Abstract
Purpose
To explore the subfoveal thickness of sclera (SST), choroid (SCT) and retina (SRT) as well as their relationship in healthy Chinese children with varying levels of refractive error.
Methods
A total of 810 healthy Chinese schoolchildren and adolescents underwent a series of comprehensive ocular examinations, as well as swept‐source optical coherence tomography (OCT) after induced cycloplegia. The thicknesses of the sclera, choroid and retina below the central fovea were measured manually, and each measurement was compared across different refractive statuses. Independent factors associated with the thickness of each layer were analysed.
Results
The mean SST, SCT and SRT were 524 ± 57 μm, 195 ± 49 μm and 224 ± 19 μm, respectively. The SSTs and SCTs of myopes were significantly thinner than those of emmetropes and hyperopes (all p < 0.001). Although the choroid seemed to be thicker in hyperopes (225 ± 46 μm) than in emmetropes (211 ± 45 μm), no statistically significant difference was observed between emmetropes and hyperopes in the sclera and choroid. Thinner SSTs and SCTs were associated with greater levels of myopia, whereas the SRT was similar in children with different myopic levels. SST (p < 0.001) and SCT (p = 0.003) as well as age (p < 0.001), sex (p < 0.001) and axial length (p < 0.001) were independently associated with spherical equivalent refraction (SER). Older age (p = 0.013), myopic‐shifted SER (p < 0.001), thicker SCT (p < 0.001) and thinner SRT (p = 0.012) were independently associated with a thinner SST.
Conclusion
The subfoveal sclera and choroid were thinner in myopes than in emmetropes and hyperopes, while the retina remains constant. Age, refractive error and choroidal and retinal thicknesses are related to subfoveal scleral thickness.
Keywords: children, Chinese, choroid, sclera, swept‐source optical coherence tomography
Introduction
Myopia is the most common refractive disorder in children and adolescents worldwide. East and South‐East Asian countries, such as China and Singapore, have the highest incidence and prevalence of myopia (Morgan et al. 2012; Pan et al. 2012). Predictions indicate that by the year 2050, nearly five billion people will be myopic globally, with one billion of them being highly myopic (Holden et al. 2016). In Taiwan, more than 80% of children who finish high school suffer from myopia, and over 10% of these children may be highly myopic (Lin et al. 2004). Another study in China reported that 95.5% of university students were myopic, and 19.5% of these students were highly myopic (Sun et al. 2012). High myopia is closely associated with a variety of pathological changes, such as posterior staphyloma, chorioretinal atrophy, lacquer crack, choroidal neovascularization, retinoschisis and retinal detachment. These pathological changes may lead to visual impairment or even blindness. Some studies have reported that myopic macular degeneration, also known as degenerative myopia, is the leading cause of blindness and low vision (Hsu et al. 2004; Iwase et al. 2006; Xu et al. 2006; Wu et al. 2011; Jonas & Xu 2014).
Although the aetiology and pathogenesis of myopia is still unclear, excessive and progressive axial length (AL) elongation, mechanical stretching of the eyeball, and chorioretinal and scleral degeneration are considered the principle causes of the visual impairment associated with myopia (Verkicharla et al. 2015). In recent years, the role of the sclera in the development of myopia has attracted increased attention. The sclera, once considered the static outer coat of the eye, is now known to play an active part in the development of myopia, as its biomechanical and biochemical properties are actively modulated for the purpose of adjusting refractive status throughout life (Norton & Rada 1995; Phillips et al. 2000; Rada et al. 2000; Gentle et al. 2003). Hence, exploring the morphology of the sclera throughout the life span would therefore provide important information about the development of myopia. However, due to the limitations in imaging technology, past studies of scleral morphology primarily depended on microscopic examination of post‐mortem eyes (Curtin & Teng 1958; Curtin et al. 1979; McBrien et al. 2001).
Enhanced depth imaging optical coherence tomography and swept‐source optical coherence tomography (SS‐OCT) are used to study the morphology of posterior sclera and choroid in vivo nowadays, with SS‐OCT considered the quickest and most efficient technique (Unterhuber et al. 2005; Spaide et al. 2008; Park et al. 2014). Morphological changes in the choroid and retina of patients with myopia have been relatively well studied with OCT, but few studies of the posterior scleral morphology, especially the thickness, have been performed (Maruko et al. 2012; Ohno‐Matsui et al. 2012; Ellabban et al. 2013, 2014; Lopilly Park et al. 2014). So far, most of these studies to explore posterior scleral thickness with SS‐OCT were all carried out in highly myopic adults many years after the onset of myopia and most of them focused on scleral thickness only. It is still a mystery how the scleral thickness changes during the early development of myopia as well as its relationship with the choroidal thickness, the retinal thickness and the other ocular variables. Also, the scleral thickness in emmetropes and hyperopes remains unknown.
Thus, the aim of our study was to make up for the vacancy of subfoveal scleral thickness and its relationship with the subfoveal thickness of choroid and retina in children and adolescents with different refractive statuses by SS‐OCT measurements, and we tried to clarify the relationship between scleral thickness and other ocular parameters in the early development of refractive error, especially in myopia.
Materials and Methods
Setting and participants
This was a cross‐sectional study performed with children and adolescents. Children from 12 primary and middle schools in the Jiading and Songjiang districts in Shanghai, China, were enrolled via cluster sampling. The study was performed in accordance with the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of Shanghai General Hospital, Shanghai Jiao Tong University. The study protocol was explained clearly to all of the children who participated, and written consent was obtained from their parents or other guardians. Oral consent was also obtained from the children before examination. Children who exhibited a clear outer scleral border in OCT images were included in the analysis. Those with previous or current severe ocular diseases, such as amblyopia (best corrected visual acuity < 20/25), strabismus, ptosis, congenital glaucoma or cataract, retinopathy or severe ocular infections, were excluded from the study.
The investigation was conducted from November 2015 to January 2016. The field examination team consisted of one ophthalmologist, five optometrists, two public health physicians and two nurses. All researchers adhered to the approved protocol.
Research methods
The age and sex of all children who participated in the study were acquired from their identification cards in advance, and their heights and weights were measured at the research site. All participants then underwent a series of detailed ocular examinations, including an ocular dominance test, uncorrected and corrected visual acuity tests, AL and intraocular pressure measurements, slit‐lamp examination of the anterior ocular segment, refraction and corneal curvature measurements with and without cycloplegia, subjective optometry and SS‐OCT.
