariate analyses of cardiovascular risk factors, total population
| LDL‐cholesterol | Non HDL‐cholesterol | Elevated blood pressure | Metabolic syndrome | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
|
Model 1 Nationality | ||||||||||||
| Norwegian | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| German | 1.00 | 0.75–1.33 | 1.00 | 0.81 | 0.61–1.08 | 0.14 | 10.74 | 7.45–15.49 | <0.001 | 2.06 | 1.50–2.82 | <0.001 |
| Italian | 1.10 | 0.88–1.43 | 0.49 | 0.55 | 0.43–0.72 | <0.001 | 4.76 | 3.38–6.73 | <0.001 | 1.12 | 0.83–1.51 | 0.45 |
|
Model 2 Nationality | ||||||||||||
| Norwegian | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| German | 1.01 | 0.76–1.35 | 0.93 | 0.82 | 0.62–1.09 | 0.17 | 11.87 | 8.19–17.20 | <0.001 | 2.14 | 1.55–2.94 | <0.001 |
| Italian | 1.12 | 0.86–1.45 | 0.42 | 0.56 | 0.43–0.73 | <0.001 | 5.22 | 3.68–7.40 | <0.001 | 1.17 | 0.86–1.58 | 0.31 |
| Gender | ||||||||||||
| Female | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Male | 1.21 | 1.02–1.43 | 0.03 | 1.18 | 1.00–1.39 | 0.051 | 1.92 | 1.61–2.28 | <0.001 | 1.54 | 1.28–1.84 | <0.001 |
|
Model 3 Nationality | ||||||||||||
| Norwegian | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| German | 1.01 | 0.76–1.35 | 0.93 | 0.81 | 0.61–1.08 | 0.15 | 11.70 | 8.06–17.0 | <0.001 | 2.05 | 1.48–2.82 | <0.001 |
| Italian | 1.12 | 0.86–1.46 | 0.41 | 0.55 | 0.43–0.72 | <0.001 | 4.96 | 3.49–7.04 | <0.001 | 1.06 | 0.78–1.44 | 0.72 |
| Gender | ||||||||||||
| Female | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Male | 1.20 | 1.02–1.42 | 0.03 | 1.19 | 1.01–1.41 | 0.042 | 2.01 | 1.68–2.41 | <0.001 | 1.65 | 1.37–1.98 | <0.001 |
| Age (year) | 1.00 | 0.95–1.05 | 0.88 | 1.03 | 0.98–1.09 | 0.23 | 1.19 | 1.13–1.26 | <0.001 | 1.28 | 1.21–1.36 | <0.001 |
|
Model 4 Nationality | ||||||||||||
| Norwegian | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| German | 0.98 | 0.73–1.31 | 0.87 | 0.75 | 0.56–1.00 | 0.048 | 9.17 | 6.27–13.40 | <0.001 | 1.46 | 1.05–2.05 | 0.027 |
| Italian | 1.11 | 0.85–1.44 | 0.45 | 0.54 | 0.42–0.71 | <0.001 | 4.82 | 3.38–6.87 | <0.001 | 0.97 | 0.71–1.32 | 0.84 |
| Gender | ||||||||||||
| Female | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Male | 1.17 | 0.98–1.39 | 0.09 | 1.11 | 0.93–1.32 | 0.26 | 1.56 | 1.29–1.88 | <0.001 | 1.23 | 1.01–1.50 | 0.04 |
| Age (year) | 0.99 | 0.94–1.04 | 0.64 | 1.01 | 0.96–1.07 | 0.67 | 1.11 | 1.05–1.18 | <0.001 | 1.19 | 1.11–1.26 | <0.001 |
| Waist (cm) | 1.00 | 1.00–1.01 | 0.22 | 1.01 | 1.00–1.01 | 0.006 | 1.03 | 1.03–1.04 | <0.001 | 1.04 | 1.03–1.04 | <0.001 |