Visual acuity was tested at a 4‐metre distance with a retro‐illuminated Early Treatment Diabetic Retinopathy Study chart. The visual acuity of children who wore glasses was tested twice, once with and once without glasses. AL measurement was conducted via ocular biometry (iol master, version 5.02; Carl Zeiss Meditec, Oberkochen, Germany). Intraocular pressure was measured using a noncontact tonometer (model NT‐4000; Nidek Inc., Fremont, CA, USA). Refraction and corneal curvature were both measured before and after cycloplegia using an autorefractor (model KR‐8900; Topcon, Tokyo, Japan). Cycloplegia was induced by administering one drop of topical 0.5% proparacaine (Alcaine; Alcon), followed by two drops of 1% cyclopentolate (Cyclogyl; Alcon, Fort Worth, TX, USA) in each eye, each drop 5 min apart. Pupil size and the pupillary light reflex were examined at least 30 min after the last drop of cyclopentolate was administered. Cycloplegia was defined as the absence of a pupillary light reflex and a pupil size larger than 6 mm.
Swept‐source optical coherence tomography (model DRI OCT‐1 Atlantis; Topcon) was used to measure scleral, choroidal and retinal thicknesses. All OCT examinations were performed by a single trained examiner after cycloplegia was induced. The SS‐OCT parameters were as follows: wavelength of the SS‐OCT light source = 1050 nm; repetition rate = 100 000 Hz; depth of the scan window = 2.6 mm; axial resolution = 8 μm; and transverse resolution = 10 μm. According to the protocol, each OCT examination included 12 radial OCT scan lines focused on the fovea, with each scan line separated by 15° and each OCT scan line being 12 mm. For each radial scan line, 32 consecutive, overlapping single B‐scan OCT images were automatically averaged by the built‐in software to create a multi‐averaged single image.
Measurement of scleral, choroidal and retinal thicknesses
The thickness of each layer at the centre of the fovea was measured manually based on the multi‐averaged images obtained from the horizontal and vertical radial OCT scan lines. Retinal thickness was defined as the vertical distance between the internal limiting membrane (ILM) and the outer border of the retinal pigment epithelium (RPE) (Fig. 1A). Choroidal thickness was defined as the vertical distance between the outer border of the RPE and the choroidal–scleral interface (Fig. 1B). Scleral thickness was defined as the vertical distance between the choroidal–scleral interface and the outer scleral border (Fig. 1C). The ILM, the outer border of the RPE and the choroidal–scleral interface were delineated by the built‐in software, and manual correction was performed if the software misjudged the interface. The posterior scleral border was carefully identified by an experienced technician and was reconfirmed by another experienced technician before measurement according to the lamellar structure, continuity and high reflectivity value of the retrobulbar tissue. In addition, images with clear and unclear posterior scleral border were compared together (Fig. 1C,E). To ensure the reproducibility of posterior scleral border, 20 OCT images were randomly selected from the database in advance and the two experienced technicians were asked to outline the posterior scleral border and measure the subfoveal scleral thickness. Intraobserver and interobserver correlations and Bland–Altman analyses were used to confirm their agreements. After examinations of reproducibility, measurements were performed by two skilled observers blinded to the study conditions after the borderlines were identified. The average of these measurements was calculated and included in the analysis. If the absolute difference between the two measurements was larger than 20 μm for the sclera, 10 μm for the choroid or 20 μm for the whole fundus, measurements were repeated until the absolute difference was within the set limits.
Figure 1.
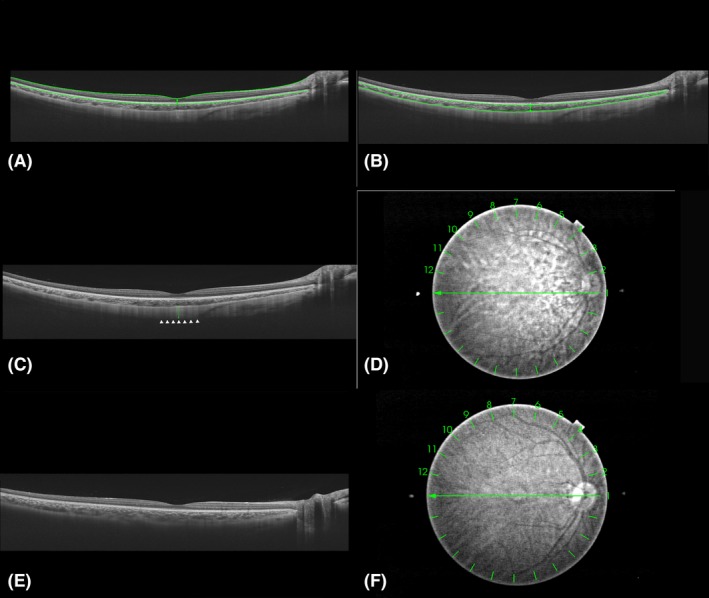
Cross‐sectional images collected by swept‐source optical coherence tomography and thickness measurements of each layer at the centre of the fovea. Horizontal cross‐sectional images of the posterior pole of the same participant were collected by swept‐source optical coherence tomography (SS‐OCT). Subfoveal retinal, choroidal and scleral thicknesses were manually measured at the centre of the fovea. (A) The inner border (internal limiting membrane, indicated by the upper horizontal green line) and outer border [the outer border of the retinal pigment epithelium (RPE), indicated by the lower horizontal green line] of the retina. Retinal thickness was defined as the vertical distance between the two borders (indicated by the vertical green line). (B) The inner border (the outer border of the RPE, indicated by the upper horizontal green line) and outer border (choroidal–scleral interface, indicated by the lower horizontal green line) of the choroid. Choroidal thickness was defined as the vertical distance between the two borders (indicated by the vertical green line). (C) The outer border of sclera (indicated by the white arrowheads). Scleral thickness was defined as the vertical distance between the choroidal–scleral interface and the outer scleral border (indicated by the vertical green line). (D) The fundus of A, B and C. Measurements were performed on the vertical and horizontal lines (line 1 and line 7). (E) Image with unclear scleral border. (F) The fundus of E.
Statistical analyses
Thickness data were input into an Excel spreadsheet by two trained staff members independently, and all discrepancies were adjudicated by the third staff member to make sure that the data input was correct. Other research data were entered into an online database system when the examinations were performed at the schools. The accuracy and completeness of the data were automatically checked by software. All statistical analyses were conducted using sas (version 9.3; SAS Institute, Cary, NC, USA). Only data from the right eyes were used for statistical analysis.
Spherical equivalent refraction (SER) values were used to categorize different refractive levels, where SER = sphere + 0.5 × cylinder. Hyperopia, emmetropia and myopia were defined as SER ≥ + 0.5 dioptres (D), −0.5 D < SER < +0.5 D and SER ≤ −0.5 D, respectively. Severe myopia, moderate myopia and mild myopia were defined as SER ≤ −5.0 D, −5.0 D < SER ≤ −3.0 D and −3.0 D < SER ≤ −0.5 D, respectively.
Quantitative variables are presented as mean ± standard deviation (SD). The data distribution was tested with a Kolmogorov–Smirnov test. Intergroup differences were examined with a t‐test or an analysis of variance (anova), and the Bonferroni method was used for post hoc tests. Stepwise multiple regression analysis was used to explore the independent factors affecting SER, subfoveal scleral thickness (SST), subfoveal choroidal thickness (SCT) and subfoveal retinal thickness (SRT). A linear correlation test was used to analyse the relationship between scleral thickness and other variables. Simple linear regression was used to determine the relationship between the thickness of each layer and the SER, as well as the AL. A p value <0.05 was considered to be statistically significant (two‐tailed).
Results
General characteristics
In total, 810 children with clear outer scleral border were included in the final analyses. Comparisons of general characteristics between children with clear and unclear posterior scleral border were performed (see Table 1). Children with clear posterior border were significantly older and more myopic and had longer axial length. Detailed general characteristics of the 810 children and the differences between sexes are shown in Table 2. The SST was normally distributed, whereas the SCT and SRT were not. As the sample size is large enough, t‐tests were also applied in data that were not normally distributed. The mean SST, SCT and SRT were 524 ± 57 μm (range: 347−697 μm), 195 ± 49 μm (range: 56−374 μm) and 224 ± 19 μm (range: 160−299 μm), respectively. The mean AL was 24.91 ± 1.25 mm, and the mean SER was −2.91 ± 2.46 D. Girls had significantly shorter AL, thinner SST, thinner SRT and thinner total thickness (sclera + choroid + retina) (t‐tests, ps < 0.05).
Table 1.
Comparisons of general characteristics between children with clear and unclear posterior scleral border
| Parameter | Clear scleral border | Unclear scleral border | Total | T value | P a |
|---|---|---|---|---|---|
| n=(810) | n=(2034) | n=(2844) | |||
| Age (years) | 12.79 ± 3.068 | 10.98 ± 3.433 | 11.49 ± 3.433 | 13.13 | <0.001 |
| AL (mm) | 24.91 ± 1.246 | 23.69 ± 1.164 | 24.04 ± 1.308 | 24.56 | <0.001 |
| Refractive error (SE) (D) | −2.91 ± 2.458 | −0.51 ± 2.162 | −1.19 ± 2.497 | –25.68 | <0.001 |
| IOP (mmHg) | 16.68 ± 2.517 | 16.44 ± 2.552 | 16.51 ± 2.544 | 2.32 | 0.020 |
| Weight (kg) | 47.21 ± 14.729 | 40.85 ± 16.600 | 42.66 ± 16.341 | 9.52 | <0.001 |
| Height (cm) | 154.52 ± 14.962 | 145.15 ± 17.158 | 147.82 ± 17.091 | 13.61 | <0.001 |
| BMI (kg/m2) | 19.28 ± 3.471 | 18.59 ± 3.896 | 18.79 ± 3.793 | 4.42 | <0.001 |
AL = axial length, BMI = body mass index, IOP = intraocular pressure.
Statistical significance was tested using t‐tests.
Table 2.
General characteristics of the 810 participants and comparison between the sexes
| Parameters | Range | Mean±SD | K‐Sa | Boys | Girls | pb | |
|---|---|---|---|---|---|---|---|
| z | p | Mean ± SD | Mean ± SD | ||||
| Age, years | 6–19 | 12.8 ± 3.1 | 0.11 | <0.001 | 12.4 ± 3.1 | 13.1 ± 3.0 | 0.002 |
| SST, μm | 347–697 | 524 ± 57 | 0.02 | 0.15 | 529 ± 57 | 519 ± 56 | 0.014 |
| SCT, μm | 56–374 | 195 ± 49 | 0.03 | 0.032 | 191 ± 48 | 198 ± 51 | 0.078 |
| SRT, μm | 160–299 | 224 ± 19 | 0.05 | <0.001 | 228 ± 20 | 220 ± 18 | <0.001 |
| SRT+SCT, μm | 275–580 | 418 ± 51 | 0.03 | 0.091 | 420 ± 49 | 417 ± 53 | 0.541 |
| SST + SCT + SRT, μm | 708–1207 | 942 ± 77 | 0.02 | 0.15 | 949 ± 74 | 937 ± 80 | 0.027 |
| SER, D | −11.38–2.38 | −2.91 ± 2.46 | 0.04 | <0.001 | −2.80 ± 2.51 | −3.00 ± 2.41 | 0.255 |
| AL, mm | 21.78–29.30 | 24.91 ± 1.25 | 0.02 | 0.15 | 25.23 ± 1.28 | 24.63 ± 1.15 | <0.001 |
| UVA | 0.03–2.00 | 0.37 ± 0.32 | 0.22 | <0.001 | 0.40 ± 0.32 | 0.35 ± 0.31 | 0.032 |
| IOP, mmHg | 10.0–25.0 | 16.7 ± 2.5 | 0.1 | <0.001 | 16.6 ± 2.4 | 16.8 ± 2.6 | 0.184 |
| Weight, kg | 19.0–112.0 | 47.2 ± 14.7 | 0.05 | <0.001 | 49.3 ± 17.0 | 45.5 ± 12.2 | <0.001 |
| Height, cm | 114.0–193.0 | 154.5 ± 15.0 | 0.08 | <0.001 | 155.5 ± 17.1 | 153.7 ± 12.8 | 0.096 |
| BMI, kg/m2 | 12.8–31.9 | 19.3 ± 3.5 | 0.06 | <0.001 | 19.7 ± 3.7 | 18.9 ± 3.2 | <0.001 |
AL = axial length, BMI = body mass index, IOP = intraocular pressure, SCT = subfoveal choroidal thickness, SER = spherical equivalent refraction, SRT = subfoveal retinal thickness; SST = subfoveal scleral thickness, UVA = uncorrected visual acuity.