|
Model 5 Nationality | ||||||||||||
| Norwegian | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| German | 0.97 | 0.72–1.31 | 0.84 | 0.73 | 0.54–0.98 | 0.037 | 11.20 | 7.58–16.54 | <0.001 | 1.52 | 1.08–2.13 | 0.016 |
| Italian | 1.10 | 0.84–1.44 | 0.47 | 0.53 | 0.41–0.70 | <0.001 | 5.68 | 3.95–8.16 | <0.001 | 1.00 | 0.73–1.37 | 0.99 |
| Gender | ||||||||||||
| Female | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Male | 1.16 | 0.96–1.39 | 0.12 | 1.08 | 0.90–1.30 | 0.41 | 1.86 | 1.52–2.27 | <0.001 | 1.28 | 1.04–1.58 | 0.018 |
| Age (year) | 0.99 | 0.94–1.04 | 0.65 | 1.01 | 0.96–1.07 | 0.64 | 1.10 | 1.04–1.17 | 0.001 | 1.19 | 1.11–1.26 | <0.001 |
| Waist (cm) | 1.01 | 1.00–1.01 | 0.34 | 1.01 | 1.00–1.02 | 0.015 | 1.01 | 1.00–1.02 | 0.24 | 1.03 | 1.02–1.04 | <0.001 |
| BMI (kg/m2) | 1.00 | 0.98–1.02 | 0.81 | 0.99 | 0.97–1.01 | 0.35 | 1.08 | 1.06–1.11 | <0.001 | 1.02 | 0.99–1.04 | 0.17 |
OR = odds ratio; 95% CI = 95% confidence intervals.
Figure 2.
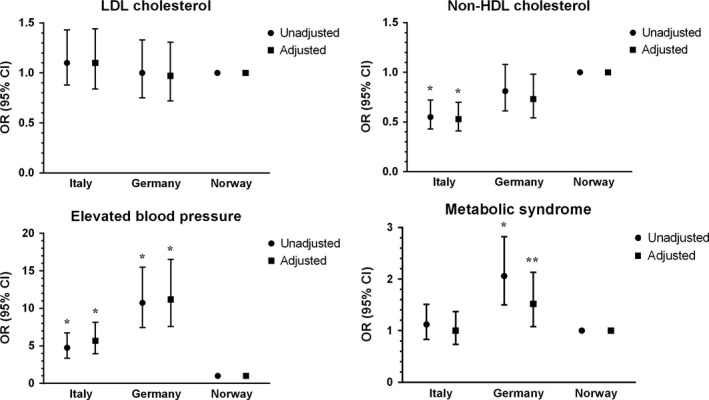
Unadjusted (circles) and adjusted (squares) odds ratio with 95% confidence intervals for having elevated LDL‐cholesterol (≥2.8 mmol/L), non HDL‐cholesterol (≥3.1 mmol/L), elevated blood pressure (systolic BP ≥130 or diastolic BP≥85 mmHg) and metabolic syndrome among the cohorts of adolescents from Italy and Germany. Norway is used as the reference category. *p < 0.001, **p < 0.05.
Discussion
This study of treatment‐seeking adolescents with obesity confirmed the hypothesis that the prevalence of clinically important cardiometabolic risk factors differed between three European countries. First, the prevalence of elevated non HDL‐C was highest in Norway (60%) and lowest in Italy (45%), and, accordingly, Italian adolescents had lower adjusted odds (OR: 0.5, 95% CI: 0.4–0.7) for elevated non HDL‐C levels, as compared with their Norwegian counterparts. Second, the prevalence of elevated blood pressure was highest in Germany (66%) and lowest in Norway (15%), and German and Italian adolescents had higher adjusted odds for elevated blood pressure, OR 11 (95% CI: 8–17) and OR 6 (95% CI: 4–8) as compared with their Norwegian counterparts (Table 2, Fig. 2).
A south‐north gradient was revealed in the case of non HDL‐C and triglyceride levels, which were found to be highest in Norway and lowest in Italy. There were, however, no statistically significant differences among the cohorts regarding the prevalence of elevated LDL‐C (40–43%, Table 1); while a larger proportion of Italian adolescents had low HDL‐C levels (Table S2).