K‐S, Kolmogorov–Smirnov test for normality.
Statistical significance was tested using t‐tests.
The results of Bland–Altman analyses are shown in Fig. 2. The intraobserver and interobserver mean differences for the SST were 9 μm (95% limits: −31 to 49 μm) and 5 μm (95% limits: −35 to 45 μm), respectively. The intraobserver and interobserver correlations of SST were 0.973 and 0.974 (both ps<0.001), respectively. As the mean thickness of sclera reached 524 ± 57 μm, the intraobserver and interobserver mean differences were considered to be acceptable (less than 2% of the scleral thickness) and the outer scleral borders drawn manually were reliable.
Figure 2.
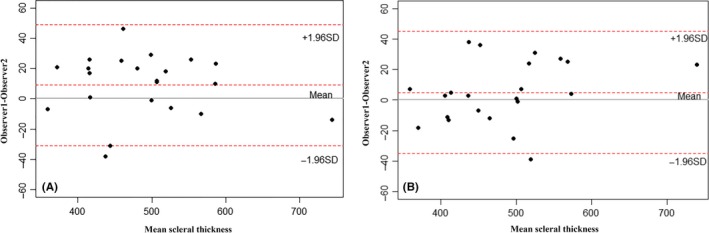
Bland–Altman plots show the intraobserver and interobserver agreements between the subfoveal scleral thickness measurements. (A) The intraobserver agreement between the subfoveal scleral thickness measurements. (B) The interobserver agreement between the subfoveal scleral thickness measurements.
Myopic refractive levels were associated with thinner subfoveal sclera and choroid layers
Next, we asked whether SST, SCT and SRT varied across the different refractive groups. As the sample size is large enough, a one‐way analysis of variance (anova) was also used in data that were not normally distributed. As shown in Table 3, myopes had significantly thinner sclera and choroid layers compared to emmetropes and hyperopes. No significant difference was observed between emmetropes and hyperopes in the sclera (Bonferroni method for post hoc test, p = 1.000) or in the choroid (Bonferroni method for post hoc test, p = 0.260), although the choroid seemed to be thicker in hyperopes (mean difference 14 μm). While myopes had a significantly larger SRT than hyperopes (Bonferroni method for post hoc test, p = 0.016), no significant difference in retinal thickness was observed between myopes and emmetropes. Similar to the SST and SCT, SRT did not differ between hyperopes and emmetropes. In the three subgroups of myopic children, both the SST and SCT were significantly smaller in high myopes than in mild or moderate myopes (all ps<0.001). In contrast, SRT was similar across levels of myopia, although SRT was statistically significant thicker in mild myopes than in high myopes (only with a mean difference of 7 μm) (p < 0.001) (Fig. 3). Taken together, these results suggest that the subfoveal thickness of sclera and choroid may get much thinner as myopic degree increases, while the subfoveal retinal thickness is similar in children with different myopic levels.
Table 3.
Thicknesses of the different layers for children with different refractive statuses (mean±SD)
| Refractive status | Mean SER, dioptres | n | SST, μm | SCT, μm | SRT, μm | SCT + SRT, μm | SST + SCT + SRT, μm |
|---|---|---|---|---|---|---|---|
| Hyperopes | 0.95 ± 0.42 | 78 | 556 ± 50 | 225 ± 46 | 218 ± 20 | 444 ± 50 | 999 ± 65 |
| Emmetropes | −0.04 ± 0.23 | 62 | 555 ± 50 | 211 ± 45 | 220 ± 18 | 431 ± 44 | 986 ± 56 |
| Myopes | −3.62 ± 2.07 | 670 | 517 ± 56 | 190 ± 49 | 225 ± 19 | 414 ± 51 | 931 ± 76 |
| F | 27.880 | 23.400 | 5.337 | 14.064 | 41.410 | ||
| pa | <0.001 | <0.001 | 0.005 | <0.001 | <0.001 | ||
| Myopes versus hyperopesb | <0.001 | <0.001 | 0.016 | <0.001 | <0.001 | ||
| Myopes versus emmetropesb | <0.001 | 0.002 | 0.151 | 0.037 | <0.001 | ||
| Hyperopes versus emmetropesb | 1.000 | 0.260 | 1.000 | 0.421 | 0.894 | ||
| Mild myopes | −1.83 ± 0.72 | 295 | 534 ± 51 | 199 ± 48 | 222 ± 18 | 421 ± 50 | 954 ± 69 |
| Moderate myopes | −3.89 ± 0.56 | 213 | 515 ± 53 | 191 ± 48 | 225 ± 19 | 416 ± 49 | 931 ± 71 |
| High myopes | −6.54 ± 1.35 | 162 | 491 ± 57 | 171 ± 45 | 229 ± 21 | 400 ± 51 | 890 ± 78 |
| F | 34.327 | 18.345 | 7.125 | 9.463 | 41.698 | ||
| pa | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| High myopes versus mild myopesb | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| High myopes versus moderate myopesb | <0.001 | <0.001 | 0.170 | 0.009 | <0.001 | ||
| Mild myopes versus moderate myopesb | <0.001 | 0.110 | 0.100 | 0.683 | <0.001 |
SCT = subfoveal choroidal thickness, SER = spherical equivalent refraction, SRT = subfoveal retinal thickness, SST = subfoveal scleral thickness.
A one‐way analysis of variance (anova) was used to determine whether the mean thicknesses differed across refractive statuses.
The Bonferroni method was used for post hoc tests.
Figure 3.
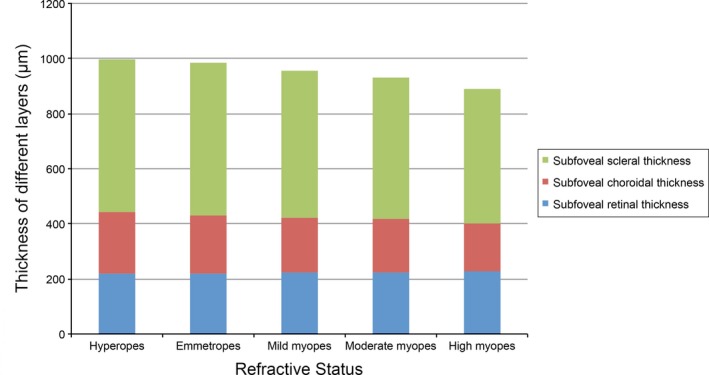
Average thicknesses for the different layers in participants with differing levels of refractive errors.
Factors related to the SER and AL
To determine the relationship between the subfoveal thickness of each layer and SER as well as AL, we further performed a simple linear regression analysis and a stepwise regression analysis. The simple linear regression analysis showed that the SER was positively associated with the SST and SCT and negatively associated with the SRT (Fig. 4A). In contrast, AL was positively associated with SRT and negatively associated with SST and SCT (Fig. 4B). Results from the stepwise regression analysis indicated that SST, SCT, age, gender and AL were independently associated with SER, whereas SRT was not included in the model (Table 4). The overall R 2 of the regression model was 0.699 (p < 0.001). More of the variation in SER could be explained by SST than by SCT. However, the factor that explained the greatest amount of variation in SER was AL (standard coefficients in the regression model: 0.110, 0.063 and −0.673 for SST, SCT and AL, respectively).
Figure 4.
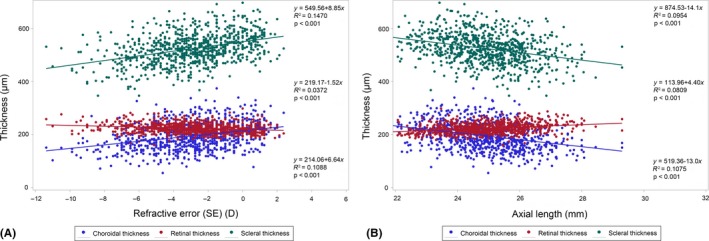
(A) Correlations between subfoveal scleral thickness, subfoveal choroidal thickness, subfoveal retinal thickness and refractive error. (B) Correlations between subfoveal scleral thickness, subfoveal choroidal thickness, subfoveal retinal thickness and axial length.
Table 4.
Systematic/ocular independent variables associated with spherical equivalent refraction (SER)
| Parameter | Unstandardized coefficients | Standardized coefficients | 95% CI | VIF | t value | p value |
|---|---|---|---|---|---|---|
| Intercept | 30.624 | 0 | 27.614, 33.633 | 0 | 19.97 | <0.001 |
| Age (yrs) | −0.130 | −0.162 | −0.171, −0.090 | 1.76 | −6.30 | <0.001 |
| Gender (1 = boys/2 = girls) | −0.901 | −0.183 | −1.110, −0.693 | 1.22 | −8.49 | <0.001 |
| AL (mm) | −1.329 | −0.673 | −1.431, −1.226 | 1.83 | −25.54 | <0.001 |
| IOP (mmHg) | 0.023 | 0.023 | −0.015, 0.061 | 1.02 | 1.19 | 0.235 |
| SST (μm) | 0.005 | 0.110 | 0.003, 0.007 | 1.18 | 5.20 | <0.001 |
| SCT (μm) | 0.003 | 0.063 | 0.001, 0.005 | 1.18 | 2.98 | 0.003 |
| SRT (μm) | −0.003 | −0.021 | −0.008, 0.002 | 1.12 | −1.02 | 0.309 |
| BMI (kg/m2) | −0.015 | −0.021 | −0.046, 0.016 | 1.33 | −0.94 | 0.348 |
AL = axial length, BMI = body mass index, IOP = intraocular pressure, SCT = subfoveal choroidal thickness, SRT = subfoveal retinal thickness, SST = subfoveal scleral thickness.
Stepwise regression model: F = 231.07, R 2 = 0.6993, P < 0.001.
Factors correlated with subfoveal scleral thickness
Next, we sought to determine which ocular factors may be related to SST. Spearman's correlation analysis demonstrated that SST was positively correlated with SER (r = 0.372, p < 0.001), as was the total thickness of the retina, choroid and sclera (r = 0.725, p < 0.001). In addition, SST was negatively correlated with AL (r = −0.312, p < 0.001). No significant correlation was observed between SST and SCT, SRT, the total thickness of the retina and choroid or intraocular pressure.
Further, we tried to find out the correlated demographic and physical factors of SST. Grouping children by age and sex indicated that the SST was thinner in older children, both for boys and for girls (Fig. 5). The mean SSTs for the age groups 6–8 years, 9–11 years, 12–14 years and 15–19 years were 553 ± 46 μm, 540 ± 51 μm, 520 ± 52 μm and 505 ± 62 μm, respectively. This conclusion was confirmed by correlation analysis (r = −0.287, p < 0.001). Additionally, SST was negatively correlated with sex (male = 1, female = 2, r = −0.071, p = 0.045), height (r = −0.232, p < 0.001), weight (r = −0.234, p < 0.001) and body mass index (BMI) (r = −0.168, p < 0.001).
Figure 5.
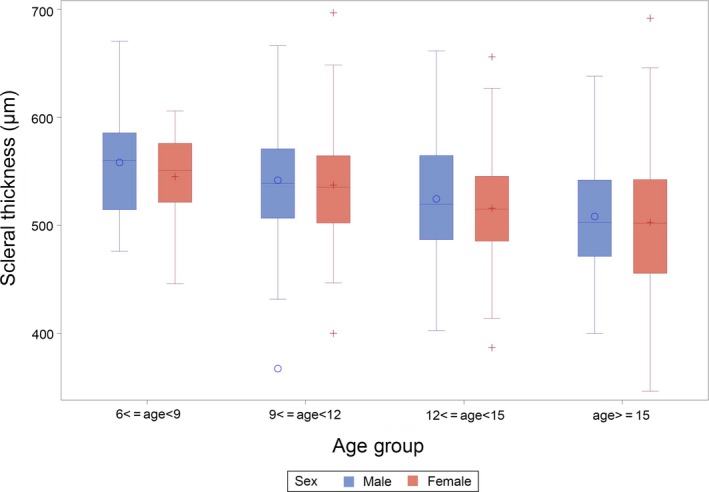
Subfoveal scleral thickness of children in different age groups.
Independent factors associated with subfoveal scleral, choroidal and retinal thicknesses
To further explore the independent factors associated with the subfoveal thickness of sclera, choroid and retina, we carried out a stepwise multiple regression analysis. Table 5 shows the independent factors that were related to the thickness of the sclera, choroid or retina. Among all the demographic, physical and ocular factors included in the stepwise multiple regression analysis, only age, SER, SCT and SRT were independently and significantly associated with SST. Subfoveal scleral thickness (SST) decreased as age and SCT increased, and increased as SER and SRT increased. According to the model, every 100‐μm decrease in SCT and SRT was associated with a 15‐μm increase in SST and a 24‐μm decrease in SST, and every 1 D unit of SER change was associated with an 8.8‐μm change in SST. Independent factors related to SCT included age, AL, SER and SST, whereas those related to SRT included AL, SST and sex, but not age or SER. The overall R 2 values given by the regression model for the sclera, choroid and retina were 0.168, 0.150 and 0.106, respectively.