Possible reasons for regional differences
The revealed differences in dyslipidemia and blood pressure might have several explanations. Regional differences in diet, for example salt intake 13, and the traditional Mediterranean‐style diet 14, might have affected metabolic risk factors – such as blood pressure and blood lipids. The same risk factors might also have been influenced by differences in physical activity levels 11, 15. Regional differences have been demonstrated in favour of Northern‐Central versus Southern Europe with regards to physical activity and sedentary time among the general adolescent populations 16, 17. An international, survey‐based, report from the WHO from 2013/2014 on Health Behaviour in school‐aged children, showed that 75% of Norwegian 15‐year olds engaged vigorous physical activity ≥2 hours a week, versus 52% in Italy and 64% in Germany, and 32% had a daily intake of vegetables, versus 29% in Italy and 24% in Germany. Fewer Norwegian 15‐year olds reported daily intake of sweets, 7% in Norway, 27% in Italy and 26% in Germany, although 74% of Norwegian and 66% of German adolescents reported spending ≥2 hours per weekday using a computer during their spare time, somewhat more frequently than the 55% of their Italian 15‐year old counterparts 17. An Italian study of 9–11‐year‐old children reported that 88% used olive oil as a condiment when eating at home 18. Vegetable oils contain phytosterols, which can have beneficial effects on blood lipids. The intake is on average 250 mg/day in Northern Europe compared to approximately the double in Mediterranean countries for adults 19. However, it should be noted that the above‐mentioned studies were based on adolescents in the general population, and the same tendencies might not apply to adolescents with obesity.
High sodium intake increases the risk of hypertension 13. Adolescents with obesity have greater BP sensitivity to sodium intake than adolescents with a normal range weight. The relative hyperinsulinemia associated with obesity can contribute to upregulate renal tubular sodium transport, and hence increase the sodium reabsorption in the distal tubules and increase BP 20. Salt intake among German 14–18‐year‐olds was 6.2 g/day for girls and 8.2 g/day for boys 21. In comparison, a recent Norwegian study of 13‐year olds, reported a mean salt intake of 5.8 g/day for girls and 6.8 g/day for boys 22, and an Italian study group reported a mean daily salt intake of 6.7 g/day for girls and 7.4 g/day for boys aged between 7 and 18 years, with a trend towards higher values in adolescents 23.
The high blood pressure levels observed in the present study in the German cohort were in line with a European health interview survey, which reported higher prevalence of hypertensive diseases in the population aged 15 years and over in Germany (28.5%) than in Norway (12.7%) and Italy (20.6%) 24. A study published in 2017 also showed a very high frequency of elevated BP in German adolescents with obesity 25, although using different cut‐off values than this study. Our findings of lowest prevalence of metabolic syndrome in the Norwegian cohort are also in consistence with an international comparison that showed children from Germany in the highest cardiovascular risk tertile, Italy in the middle and children from Norway in the tertile with most favourable cardiovascular risk profile overall 5.
The Norwegian adolescents had high levels of triglycerides. This might be a result of a high intake of refined sugar or a diet high in saturated fat or trans fat 19, which may lead to an increase in visceral fat and triglyceride levels 19.