Table 5.
Independent systematic/ocular variables associated with subfoveal scleral, choroidal and retinal thicknesses
| Independent variables | Unstandardized coefficients (95% CI) | Standardized coefficients | VIF | Variable's p value | Equation's p value | R 2 |
|---|---|---|---|---|---|---|
| Subfoveal scleral thickness (SST) | ||||||
| Intercept | 547 (498 to 597) | 0 | 0 | <0.001 | <0.001 | 0.168 |
| SCT | −0.15 (−0.22 to−0.07) | −0.13 | 1.15 | <0.001 | ||
| SRT | 0.24 (0.05 to 0.43) | 0.08 | 1.04 | 0.012 | ||
| SER | 8.8 (6.94 to 10.66) | 0.38 | 1.63 | <0.001 | ||
| Age | −1.85 (−3.32 to −0.39) | −0.1 | 1.57 | 0.013 | ||
| SCT | ||||||
| Intercept | 464 (361 to 566) | 0 | 1.55 | <0.001 | <0.001 | 0.15 |
| Age | −2.84 (−4.14 to −1.55) | −0.16 | 2.55 | 0.002 | ||
| AL | −6.49 (−10.56 to −2.42) | 0.15 | 3.02 | 0.008 | ||
| SER | 3.03 (0.79 to 5.28) | −0.14 | 1.18 | <0.001 | ||
| SST | −0.12 (−0.18 to −0.06) | −0.18 | 1.55 | <0.001 | ||
| SRT | ||||||
| Intercept | 109 (73 to 144) | 0 | 0 | <0.0001 | <0.001 | 0.106 |
| AL | 4.31 (3.20 to 5.42) | 0.28 | 1.2 | <0.0001 | ||
| SST | 0.03 (0.01 to 0.05) | 0.09 | 1.14 | 0.015 | ||
| Gender | −5.32 (−7.98 to −2.66) | −0.14 | 1.09 | <0.0001 | ||
AL = axial length, SCT = subfoveal choroidal thickness, SER = spherical equivalent refraction, SRT = subfoveal retinal thickness.
Discussion
To our knowledge, this study is the first to explore SST in a paediatric population using SS‐OCT and to consider different refraction levels. Our results indicated that in Chinese children and adolescents, the sclera and choroid layers are thinner in myopes than in emmetropes and hyperopes, but that neither differed between emmetropes and hyperopes. For the retinal layer, a significant difference was observed between myopes and hyperopes, while no significant difference was observed between emmetropes and hyperopes or between myopes and emmetropes. The SST accounted for more of the variation in SER than the SCT or SRT, and older age, myopic‐shifted SER, a thicker SCT and a thinner SRT were independently associated with a thinner SST. Additionally, older age and myopic‐shifted SER are common influencing factors of thinner SST and thinner SCT.
Thickness of the sclera
Our study showed that the subfoveal sclera was much thinner in the older age groups and thinner in girls than in boys. This might be related to age as the girls were significantly older than the boys. Also the differences between figures might lead to differences in the ocular parameters, as the boys were significantly heavier than the girls and the mean BMI of boys was also significantly larger.
Previous studies of older patients with severe myopia have reported that the mean SST ranged from 228 μm to 659 μm (Maruko et al. 2012; Ohno‐Matsui et al. 2012; Ellabban et al. 2013, 2014; Lopilly Park et al. 2014; Park et al. 2014). In our study, the mean SST of the high myopes was 491 μm, which falls within the range reported in these earlier adult studies, and it was thicker than most of the SSTs that have been reported. The differences between our study and those earlier studies may be due to different age, refractive levels and ethnicities, as older age and greater myopic level may lead to thinner sclera according to the regression model shown in Table 5. Further studies are needed to confirm our results.
Park et al. (2014) reported that the mean SST for adults with relatively mild myopia (mean age: 51.62 years, mean SER: −5.54 ± 4.86 D, median SER: −3.25D) was approximately 650 μm. In our study, the sclera of the children with mild and moderate myopia was thinner (534 μm and 515 μm, respectively). Variation between the results of the Park et al. study and ours may be due to different ethnicities. In addition, the sample size of the previous study was quite small (only 32 patients). So far, a majority of the studies to explore scleral thickness have been focused on highly myopic patients and few studies have been performed to explore the SST in relatively mild myopes. Further explorations are needed to confirm our results and to elucidate the changes in scleral thickness during the early development of myopia.
However, it is worth noting that in autopsied eyes, the mean scleral thickness at the posterior pole has been reported to be 0.7–0.9 mm, which is much thicker than the scleral thickness measured by SS‐OCT (Vurgese et al. 2012; Shen et al. 2015). These differences may be caused by tissue oedema after enucleation.
So far, results from different studies are not uniform. Differences in age, refractive level and ethnicity may lead to different scleral thickness and make comparison between these studies much more difficult. Thus, further investigation is needed to establish data sets of SST in vivo that cover all age groups, different refractive levels and different ethnicities.
The SST and SCT differed between myopic children and the other children
In our study, the SSTs of hyperopic and emmetropic children were thicker than that in myopic children, but were not different from each other (mean difference 1 μm). To our knowledge, this is the first study to report the SSTs in hyperopes and emmetropes. Results from our study indicated that the mean SST was relatively constant in hyperopes and emmetropes, but the SST would get thinner once the refraction status became myopic, even in childhood and in the early stage of myopia. It is a breakthrough as the previous studies that have reported thinner sclera in high myopes are the results many years after the onset of myopia, not the early stage. The specified pathogenesis of such changes during the early development of myopia is still unknown, maybe for the purpose of adapting the changes in refraction status and the increased AL. More research should be performed to find out the exact cut point and follow up the changes in the SSTs during the early development of myopia.
The SCT for the different refraction levels was similar to the SST. Although the SCT did not differ significantly between hyperopes and emmetropes, the absolute mean difference reached 14 μm (nearly 7% of the choroidal thickness), which could be argued to be a meaningful difference when one considers the small sample sizes of the hyperopic and emmetropic groups (78 hyperopes and 62 emmetropes, respectively). Indeed, we previously examined 276 children aged 7–13 years and determined that the mean central foveal choroidal thicknesses were 227 μm, 253 μm and 271 μm for myopes, emmetropes and hyperopes, respectively, and the differences between the emmetropic and hyperopic groups were significant (Jin et al. 2016). Read et al. reported that the SCTs in 104 children aged 10–15 years were 303 μm and 359 μm in myopes and nonmyopes, respectively. However, the results for emmetropes and hyperopes were not available for their study (Read et al. 2013).
In summary, these results indicate that the subfoveal thickness of both the sclera and the choroid may get thinner even in childhood and in the early stage of myopia. Moreover, the SCT may even get thinner when the refraction status shifts from hyperopia to emmetropia and this process continues as the refractive status becomes more myopic‐shifted.
Independent factors related to scleral thickness
Here, we tried to make a comparison between the factors found in our study that are related to scleral thickness and the factors from the previous ones. Our study indicated that SER, SCT, SRT and age were independent factors associated with SST. The SST decreased as age and SCT increased, and SST increased as SER and SRT increased.
AL was excluded from the regression model, although it had a correlation coefficient of −0.312 with SST when using Spearman's rank correlation coefficient analysis. Previous studies have also shown that AL was negatively correlated with scleral thickness (Ren et al. 2009; Maruko et al. 2012; Shen et al. 2015; Jin et al. 2016). However, Hayashi et al. (2013) reported that according to a simple regression analysis, AL was not statistically correlated with scleral thickness in highly myopic adults. Thus, taking the results from our study and those previous studies together, we may put forward a hypothesis that both children and adults may have thinner SSTs with the increase in AL. This process may start at the early stage of myopia when the AL gradually gets longer. To confirm this hypothesis, more research needs to be carried out in paediatric groups with very early stage of myopia and adult groups with different levels of myopia.
As with AL, the relationships between scleral thickness and age, SCT and SRT are also conflicting. Hayashi et al. (2013) found that age was not significantly correlated with scleral thickness in a simple regression analysis, and Shen et al. (2015) reported that a multivariate analysis showed that scleral thickness was independent of age for individuals older than 5 years. However, using univariate analyses, other researchers have found that age was negatively correlated with scleral thickness (Maruko et al. 2012; Ellabban et al. 2014), and Maruko et al. (2012) used a multiple linear regression analysis to show that age was negatively correlated with SST in adults. Maruko et al. (2012) also found that SCT was positively correlated with SST in both simple and multiple linear regression analyses, whereas Hayashi et al. (2013) performed simple regression analyses and found no statistical correlation between SCT and SST or between SRT and SST. In our study, simple regression analyses showed that both the SST and SCT were thinner in children with higher degree of myopia, while multiple linear regression analysis indicated that SST was negatively correlated with SCT. Such discrepancy may be because SE is just one of the influencing factors of SST and SCT, and other factors such as the growth and development of the ocular wall with increased age in children make the relationship between SST and SCT complicated. Thus, additional studies including variable age, refraction statuses and ethnicities are needed to further elucidate the related factors of SST, as most of the current studies are performed in highly myopic and adult patients solely.
Limitations
The current study has several limitations. First, although SS‐OCT is currently the optimal imaging tool, the posterior scleral border could not be accurately detected in a large number of participants, and thus, they were excluded from the final analysis. A large portion of the excluded children were hyperopic, emmetropic or mild myopic, and they might have thicker mean SST as compared with the mean SST of the 810 children. Thus, we might underestimate the mean SST. This issue may also partially explain the differences between our results and those from autopsy. However, this limitation does not influence the relationship between scleral thickness and other ocular parameters. Second, the present study was cross‐sectional; therefore, attribution of a causal relationship between scleral thickness and refractive error or other parameters must be made with caution. We will follow this cohort of paediatric patients over time, which may help to further elucidate the role that the sclera plays in the development of myopia. Third, the independent factors of SST were complicated and the R 2 of the current regression model was small, suggesting that other influencing factors of SST should be explored in future studies. In addition, linear model may not be the most suitable model to show such relationship. Nevertheless, linear model was relatively easy to understand for other clinicians and researchers. Other nonlinear models that can better explain such relationship need to be explored in the future. Finally, our findings may only be applicable to 6‐ to 19‐year‐old Chinese paediatric and adolescent patients. These results may not be applicable to children younger than 6 years or children from the other ethnic groups. Further studies should be carried out to compensate for such vacancies.
Conclusion
Despite the limitations, this study is the first to compare scleral thickness in a paediatric population with different refraction statuses, particularly in emmetropes and hyperopes, in vivo. Also, this is the first study to include all the three layers in the regression model and clarify the independent factors of SST, especially its relationship with SCT and SRT. This may be useful to elucidate the pathogenesis of myopia if confirmed by further studies.
This study was supported by the Three‐year Action Program of Shanghai Municipality for Strengthening the Construction of the Public Health System (2015–2017) (Grant No. GWIV‐13.2); the National Natural Science Foundation of China for Young Staff (Grant No. 81402695); the Shanghai Natural Science Foundation (Grant No. 15ZR1438400); the Key Discipline of Public Health–Eye Health in Shanghai (Grant No. 15GWZK0601); and the Overseas High‐end Research Team–Eye Health in Shanghai (Grant No. GWTD2015S08). The sponsor or funding organization had no role in the design or conduct of this research.