Strengths and limitations
One strength of this study was the measurement of specific cardiovascular risk factors, atherogenic lipids and high blood pressure, rather than metabolic syndrome, a composite risk factor. This might possibly have provided a more valid estimate of cardiovascular health 2, 9. Similar laboratory assays were used in Germany and Italy (Table S1). Although different assays were used to analyse the samples from the Norwegian cohort, the Norwegian laboratory changed their method to the one used in Italy and Germany after completion of the current study, and validation analyses showed that these assays were comparable. However, this study had some limitations that need to be addressed. First, the cross‐sectional design does not allow for drawing conclusions about causality. Second, heterogeneity in the measuring techniques of BP might have biased the results. The Italian and German cohorts had their BP measured twice and averaged, while the Norwegians had their BP measured four times, and the last three readings were averaged. This is likely to have affected the Norwegian BPs to be lower on average than their European counterparts, as each of three consecutive BP measurement in children with overweight have been shown to be lower than the previous one, with the third measurement being on average 4.3 and 2.6 mmHg lower for systolic BP and diastolic BP than the first one, respectively 26. Further, BP was measured with aneroid manual sphygmomanometers (auscultation) in Italy and Germany, whereas in Norway an automated oscillometric device (measures vibrations in the artery wall and converts the measurements into electrical signals) was used. Oscillometric devices in general systematically overestimate systolic BP, and might also overestimate diastolic BP compared with values obtained with auscultation 13, 27. In best case, these flaws in different directions might have neutralised each other to some degree. Third, age, body weight and gender balance differed slightly between clinics, but multivariate analyses were implemented to adjust for these differences. Fourth, the German cohort had a lower percentage of complete datasets for analysis, which could have biased the results. Fifth, objective measures of physical activity and nutritional intake were not assessed, which limits the interpretation of the findings.
Finally, the cohorts were measured at different time periods; the German cohort was examined from 1999 to 2008, while the Italian and the Norwegian cohorts were examined between 2010 and 2013 and between 2009 and 2015, respectively. Both changes in dietary components and patterns, physical activity, sleep patterns and prevalence of obesity among adolescents might have occurred during this time period, and thereby might have biased the results.
Implications for practice
Conservative treatment of adolescents with obesity is difficult, and it often has small or negligible effects on body weight 28. However, even a small weight reduction of 5–10%, improves cardiovascular risk factors in adults 19, and small changes in BMI SDS may improve blood pressure, triglycerides and LDL‐C levels in children and adolescents 29. For those not able to reduce their overweight, it might also be helpful to address elevated blood pressure or dyslipidemia in other ways than just focusing on BMI, helping to reduce and postpone the future burden of disease. In this study, the German and Italian adolescents showed a worryingly high prevalence of elevated blood pressure, while the Norwegian adolescents had a high prevalence of elevated non HDL‐C. To reduce blood pressure, it is recommended to eat a diet rich in fruits and vegetables, whole grains and lean proteins, and to reduce the intake of saturated fat, sugar‐sweetened beverages and salt 13, 20. To reduce dyslipidemia, reducing the intake of total and saturated fat, minimising intake of trans fat, and reducing intake of added sugars to below 10% of daily energy consumption is recommended, as is increasing the intake of vegetables, legumes, fruits, nuts, fish (especially oily) and whole grains 19. Finally, engaging in more physical activity 13, 19, – especially high‐intensity interval training has been shown to be beneficial for cardiorespiratory fitness 30, while having a regular sleep pattern 31 may be beneficial for both high BP and dyslipidemia.
Conclusion
The current study revealed significant differences in blood pressure and non HDL‐C levels among European adolescents seeking treatment for obesity. The Norwegian cohort had the highest prevalence of elevated non HDL‐C (60%), followed by Germany (54%) and Italy (45%). The prevalence of high blood pressure was higher in the German cohort (66%) than in the Italian (46%) and Norwegian (15%) cohorts. These findings suggest that cardiometabolic risk factors among adolescents with obesity might differ across populations, which might imply that preventive clinical work should reflect such differences.
Finance
This study did not receive any specific funding.
Conflict of interests
The authors have no conflict of interests to declare.
Supporting information
Table S1 Details of measurements in the three cohorts.
Table S2 Prevalence of metabolic syndrome and its components.
Acknowledgements
We are grateful for the contributions of all healthcare professionals involved at the three obesity centres and all the adolescents who participated in this study. Finally, we thank Matthew McGee for proofreading the manuscript.