References
- Curtin BJ & Teng CC (1958): Scleral changes in pathological myopia. Trans Am Acad ophthalmol otolaryngol 62: 777–788. [PubMed] [Google Scholar]
- Curtin BJ, Iwamoto T & Renaldo DP (1979): Normal and staphylomatous sclera of high myopia. An electron microscopic study. Arch Ophthalmol 97: 912–915. [DOI] [PubMed] [Google Scholar]
- Ellabban AA, Tsujikawa A, Matsumoto A et al. (2013): Three‐dimensional tomographic features of Dome‐shaped macula by swept‐source optical coherence tomography. Am J Ophthalmol 155: 320–328. [DOI] [PubMed] [Google Scholar]
- Ellabban AA, Tsujikawa A, Muraoka Y et al. (2014): Dome‐shaped macular configuration: longitudinal changes in the sclera and choroid by swept‐source optical coherence tomography over two years. Am J Ophthalmol 158: 1062–1070. [DOI] [PubMed] [Google Scholar]
- Gentle A, Liu Y, Martin JE, Conti GL & McBrien NA (2003): Collagen gene expression and the altered accumulation of scleral collagen during the development of high myopia. J Biol Chem 278: 16587–16594. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Ito Y, Takahashi A, Kawano K & Terasaki H (2013): Scleral thickness in highly myopic eyes measured by enhanced depth imaging optical coherence tomography. Eye 27: 410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden BA, Fricke TR, Wilson DA et al. (2016): Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 123: 1036–1042. [DOI] [PubMed] [Google Scholar]
- Hsu WM, Cheng CY, Liu JH, Tsai SY & Chou P (2004): Prevalence and causes of visual impairment in an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology 111: 62–69. [DOI] [PubMed] [Google Scholar]
- Iwase A, Araie M, Tomidokoro A, Yamamoto T, Shimizu H, Kitazawa Y & Tajimi Study Group (2006): Prevalence and causes of low vision and blindness in a Japanese adult population: the Tajimi Study. Ophthalmology 113: 1354–1362. [DOI] [PubMed] [Google Scholar]
- Jin P, Zou H, Zhu J et al. (2016): Choroidal and retinal thickness in children with different refractive status measured by swept‐source optical coherence tomography. Am J Ophthalmol 168: 164–176. [DOI] [PubMed] [Google Scholar]
- Jonas JB & Xu L (2014): Histological changes of high axial myopia. Eye 28: 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LL, Shih YF, Hsiao CK & Chen CJ (2004): Prevalence of myopia in Taiwanese schoolchildren: 1983 to 2000. Ann Acad Med Singapore 33: 27–33. [PubMed] [Google Scholar]
- Lopilly Park HY, Lee NY, Choi JA & Park CK (2014): Measurement of scleral thickness using swept‐source optical coherence tomography in patients with Open‐Angle glaucoma and myopia. Am J Ophthalmol 157: 876–884. [DOI] [PubMed] [Google Scholar]
- Maruko I, Iida T, Sugano Y, Oyamada H, Akiba M & Sekiryu T (2012): Morphologic analysis in pathologic myopia using high‐penetration optical coherence tomography. Invest Ophthalmol Vis Sci 53: 3834–3838. [DOI] [PubMed] [Google Scholar]
- McBrien NA, Cornell LM & Gentle A (2001): Structural and ultrastructural changes to the sclera in a mammalian model of high myopia. Invest Ophthalmol Vis Sci 42: 2179–2187. [PubMed] [Google Scholar]
- Morgan IG, Ohno‐Matsui K & Saw SM (2012): Myopia. Lancet 379: 1739–1748. [DOI] [PubMed] [Google Scholar]
- Norton TT & Rada JA (1995): Reduced extracellular matrix in mammalian sclera with induced myopia. Vision Res 35: 1271–1281. [DOI] [PubMed] [Google Scholar]
- Ohno‐Matsui K, Akiba M, Modegi T, Tomita M, Ishibashi T, Tokoro T & Moriyama M (2012): Association between shape of sclera and myopic retinochoroidal lesions in patients with pathologic myopia. Invest Ophthalmol Vis Sci 53: 6046–6061. [DOI] [PubMed] [Google Scholar]
- Pan CW, Ramamurthy D & Saw SM (2012): Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt 32: 3–16. [DOI] [PubMed] [Google Scholar]
- Park HY, Shin HY & Park CK (2014): Imaging the posterior segment of the eye using swept‐source optical coherence tomography in myopic glaucoma eyes: comparison with enhanced‐depth imaging. Am J Ophthalmol 157: 550–557. [DOI] [PubMed] [Google Scholar]
- Phillips JR, Khalaj M & McBrien NA (2000): Induced myopia associated with increased scleral creep in chick and tree shrew eyes. Invest Ophthalmol Vis Sci 41: 2028–2034. [PubMed] [Google Scholar]
- Rada JA, Nickla DL & Troilo D (2000): Decreased proteoglycan synthesis associated with form deprivation myopia in mature primate eyes. Invest Ophthalmol Vis Sci 41: 2050–2058. [PubMed] [Google Scholar]
- Read SA, Collins MJ, Vincent SJ & Alonso‐Caneiro D (2013): Choroidal thickness in myopic and nonmyopic children assessed with enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci 54: 7578–7586. [DOI] [PubMed] [Google Scholar]
- Ren R, Wang N, Li B, Li L, Gao F, Xu X & Jonas JB (2009): Lamina cribrosa and peripapillary sclera histomorphometry in normal and advanced glaucomatous Chinese Eyes with various axial length. Invest Ophthalmol Vis Sci 50: 2175–2184. [DOI] [PubMed] [Google Scholar]
- Shen L, You QS, Xu X, Gao F, Zhang Z, Li B & Jonas JB (2015): Scleral thickness in Chinese eyes. Invest Ophthalmol Vis Sci 56: 2720–2727. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Koizumi H & Pozzoni MC (2008): Enhanced depth imaging spectral‐domain optical coherence tomography. Am J Ophthalmol 146: 496–500. [DOI] [PubMed] [Google Scholar]
- Sun J, Zhou J, Zhao P et al. (2012): High prevalence of myopia and high myopia in 5060 Chinese university students in Shanghai. Invest Ophthalmol Vis Sci 53: 7504–7509. [DOI] [PubMed] [Google Scholar]
- Unterhuber A, Povazay B, Hermann B, Sattmann H, Chavez‐Pirson A & Drexler W (2005): In vivo retinal optical coherence tomography at 1040 nm – enhanced penetration into the choroid. Opt Express 13: 3252–3258. [DOI] [PubMed] [Google Scholar]
- Verkicharla PK, Ohno‐Matsui K & Saw SM (2015): Current and predicted demographics of high myopia and an update of its associated pathological changes. Ophthalmic Physiol Opt 35: 465–475. [DOI] [PubMed] [Google Scholar]
- Vurgese S, Panda‐Jonas S & Jonas JB (2012): Scleral thickness in human eyes. PLoS One 7: e29692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Sun X, Zhou X & Weng C (2011): Causes and 3‐year‐incidence of blindness in Jing‐An district, Shanghai, China 2001–2009. BMC Ophthalmol 11: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Wang Y, Li Y, Wang Y, Cui T, Li J & Jonas JB (2006): Causes of blindness and visual impairment in urban and rural areas in Beijing: the Beijing Eye Study. Ophthalmology 113: e1–e11. [DOI] [PubMed] [Google Scholar]