References
- 1. The GBD 2015 Obesity Collaborators ; Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health effects of overweight and obesity in 195 countries over 25 Years. N Engl J Med 2017; 377: 13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koskinen J, Magnussen CG, Sinaiko A, Woo J, Urbina E, Jacobs DR Jr, et al. Childhood age and associations between childhood metabolic syndrome and adult risk for metabolic syndrome, type 2 diabetes mellitus and carotid intima media thickness: The International Childhood Cardiovascular Cohort Consortium. J Am Heart Assoc 2017; 6: e005632 10.1161/JAHA.117.005632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ho M, Garnett SP, Baur L, Burrows T, Stewart L, Neve M, et al. Effectiveness of lifestyle interventions in child obesity: systematic review with meta‐analysis. Pediatrics 2012; 130: e1647–71. [DOI] [PubMed] [Google Scholar]
- 4. Lafortuna CL, Adorni F, Agosti F, De Col A, Sievert K, Siegfried W, et al. Prevalence of the metabolic syndrome among extremely obese adolescents in Italy and Germany. Diabetes Res Clin Pract 2010; 88: 14–21. [DOI] [PubMed] [Google Scholar]
- 5. van Vliet M, Heymans MW, von Rosenstiel IA, Brandjes DP, Beijnen JH, Diamant M. Cardiometabolic risk variables in overweight and obese children: a worldwide comparison. Cardiovasc Diabetol 2011; 10: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Srinivasan SR, Frontini MG, Xu J, Berenson GS. Utility of childhood non‐high‐density lipoprotein cholesterol levels in predicting adult dyslipidemia and other cardiovascular risks: the Bogalusa Heart Study. Pediatrics 2006; 118: 201–6. [DOI] [PubMed] [Google Scholar]
- 7. Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, et al. Association between lowering LDL‐C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta‐analysis. JAMA 2016; 316: 1289–97. [DOI] [PubMed] [Google Scholar]
- 8. Zimmet P, Alberti G, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents. Lancet 2007; 369: 2059–61. [DOI] [PubMed] [Google Scholar]
- 9. Magge SN, Goodman E, Armstrong SC; Committee on Nutrition; Section on Endocrinology; Section on Obesity . The metabolic syndrome in children and adolescents: shifting the focus to cardiometabolic risk factor clustering. Pediatrics 2017; 140: e20171603 10.1542/peds/.2017-1603. [DOI] [PubMed] [Google Scholar]
- 10. Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut‐offs for thinness, overweight and obesity. Pediatr Obes 2012; 7: 284–94. [DOI] [PubMed] [Google Scholar]
- 11. Expert Panel on Integrated Guidelines for Cardiovascular Health, and Risk Reduction in Children and Adolescents; National Heart Lung and Blood Institute . Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 2011; 128(Suppl 5): S213–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li C, Ford ES, Mokdad AH, Cook S. Recent trends in waist circumference and waist‐height ratio among US children and adolescents. Pediatrics 2006; 118: e1390–8. [DOI] [PubMed] [Google Scholar]
- 13. Flynn JT, Kaelber DC, Baker‐Smith CM, Blowey D, Carroll AE, Daniels SR, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 2017; 140: e20171904 10.1542/peds.2017-1904. [DOI] [PubMed] [Google Scholar]
- 14. Garcia M, Bihuniak JD, Shook J, Kenny A, Kerstetter J, Huedo‐Medina TB. The effect of the traditional Mediterranean‐style diet on metabolic risk factors: a meta‐analysis. Nutrients 2016; 8: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farpour‐Lambert NJ, Aggoun Y, Marchand LM, Martin XE, Herrmann FR, Beghetti M. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre‐pubertal obese children. J Am Coll Cardiol 2009; 54: 2396–406. [DOI] [PubMed] [Google Scholar]
- 16. Ruiz JR, Ortega FB, Martinez‐Gomez D, Labayen I, Moreno LA, De Bourdeaudhuij I, et al. Objectively measured physical activity and sedentary time in European adolescents: the HELENA study. Am J Epidemiol 2011; 174: 173–84. [DOI] [PubMed] [Google Scholar]
- 17. Inchley J, Currie D, Young T, Samdal O, Torsheim T, Augustson L, et al. Growing up unequal: gender and socioeconomic differences in young people`s health and well‐being. International report from the 2013/2014 HBSC‐survey. WHO, Regional office for Europe 2016. ISBN 978 92 890 5136 1. Available at: http://www.euro.who.int/__data/assets/pdf_file/0003/303438/HSBC-No.7-Growing-up-unequal-Full-Report.pdf?ua= (accessed on November 6, 2017).
- 18. Rosi A, Calestani MV, Parrino L, Milioli G, Palla L, Volta E, et al. Weight status is related with gender and sleep duration but not with dietary habits and physical activity in primary school Italian children. Nutrients 2017; 9: 579 10.3390/nu9060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. 2016 ESC/EAS Guidelines for the management of dyslipidaemias. Eur Heart J 2016; 37: 2999–3058. [DOI] [PubMed] [Google Scholar]
- 20. Falkner B. Monitoring and management of hypertension with obesity in adolescents. Integr Blood Press Control 2017; 10: 33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Strohm D, Boeing H, Leschik‐Bonnet E, Heseker H, Arens‐Azevedo U, Bechthold A, et al. Salt intake in Germany, health consequences, and resulting recommendations for action. A scientific statement from the German Nutrition Society (DGE). Ernahrungs . Umschau 2016; 63: 62–70. [Google Scholar]
- 22. Ungkost 3 . 2016. ed. The Norwegian Institute of Public Health 2016. Available at: https://www.fhi.no/globalassets/dokumenterfiler/rapporter/ungkost-rapport-24.06.16.pdf (accessed on October 10, 2017).
- 23. Campanozzi A, Avallone S, Barbato A, Iacone R, Russo O, De Filippo G, et al. High sodium and low potassium intake among Italian children: relationship with age, body mass and blood pressure. PLoS ONE 2015; 10: 1183 10.1371/journal.pone.0121183.eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eurostat Cardiovascular Diseases Statistics . Eurostat. Available at: http://ec.europa.eu/eurostat/statistics-explained/index.php/Cardiovascular_diseases_statistics (accessed on November 23, 2017).
- 25. Bohn B, Wiegand S, Kiess W, Reinehr T, Stachow R, Oepen J, et al. Changing characteristics of obese children and adolescents entering pediatric lifestyle intervention programs in Germany over the last 11 years: an adiposity patients registry multicenter analysis of 65,453 children and adolescents. Obes Facts 2017; 10: 517–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wirix AJ, Nauta J, Groothoff JW, Rabelink TJ, HiraSing RA, Chinapaw MJ, et al. Is the prevalence of hypertension in overweight children overestimated? Arch Dis Child 2016; 101: 998–1003. [DOI] [PubMed] [Google Scholar]
- 27. Duncombe SL, Voss C, Harris KC. Oscillometric and auscultatory blood pressure measurement methods in children: a systematic review and meta‐analysis. J Hypertens 2017; 35: 213–24. [DOI] [PubMed] [Google Scholar]
- 28. Al‐Khudairy L, Loveman E, Colquitt JL, Mead E, Johnson RE, Fraser H, et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese adolescents aged 12–17 years. Cochrane Database Syst Rev 2017; 6: CD012691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ford AL, Hunt LP, Cooper A, Shield JP. What reduction in BMI SDS is required in obese adolescents to improve body composition and cardiometabolic health? Arch Dis Child 2010; 95: 256–61. [DOI] [PubMed] [Google Scholar]
- 30. Dias KA, Ingul CB, Tjonna AE, Keating SE, Gomersall SR, Follestad T, et al. Effect of high‐intensity interval training on fitness, fat mass and cardiometabolic biomarkers in children with obesity: a randomised controlled trial. Sports Med 2018; 48: 733–46. [DOI] [PubMed] [Google Scholar]
- 31. He F, Bixler EO, Berg A, Imamura Kawasawa Y, Vgontzas AN, Fernandez‐Mendoza J, et al. Habitual sleep variability, not sleep duration, is associated with caloric intake in adolescents. Sleep Med 2015; 16: 856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Details of measurements in the three cohorts.
Table S2 Prevalence of metabolic syndrome and its components.


